Abstract
The substrate of nitric oxide synthase (NOS), L-arginine (L-Arg, 0.01 μM−1 mM), induced endothelium-independent relaxations in segments of middle cerebral arteries (MCAs) from normotensive Wistar-Kyoto (WKY) and hypertensive rats (SHR) precontracted with prostaglandin F2α (PGF2α). These relaxations were higher in SHR than WKY arteries.
L-NG-nitroarginine methyl ester (L-NAME) and 2-amine-5,6-dihydro-6-methyl-4H-1,3-tiazine (AMT), unspecific and inducible NOS (iNOS) inhibitors, respectively, reduced those relaxations, specially in SHR.
Four- and seven-hours incubation with dexamethasone reduced the relaxations in MCAs from WKY and SHR, respectively.
Polymyxin B and calphostin C, protein kinase C (PKC) inhibitors, reduced the L-Arg-induced relaxation.
Lipopolysaccharide (LPS, 7 h incubation) unaltered and inhibited these relaxations in WKY and SHR segments, respectively. LPS antagonized the effect polymyxin B in WKY and potentiated L-Arg-induced relaxations in SHR in the presence of polymyxin B.
The contraction induced by PGF2α was greater in SHR than WKY arteries. This contraction was potentiated by dexamethasone and polymyxin B although the effect of polymyxin B was higher in SHR segments. LPS reduced that contraction and antagonized dexamethasone- and polymyxin B-induced potentiation, these effects being greater in arteries from SHR.
These results suggest that in MCAs: (1) the induction of iNOS participates in the L-Arg relaxation and modulates the contraction to PGF2α; (2) that induction is partially mediated by a PKC-dependent mechanism; and (3) the involvement of iNOS in such responses is greater in the hypertensive strain.
Keywords: Rat middle cerebral artery, inducible NO synthase, L-arginine, lipopolysaccharide, dexamethasone, polymyxin B, protein kinase C
Introduction
The generation of nitric oxide (NO) from L-arginine (L-Arg) by NO synthase (NOS) plays an essential role in the regulation of cerebrovascular tone under both physiological and pathological conditions (Faraci, 1993; Iadecola et al., 1994). Three distinct genes encoding NOS isoforms have been cloned (Moncada & Higgs, 1993; Faraci & Brian, 1994; Marín & Rodríguez-Martínez, 1997). In cerebral arteries, the NOS constitutive (cNOS), present in endothelium and perivascular nerves (Katusic et al., 1993; Chen & Lee, 1995), produce NO in basal conditions and in response to several physiological stimuli (Faraci, 1993). In contrast, the inducible form of NOS (iNOS) produces greater quantities of NO for long periods in response to bacterial lipopolysaccharide (LPS) and cytokines in smooth muscle, macrophages, neutrophils and endothelium (Faraci & Brian, 1994; Marín & Rodríguez-Martínez, 1997).
Because of the role of NO in the physiological control of blood pressure, alterations in NO synthesis may be an important factor in the pathogenesis of hypertension (Moncada et al., 1991; Welch & Loscalzo, 1994; Marín & Rodríguez-Martínez, 1997). Thus, an impairment of endothelium-dependent relaxations in some vessels, including the cerebral ones, from spontaneously hypertensive rats (SHR) has been reported (see Marín & Rodríguez-Martínez, 1997). This suggests that endothelial function could be affected in hypertension. Moreover, it has been reported that L-Arg induces inhibition of vasoconstriction and relaxation of precontracted vessels by an endothelium-independent mechanism mediated by NO formation (Schini & Vanhoutte, 1991; Moritoki et al., 1992; Jovanovic et al., 1994; Laing et al., 1995; Alonso et al., 1998). The NOS isoform responsible for this NO production has been suggested to be of inducible type (Moritoki et al., 1992; Jovanovic et al., 1994; Laing et al., 1995; Alonso et al., 1998). The involvement of the iNOS in the physiological control of blood pressure is still unclear. Some authors have described an increase of both basal iNOS expression and activity in SHR (Junquero et al., 1992; Wu et al., 1996; Chou et al., 1998) probably to compensate the blood pressure increase. However, other investigators have suggested that although iNOS expression is similar in vascular smooth muscle of SHR and Wistar-Kyoto (WKY) rats, there are differences in the regulation of transcription of iNOS mRNA (Singh et al., 1996). It has been also proposed that changes in vascular tone regulation observed in hypertension, may be due to a deficiency of NOS substrate, as L-Arg supplied to hypertensive patients reduces the high blood pressure (Marín & Rodríguez-Martínez, 1997). It is also possible that these changes are due to an alteration of iNOS that modifies its ability to synthesize NO from L-Arg in arteries subjected to hypertension, a mechanism still unexplored in cerebral vessels.
The aim of the present study was to further study the capacity of L-Arg to induce relaxations in segments of the middle cerebral artery (MCA) from WKY and SHR and the mechanisms involved in such responses, specially the iNOS involvement.
Methods
Male WKY and SHR 6-month-old rats were obtained from colonies maintained at the Animal Quarter of the Facultad de Medicina of the Universidad Autonoma of Madrid. Rats were anaesthetized with diethyl ether (Panreac, Barcelona, Spain) and systolic arterial pressure was registered by cannulating the carotid artery, and connecting the cannula to a pressure transducer (Letica, Barcelona, Spain) and this in turn to a recorder (Letica Polygraph 2006). The average systolic pressure of the animals (mean±s.e.mean in mm of Hg) was 169.7±6.9 and 111.7±20.8 for SHR and WKY rats, respectively (P<0.05). The rats were sacrificed by bleeding and the brain removed and immersed in cold Krebs-Henseleit solution (KHS) bubbled with a 95% O2–5% CO2 mixture. Then, the right and left MCAs were carefully dissected, divided in segments of 2 mm in length and transferred to the organ bath of a dual isometric myograph (JP-Trading, Denmark). Two tungsten wires (40 μm diameter) were introduced through the lumen of the segments and mounted according to the method described by Mulvany & Halpern (1976).
After a 30 min equilibration period in oxygenated KHS at 37°C and pH=7.4, segments were stretched to their optimal lumen diameter for active tension development. This was determined based on the internal circumference-wall tension ratio of the segments by setting their internal circumference, Lo, to 90% of which the vessels would have had if they were exposed to a passive tension equivalent to that produced by a transmural pressure of 100 mm Hg (Mulvany & Halpern, 1977). The effective lumen diameter was calculated as Lo π−1.
Afterwards, segments were washed three times and left to equilibrate for 30 min; then, segments contractility was tested by an initial exposure to a high K+ solution (120 mM K+-KHS).
At the end of the experiment, segments were washed and when basal tone was reached, KHS was replaced by 120 mM K+-KHS; once the contraction was stable, 0.1 mM papaverine was added.
The presence of endothelium was determined by the ability of 1 μM bradykinin (BK) to produce relaxation in segments precontracted with 10 μM prostaglandin F2α (PGF2α). In some experiments, endothelium was removed by the technique described by Osol et al. (1989). A human hair was washed in ethanol and rinsed in KHS before being inserted into the lumen of the segment mounted under tension in the myograph.
Experimental design
Once determined the presence of endothelium, rings were contracted with 10 μM PGF2α and after a stable plateau was reached, a cumulative concentration-response curve to L-Arg (0.01 μM–1 mM) was performed in the presence or absence of different drugs. Steady-state response at each concentration was reached at 3 min, and this was the response measured. Only one concentration-response curve to L-Arg was achieved with each segment because of the reduction of responses in successive concentration-response curves. Control and treated rings obtained from the same animal were studied in parallel.
To determine the role of prostanoids or superoxide anions on the response induced by L-Arg, segments were incubated for 30 min with indomethacin, a cyclo-oxygenase inhibitor, or with the scavenger of these anions, superoxide dismutase (SOD) before the concentration-response curve to L-Arg was performed.
To assess the involvement of NO on the response to L-Arg, some segments were incubated for 30 min with the unspecific NOS inhibitor L-NG-nitroarginine methyl ester (L-NAME) or with the selective inhibitor of iNOS, 2-amine-5,6-dihydro-6-methyl-4H-1,3-tiazine (AMT), before the concentration-response curve to L-Arg was performed.
The participation of iNOS on this response was also checked by incubation of segments with dexamethasone, a compound able to inhibit NOS induction or with LPS, a stimulator of iNOS. The involvement of PKC was checked by incubation with polymyxin B or calphostin C, inhibitors of this enzyme. To test the hypothesis that small contaminant endotoxin concentrations in KHS can contribute to the relaxation induced by L-Arg, experiments were performed in the presence of gentamycin plus ampicillin. The ability of LPS to reverse the effect of dexamethasone or polymyxin B was also tested in experiments in which it was added associated with dexamethasone or polymyxin B. Dexamethasone, LPS, polymyxin B and gentamycin plus ampicillin were added to the bath since the time the brain was removed from the animal and maintained throughout the experiment. The period of incubation of the arteries with these drugs, before the concentration-response curve to L-Arg, was of 4 or 7 h. Calphostin C was added to the bath 1 h before the curve to L-Arg; higher incubation times were not used because of the marked inhibition of contractile responses.
Data analysis and statistics
Vasoconstrictor responses to PGF2α were expressed as active wall tension (calculated as the increase in vessel wall force above resting level divided by twice the vessel segment length), as a percentage of the maximum contraction (determined by the difference between the tone generated by 120 mM K+ and that produced by 0.1 mM papaverine) or as a percentage of the corresponding parallel control response. Vasodilator responses to L-Arg were expressed as a percentage of the previous tone induced by PGF2α. The concentrations of L-Arg producing 50% of maximum response (EC50 values) were calculated from individual concentration-response curves according to the method of Fleming et al. (1972). The concentration-ratio (EC50 value in the presence of antagonist/EC50 in the absence of antagonist) was also determined.
Results are expressed as mean±s.e.mean of the number of arterial segments indicated in each case. Statistically significant differences were calculated by means of Student's t-test for paired or unpaired experiments, with Bonferroni corrections for multiple comparisons, or by two way analysis of variance (ANOVA) to compare groups; a probability value of less than 5% was considered significant. At least four rats were used in each set of reactivity experiments.
Drugs and solutions
KHS contained (mM): NaCl 115, NaHCO3 25, KCl 4.7, MgSO4.7H2O 1.2, CaCl2 2.5, KH2PO4 1.2, glucose 11.1 and Na2EDTA 0.01. The high K+ solution was identical to KHS except that NaCl was replaced by KCl in an equimolar basis. Drugs used were: PGF2α tris salt, L-Arg hydrochloride, D-Arg hydrochloride, calphostin C, dexamethasone 21-acetate, BK acetate, indomethacin, L-NAME dihydrochloride, SOD, papaverine hydrochloride, LPS (Escherichia coli, serotype 055:B5), polymyxin B sulphate and ampicillin sodium salt (Sigma Chemical, Co., St. Louis, MO, U.S.A.), gentamycin sulphate (Biological Industries, Kibbutz Beit-Haemek, Israel) and AMT hydrochloride (Research Biochemicals International, Natick, MA, U.S.A.). Drug solutions were made in bidistilled water, except PGF2α which was dissolved in absolute ethanol, calphostin C in dimethylsulphoxide and indomethacin in water containing sodium carbonate 1.5 mM; these solvents did not modify basal tone or the vasoconstrictor responses. Stock solutions were kept at −20°C, and appropriate dilutions were made on the day of the experiment.
Results
Data of effective lumen diameter, maximum response, contraction induced by the first administration of 10 μM PGF2α and relaxation induced by 1 μM BK in segments of MCAs from WKY and SHR are shown in Table 1. The effective lumen diameter, maximum response and vasoconstriction induced by PGF2α were higher in segments from normotensive than hypertensive strain, whereas the relaxation elicited by BK was similar in both strains. The increase in contractions to PGF2α in segments from WKY was also observed when the responses were compared in active wall tension values (mN mm−1, Table 1). Successive administration of PGF2α decreased contractile responses in a similar degree in normotensive and hypertensive strain (Figure 1A). Thus, after 7 h incubation, the responses to PGF2α were of 73.6±5.0% and 74.3±4.0% of the initial value, in the normotensive and hypertensive strain (P>0.05), respectively.
Table 1.
Data of effective lumen diameter, maximum response and responses to PGF2α and BK in segments of MCA from WKY and SHR

Figure 1.
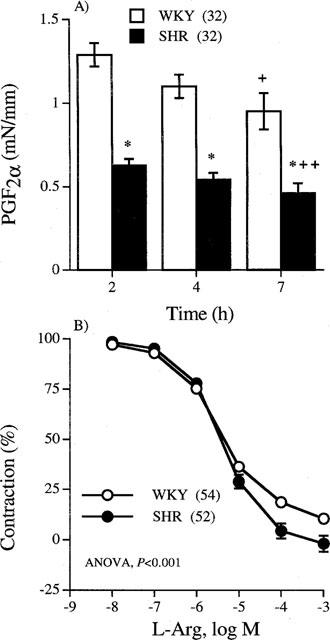
(A) Vasoconstrictor responses (mN mm−1) induced by successive administration of 10 μM PGF2α at different incubation times. (B) Concentration-response curves to L-Arg in middle cerebral arteries from WKY and SHR previously contracted with 10 μM PGF2α. Results (mean±s.e.mean) are expressed as active wall tension (mN mm−1) or as a percentage of the contraction induced by PGF2α. Number of arterial segments is indicated in parentheses. *P<0.05 vs WKY. +P<0.05 and ++P<0.01 vs response at 2 h (paired Student's t-test).
Effect of L-arginine
L-Arg (0.01 μM–1 mM) induced concentration-dependent relaxations in MCAs from both WKY and SHR precontracted with 10 μM PGF2α (Figure 1B). The EC50 values were not different in arteries from both strains [WKY: 2.8 (2.3–3.4) μM; SHR: 3.7 (2.9–4.8) μM, in parentheses 95% confidence interval], but the maximum relaxation evoked by L-Arg was greater in segments from SHR than from WKY (WKY: 89.5±1.7%; SHR: 101.9±3.9% of previous tone, P<0.05). The relaxation obtained in both strains was similar to that observed in the absence of endothelium (EC50 values: WKY, 3.0 (2.5–3.6) μM, n=10; SHR, 4.1 (3.1–5.4) μM, n=8). The effect of L-Arg was stereospecific since the inactive enantiomer D-Arg did not cause any effect (data not shown).
Effect of indomethacin and SOD
Basal tone, PGF2α-induced contraction and L-Arg-caused relaxation were not modified in the presence of 10 μM indomethacin or SOD (60 u ml−1) in segments from both strains (data not shown).
Effect of NOS inhibitors
To determine the role of NO on the L-Arg-induced relaxation, segments from MCA were incubated with 0.1 mM L-NAME or 5 and 10 nM AMT. In the presence of 0.1 mM L-NAME, the concentration-dependent curve to L-Arg was displaced to the right in segments from both WKY and SHR, this displacement being greater in the hypertensive strain (concentration-ratio WKY: 3.1±0.9; SHR: 18.5±7.0, P<0.05) (Figure 2). The L-Arg-induced relaxation was reduced by 10 nM AMT in MCAs from both WKY and SHR in a similar extent (concentration-ratio WKY: 2.6±0.4; SHR: 1.9±0.2, P>0.05). However, 5 nM AMT shifted to the right the curve to L-Arg only in segments from hypertensive animals (Figure 2).
Figure 2.

Effect of L-NAME and AMT on the concentration-response curves to L-Arg in middle cerebral arteries from WKY and SHR previously contracted with 10 μM PGF2α. Results (mean±s.e.mean) are expressed as a percentage of the contraction induced by PGF2α in each case (WKY: 1.29±0.14, 1.95±0.08 and 1.69±0.26 mN mm−1; SHR: 0.66±0.05, 1.05±0.80 and 0.84±0.11 mN mm−1, in control, L-NAME or AMT treated segments, respectively). Number of arterial segments is indicated in parentheses.
L-NAME induced a basal tone increase of 33.2±8.4% and 24.9±6.8% of the maximum response, in segments from WKY and SHR, respectively. In this situation the tone reached by 10 μM PGF2α was significantly potentiated (WKY: control, 65.6±6.7, L-NAME, 90.7±5.0%, P<0.05; SHR: control, 39.6±2.0, L-NAME, 62.7±6.0%, P<0.05). AMT induced slight contractile responses, which were similar in segments from normotensive and hypertensive animals (5 nM: 9.0±2.6 and 5.4±1.5%, P>0.05, and 10 nM: 14.7±2.8 (n=10) and 8.8±3.6% (n=8), P>0.05, of the maximum response in segments from WKY and SHR, respectively). In addition, the contraction to 10 μM PGF2α was significantly increased in the presence of both 5 nM AMT (WKY: control, 60.6±3.5, AMT, 78.6±7.1%, P<0.05; SHR: control, 33.9±4.8, AMT, 50.1±4.5%, P<0.05) and 10 nM AMT (WKY: control, 55.2±6.2, AMT, 74.5±8.4%, P<0.05; SHR: control, 37.4±4.3, AMT, 52.1±3.2%, P<0.05).
Effect of dexamethasone and LPS
L-Arg-induced relaxation was reduced after 4 or 7 h incubation of segments from WKY rats with dexamethasone, whereas the reduction was only observed after a 7 h incubation in segments from SHR (Figures 3 and 4). The relaxation induced by L-Arg was not modified by LPS in segments from WKY whereas it was slightly inhibited in those from SHR (Figure 4). The presence of both drugs reduced the inhibitory effect of dexamethasone on L-Arg-induced relaxation, only in segments from WKY rats (Figure 4).
Figure 3.

Effect of preincubation for 4 h with dexamethasone on the concentration-response curves to L-Arg in middle cerebral arteries from WKY and SHR previously contracted with 10 μM PGF2α. Results (mean±s.e.mean) are expressed as a percentage of the contraction induced by PGF2α in each case (WKY: 0.92±0.09 and 1.21±0.07 mN mm−1, SHR: 0.40±0.06 and 0.66±0.06 mN mm−1, in control and dexamethasone treated segments, respectively). Number of arterial segments is indicated in parentheses.
Figure 4.

Effect of incubation for 7 h with LPS, dexamethasone, and dexamethasone plus LPS on the concentration-response curves to L-Arg in middle cerebral arteries from WKY and SHR previously contracted with 10 μM PGF2α. Results (mean±s.e.mean) are expressed as a percentage of the contraction induced by PGF2α in each case (WKY: 0.95±0.11, 0.75±0.13, 1.15±0.14 and 1.04±0.13 mN mm−1; SHR: 0.46±0.07, 0.26±0.08, 0.67±0.12 and 0.36±0.08 mN mm−1, in control, LPS, dexamethasone and LPS plus dexamethasone treated segments, respectively). Number of arterial segments is indicated in parentheses.
Incubation of segments for 4 or 7 h with 1 μM dexamethasone did not modify the maximum response (results not shown), whereas the contraction caused by PGF2α was similarly potentiated in both WKY and SHR (Figure 5). The presence of 10 μg ml−1 LPS since the time the brain was removed from the animal and for 7 h did not modify the maximum response in MCAs from WKY and SHR (results not shown). However, in these conditions, the contraction induced by PGF2α was reduced in segments from normotensive and hypertensive animals, this reduction being greater in SHR (Figure 5). In the presence of LPS plus dexamethasone for 7 h the contraction to PGF2α was unmodified in the normotensive strain and reduced in the hypertensive one (Figure 5).
Figure 5.

Vasoconstrictor responses induced by 10 μM PGF2α in middle cerebral arteries from WKY and SHR treated for 4 or 7 h with 1 μM dexamethasone (Dex) and for 7 h with 10 μg ml−1 LPS, Dex plus LPS, 10 μg ml−1 polymyxin B (Polym) and polymyxin B plus LPS. Results (mean±s.e.mean) are expressed as a percentage of the contraction induced by PGF2α in a parallel control. Number of arterial segments is indicated in parentheses. aP<0.05 vs control; bP<0.05 vs WKY; cP<0.05 vs Dex or Polym (unpaired Student's t-test with Bonferroni correction).
Effect of PKC inhibitors
The incubation for 7 h with 10 μg ml−1 polymyxin B since the time the brain was removed from the animal, induced a significant increase of basal tone, which was greater in segments from SHR than WKY (percentage of maximum response: WKY, 12.8±3.1; SHR, 31.4±7.7, P<0.05). In these conditions, the contraction to 10 μM PGF2α was potentiated in segments from both strains, this potentiation being greater in SHR (Figure 5). In addition, the relaxation induced by L-Arg in segments from both types of animals was significantly reduced (Figure 6). Calphostin C (0.5 μM) produced a reduction of the L-Arg relaxation (ANOVA, WKY: P<0.05, n=6; SHR : P<0.005, n=7), whereas the contraction to PGF2α was not modified (results not shown).
Figure 6.

Effect of 10 μg ml−1 polymyxin B, and polymyxin B plus 10 μg ml−1 LPS on the concentration-response curves to L-Arg in middle cerebral arteries from WKY and SHR rats previously contracted with 10 μM PGF2α. Results (mean±s.e.mean) are expressed as percentage of the contraction induced by PGF2α in each case (WKY: 0.90±0.10, 1.19±0.08 and 0.66±0.10 mN mm−1; SHR: 0.42±0.06, 0.83±0.18 and 0.21±0.02 mN mm−1, in control polymyxin B and LPS plus polymyxin B treated segments, respectively). Number of arterial segments is in parentheses.
LPS abolished the increase in basal tone induced by polymyxin B. In addition, the contraction caused by PGF2α in the presence of LPS plus polymyxin B was lower than that obtained in the presence of polymyxin B alone and similar or lower than the control in WKY and SHR, respectively (Figure 5). The inhibition L-Arg relaxation induced by polymyxin B in segments from both types of rats was abolished by LPS, but in the case of segments from SHR the relaxation obtained was higher than that found in control situation (Figure 6).
As polymyxin B is an antibiotic, in addition of PKC inhibitor, experiments were achieved with other antibiotics to analyse the specificity of the inhibitory effect of polymyxin B. Incubation of segments with gentamycin plus ampicillin for 7 h, both at 10 μg ml−1, did modify neither basal tone, the maximum response nor the contraction induced by 10 μM PGF2α. In addition, the concentration-response curve to L-Arg was not affected by these antibiotics in segments from both WKY and SHR (data not shown).
Discussion
This study investigates some functional consequences that hypertension produces in the rat MCA. The major and new finding of the present study is that the vasodilator responses induced by L-Arg are augmented in SHR-cerebral segments compared with WKY ones, whereas the vasoconstrictor responses induced by PGF2α are reduced. A different functional expression and/or participation of iNOS through a PKC-dependent mechanism seems to be involved in these effects.
Vasodilator response to L-arginine
Previous studies have shown that the substrate of NO synthesis, L-Arg, has a weak effect in the vascular tone regulation of cerebral arteries and arterioles (Faraci, 1993; Kitazono et al., 1996), and mild dilating effect on pial circulation under physiological conditions (Haberl et al., 1991; Morikowa et al., 1992; Rosenblum et al., 1990; Riedel et al., 1995). Recently, we have found that L-Arg induces an inhibitory effect of vasoconstrictor responses induced by PGF2α and K+ in the rat middle cerebral artery through a mechanism that involves the iNOS activation (Alonso et al., 1998).
In the present study, L-Arg induced an endothelium-independent relaxation that was more pronounced in SHR than in WKY, whereas EC50 values were not altered, suggesting that the sensitivity of both arteries to L-Arg is similar. Other investigators have also found that hypertension increases the vasodilator responses to L-Arg. Thus, Riedel et al. (1995) reported that the increase of diameter induced by L-Arg in pial arterioles was greater in the hypertensive than in normotensive rats. In peripheral arteries, hypertension may modify the responses to L-Arg, e.g., Puci et al. (1995) and Wu et al. (1996) observed that L-Arg caused relaxation in aortic rings from hypertensive but not from normotensive rats. In contrast, the endothelium-dependent relaxation elicited by BK was not modified by hypertension. Other investigators, using the cranial window method, observed that the dilatation of the basilar artery in response to acetylcholine and BK is impaired in SHR and SHR stroke-prone (SHRSP) (Faraci, 1993; Kitazono et al., 1996). Generally, hypertension impairs the endothelium-dependent vasodilator responses to different agonists in isolated vascular preparations (Lüscher, 1992), although unchanged, an even, improved responses have been also reported (Marín & Rodríguez-Martínez, 1997).
In some vessels, the generation of prostanoids and/or free radicals has been described as a possible cause of L-Arg-induced dilatation (Brian et al., 1995; Riedel et al., 1995). However, in our study, their participation is ruled out as indomethacin and SOD failed to modify the responses to L-Arg in both strains.
The vasodilator responses induced by L-Arg were inhibited by L-NAME in segments from both WKY and SHR. The effects of L-Arg were enantiomerically specific, as D-Arg has no effect, and L-NAME is a competitive inhibitor of NOS. These results indicate that the synthesis of NO is a necessary step for the vasodilatation induced by L-Arg in both strains. Similar results with unspecific inhibitors of NOS have been described (Morikowa et al., 1992; Moritoki et al., 1992; Riedel et al., 1995; Puci et al., 1995). The specific inhibitor of iNOS, AMT (Nakane et al., 1995; Tracey et al., 1995) inhibited the L-Arg relaxation in both strains, indicating the participation of this isoform in such responses. However, the fact that the effect of 5 nM AMT on L-Arg relaxation was observed only in MCAs from SHR, and that the inhibitory effect of L-NAME was higher in SHR, suggests that the participation of iNOS in the vasodilator responses induced by L-Arg is higher in the hypertensive than in the normotensive strain. Wu et al. (1996) and Chou et al. (1998) found that the degree of both iNOS expression and activity, in basal conditions or after LPS stimulation, was greater in aorta homogenates from SHR than from WKY. These differences in the expression of iNOS could also explain the higher relaxation to L-Arg found in the hypertensive strain.
Previous studies in peripheral arteries from normotensive rats demonstrated that L-Arg relaxed only precontracted arterial vessels subjected to prolonged incubation in physiological buffers or after LPS treatments (Rees et al., 1990; Schini & Vanhoutte, 1991; Moritoki et al., 1992; Martínez et al., 1996). In our study, however, L-Arg induced an early and marked relaxation of segments from normotensive and hypertensive strain in the absence of iNOS activators. Ex vivo induction of NO synthases by low levels of endotoxin present in the incubation medium was thought to be involved in this enhanced responsiveness to L-Arg (Rees et al., 1990). It is unlikey that this mechanism was involved in the relaxant responses to L-Arg in MCAs, since these responses were not modified in the presence of the antibiotics ampicillin plus gentamycin. This finding is compatible with the notion that the important vasodilator responses observed in the MCA without previous exposure to endotoxin could be due, at least in part, to an in vivo induction of iNOS. The antibiotic polymyxin B induced a basal tone increase, potentiated the contraction induced by PGF2α and reduced the vasodilator effect induced by L-Arg on the MCA from both strains. These effects, that were antagonized by LPS, could not be attributed to the presence of bacterial contaminants in the medium by the lack of effect of ampicillin plus gentamycin. The ability of polymyxin B to inhibit PKC has been extensively described and is currently used as PKC inhibitor (Kuo et al., 1984; Yoon et al., 1994). In vascular smooth muscle, PKC is involved in the contractile responses to different agonists (Salaices et al., 1990; Singer et al., 1996) and in the iNOS expression (McKenna et al., 1994; Paul et al., 1997). The results obtained in the present study with polymyxin B suggest the involvement of PKC in the activation of iNOS in the MCA from hypertensive and normotensive rats. The fact that calphostin C induced a similar reduction in L-Arg relaxation to that caused by polymyxin B supports this assumption.
Dexamethasone, an inhibitor of NOS induction (Radomski et al., 1990), reduced, after a 4 h incubation, the relaxation induced by L-Arg in WKY, whereas in segments from SHR it was necessary to increase the incubation time up to 7 h to obtain such a reduction. The results obtained with dexamethasone suggest that a continuous protein synthesis is necessary for induction of L-Arg pathway, as previously indicated (Fleming et al., 1993) and further confirm the participation of the iNOS in the vasodilator responses induced by L-Arg.
It has been described that LPS decreases vascular resistance, produces vascular hyporeactivity to different vasoconstrictors and potentiates the inhibitory effect of L-Arg by overproduction of NO mediated by activation of iNOS (Ueno & Lee, 1993; Berrazueta et al., 1994; Brian et al., 1995; Villamor et al., 1995; Alonso et al., 1998). In our study, LPS inhibited the vasoconstrictor responses induced by PGF2α and the effect of dexamethasone and polymyxin B on basal tone, the contraction induced by PGF2α and on the L-Arg-induced vasodilatation. These results confirm that the effects of LPS are mediated by activation of iNOS. Unexpectedly, LPS inhibited and unaltered the vasodilator response to L-Arg in SHR and WKY, respectively. The cause of this effect is unclear but it is possible that in normal conditions the iNOS was maximally induced in the presence of L-Arg in these arteries, and an ulterior induction by LPS was impossible. However, when the iNOS expression is inhibited by polymyxin B, the presence of LPS potentiated the L-Arg vasodilatation in SHR.
Contractile responses induced by PGF2α
The contractions elicited by PGF2α were markedly attenuated in segments from SHR compared with those from WKY. Thus, we obtained not only a reduction in the active force generated (the contraction in mN mm−1 was approximately a half of that obtained in MCA from WKY rats), but also in the percentage of maximum responses of the arteries. These changes could be independent, at least in part, of receptor-signal transduction mechanisms, since the maximum responses were also reduced. The reduction of the contractions induced by K+, a non specific stimulus, suggests a decreased vasoconstrictor ability of the smooth muscle cells in the cerebral vessels of the hypertensive strain. In some peripheral vessels an increase of vasoconstrictor responses in hypertension has been reported (Vila et al., 1993; Tabernero et al., 1996; Dickhout & Lee, 1997). However, a similar reduction of vasoconstrictor responses has been obtained in cerebral arteries of SHR and SHRSP compared with WKY (Winquist & Bohr, 1983; Mulvany, 1986; Yokota et al., 1994; Arribas et al., 1996). Alterations of vascular structure associated with vascular remodelling and vascular smooth muscle cell geometric disorganization observed in rat basilar arteries (Arribas et al., 1996), could explain, in part, the impairment of the contraction.
The vasoconstrictor responses to PGF2α after 7 h of incubation were about a 70% of those obtained in the first administration, in segments from SHR and WKY. This reduction could be due to an induction of iNOS because it was potentiated by LPS. Similar results have been obtained in the porcine basilar artery (Ueno & Lee, 1993), the rat aorta (Rees et al., 1990) and the human mammary artery (Berrazueta et al., 1994) in normotension. The participation of iNOS in this time-dependent reduction of PGF2α contraction was also supported by the fact that in the presence of dexamethasone or polymyxin B, such a reduction was transformed in an increase of contraction that was antagonized by LPS. An inhibition by dexamethasone and polymyxin B of the time-dependent loss of agonist-induced tone has been reported (Rees et al., 1990; Berrazueta et al., 1994). The presence of gentamycin plus ampicillin did not alter the contractile response reduction which indicates that this reduction is not due to the presence of bacterial contaminant in the medium. Therefore, the effect of polymyxin B is independent of its antibiotic activity and probably related to its capacity to inhibit PKC (Kuo et al., 1984; Yoon et al., 1994), and therefore to the inhibition of iNOS expression. The fact that polymyxin B induced an increase in basal tone that was antagonized by LPS, suggests a continuous production of NO mediated by iNOS activation in basal condition. The increase in basal tone induced by L-NAME and AMT also supports this assumption.
Both the reduction and the potentiation of PGF2α-induced vasoconstrictor responses caused by incubation for 7 h with LPS and polymyxin B, respectively, were greater in the MCA from SHR than from WKY. In addition, LPS antagonized the increase of PGF2α response elicited by polymyxin B and dexamethasone in a greater extent in segments from SHR. These results agree with those obtained by Wu et al. (1996) and Ghou et al. (1998) in aortic homogenates from SHR and WKY in which a higher expression of iNOS in the hypertensive strain was observed. In addition, Junquero et al. (1992) using cultured aortic smooth muscle cells from 20 week old age-matched SHR and WKY, reported that cells obtained from the hypertensive strain produce three times more NO than those from WKY rats in response to interleukin-1β. These authors suggested that the quantitative differences in NO production between these strains may reflect differences in the induction process by cytokines or a differential modulation of the L-Arg pathway. However, Singh et al. (1996) did not find significant differences in either the level of iNOS mRNA expression or the amount of NO generated in vascular smooth muscle cells from prehypertensive SHR and WKY after stimulation with interleukin-1β, whereas the cyclic GMP concentration was smaller in SHR cells than in WKY cells. The results obtained in the present study suggest that an increase in the NO production in the MCA vascular wall from SHR could be involved in its hyporesponsiveness to PGF2α. Similar conclusion was obtained by Michielsen et al. (1995) in aortic rings from portal hypertensive rats. One alternative is a greater sensitivity of the MCA from SHR than WKY to NO produced through iNOS activation.
In conclusion, the results obtained in the present study suggest that the induction of iNOS, mediated by a PKC-dependent mechanism, participates in both the L-Arg-induced relaxation and the hyporeactivity to PGF2α in MCA from WKY and SHR rats, the participation of iNOS in such responses being greater in the hypertensive strain.
Acknowledgments
This study has been supported by Grants from DGYCYT (PM 97-0008), FISS (98/0074-02), Comunidad de Madrid (08.3/0002/1997) and Bayer España. We thank Dr M. Carmen Fernández-Criado for the care of animals and Dr Juliana Redondo for critical revision of the manuscript.
Abbreviations
- AMT
2-amine-5,6-dihydro-6-methyl-4H-1,3-tiazine
- BK
bradykinin
- iNOS
inducible nitric oxide synthase
- KHS
Krebs-Henseleit solution
- L-Arg
L-arginine
- LPS
lipopolysaccharide, L-NAME, L-NG-nitroarginine methyl ester
- MCA
middle cerebral artery
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGF2α
Prostaglandin F2α
- PKC
Protein Kinase C
- SHR
spontaneously hypertensive rats
- WKY
Wistar Kyoto rats
References
- ALONSO M.J., RODRÍGUEZ-MARTÍNEZ A., MARTÍNEZ ORGADO J., MARÍN J., SALAICES M. The L-arginine inhibition of rat middle cerebral artery contractile responses is mediated by inducible nitric oxide synthase. J. Auton. Pharmacol. 1998;18:105–113. doi: 10.1046/j.1365-2680.1998.1820105.x. [DOI] [PubMed] [Google Scholar]
- ARRIBAS S., GORDON J., DALY C.J., DOMINICZAK A.F., MCGRATH J.C. Confocal microscopic characterization of a lesion in cerebral vessel of the stroke-prone spontaneously hypertensive rat. Stroke. 1996;27:1118–1123. doi: 10.1161/01.str.27.6.1118. [DOI] [PubMed] [Google Scholar]
- BERRAZUETA J.R., SALAS E., AMADO J.A., SÁNCHEZ DE VEGA M.J., POVEDA J.J. Induction of nitric oxide synthase in human mammary arteries in vitro. Eur. J. Pharmacol. 1994;251:303–305. doi: 10.1016/0014-2999(94)90414-6. [DOI] [PubMed] [Google Scholar]
- BRIAN J.E., HEISTAD D.D., FARACI F.M. Dilatation of cerebral arterioles in response to lipopolysaccharide in vivo. Stroke. 1995;26:277–281. doi: 10.1161/01.str.26.2.277. [DOI] [PubMed] [Google Scholar]
- CHEN F.Y., LEE T.J-F. Arginine synthesis from citrulline in perivascular nerves of cerebral artery. J. Pharmacol. Exp. Ther. 1995;273:895–901. [PubMed] [Google Scholar]
- CHOU T-C., YEN M.-H., LI C.-Y., DING Y.-A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- DICKHOUT J.G., LEE R.M.K.W. Structural and functional analysis of small arteries from young spontaneously hypertensive rats. Hypertension. 1997;29:781–789. doi: 10.1161/01.hyp.29.3.781. [DOI] [PubMed] [Google Scholar]
- FARACI F.M. Endothelium-derived vasoactive factors and regulation of the cerebral circulation. Neurosurgery. 1993;33:648–659. doi: 10.1227/00006123-199310000-00014. [DOI] [PubMed] [Google Scholar]
- FARACI F.M., BRIAN J.E. Nitric oxide synthase: from molecular biology to cerebrovascular biology. News Physiol. Sci. 1994;9:64–67. [Google Scholar]
- FLEMING I., GRAY G.A., STOCLET J-C. Influence of endothelium on induction of the L-arginine-nitric oxide pathway in rat aortas. Am. J. Physiol. 1993;264:H1200–H1207. doi: 10.1152/ajpheart.1993.264.4.H1200. [DOI] [PubMed] [Google Scholar]
- FLEMING W.W., WESTFALL D.P., DE LA LANDE I.S., JELLET L.B. Long-normal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J. Pharmacol. Exp. Ther. 1972;181:330–345. [PubMed] [Google Scholar]
- HABERL R.L., DECKER P.J., EIINHÄUPL K.M. Angiotensin degradation products mediate endothelium-dependent dilation of rabbit brain arterioles. Circ. Res. 1991;68:1621–1627. doi: 10.1161/01.res.68.6.1621. [DOI] [PubMed] [Google Scholar]
- IADECOLA C., PELLIGRINO D.A., MOSKOWITZ M.A., LASSEN N.A. Nitric oxide synthase inhibition and cerebrovascular regulation. J. Cereb. Blood Flow Metab. 1994;14:175–192. doi: 10.1038/jcbfm.1994.25. [DOI] [PubMed] [Google Scholar]
- JOVANOVIC A., GRBOVIC L., TULIC I. L-Arginine induces relaxation of human uterine artery with both intact and denuded endothelium. Eur. J. Pharmacol. 1994;256:103–107. doi: 10.1016/0014-2999(94)90623-8. [DOI] [PubMed] [Google Scholar]
- JUNQUERO D.C., SCOTT-BURDEN T., SCHINI V.B., VANHOUTTE P.M.The production of nitric oxide by interleukin-1 in cultured aortic smooth cells from spontaneously hypertensive rats is greater than that from normotensive rats Genetic Hypertension 1992vol. 218Paris: Colloque Inserm/John Libbey Eurotex Ltd; 3–5.(ed.) Sassard, J [Google Scholar]
- KATUSIC Z.S., MILDE J.H., COSENTINO F., MITROVIC B.S. Subarachnoid hemorrhage and endothelial L-arginine pathway in small brain stem arteries in dogs. Stroke. 1993;24:392–399. doi: 10.1161/01.str.24.3.392. [DOI] [PubMed] [Google Scholar]
- KITAZONO T., FARACI M., HEISTAD D.D. L-Arginine restores dilator responses of the basilar artery to acetylcholine during chronic hypertension. Hypertension. 1996;27:893–896. doi: 10.1161/01.hyp.27.4.893. [DOI] [PubMed] [Google Scholar]
- KUO J.F., SCHATZMAN R.L., TURNER R.S., MAZZEI G.J. Phospholipid-sensitive Ca2+-dependent protein kinase: a major protein phosphorylation system. Mol. Cell Endocrinol. 1984;35:65–73. doi: 10.1016/0303-7207(84)90001-7. [DOI] [PubMed] [Google Scholar]
- LAING R.L., JAKUBOWSKI J., MORICE A.L. An in vitro study of the pharmacological responses of rat middle cerebral artery: Effects of overnight storage. J. Vasc. Res. 1995;32:230–236. doi: 10.1159/000159097. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F. Heterogeneity of endothelial dysfunction in hypertension. Eur. Heart J. 1992;13:50–55. doi: 10.1093/eurheartj/13.suppl_d.50. [DOI] [PubMed] [Google Scholar]
- MARÍN J., RODRÍGUEZ-MARTÍNEZ M.A. Role of nitric oxide in physiological and pathological conditions. Pharmacol. Ther. 1997;75:111–134. doi: 10.1016/s0163-7258(97)00051-x. [DOI] [PubMed] [Google Scholar]
- MARTÍNEZ M.C., MULLER B., STOCLET J.C., ADRIANTSITOHAINA R. Alteration by lipopolysaccharide of the relationship between intracellular calcium levels and contraction in rat mesenteric artery. Br. J. Pharmacol. 1996;118:1218–1222. doi: 10.1111/j.1476-5381.1996.tb15526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKENNA T.M., CLEGG J.M., WILLIAMS T.J. Protein kinase C is a mediator of lipopolysaccharide-induced vascular supression in the rat aorta. Shock. 1994;2:84–88. doi: 10.1097/00024382-199408000-00002. [DOI] [PubMed] [Google Scholar]
- MICHIELSEN P.P., BOECKXSTAENS G.E., SYS S.U., HERMAN A.G., PELCKMANS P.A. Role of nitric oxide in hyporeactivity to noradrenaline of isolated aortic rings in portal hypertension. Eur. J. Pharmacol. 1995;273:167–174. doi: 10.1016/0014-2999(94)00691-y. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HIGGS E.A. The L-arginine-nitric oxide pathway. New Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MORIKOWA E., ROSENBLATT S., MOSKOWITZ M.A. L-arginine dilates rat pial arterioles by nitric oxide-dependent mechanisms and increases blood flow during focal cerebral ischaemia. Br. J. Pharmacol. 1992;107:905–907. doi: 10.1111/j.1476-5381.1992.tb13382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORITOKI H., TAKEHUCHI S., HISAYAMA T., KONDOH W. Nitric oxide synthase responsible for L-arginine-induced relaxation of rat aortic rings in vitro may be an inducible type. Br. J. Pharmacol. 1992;107:361–366. doi: 10.1111/j.1476-5381.1992.tb12752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J. Role of vascular structure in blood pressure development of the spontaneously hypertensive rat. J. Hypertens. 1986;4:S61–S63. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Mechanical properties of vascular muscle cells in situ. Nature. 1976;260:617–619. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arteries vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NAKANE M., KLINGHOFER V., KUK J.E., DONNELLY J.L., BUDZIK G.B., POLLOCK J.S., BASA F., CARTER G. Novel potent and selective inhibitors of inducible nitric oxide synthase. Mol. Pharmacol. 1995;47:831–834. [PubMed] [Google Scholar]
- OSOL G., CIPOLLA M., KNUTSON S. A new method for mechanically denuding the endothelium of small (50–150 μm) arteries with human hair. Blood Vessels. 1989;26:320–324. doi: 10.1159/000158781. [DOI] [PubMed] [Google Scholar]
- PAUL A., DOHERTY K., PLEVIN R. Differential regulation by protein kinase C isoforms of nitric oxide synthase induction in Raw 264.7 macrophages and aortic smooth muscle cell. Br. J. Pharmacol. 1997;120:940–946. doi: 10.1038/sj.bjp.0700976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCI M.L., DICK L.B., MILLER K.B., SMITH C.J., NASJLETTI A. Enhanced responses to L-arginine in aortic rings from rats with angiotensin-dependent hypertension. J. Pharmacol. Exp. Ther. 1995;274:1–7. [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive nitric oxide synthase in vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES D.D., CELLEK S., PALMER R.M.J., MONCADA S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem. Biophys. Res. Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- RIEDEL M.W., ANNESER F., HABERL R.L. Different mechanisms of L-arginine induced dilatation of brain arterioles in normotensive and hypertensive rats. Brain Res. 1995;671:21–26. doi: 10.1016/0006-8993(94)01292-p. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM W.I., NISHIMURA H., NELSON G.H. Endothelium-dependent L-Arg- and L-NMMA-sensitive mechanisms regulate tone in brain microvessels. Am. J. Physiol. 1990;259:H1396–H1401. doi: 10.1152/ajpheart.1990.259.5.H1396. [DOI] [PubMed] [Google Scholar]
- SALAICES M., BALFAGON G., ARRIBAS S., SAGARRA R., MARIN J. Effects of phorbol 12,13-dibutyrate on the vascular tone and on norepinephrine- and potassium-induced contractions of cat cerebral arteries. J. Pharmacol. Exp. Ther. 1990;255:66–73. [PubMed] [Google Scholar]
- SCHINI V., VANHOUTTE P.M. L-arginine evokes both endothelium-dependent and -independent relaxations in L-arginine-depleted aortas of the rat. Circ. Res. 1991;68:209–216. doi: 10.1161/01.res.68.1.209. [DOI] [PubMed] [Google Scholar]
- SINGER H.A.Protein Kinase C Biochemistry of smooth muscle contraction 1996San Diego: Academic Press Inc; 155–165.Ed. Barany, M. pp [Google Scholar]
- SINGH A., SVENTEK P., LARIVIÈRE R., THIBAULT G., SCHIFFRIN E.L. Inducible nitric oxide synthase in vascular smooth muscle cells from prehypertensive spontaneously hypertensive rats. Am. J. Hypertens. 1996;9:867–877. doi: 10.1016/s0895-7061(96)00104-5. [DOI] [PubMed] [Google Scholar]
- TABERNERO A., GIRALDO J., VIVAS N.M., BADIA A., VILA E. Endothelial modulation of α1-adrenoceptor contractile responses in the tail artery of spontaneously hypertensive rats. Br. J. Pharmacol. 1996;119:765–771. doi: 10.1111/j.1476-5381.1996.tb15738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRACEY W.R., NAKANE M., BASHA F., CARTER C. In vivo pharmacological evaluation of two novel type II inducible nitric oxide synthase inhibitors. J. Physiol. Pharmacol. 1995;73:665–669. doi: 10.1139/y95-085. [DOI] [PubMed] [Google Scholar]
- UENO M., LEE J.-F.T. Endotoxin decreases the contractile responses of the porcine basilar artery to vasoactive substances. J. Cereb. Blood Flow Metab. 1993;13:712–719. doi: 10.1038/jcbfm.1993.90. [DOI] [PubMed] [Google Scholar]
- VILA E., TABERNERO A., IVORRA D. Inositol formation and contractile response linked to α1-adrenoceptor in tail artery and aorta from spontaneously hypertensive and Wistar-Kyoto rats. J. Cardiovasc. Pharmacol. 1993;22:191–197. doi: 10.1097/00005344-199308000-00003. [DOI] [PubMed] [Google Scholar]
- VILLAMOR E., PÉREZ-VIZCAÍNO F., RUIZ T., LEZA J.C., MORO M., TAMARGO J. Group B Streptococcus and E. coli LPS-induced NO-dependent hyporesponsiveness to noradrenaline in isolated intrapulmonary arteries of neonatal piglets. Br. J. Pharmacol. 1995;115:261–266. doi: 10.1111/j.1476-5381.1995.tb15872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELCH G., LOSCALZO J. Nitric oxide and the cardiovascular system. J. Cardiovasc. Surg. 1994;9:361–371. doi: 10.1111/j.1540-8191.1994.tb00857.x. [DOI] [PubMed] [Google Scholar]
- WINQUIST R.J., BOHR D.F. Structural and functional changes in cerebral arteries from spontaneously hypertensive rats. Hypertension. 1983;5:292–297. doi: 10.1161/01.hyp.5.3.292. [DOI] [PubMed] [Google Scholar]
- WU C.-C., HONG H.-J., CHOU T.-C., DING Y.-A., YEN M.-H. Evidence for inducible nitric oxide synthase in spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 1996;228:459–466. doi: 10.1006/bbrc.1996.1682. [DOI] [PubMed] [Google Scholar]
- YOKOTA Y., IMAIZUMI Y., ASANO M., MATSUDA T., WATANABE M. Endothelium derived relaxing factor released by 5-HT: distinct from nitric oxide in basilar arteries of normotensive and hypertensive rats. Br. J. Pharmacol. 1994;113:324–330. doi: 10.1111/j.1476-5381.1994.tb16212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOON H.J., JUN C.D., KIM J.M., RIM G.M., KIM H.M., CHUNG H.T. Phorbol ester synergistically increases interferon-gamma-induced nitric oxide synthesis in murine microglial cells. Neuroimmunomodulation. 1994;1:377–388. doi: 10.1159/000097191. [DOI] [PubMed] [Google Scholar]


