Abstract
The hypothesis that endogenous superoxide dismutase (SOD) protects the nitrergic transmitter from inactivation by superoxide and that this explains the lack of sensitivity of the transmitter to superoxide generators was tested in the rat isolated anococcygeus muscle.
Responses to nitrergic nerve stimulation or to NO were not significantly affected by exogenous SOD or by the Cu/Zn SOD inhibitor diethyldithiocarbamic acid (DETCA).
Hydroquinone produced a concentration-dependent reduction of responses to NO with an IC50 of 27 μM, and higher concentrations reduced relaxant responses to nitrergic nerve stimulation with an IC50 of 612 μM. The effects of hydroquinone were only slightly reversed by SOD, so it does not appear to be acting as a superoxide generator.
Pyrogallol produced a concentration-dependent reduction in responses to NO with an IC50 value of 39 μM and this effect was reversed by SOD (100–1000 u ml−1). Pyrogallol did not affect responses to nitrergic nerve stimulation. Treatment with DETCA did not alter the differentiating action of pyrogallol.
Duroquinone produced a concentration-dependent reduction of relaxations to NO with an IC50 value of 240 μM and 100 μM slightly decreased nitrergic relaxations. After treatment with DETCA, duroquinone produced greater reductions of relaxant responses to NO and to nitrergic stimulation, the IC50 values being 8.5 μM for NO and 40 μM for nitrergic nerve stimulation: these reductions were reversed by SOD.
The findings do not support the hypothesis that the presence of Cu/Zn SOD explains the greater susceptibility of NO than the nitrergic transmitter to the superoxide generator pyrogallol, but suggest that it may play a role in the effects of duroquinone.
Keywords: Anococcygeus muscle (rat), diethyldithiocarbamic acid (DETCA), duroquinone, hydroquinone, nitrergic transmission, nitric oxide, pyrogallol, superoxide dismutase
Introduction
There is indisputable evidence that the functional integrity of neuronal nitric oxide synthase (nNOS) is essential for nitrergic transmission and it is generally thought that this enzyme generates free radical nitric oxide (NO) from L-arginine; however, it is difficult to reconcile the postulate that the actual transmitter is NO since several substances differentiate between the actions of the nitrergic transmitter and exogenous NO (or at least an aqueous solution of nitric oxide gas) at a number of neuroeffector junctions (Rand & Li, 1995a,1995b). Thus, NO does not meet one of the essential criteria for acceptance as the transmitter, namely, that responses to the putative transmitter and those produced by nerve stimulation should be affected in comparable ways by various interacting agents.
Superoxide generators are one of the groups of differentiating agents. They rapidly inactivate NO and block responses to it and to endothelium derived relaxing factor (Rubyani & Vanhoutte, 1986). However, a number of superoxide generators do not affect responses to nitrergic nerve stimulation although responses to exogenous nitric oxide in the same tissues are blocked. This has been shown with pyrogallol in the rat gastric fundus (De Man et al., 1996), duroquinone in the mouse anococcygeus muscle (Lilley & Gibson, 1995) and rat gastric fundus (De Man et al., 1996), 7-ethoxyresorufin in the rat anococcygeus muscle (Li & Rand, 1996), LY83583 in the rat gastric fundus (Barbier & Lefebvre, 1992; Lefebvre, 1996), xanthine or hypoxanthine in the presence of xanthine oxidase in the mouse anococcygeus muscle (Gibson et al., 1994) and rat gastric fundus (Lefebvre, 1996), and hydroquinone, which may act as superoxide generator or NO-scavenger depending on conditions, in the bovine retractor penis muscle (Paisley & Martin, 1996), mouse anococcygeus muscle (Hobbs et al., 1991; Lilley & Gibson, 1995) and rat gastric fundus (Lefebvre, 1996).
It has been postulated that elimination of superoxide by endogenous superoxide dismutase (SOD) protects transmitter NO from inactivation since inhibition of Cu/Zn SOD with diethyldithiocarbamic acid (DETCA) brought about increases in the sensitivity of the transmitter to hydroquinone, duroquinone and LY83583 in the bovine retractor penis (Martin et al., 1994; Paisely & Martin, 1996) and mouse anococcygeus muscles (Lilley & Gibson, 1996), to pyrogallol and duroquinone in the rat gastric fundus (DE Man et al., 1996) and to pyrogallol in the rat anococcygeus muscle (Liu et al., 1997). However, Lefebvre (1996) showed that although DETCA slightly increased the transmitter-blocking action of LY83583, it did not affect that of hydroquinone or hypoxanthine : xanthine oxidase on the rat gastric fundus. Furthermore, DETCA treatment did not render the nitrergic transmitter in the mouse anococcygeus sensitive to xanthine : xanthine oxidase (Lilley & Gibson, 1996).
The aim of the present study was to determine whether DETCA affected the differentiating actions of the superoxide generators pyrogallol and duroquinone in the rat anococcygeus muscle; hydroquinone was also used but turned out not to be acting as a superoxide generator.
Methods
Tissue preparation and experimental protocols
Male Sprague-Dawley rats (300–450 g) were killed by decapitation and the anococcygeus muscles were removed and set up as previously described (Gillespie, 1972; Li & Rand, 1989). Briefly, muscles were mounted under a resting tension of 1 g and equilibrated for a period of 30 min before commencing experimental intervention. After the tone was raised by guanethidine (10–30 μM), and clonidine (0.05 μM) was added to help stabilize the contraction, relaxant responses to either nitric oxide in aqueous solution (NO) or electrical field stimulation (EFS; 1 ms pulses at 1 Hz for 10 s) were obtained. Once these responses had reached a steady extent, hydroquinone, pyrogallol, duroquinone or superoxide dismutase (SOD) was added in cumulatively increasing concentrations and the effect on NO- or EFS-induced relaxations determined. Each concentration was left in contact with the tissue for at least 20 min before the addition of the next concentration.
NO (0.4, 0.8 and 2 μM) was added in successively increasing concentrations at 1–3 min intervals, during which the relaxant action of the previous concentration of NO had completely dissipated. At least two concentration-response curves to NO were obtained before the addition of other agents and at least 5 min was allowed to elapse before constructing another concentration-response curve to NO.
Parallel experiments without addition of the substances tested were carried out on a second anococcygeus muscle from the same donor rat to serve as a time control in each set of experiments.
Inhibition of superoxide dismutase with DETCA
In some experiments, the consequences of inhibition of Cu/Zn superoxide dismutase on the effects of pyrogallol or duroquinone on EFS and NO were determined. In these, the anococcygeus was incubated with DETCA (3 mM) for 1 h followed by a washout period of 30 min. Then guanethidine and clonidine were added to the organ bath to raise the tone, as in other experiments. Responses to EFS (1–5 Hz) and NO (0.4–2 μM) were obtained, and once these had stabilized, the effects of pyrogallol (100 μM) or duroquinone (10–100 μM) on them were determined. Only one frequency of stimulation was used in each preparation. Parallel experiments without DETCA were carried out on a second muscle from the same donor rat to serve as a control.
Drugs and solutions
The PSS had the following composition (mM): NaCl, 118; KCl, 4.7; NaHCO3, 25; MgSO4, 0.45; KH2PO4, 1.03; CaCl2, 2.5; D-(+)-glucose, 11.1. The PSS was gassed with 5% CO2 and 95% O2 and maintained at 37°C.
The drugs used and their sources were: clonidine hydrochloride, guanethidine sulphate, diethyldithiocarbamic acid (DETCA), hydroquinone, pyrogallol, superoxide dismutase (Sigma, St. Louis, MO, U.S.A.); duroquinone (Aldrich Chemical Co., Castle Hill, NSW, Australia), nitric oxide (compressed gas; CIG, Melbourne, Victoria, Australia). Saturated aqueous solutions of NO were prepared from NO gas as previously described (Rajanayagam et al., 1993). All drugs were dissolved in distilled water prior to dilution with PSS with the exception of duroquinone, which was dissolved in 100% DMSO. The maximum concentration of DMSO in the organ bath was 0.01% v/v, which was without effect on responses to NO or EFS in control experiments.
Analysis of results
Responses to NO and EFS were expressed as reductions of tension. Data are expressed as means±s.e.means and n indicates the number of animals contributing tissues. Differences between means were assessed by Student's t-test (paired or unpaired, as appropriate) or analysis of variance (ANOVA; two-way or repeated measurements). Probability values of P<0.05 were considered significant.
The IC50 values for duroquinone, pyrogallol and hydroquinone were calculated from the falls in tension caused by the maximal concentrations of NO used (2 μM) or the maximal frequency of EFS plotted against increasing concentration of drugs. An IC50 value was determined for each experiment and the mean±s.e. of IC50 values was then calculated.
Ethical considerations
The experiments reported here were approved by the Animal Experimentation Ethics Committee of the Royal Melbourne Institute of Technology and conformed to the guidelines of the Australian National Health and Medical Research.
Results
SOD
Exogenous SOD (100–1000 u ml−1) had no effect on the tone of the anococcygeus muscle and did not significantly affect responses to EFS or NO.
DETCA
Incubation with DETCA (3 mM) for 1 h did not significantly alter the contraction induced by guanethidine and clonidine, which was 8.7±0.3 g (n=21) compared with 8.7±0.4 (n=21) in control untreated tissues, and did not significantly affect relaxant responses to NO or EFS.
Hydroquinone
Hydroquinone (10–100 μM) had no effect on tone but produced concentration-dependent reductions in responses to NO (Figure 1) with an IC50 value of 27±4.7 μM (n=5). A concentration of 100 μM had no effect on responses to EFS, but higher concentrations (300 and 1000 μM) significantly reduced them (Figure 2), the IC50 value for the reduction being 612±98 μM (n=6). Thus it had a 30 fold greater potency in discriminating against NO compared with the nitrergic transmitter.
Figure 1.

Effects of hydroquinone (n=4) and pyrogallol (n=5) on relaxant responses to NO (0.4–2 μM) in anococcygeus muscles in which tone was raised by guanethidine plus clonidine. Control responses are in the absence of pyrogallol or hydroquinone. Symbols represent means and I-bars are s.e.means. Symbols below * are significantly less than corresponding control value.
Figure 2.

Effects of hydroquinone on relaxant responses to electrical field stimulation (EFS, 1–5 Hz) in rat anococcygeus muscles in which tone was raised by guanethidine plus clonidine. Symbols represent means and I-bars are s.e.means (n=3–6). *Values in curves marked thus are significantly less than in control curve (ANOVA; P<0.05).
The reduction by hydroquinone (100 μM) of responses to NO was only slightly reversed by the addition of SOD (100–1000 u ml−1) and the reversal was only significant with the highest concentration of 1000 u ml−1 SOD (Figure 3). Thus it is unlikely to be acting predominantly as a superoxide generator in this situation.
Figure 3.
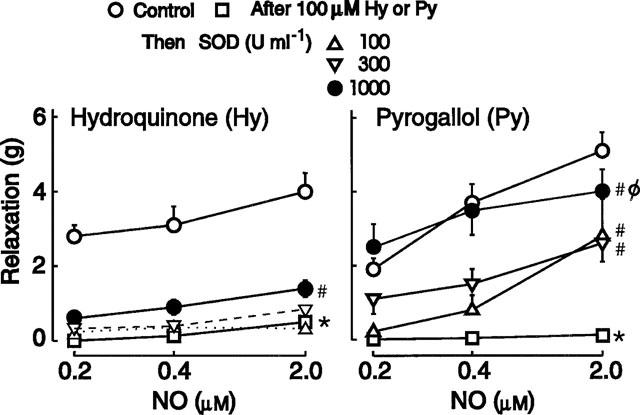
Relaxant responses to NO in the absence and presence of hydroquinone or pyrogallol (for each: 100 μM; n=4). Then increasing concentrations of SOD were added. In the left-hand panel, the responses in the presence of 100 and 300 u ml−1 of SOD are indicated by broken lines (.. and - - -, respectively). Symbols represent means and I-bars are s.e.means, which were smaller than the size of the symbol in some cases. *Values in these curves significantly less than control (ANOVA, P<0.01). #Values in these curves significantly greater than in absence of SOD (ANOVA, P<0.05). ΦCurve not significantly different from control (ANOVA, P>0.5).
Pyrogallol
Pyrogallol (10–100 μM) produced concentration-dependent reductions in responses to NO (Figure 1) with an IC50 value of 39±7 μM (n=5) but did not affect responses to EFS (1 Hz): the responses in its absence and in the presence of 10, 30 and 100 μM pyrogallol being (in g) 3.1±0.2, 3.3±0.4, 3.5±0.4 and 3.3±0.3 (n=6), respectively. Higher concentrations of pyrogallol were not used in this series of experiments as it generally produced spontaneous activity in the muscles and altered the basal tone.
The addition of SOD (100–1000 u ml−1) significantly reversed in a concentration-dependent manner the reduction by pyrogallol (100 μM) of responses to NO, and at a concentration of 1000 u ml−1 of SOD the responses to NO were restored to the control level (Figure 3).
Treatment with DETCA did not alter the differentiating action of pyrogallol (100 μM) in that the response to EFS (1 Hz) was not significantly changed and the response to NO (0.4 μM) was reduced to the same extent as in the absence of DETCA-pretreatment (Figure 4).
Figure 4.
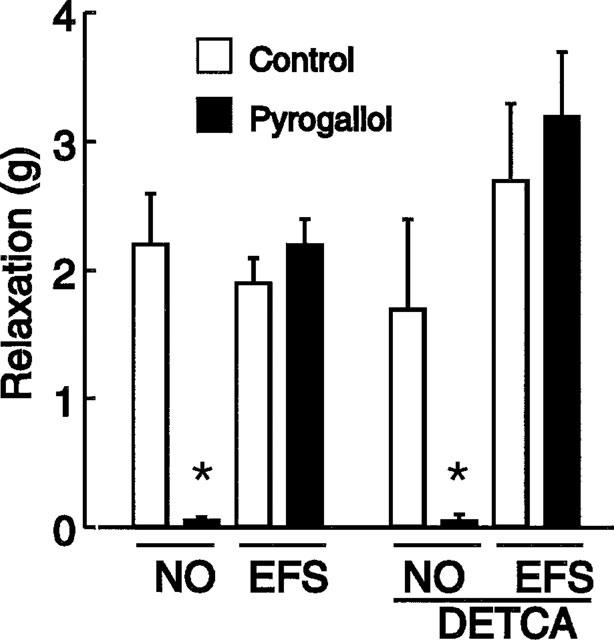
Effects of pyrogallol (100 μM) on relaxant responses to electrical field stimulation (EFS, 1 Hz) and NO (0.4 μM) in the absence of (left columns) and after treatment with DETCA (3 mM) (right columns). Columns represent means and I-bars are s.e.means, n=4. *Indicates significantly less than corresponding control (paired t-test; P<0.01).
Duroquinone
In time control experiments, the solvent for duroquinone, dimethylsulphoxide, had no effect on relaxations elicited by NO or EFS. Duroquinone had no effect in low concentrations (10 and 30 μM), but the highest concentration used (100 μM) produced significant reductions of responses to NO (Figure 5a) and EFS (Figure 5b). The estimated IC50 of duroquinone against NO was 240±56 μM (n=4).
Figure 5.
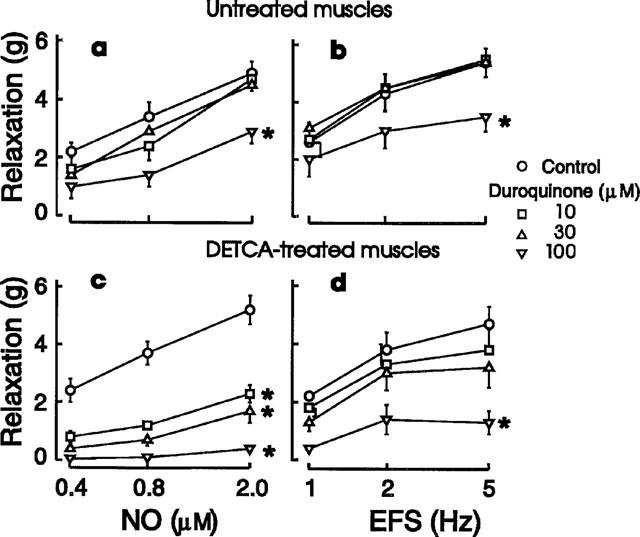
Effects of duroquinone on relaxant responses to NO (a and c, 0.4–2 μM) and electrical field stimulation (EFS: b and d, 1–5 Hz) in control (a and b) and DETCA-treated muscles (c and d). Symbol represents means and I-bars are s.e.means, n=5 for a and c, and n=4 for b and d. *Curves significantly different from control (ANOVA, P<0.01).
Treatment with DETCA enhanced the blocking action of duroquinone on both NO (Figure 5c) and EFS (Figure 5d). The IC50 values were 8.5±0.9 μM (n=4) for NO and 40±7 μM (n=5) for EFS. The reductions of responses to NO and EFS (1 Hz) produced by duroquinone after treatment with DETCA were partially reversed by the addition of exogenous SOD (1000 u ml−1) (Figure 6).
Figure 6.
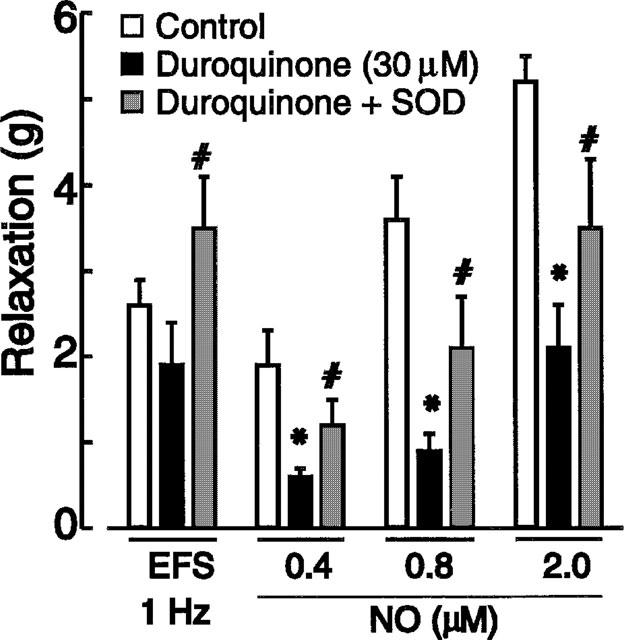
Effects of 30 μM duroquinone in DETCA-treated muscles in the absence and presence of 1000 u ml−1 SOD on relaxant responses to electrical field stimulation (EFS, 1 Hz; n=3) and NO (n=4). Column heights represent means and I-bars are s.e.means. *Indicates significant reduction from control. #Indicates significant increase produced by SOD.
Discussion
The addition of exogenous SOD had no significant effect on responses to NO or to the nitrergic transmitter in the rat anococcygeus muscle in the present work and as reported by Liu et al. (1997), or on rat gastric fundus strips (Lefebvre, 1996); therefore, if endogenous SOD does have an influence, its activity must be maximal. However, DETCA, which inhibits extracellular and intracellular endogenous Cu/Zn SOD (Kelner et al., 1989) also had no effect on these responses. Liu et al. (1997) reported that DETCA slightly reduced EFS-induced relaxations from 61.5±10.5% (n=10) to 51.7±10.8% (n=10), but this difference is not statistically significant when tested by unpaired t-test (P=0.52). These findings suggest either that the anococcygeus muscle does not normally produce superoxide or that the endogenous Cu/Zn SOD which is present in nitrergic nerves in the rat anococcygeus muscle (Liu et al., 1997) has little or no influence on exogenous NO or the nitrergic transmitter. However, in addition to Cu/Zn SOD, as De Man et al. (1996) pointed out, there is a mitochondrial Mn SOD that is not inhibited by DETCA and is present in the rat gastrointestinal tract muscle and nerve cells, where it is colocalized with NADPH diaphorase, a marker for NO synthase (Ceilley et al., 1995; Fang & Christensen, 1995). Although the anococcygeus muscle is not part of the gastrointestinal tract, it may nevertheless contain Mn SOD and the effect of inhibiting this enzyme remains to be tested.
Hydroquinone (10–100 μM) had no effect on nitrergic transmission but significantly reduced responses to exogenous NO in a concentration-dependent manner, being slightly more potent than pyrogallol. In contrast to the finding with pyrogallol, the effect of hydroquinone was only slightly reversed by SOD, even at a high concentration. This finding is in agreement with the view of Hobbs et al. (1991), Lefebvre (1996) and Lilley & Gibson (1996) that hydroquinone in this situation acts mainly as a free radical scavenger rather than as a superoxide generator. For this reason, the effect of inhibiting Cu/Zn SOD with DETCA on the activity of hydroquinone was not investigated.
Pyrogallol significantly reduced responses to exogenous NO in a concentration-dependent manner and this effect was reversed by addition of SOD, thus supporting the view that the reduction was due to the superoxide-generating activity of pyrogallol. However, pyrogallol (100 μM) had no effect on nitrergic transmission, and Liu et al. (1997) also found that pyrogallol (30 μM) had no effect on nitrergic relaxations of the rat anococcygeus muscle. These findings are in contrast with those of Gillespie & Sheng (1990) using the same tissue, who reported that 30 μM pyrogallol significantly reduced nitrergic relaxation at frequencies above 1 Hz and 100 μM significantly reduced those at frequencies above 0.5 Hz. We can offer no explanation for the discrepancy.
In the present experiments, inhibition of endogenous Cu/Zn SOD with DETCA did not significantly alter the effects of pyrogallol on NO or EFS-induced relaxations. In similar experiments with DETCA-treated rat anococcygeus muscles, Liu et al. (1997) reported that EFS-induced relaxations were 51.7±10.8% (n=10) before pyrogallol and were reduced about 40% to 30.7±7.6% (n=7) in the presence of pyrogallol. Although the difference they reported is not statistically significant by unpaired t-test; P=0.17, it may nevertheless have been a real effect since it was reversed by SOD. In contrast, we observed that pyrogallol produced a slight, but not significant increase in EFS-induced relaxations after DETCA treatment. The possible discrepancy between our findings and those of Liu et al. (1997) would require further experiments for its resolution.
Duroquinone weakly reduced responses to NO with an IC50 value of 240±56 μM, but this effect was markedly enhanced after treatment with DETCA, the IC50 value then being 8.5±0.9 μM. Treatment with DETCA likewise enhanced the reduction of responses to nitrergic nerve stimulation, the IC50 value being 40±7 μM. However, it should be noted that duroquinone still differentiated quantitatively between relaxant responses to EFS and NO after treatment with DETCA with a 4.7 fold difference in IC50 values. The findings provide some support for the hypothesis that the relative resistance of the nitrergic transmitter in the rat anococcygeus muscle to duroquinone could be due to elimination of the superoxide generated by it by Cu/Zn SOD, but this does not account fully for the differentiating action.
Recently, Lilley & Gibson (1997) reported that ascorbate is released by EFS, and it is possible that it acts as an antioxidant to protect NO from attack by scavenger molecules, and might modify the effects of superoxide generators on nitrergic transmission and NO-induced relaxations. Furthermore, the local concentration of neuronal ascorbate might preferentially protect the nitrergic transmitter. On the other hand, we have not observed any differences in responses to NO or EFS between experiments in which ascorbate (0.14 mM) was present or absent (unpublished data).
One explanation for the differential actions of the superoxide generator pyrogallol and the free radical scavenger hydroquinone is that the nitrergic transmitter is not NO but may be a NO-donating species. Another view is that it is NO but it is not appreciably affected by inactivating agents in the short time it would take for NO to diffuse from its site of generation to its site of action (Wood & Garthwaite, 1994). However, the rate of reaction of NO with superoxide to form peroxynitrite (ONOO−) is 6.7×109 M−1 s−1, and is described as near-diffusion limited (Huie & Padmaja, 1993). Furthermore, the interaction between NO and superoxide to produce peroxynitrite occurs 3.5 times faster than the dismutation of superoxide by SOD (Beckman & Koppenol, 1996). Thus, it is likely that any NO acting as a transmitter would be appreciably affected by superoxide, even in the presence of SOD, in its rapid diffusion across the neuroeffector junction from its site of formation to its site of action. This favours the view that the transmitter is not NO, but a contribution of the effect envisaged by Wood & Garthwaite (1994) can not be excluded.
It has recently been suggested that the product of NOS is the nitrosyl anion (NO−) rather than free radical NO (Schmidt et al., 1996). If the nitrergic transmitter were nitrosyl, it would not be expected to be scavenged by hydroquinone or to react rapidly with free radical superoxide generated by pyrogallol or duroquinone. It is of some interest that Schmidt et al. (1996) found that the Cu (II) of SOD reacts with the nitrosyl ion to form Cu (I) and NO free radical. Since the SOD inhibitor DETCA is a copper chelator, it may stabilize nitrosyl ions acting as the nitrergic transmitter.
Acknowledgments
This work was supported by grants from the Smoking & Health Research Foundation of Australia and the National Health & Medical Research Council.
Abbreviations
- ANOVA
analysis of variance
- DETCA
diethyldithiocarbamic acid
- DMSO
dimethylsulphoxide
- EFS
electrical field stimulation
- NADPH
nicotinamide dinucleotide phosphate (reduced)
- NO
nitric oxide
- NOS
nitric oxide synthase
- SOD
superoxide dismutase
References
- BARBIER A.J.M., LEFEBVRE R.A. Effects of LY83583 on relaxation induced by non-adrenergic non-cholinergic nerve stimulation and exogenous nitric oxide in the rat gastric fundus. Eur. J. Pharmacol. 1992;219:1280–1286. doi: 10.1016/0014-2999(92)90315-u. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide and peroxynitrite: the good, the bad and the ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- CEILLEY J.W., FANG S., CHRISTENSEN J. Colocalizations of Mn superoxide dismutase (SOD) and nitric oxide (NO) by combined diaphorase biochemistry and ABC immunostaining in enteric nerves. Gastroenterology. 1995;108:A579. [Google Scholar]
- De MAN J.G., De WINTER B.Y., BOECKXSTAENS G.E., HERMAN A.G., PELCKMANS P.A. Effect of thiol modulators and Cu/Zn superoxide dismutase inhibition on nitrergic relaxations in the rat gastric fundus. Br. J. Pharmacol. 1996;119:1022–1028. doi: 10.1111/j.1476-5381.1996.tb15773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANG S., CHRISTENSEN J. Manganese superoxide dismutase and reduced nicotinamide adenine dinucleotide diaphorase colocalize in the rat gut. Gastroenterology. 1995;109:1429–1436. doi: 10.1016/0016-5085(95)90627-4. [DOI] [PubMed] [Google Scholar]
- GIBSON A., BRAVE S.R., TUCKER J.F. Differential effect of xanthine : xanthine oxidase on NANC and NO-induced relaxations of the mouse anococcygeus. Can. J. Physiol. Pharmacol. 1994. p. 475.
- GILLESPIE J.S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br. J. Pharmacol. 1972;45:404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J.S., SHENG H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br. J. Pharmacol. 1990;99:194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBBS A.J., TUCKER J.F., GIBSON A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br. J. Pharmacol. 1991;104:145–152. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Rad. Res. Comm. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- KELNER M.J., BAGNELL R., HALE B., ALEXANDER N.M. Inactivation of intracellular copper-zinc superoxide dismutase by copper chelating agents without glutathione depletion and methemoglobin formation. Free Rad. Biol. Med. 1989;6:355–360. doi: 10.1016/0891-5849(89)90079-8. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Influence of superoxide dismutase inhibition on the discrimination between NO and the nitrergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 1996;118:2171–2177. doi: 10.1111/j.1476-5381.1996.tb15659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin. Exp. Pharmacol. Physiol. 1989;16:933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Inhibition of NO-mediated responses by 7-ethoxyresorufin, a substrate and competitive inhibitor of cytochrome P450. Br. J. Pharmacol. 1996;118:57–62. doi: 10.1111/j.1476-5381.1996.tb15366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Inhibition of relaxations to nitrergic stimulation in the mouse anococcygeus by duroquinone. Br. J. Pharmacol. 1995;116:3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus against inhibition of superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of the antioxidant ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU X., MILLER S.M., SZURSZEWSKI J.H. Protection of nitrergic transmission by colocalization of neural nitric oxide synthase with copper zinc superoxide dismutase. J. Auton. Nerv. Syst. 1997;62:126–133. doi: 10.1016/s0165-1838(96)00113-0. [DOI] [PubMed] [Google Scholar]
- MARTIN W., MCALLISTER K.H.M., PAISLEY K. NANC neurotransmission in the bovine retractor penis is blocked by superoxide anion following inhibition of superoxide dismutase with diethylthiocarbamate. Neuropharmacology. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- PAISLEY K., MARTIN W. Blockade of nitrergic transmission by hydroquinone, bydroxocobalamin and carboxy-PTIO in bovine retractor penis: role of superoxide anion. Br. J. Pharmacol. 1996;117:1633–1638. doi: 10.1111/j.1476-5381.1996.tb15333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJANAYAGAM M.A.S., LI C.G., RAND M.J. Differential effects of hydroxocobalamin on NO-mediated relaxations is rat aorta and anococcygeus muscle. Br. J. Pharmacol. 1993;108:3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Ann. Rev. Physiol. 1995a;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G.Nitric oxide in the autonomic and enteric nervous systems Nitric Oxide in the Nervous System 1995bAcademic Press: London; 228–279.ed. Vincent S.R. pp [Google Scholar]
- RUBYANI G.M., VANHOUTTE P. M. Superoxide and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., HOFMANN H., SCHINDLER U., SHUTENKO Z.S., CUNNINGHAM D.D., FEELISCH M. No•NO from NO synthase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD J., GARTHWAITE J. Models of diffusional spread of nitric oxide (NO): Implications for neuronal NO signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]


