Abstract
NMDA-induced changes in free intracellular Ca2+ concentration ([Ca2+]i) were determined in individual cultured rat mesencephalic neurones by the fura-2 method. mRNA expression encoding NMDA receptor subunits (NR1, NR2A-D) was examined by RT–PCR.
NMDA (1–100 μM, plus 10 μM glycine) induced a concentration-dependent increase in [Ca2+]i (EC50=5.7 μM). The effect of NMDA was virtually insensitive to tetrodotoxin (0.3 μM) and nitrendipine (1 μM), but dependent on extracellular Ca2+. 5,7-Dichlorokynurenic acid (10 μM), a specific antagonist at the glycine binding site on the NMDA receptor, abolished the NMDA response.
Memantine, an open-channel blocker, and ifenprodil, a preferential non-competitive NR1/NR2B receptor antagonist diminished the NMDA effect with an IC50 value of 0.17 and 1 μM, respectively. Ethanol at 50 and 100 mM caused about 25 and 45%-inhibition, respectively.
Agarose gel analysis of the PCR products followed by ethidium bromide fluorescence or CSPD chemiluminescence detection revealed an almost exclusive expression of the NR1 splice variants lacking exon (E) 5 and E22. The 3′ splice form without both E21 and E22 exceeded that containing E21 by approximately 4 fold. The relative amounts of NR2A, NR2B, NR2C corresponded to approximately 1:2:1. NR2D mRNA was also detectable.
In conclusion, mesencephalic neurones bear ethanol-sensitive NMDA receptors which might be involved in the development of ethanol dependence and withdrawal. The high affinity of NMDA to this receptor, its sensitivity to ifenprodil and memantine may suggest that the mesencephalic NMDA receptor comprises the NR1 splice variant lacking E5, NR2B, and NR2C, respectively.
Keywords: Cultured mesencephalic neurones, NMDA receptors, intracellular Ca2+, ethanol, ifenprodil, memantine, fura-2 method, RT–PCR, NR1 splice variants, NR2 subunits
Introduction
The N-methyl-D-aspartate (NMDA) receptor is essentially involved in a number of important central processes such as neuronal development, long-term-potentiation, and neuronal death (excitotoxicity) (Nakanishi, 1992). Moreover, this receptor mediates some of the acute and chronic CNS effects of ethanol (Harris et al., 1992; Hoffman, 1995; Grant & Lovinger, 1995). At concentrations associated with moderate to severe intoxications [10–100 mM, corresponding to 0.046–0.46% (w/v) in blood] ethanol acutely inhibits NMDA receptor-mediated responses in cultured neurones (Lovinger et al., 1989), brain slices (Göthert & Fink, 1989; Woodward & Gonzales, 1990; Poelchen et al., 1997; Darstein et al., 1998; Nieber et al., 1998), and transfected expression systems (Koltchine et al., 1993; Mirshahi & Woodward, 1995).
The NMDA receptor is a ligand-gated ion channel highly permeable to Ca2+. In addition to glutamate, glycine is required as a co-agonist (Kleckner & Dingledine, 1988; Kemp & Leeson, 1993). Several NMDA receptor subunits (NR1, NR2A-D) have been cloned (Monyer et al., 1992; Laurie & Seeburg, 1994; Zukin & Bennett, 1995). The NR1 subunit with eight known splice variants is expressed throughout the brain, while the NR2A-D subunits display regional differences. Xenopus oocyte expression studies have shown that only the NR1 subunit is required to generate functional NMDA receptors and that co-expression with various NR2 subunits results in the assembly of ion channels with conductances that resemble native NMDA receptors. Natively expressed receptors presumably comprise two NR1 subunits (Behe et al., 1995) and at least one member of the NR2 family (Schoepfer et al., 1992). Heteromers containing different NR2 subunits display distinct pharmacological and physiological characteristics (Monyer et al., 1992). For example, ifenprodil blocks the NR1/NR2B heteromer with considerably higher potency than the NR1/NR2A heteromer (Williams, 1993; Lovinger, 1995), while ethanol preferentially affects both NR1/NR2A and NR1/NR2B receptors (Masood et al., 1994; Mirshahi & Woodward, 1995).
There is considerable evidence that the ventral tegmental area (VTA)-nucleus accumbens system is implicated in the stimulant and reinforcing properties of ethanol and other drugs of abuse (Harris et al., 1992). Like morphine and nicotine, acutely administered ethanol has been shown to increase the firing rate of dopaminergic principal neurones of the VTA by a mechanism which is not completely understood. Ethanol might directly act on the dopamine neurones or inhibit GABAergic interneurones. In the present study, NMDA receptor function and mRNA subunit expression were studied on cultured rat ventral mesencephalic neurones by means of single-cell microfluorimetry (fura-2 method) and reverse transcriptase-polymerase chain reaction (RT–PCR). According to electrophysiological data, burst firing in dopamine neurones is induced by activation of somatodendritic NMDA receptors (Johnson et al., 1992). Preliminary accounts of the present results were given previously (Scheibler et al., 1998).
Methods
Mesencephalic cultures
For a detailed description of tissue preparation and cell culture conditions see Shimoda et al. (1992) and Krieglstein & Unsicker (1994), respectively. In brief, 1 mm3 of ventral mesencephalic tissue was dissected out from Wistar rat foetuses of gestational day 14 and pooled in ice-cold Hanks buffered salt solution. The tissue was dissociated by a consecutive treatment with trypsin (0.25%) and DNAse (≈amp;2000 u), followed by careful trituration. Subsequent to centrifugation, the supernatant was discarded and the pellet was dispersed in a 1 : 1 mixture of Dulbecco's modified Eagle's Medium and Nutrient F12 supplemented with 20% foetal calf serum, 2 mM L-glutamine, 50 μg ml−1 gentamycin, and 36 mM D-glucose. The cell suspension was seeded in the centre of circular glass coverslips (30 mm in diameter) coated with poly-L-lysine at a density of 250,000 cells cm−2. Medium was replaced every 3 days.
[Ca2+]i measurement
Usually, 7–10 day-old cultures (DIV 7–11) were loaded with fura-2 acetoxymethyl ester (5 μM) for 30 min at 37°C, then rinsed with HEPES buffer (composition in mM: NaCl 133, KCl 4.8, CaCl2 1.3, KH2PO4 1.2, HEPES 10; D-glucose 10; pH 7.4 adjusted with NaOH) and left to equilibrate for approximately 30 min. Subsequently, the preparation was placed in a closed superfusion chamber, mounted onto the stage of an inverted microscope (Diaphot 200, Nikon) and superfused with HEPES-buffer at a flow rate of 0.6 ml min−1 for 15 min. During the washout period, the preparation was focused in normal light using a 1000 fold magnification (Fluor 100× oil immersion objective, Nikon). Fura-2 fluorescence excited alternately at 340 and 380 nm by the light of a 75-W xenon bulb was recorded at 510/20 nm with a photomultiplier. The fluorescence field chosen by means of an adjustable rectangular diaphragm was slightly larger than the bipolar neurone in focus. Monochromator settings, chopper frequency, and complete data acquisition were computer-controlled (PTI, Wedel, Germany). The cells were stimulated by the application of NMDA for 1 min (usually in the presence of 10 μM glycine), and in some experiments by electrical stimulation (30 pulses/3 Hz; voltage drop: 37.5 V cm−1; current strength: 58 mA; pulse width: 0.5 ms); the latter was mainly done to check the contribution of voltage-dependent sodium channels to the NMDA response. Up to three stimulations, separated by 15 min-intervals, were applied. During intervals the cells were protected from the excitation light to prevent bleaching and phototoxic action. Substances, such as NMDA receptor antagonists and ethanol were administered 10 min before and during the second stimulation period. Cells were exposed to excitation lights over a period of 3 min, starting 1 min before stimulation. Calibration was performed according to Grynkiewicz et al. (1985) at the end of the experiment; Ca2+-saturated fura-2 signals (Rmax) were measured in the presence of 10 μM ionomycin, and Ca2+-free (Rmin) signals with buffer containing no Ca2+ but 25 mM EGTA.
Reverse transcription and polymerase chain reaction (RT–PCR)
Total RNA was prepared using Ultraspec™-RNA (Biotecx Laboratories, Inc). First-strand cDNA was synthesized by reverse transcription of 1 μg total RNA with 200 u Superscript™ II reverse transcriptase (Gibco-BRL) in first strand buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2) additionally containing 10 mM dithiothreitol, 500 μM of each dNTP, and 50 ngμl−1 oligo (dT)12–18 primer (Pharmacia). Subsequent to a 10-min pre-incubation at 70°C followed by chilling on ice for denaturation, the samples were kept for 60 min at 45°C. The reaction was stopped by heating for 10 min at 65°C.
To identify mRNAs coding for the different isoforms of the NR1 receptor subunit, primer pairs flanking the regions of alternative splicing were designed. Splice variants with and without exon (E) 5 were detected using the primer pair E5 sense (5′-TCAGCGACGACCACGAGGGACG-3′) and E5 antisense (5′-TTGTAGATGCCCACTTGCACCA-3′) binding to position 495 and 1162 (position 1 represents the first nucleotide of the initiation codon of the full length isoform), respectively. PCR was initiated by a 3-min denaturation period (94°C) followed by 35 cycles of denaturation (94°C for 30 s), annealing (64°C for 30 s), and extension (72°C for 1 min), and terminated by a final extension at 72°C for 10 min. Predicted product lengths were 667 bp (+E5) and 604 bp (−E5). Alternative splicing at E21 and E22 was analysed with the primer pair E21/E22 sense (5′-AAGCTGCACGCCTTTATCTG-3′; binding position 2230) and E21/E22 antisense (5′-CTGACCGAGGGATCTGAGAG-3′; binding position 3150), giving rise to the following PCR products: +E21/+E22, 940 bp; −E21/+E22, 830 bp; +E21/−E22, 583 bp; −E21/−E22, 473 bp. PCR consisted of initial denaturation (94°C for 2 min), 30 cycles of denaturation (94°C for 30 s), annealing (58°C for 1 min), and extension (72°C for 1.5 min plus additional 2 s cycle1), and a final extension at 72°C for 10 min. The amplified PCR products allowed an estimation of the ratio of the respective NR1 splice variants.
The expression pattern of the NR2 subunits was investigated with primers common to NR2A, 2B, and 2C cDNA fragments (sense: 5′-GGGGTTCTGCATCGACATCC-3′; antisense: 5′-GACAGCAAAGAAGGCCCACAC-3′) yielding a 547 bp amplified product from each NR2A–2C (Audinat et al., 1994). Aliquots of the product were digested by restriction enzymes BpmI, BfaI, and ScaI (New England Biolabs), which selectively cut the NR2A, 2B and 2C PCR products, respectively. Cleavage by BpmI, BfaI, and ScaI yielded 226 and 321, 392 and 155, and 361 and 186 bp fragments, respectively. The primers used for the amplification of the NR2D cDNA fragment were 5′-CGATGGCGTCTGGAATGG-3′ (sense; position 1541) and 5′-CTGGCAAGAAAGATGACCGC-3′ (antisense; position 1986) yielding a 465 bp product (Audinat et al., 1994). All PCR reactions were performed in a total volume of 25 μl PCR buffer containing 1 u AmpliTaqRDNA polymerase (Perkin Elmer), 1 μl cDNA, 200 μM dNTPs, 200 nM of each primer, and 1 mM MgCl2 on a PTC-200 thermal cycler (MJ Research). Amplification products were visualized by ethidium bromide staining or Southern hybridization followed by CSPD detection subsequent to agarose gel electrophoresis (1.5–2%); semi-quantitation of ethidium bromide fluorescence and chemiluminescence intensities were performed using a Diana II Digital Image Analyzer (raytest). Southern hybridization of E21/E22 PCR products was carried out with a digoxigenin-labelled oligonucleotide (5′-GCC ACC AGC ATG AAG ACC CCT GCC ATG TTC TCA AAA GTG A-3′) after vacuum blotting of the amplification products onto positively charged nylon membranes (Qiagen). Hybridization was performed at 55°C in 5× standard saline-citrate (SSC), 1% blocking reagent (Boehringer), 0.1% lauroylsarcosine, 0.02% SDS for at least 4 h. Stringent washing was carried out for 2×10 min at 55°C in 2×SSC, 0.1% sodium dodecyl sulphate (SDS), and 2×15 min at 55°C in 0.1×SSC, 0.1% SDS. Anti-digoxigenin alkaline phosphatase conjugate and the chemiluminescence substrate CSPD were used for the detection of the hybridized probe.
Data analysis
Fluorescence ratios F340/F380 reflecting [Ca2+]i were used for statistical evaluation of drug effects. NMDA-induced changes in [Ca2+]i were calculated as difference between the fluorescence ratio subsequent to agonist application and the basal fluorescence ratio, and referred to as Δ fluorescence ratios. Drug effects on the NMDA-induced increase in [Ca2+]i were evaluated by calculating the ratio between the second and the first NMDA response, and are expressed as a percentage of the mean NMDA control ratio (i.e., Δ fluorescence ratios, expressed as per cent of control).
All data are given as arithmetic means±s.e.mean. Subsequent to one-way-analysis of variance (ANOVA), the significance of differences between the means of various groups was determined by Student's t-test. Statistical analysis of concentration response curves was performed using a 4-parameter logistic function (GraFit, Erithacus Software Ltd., U.K.) to generate estimates of EC50 and IC50 values.
Drugs and chemicals
DL-2-amino-5-phosphonopentanoic acid (AP5), 5,7-dichlorokynurenic acid, ifenprodil tartrate, nitrendipine, tetrodotoxin citrate (TTX) (Biotrend, Köln, Germany); fura-2 acetoxymethyl ester, glycine, ionomycin calcium salt, NMDA (Sigma, Deisenhofen, Germany). Memantine HCl was kindly donated by Dr G. Quack (Merz & Co., Frankfurt/Main, Germany). Stock solutions (10 mM) of nitrendipine and 5,7-dichlorokynurenic acid were made in dimethyl sulphoxide (DMSO); final concentrations of DMSO did not exceed 0.1% (v/v). AP5 was dissolved at 10 mM in 0.05 N NaOH and diluted appropriately.
Results
NMDA receptor function: intracellular Ca2+ levels
[Ca2+]i was determined in the cell bodies of bipolar rat mesencephalic neurones (DIV 7–11; Figure 1) incubated in magnesium-free HEPES buffer by means of the fura-2 method. Basal calcium concentrations averaged 95.6±13.4 nM (n=11). Application of NMDA (100 μM) plus glycine (10 μM) induced a rapid increase in [Ca2+]i which was reversible within 5 min and usually reproducible after an additional 15-min washout period (Figure 2A). The concentration dependence of the NMDA response (1–100 μM) is given in Figure 3A; the EC50 value was 5.7 μM. The maximal effect observed at 100 μM NMDA corresponded to an increase of [Ca2+]i to 320.5±28.6 nM (n=13). Two thirds of the neurones kept in culture for 4 days and longer were responsive to NMDA, whereas only one out of seven neurones exhibited a measurable response to NMDA after 2 DIV (Figure 4). The NMDA-induced increase in [Ca2+]i remained unchanged in the presence of 0.3 μM TTX (Δ fluorescence ratios above baseline were 0.71±0.18 (n=7) and 0.78±0.13 (n=9) with and without TTX, respectively), in contrast to that evoked by electrical field stimulation which was abolished by blockade of voltage-dependent Na+ channels. The effect of NMDA was prevented in the absence of extracellular Ca2+ (Figure 2B and 3B) but was virtually insensitive to nitrendipine (1 μM), a blocker of voltage-dependent L-type Ca2+ channels (Figure 3B).
Figure 1.
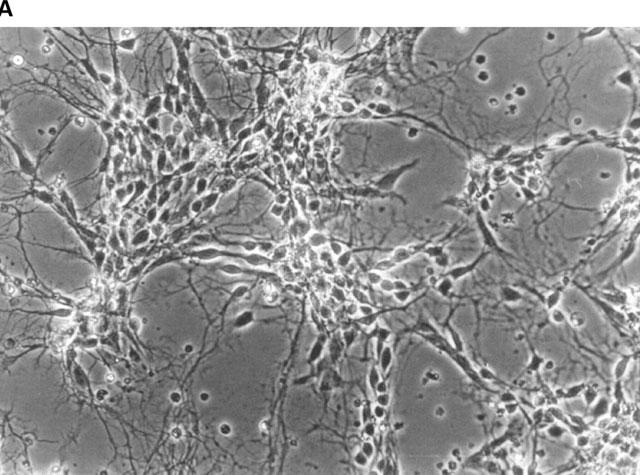
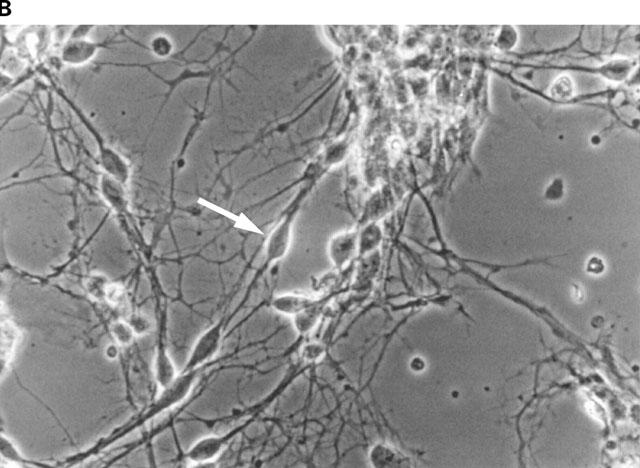
Phase-contrast photomicrographs of dissociated primary cultures of rat ventral mesencephalon after 7 days in culture. (A) overview photograph (magnification 50 fold); (B) arrow-labelled bipolar mesencephalic neurone of typical morphology as used in the fura experiments (magnification 100 fold).
Figure 2.
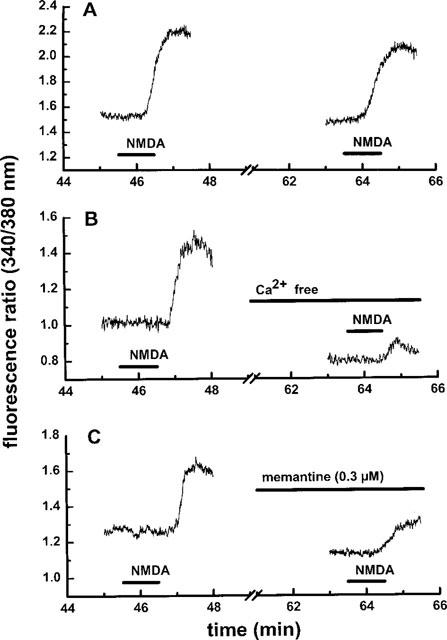
Effect of NMDA stimulation on the intracellular calcium concentration in individual mesencephalic neurones: time course of the experiment. (A) The cultures were superfused with magnesium-free HEPES buffer and stimulated twice with 100 μM NMDA (plus 10 μM glycine) for 1 min as described in Methods. (B) The original buffer containing 1.3 mM calcium was replaced by calcium-free buffer from 10 min before the second stimulation onwards. (C) Memantine (0.3 μM) was present from 10 min before the second stimulation onwards. Representative traces from single experiments are shown.
Figure 3.
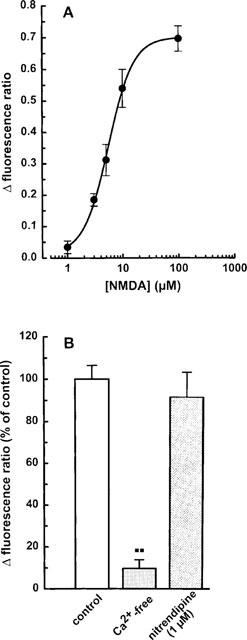
NMDA-induced enhancement of the intracellular calcium concentration in individual mesencephalic neurones: (A) concentration dependence; (B) effects of calcium deprivation and nitrendipine. The cells were superfused and stimulated twice with NMDA (1–100 μM; plus 10 μM glycine) for 1 min at increasing concentrations as described in Methods. Calcium-free buffer was used or 1 μM nitrendipine was present from 10 min before the second stimulation onwards. (A) Δ Fluorescence ratios above baseline were calculated from the difference between the peak response to each NMDA concentration and the corresponding basal value (see Figure 2). As verified in control experiments, responses to two consecutive stimulations with 100 μM NMDA were of similar magnitude. (B) Effects of calcium deprivation and nitrendipine were evaluated by calculating the ratio between the second and the first NMDA response, and expressed as a percentage of the mean NMDA control ratio. Means±s.e.mean of 4–24 (A) and 3–8 (B) observations. **P<0.01 vs control.
Figure 4.
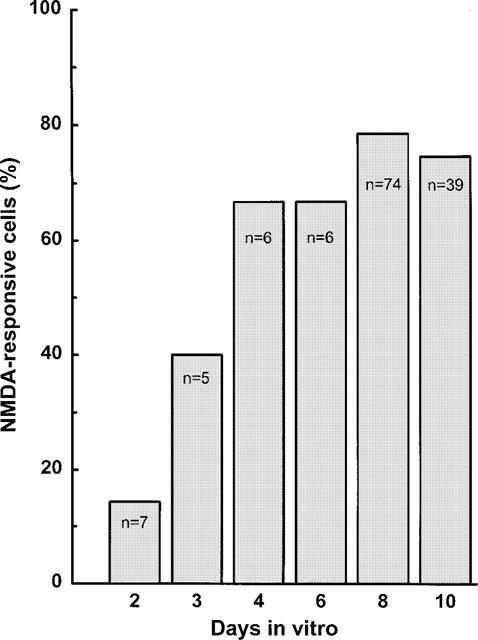
Development of NMDA receptor function in individual mesencephalic neurones. Cultures (2–10 DIV) were superfused and stimulated twice with 100 μM NMDA (plus 10 μM glycine) for 1 min. Δ Fluorescence ratios above baseline were calculated from the difference between the peak response to NMDA and the corresponding basal value. The percentage of cells exhibiting a Δ fluorescence ratio >0.6 is given.
In the absence of exogenous glycine, NMDA (100 μM) caused less increase in [Ca2+]i than that observed in the presence of the co-agonistic amino acid (Figure 5A). 5,7-dichlorokynurenic acid (10 μM), a specific antagonist at the glycine site on the NMDA receptor, abolished the NMDA response (Figure 5A).
Figure 5.
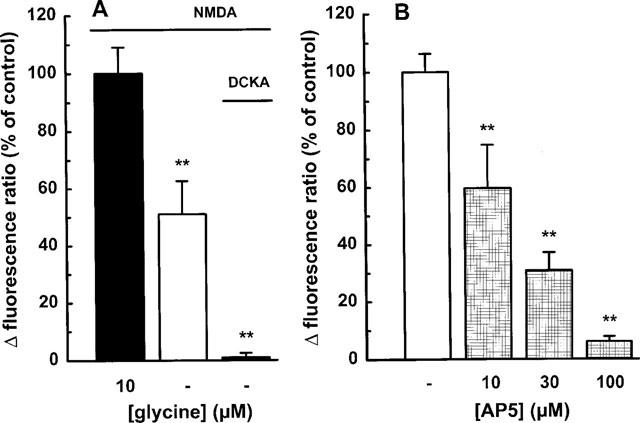
NMDA-induced enhancement of the intracellular calcium concentration in individual mesencephalic neurones: effect of (A) glycine and (B) AP5. The cells were superfused and stimulated twice with 100 μM NMDA in the (A) absence or presence of 10 μM glycine for 1 min. In some experiments, 5,7-dichlorokynurenic acid (DCKA; 10 μM) was added. (B) AP5 (10–100 μM) was present from 10 min before the second stimulation. Drug effects were evaluated by calculating the ratio between the second and the first NMDA response, and are expressed as a percentage of the mean NMDA control ratio (100 μM plus 10 μM glycine). Means±s.e.mean of 3–8 observations. **P<0.01 vs control.
Addition of AP5 (10–100 μM), a competitive NMDA receptor antagonist, attenuated the elevation of [Ca2+]i in a concentration-dependent manner (Figure 5B); the NMDA response was virtually abolished at 100 μM AP5. Memantine, an open-channel blocker antagonized the NMDA response with an IC50 value of 0.17 μM (Figure 2C and 6). Exposure to 50 and 100 mM ethanol for 10 min caused about 25% and 45%-inhibition, respectively (Figure 7A). Ifenprodil, a preferential non-competitive NR1/NR2B receptor antagonist, significantly diminished the effect of NMDA at a concentration as low as 0.1 μM; at 10 μM the inhibition amounted to approximately 75% (Figure 7B). Half-maximal inhibition occurred at ∼1 μM ifenprodil.
Figure 6.
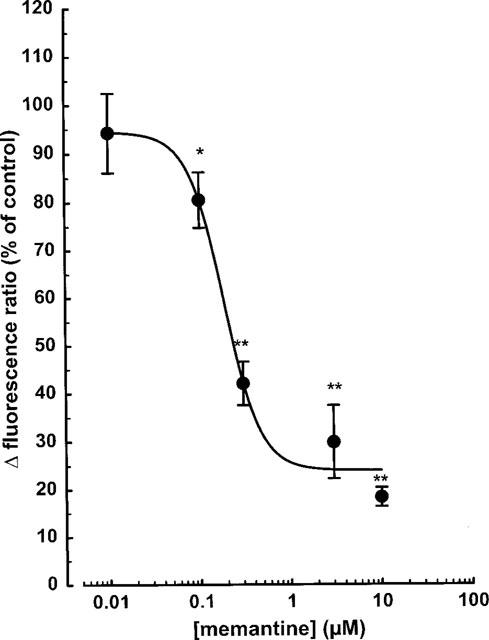
NMDA-induced enhancement of the intracellular calcium concentration in individual mesencephalic neurones: effect of memantine. The cells were superfused and stimulated twice with 100 μM NMDA (plus 10 μM glycine) for 1 min. Memantine (0.01–10 μM) was present from 10 min before the second stimulation. Drug effects were evaluated by calculating the ratio between the second and the first NMDA response, and are expressed as a percentage of the mean NMDA control ratio. Means±s.e.mean of 3–8 observations. Significant differences vs control: *P<0.05; **P<0.01.
Figure 7.
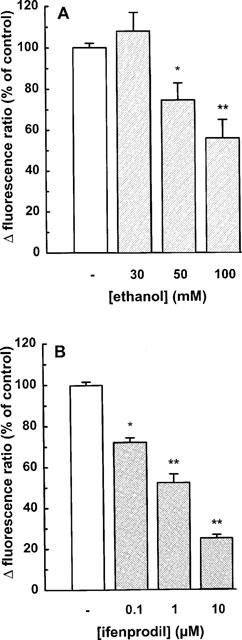
NMDA-induced enhancement of the intracellular calcium concentration in individual mesencephalic neurones: effect of (A) ethanol and (B) ifenprodil. The cells were superfused and stimulated twice with 100 μM NMDA (plus 10 μM glycine) for 1 min. Ethanol (30–100 mM) or ifenprodil (0.1–10 μM) was present from 10 min before the second stimulation. Drug effects were evaluated by calculating the ratio between the second and the first NMDA response, and are expressed as a percentage of the mean NMDA control ratio. Means±s.e.mean of 4–10 observations. Significant differences vs control: *P<0.05; **P<0.01.
mRNA expression of NMDA receptor subunits
NR1 splice forms were detected by RT–PCR from mRNA of cultured mesencephalic neurones (8 DIV) using primer pairs flanking E5 and E21/E22, respectively. Estimation of the ethidium bromide fluorescence intensities subsequent to agarose gel analysis of the PCR products representing the 5′ splice variants (±E5), revealed a virtually exclusive expression of the −E5 variant (Figure 8). Southern hybridization followed by CSPD chemiluminescence detection was used for semi-quantification of the 3′ splice variants. The −E21/−E22 isoform exceeded +E21/−E22 by approximately 4 fold (Figure 8). Only traces of the −E21/+E22 and +E21/+E22 isoforms were detectable.
Figure 8.
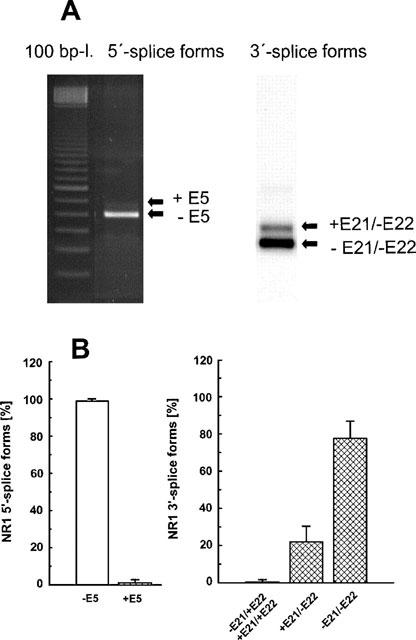
NR1 subunit mRNA expression in cultures of mesencephalic neurones (8 DIV). Subsequent to total mRNA extraction and RT–PCR amplification, cDNA products were analysed by agarose gel electrophoresis. NR1 fragments were detected using primer pairs flanking E5 (5′-splice forms) or E21/E22 (3′-splice forms). The ratios of the 5′- and 3′-splice forms were estimated after ethidium bromide staining and Southern hybridization, respectively. (A) Representative agarose gel electrophoresis and Southern blot. (B) Relative percentages of expression of each NR1 splice variant calculated from the corresponding total expression values (means±s.d. of three individual experiments).
Digestion of amplified NR2A-C fragments was analysed by agarose gel electrophoresis (Figure 9). The relative amount of the digestion products of NR2A, 2B and 2C corresponded to a ratio of approximately 1:2:1, respectively, as estimated from their ethidium bromide fluorescence intensities (Figure 9B). The occurrence of mRNA coding for the NR2D subunit was demonstrated by separate RT–PCR analysis (Figure 9C).
Figure 9.
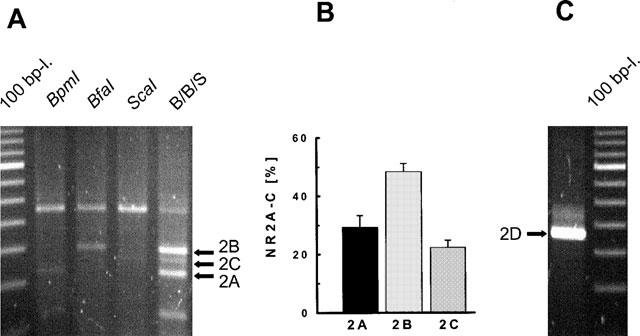
NR2A-D subunit mRNA expression in cultures of mesencephalic neurones (8 DIV). Subsequent to total mRNA extraction and RT–PCR amplification, cDNA products were analysed by agarose gel electrophoresis. (A) Restriction analysis of a NR2A-C amplification product with BpmI (lane 2), BfaI (lane 3), and ScaI (lane 4) which selectively cut the NR2A, 2B and 2C fragments, respectively; lane 5 (B/B/S) corresponds to the simultaneous digestion by the three enzymes; 100 bp ladder (lane 1). (B) Relative percentages of expression of each NR2A-C subunit calculated from the corresponding total expression values (means±s.d. of three individual experiments). (C) Representative agarose gel electrophoresis of the 2D mRNA RT–PCR amplification product.
In primary cultures of glial cells prepared for control purposes from mesencephalic tissue of rats at embryonic day 14, NR1 or NR2A-C mRNA expression was not observed (data not shown).
Discussion
Primary cultures of mesencephalic neurones were prepared from the ventral brainstem of Wistar rat foetuses. At gestational day 14, this area contains 90% of mesencephalic dopaminergic neurones representing about 20% of the total number of neurones (Shimoda et al., 1992). These observations have been corroborated for the present preparation by detecting tyrosine hydroxylase (TH) immunoreactivity by using a mouse monoclonal TH antibody and subsequently the Vectastain ABC kit in combination with DAB reagents or goat anti-mouse IgG-Cy3 as secondary antibody. About 50% of the TH-positive cells in the mature culture (DIV 7) are bipolar, and spindle to oval shaped (Shimoda et al., 1992). The cultures additionally comprise GABAergic and glutamatergic neurones (Rohrbacher et al., 1995). Astrocytes did not significantly contribute to total cell counts up to DIV 11 (Shimoda et al., 1992; own observations).
The NMDA-induced elevation of [Ca2+]i was studied in the cell bodies of bipolar mesencephalic neurones. Although it was not possible to determine the phenotype of each individual cell under investigation, it may be assumed that dopamine neurones contribute with a considerable proportion to the total number of measured cells. In response to a given NMDA concentration no larger differences between various neurones were observed. Seven to 11-day old neurones were used, since the fraction of responsive cells increased with time, reaching a plateau at about 70% from DIV 6 onwards.
Glycine has repeatedly been shown to act as a co-agonist at a strychnine-insensitive site of the NMDA receptor complex different from that at which glutamate binds (Kleckner & Dingledine, 1988; Kemp & Leeson, 1993). Since 5,7-dichlorokynurenic acid abolished the NMDA response, the NMDA receptor apparently required the occupation of both NMDA and glycine, in order to be activated. The effect of NMDA in the absence of exogenous glycine, however, may suggest the presence of endogenous glycine in the preparation investigated.
The response to NMDA was abolished by deprivation of extracellular Ca2+, but remained virtually unaffected in the presence of nitrendipine, a blocker of L-type voltage-dependent Ca2+ channels indicating that this type of Ca2+ channel does not contribute to the NMDA effect. A role of voltage dependent Na+ influx can also be ruled out, since TTX (0.3 μM) had no significant effect on the NMDA response.
NMDA antagonists strongly reduced the increase in [Ca2+]i evoked by 100 μM NMDA in mesencephalic neurones. AP5, a selective NMDA receptor antagonist which competes with NMDA at the agonist binding site, significantly decreased the NMDA response at 10 μM and more. The IC50 value of memantine, an open channel blocker of NMDA receptors with anti-parkinsonian and neuroprotective properties (Chen et al., 1992; Parsons et al., 1993; Danysz et al., 1997) was 0.17 μM in the present study. This value resembles the IC50 value obtained previously in cultured cerebellar neurones (0.2 μM) with the fura-2 method (Danysz et al., 1997). In cortical neurones, however, memantine was approximately 25 times less potent (IC50 of 4.1 μM; Black et al., 1996) than in mesencephalic or cerebellar neurones. The high potency of memantine observed in the present study may be due to a particularly high affinity of memantine to NMDA receptors comprising the NR2C subunit (Danysz et al., 1997). The highest levels of the NR2C subunit are expressed in the cerebellum (Nakanishi, 1992), but the present results show a significant expression also in mesencephalic neurones.
Ethanol, when administered acutely, impaired NMDA receptor function in intact brain tissue as well as in cultured neurones (Hoffman, 1995). For example, short-term in vitro exposure to ethanol inhibited NMDA-activated Ca2+ currents in hippocampal neurones (Lovinger et al., 1989), 45Ca2+ uptake in cerebellar granule cells (Hoffman et al., 1989), and the elevation of [Ca2+]i in cortical neurones (Lustig et al., 1992; Hu & Ticku, 1995; Bhave et al., 1996). In the latter preparation (Bhave et al., 1996) and in cerebellar granule cells (Bhave & Hoffman, 1997), ethanol inhibited the NMDA response in a concentration-dependent manner with a threshold concentration determined at 30 mM; a maximum effect of 40 and 70% in cortical neurones and cerebellar granule cells, respectively, was observed at 100 mM. In the present study on mesencephalic neurones, the inhibition of the NMDA-induced increase in [Ca2+]i caused by 100 mM ethanol amounted to 45%.
The mechanism of the acute inhibitory effect of ethanol on NMDA receptor function is still unclear. Considerable discrepancies occur between various studies. Since glycine reversed the inhibitory effect of ethanol on NMDA receptor function in striatal slices (dopamine release; Woodward & Gonzales, 1990), dissociated cells of neonatal brain (elevation of [Ca2+]i; Dildy-Mayfield & Leslie, 1991), and cerebellar granule cells (elevation of [Ca2+]i; Bhave & Hoffman, 1997), the glycine binding site was postulated as site of the action of ethanol. However, studies on cultured hippocampal neurones (Peoples & Weight, 1992), cortical and hippocampal tissue (Gonzales & Woodward, 1990; Woodward, 1994), and cultured cerebellar granule cells (glycine ⩽ 100 μM; Cebers et al., 1996) failed to demonstrate a reversal of the effect of ethanol by glycine. The reason for the discrepancies observed are not completely understood. The sensitivity to ethanol and glycine may partly depend on the NMDA receptor subunit composition. Studies performed on recombinant NMDA receptors suggest that ethanol preferentially affects heteromers containing the NR2A or NR2B subunit (Kuner et al., 1993; Sucher et al., 1996). In addition, it was reported that glycine reversal of ethanol inhibition was more pronounced when NR1/NR2A subunits were expressed, compared with NR1/NR2B (Buller et al., 1995).
Ifenprodil, an agent with potential anti-ischaemic properties, can be used to detect NMDA receptors comprising the NR2B subunit. Ifenprodil is a non-competitive NMDA receptor antagonist initially assumed to act at the polyamine binding site (Carter et al., 1989). However, at low concentrations ifenprodil promoted a non-conducting state of the receptor that did not involve the polyamine site on NMDA receptors (Legendre & Westbrook, 1991). According to recent data, ifenprodil binds to a site close to but distinct from the polyamine binding site so that the mechanism of interaction is presumably due to anti-cooperativity (Trube & Malherbe, 1998). The racemic form of ifenprodil which has been commonly used exhibited a potency for the NR1/NR2B receptor exceeding that for the NR1/NR2A receptor by more than 400 fold (Lovinger, 1995; Williams, 1993; Lovinger & Ziegelgänsberger, 1996). Similar results were observed with the pure stereoisomers of ifenprodil (Avenet et al., 1996). In rat cultured hippocampal pyramidal neurones, ifenprodil attenuated the NMDA-induced elevation of [Ca2+]i with an IC50-value of 0.7 μM (Church et al., 1994). In mesencephalic neurones, ifenprodil exhibited a similar potency indicating that the NMDA response in each preparation is mediated by NMDA receptors comprising the NR2B subunit.
Expression of NR subunit mRNA levels has been investigated in detail in the adult rat CNS using in situ hybridization or solution hybridization/RNAse protection assays (Laurie & Seeburg, 1994; Laurie et al., 1995; Zhong et al., 1995). The NR1 mRNA is abundantly expressed in every neuronal type examined. In the cortex and hippocampus, the predominant splice variants were those without E5 and with or without both E21 and E22. In cerebellum, however, the major NR1 variants were those lacking E5 and both 3′ inserts (Zhong et al., 1995). Little is known on the NR1 splice variant mRNA expression in mesencephalic structures. In the substantia nigra pars compacta, a significant expression of only the splice forms lacking E5 and both E21 and E22 has been found (Laurie et al., 1995). Similarly, the present study on cultured mesencephalic neurones using RT–PCR reveals an almost exclusive expression of the low molecular splice variants (i.e. lacking E5, E22).
Studies on recombinant NMDA receptors expressed in Xenopus oocytes and in mammalian cells revealed distinct functional properties of the different NR1 isoforms (Zukin & Bennett, 1995; Sucher et al., 1996). For example, NR1 splice variants containing E5 had approximately five-times lower affinity for the agonists glutamate and NMDA, a higher affinity for antagonists, a virtual loss of potentiation by polyamines, and altered allosteric modulation by Mg2+. The functional significance of alternative splicing of the C-terminal region of NR1 subunits has yet to be established. E21 was shown to contain most of the target sites for protein kinase C. Consequently, subcellular distribution of the NR1 subunit and NMDA channel properties such as open times and desensitization may be regulated by alternative splicing of E21 (Laurie et al., 1995; Sucher et al., 1996).
In contrast to NR1 mRNA expression, the mRNAs encoding the NR2 subunits are expressed more restrictedly, both spatially and in abundance. The NR2A mRNA is detectable throughout the brain, NR2B is confined to forebrain structures, NR2C is primarily expressed in cerebellar granule cells, and NR2D mRNA is expressed in lower abundance in structures such as midline thalamus and dentate gyrus (Monyer et al., 1994). The cell-specific and developmental regulation of NR2 subunit expression additionally accounts for the heterogeneity of NMDA receptor properties observed in different neuronal populations. If the heteromeric NR1/NR2 receptor contains the subunit 2A or 2C, potentiation by spermine will not be observed. In contrast, NR2B is permissive for potentiation by spermine. NR1/NR2A receptors exhibit a much stronger block by Mg2+ than NR1/NR2C channels do. Receptors consisting of NR1/NR2C subunits were less sensitive to inhibition by ethanol compared to receptors consisting of NR1/NR2A or NR1/NR2B, while there was only a minor difference within the sensitivity of the latter two types of receptors to ethanol (Kuner et al., 1993; Buller et al., 1995). In the present study on mesencephalic neurones, NR2A, NR2B, and NR2C subunit expression was determined at a ratio of approximately 1:2:1, respectively, by RT–PCR. In addition, the occurrence of mRNA coding for the NR2D subunit was demonstrated. The question whether the NMDA subunits are expressed in dopaminergic or GABAergic neurones remains as yet open. The answer can be presumably given by carrying out single cell RT–PCR experiments. An immunohistochemical and in situ hybridization study performed in midbrain tissue failed to detect NR2A/B subunits in dopaminergic neurones of the substantia nigra pars compacta or VTA (Paquet et al., 1997).
In conclusion, the present data indicate that mesencephalic neurones bear ethanol sensitive NMDA receptors which might be implicated in the development of ethanol dependence and withdrawal. The high affinity of NMDA and the sensitivity to ifenprodil may suggest that the NMDA receptor comprises the NR1 splice variant lacking E5, and the NR2B subunit. Due to the high potency of memantine, the NMDA receptor may additionally contain the NR2C subunit, which is significantly expressed in mesencephalic neurones.
Acknowledgments
The authors wish to express their gratitude to Dr K. Krieglstein (Institut für Anatomie und Zellbiologie, University Heidelberg) for advice in establishing the mesencephalic primary cultures and to H. Sobottka for excellent technical assistance. The support by the Bundesministerium für Bildung, Forschung und Technologie (Biologische and psychosoziale Faktoren von Drogenmißbrauch und Drogenabhängigkeit; 01 EB 9413/1 und 01 EB 9425) and the Fonds der Chemischen Industrie (FCI; C.A.) is gratefully acknowledged.
Abbreviations
- AP5
DL-2-amino-5-phosphonopentanoic acid
- DIV
days in vitro
- DMSO
dimethyl sulphoxide
- E
exon
- EC50
50%effective concentration
- IC50
50%inhibitory concentration
- NMDA
N-methyl-D-aspartate
- NR1
NMDA receptor subunit 1, NR2A-D, NMDA receptor subunits 2A-D
- RT–PCR
reverse transcriptase-polymerase chain reaction
- SDS
sodium dodecyl sulphate
- SSC
standard saline-citrate
- TTX
tetrodotoxin acetate
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
References
- AUDINAT E., LAMBOLEZ B., ROSSIER J., CRÉPEL F. Activity-dependent regulation of N-methyl-D-aspartate receptor subunit expression in rat cerebellar granule cells. Eur. J. Neurosci. 1994;6:1792–1800. doi: 10.1111/j.1460-9568.1994.tb00572.x. [DOI] [PubMed] [Google Scholar]
- AVENET P., LÉ ONARDON J., BESNARD F., GRAHAM D., FROST J., DEPOORTERE H., LANGER S.Z., SCATTON B. Antagonist properties of the stereoisomers of ifenprodil at NR1A/NR2A and NR1A/NR2B subtypes of the NMDA receptor expressed in Xenopus oocytes. Eur. J. Pharmacol. 1996;296:209–213. doi: 10.1016/0014-2999(95)00700-8. [DOI] [PubMed] [Google Scholar]
- BEHE P., STERN P., WYLLIE D.J., NASSAR M., SCHOEPFER R., COLQUHOUN D. Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc. R. Soc. Lond. (Biol.) 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- BHAVE S.V., HOFFMAN P.L. Ethanol promotes apoptosis in cerebellar granule cells by inhibiting the trophic effect of NMDA. J. Neurochem. 1997;68:578–586. doi: 10.1046/j.1471-4159.1997.68020578.x. [DOI] [PubMed] [Google Scholar]
- BHAVE S.V., SNELL L.D., TABAKOFF B., HOFFMAN P.L. Mechanism of ethanol inhibition of NMDA receptor function in primary cultures of cerebral cortical cells. Alkohol. Clin. Exp. Res. 1996;20:934–941. doi: 10.1111/j.1530-0277.1996.tb05274.x. [DOI] [PubMed] [Google Scholar]
- BLACK M., LANTHORN T., SMALL D., MEALING G., LAM V., MORLEY P. Study of potency, kinetics of block and toxicity of NMDA receptor antagonists using fura-2. Eur. J. Pharmacol. 1996;317:377–381. doi: 10.1016/s0014-2999(96)00730-3. [DOI] [PubMed] [Google Scholar]
- BULLER A.L., LARSON H.C., MORRISETT R.A., MONAGHAN D.T. Glycine modulates ethanol inhibition of heteromeric N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol. Pharmacol. 1995;48:717–723. [PubMed] [Google Scholar]
- CARTER C., RIVY J.-P., SCATTON B. Ifenprodil and SL 82.0715 are antagonists at the polyamine site of the N-methyl-D-aspartate (NMDA) receptor. Eur. J. Pharmacol. 1989;164:611–612. doi: 10.1016/0014-2999(89)90275-6. [DOI] [PubMed] [Google Scholar]
- CEBERS G., CEBERE A., ZHARKOVSKY A., LILJEQUIST S. Glycine does not reverse the inhibitory actions of ethanol on NMDA receptor functions in cerebellar granule cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:736–745. doi: 10.1007/BF00166900. [DOI] [PubMed] [Google Scholar]
- CHEN H.-S.V., PELLEGRINI J.W., AGGARWAL S.K., LEI S.Z., WARACH S., JENSEN F.E., LIPTON S.A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH J., FLETCHER E.J., BAXTER K., MACDONALD J.F. Blockade by ifenprodil of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones: comparison with N-methyl-D-aspartate receptor antagonist actions. Br. J. Pharmacol. 1994;113:499–507. doi: 10.1111/j.1476-5381.1994.tb17017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANYSZ W., PARSONS C.G., KORNHUBER J., SCHMIDT W.J., QUACK G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents–preclinical studies. Neurosci. Biobehav. Rev. 1997;21:455–468. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]
- DARSTEIN M., ALBRECHT C., LÒPEZ-FRANCOS L., KNÖRLE R., HÖLTER S.M., SPANAGL R., FEUERSTEIN T.J. Release and accumulation of neurotransmitters in the rat brain: acute effects of ethanol in vitro and effects of long-term voluntary ethanol intake. Alcohol. Clin. Exp. Res. 1998;22:704–709. [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., LESLIE S.W. Mechanism of inhibition of N-methyl-D-aspartate-stimulated increases in free intracellular Ca2+ concentration by ethanol. J. Neurochem. 1991;56:1536–1543. doi: 10.1111/j.1471-4159.1991.tb02048.x. [DOI] [PubMed] [Google Scholar]
- GONZALES R.A., WOODWARD J.J. Ethanol inhibits N-methyl-D-aspartate-stimulated [3H]-norepinephrine release from rat cortical slices. J. Pharmacol. Exp. Ther. 1990;252:1138–1144. [PubMed] [Google Scholar]
- GÖTHERT M., FINK K. Inhibition of N-methyl-D-aspartate (NMDA)- and L-glutamate-induced noradrenaline and acetylcholine release in the rat brain by ethanol. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;340:516–521. doi: 10.1007/BF00260606. [DOI] [PubMed] [Google Scholar]
- GRANT K.A., LOVINGER D.M. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin. Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HARRIS R.A., BRODIE M.S., DUNWIDDIE T.V. Possible substrates of ethanol reinforcement: GABA and dopamine. Ann. NY. Acad. Sci. 1992;654:61–69. doi: 10.1111/j.1749-6632.1992.tb25956.x. [DOI] [PubMed] [Google Scholar]
- HOFFMAN P.L.Effects of alcohol on excitatory amino acid receptor function Handbook of Experimental Pharmacology 1995vol 114Heidelberg, Springer; 75–102.Kranzler H (ed)The Pharmacology of Alcohol Abuse [Google Scholar]
- HOFFMAN P.L., RABE C.S., MOSES F., TABAKOFF B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J. Neurochem. 1989;52:1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- HU X.-J., TICKU M.K. Chronic ethanol treatment upregulates the NMDA receptor function and binding in mammalian cortical neurons. Mol. Brain Res. 1995;30:347–356. doi: 10.1016/0169-328x(95)00019-o. [DOI] [PubMed] [Google Scholar]
- JOHNSON S.W., SEUTIN V., NORTH R.A. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- KEMP J.A., LEESON P.D. The glycine site of the NMDA receptor – five years on. Trends Pharmacol. Sci. 1993;14:20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- KLECKNER N.W., DINGLEDINE R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- KOLTCHINE V., ANANTHARAM V., WILSON A., BAYLEY H., TREISTMAN S.N. Homomeric assemblies of NMDAR1 splice variants are sensitive to ethanol. Neurosci. Lett. 1993;152:13–16. doi: 10.1016/0304-3940(93)90471-v. [DOI] [PubMed] [Google Scholar]
- KRIEGLSTEIN K., UNSICKER K. Transforming growth factor-ß promotes survival of midbrain dopaminergic neurons and protects them against N-methyl-4-phenylpyridinium ion toxicity. Neuroscience. 1995;63:1189–1196. doi: 10.1016/0306-4522(94)90583-5. [DOI] [PubMed] [Google Scholar]
- KUNER T., SCHOEPFER R., KORPI E.R. Ethanol inhibits glutamate-induced currents in heteromeric NMDA receptor subtypes. NeuroReport. 1993;5:297–300. doi: 10.1097/00001756-199312000-00029. [DOI] [PubMed] [Google Scholar]
- LAURIE D.J., PUTZKE J., ZIEGLGÄNSBERGER W., SEEBURG P.H., TÖLLE T.R. The distribution of splice variants of the NMDAR1 subunit mRNA in adult rat brain. Mol. Brain. Res. 1995;32:94–108. doi: 10.1016/0169-328x(95)00067-3. [DOI] [PubMed] [Google Scholar]
- LAURIE D.J., SEEBURG P.H. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J. Neurosci. 1994;14:3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGENDRE P., WESTBROOK G.L. Ifenprodil blocks N-methyl-D-aspartate receptors by a two-component mechanism. Mol. Pharmacol. 1991;40:289–298. [PubMed] [Google Scholar]
- LOVINGER D.M. Developmental decrease in ethanol inhibition of N-methyl-D-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J. Pharmacol. Exp. Ther. 1995;274:164–172. [PubMed] [Google Scholar]
- LOVINGER D.M., WHITE G., WEIGHT F.F. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., ZIEGLGÄNSBERGER W. Interactions between ethanol and agents that act on the NMDA-type glutamate receptor. Alcohol. Clin. Exp. Res. 1996;20:187A–191A. doi: 10.1111/j.1530-0277.1996.tb01773.x. [DOI] [PubMed] [Google Scholar]
- LUSTIG H.S., VON BRAUCHITSCH K.L., CHAN J., GREENBERG D.A. Ethanol and excitotoxicity in cultured cortical neurons: differential sensitivity of N-methyl-D-aspartate and sodium nitroprusside toxicity. J. Neurochem. 1992;59:2193–2200. doi: 10.1111/j.1471-4159.1992.tb10111.x. [DOI] [PubMed] [Google Scholar]
- MASOOD K., WU C., BRAUNEIS U., WEIGHT F.F. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol. Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- MIRSHAHI T., WOODWARD J.J. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg2+-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- MONYER H., BURNASHEV N., LAURIE D.J., SAKMANN B., SEEBURG P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- MONYER H., SPRENGEL R., SCHOEPFER R., HERB A., HIGUCHI M., LOMELI H., BURNASHEV N., SAKMANN B., SEEBURG P.H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- NAKANISHI S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- NIEBER K., POELCHEN W., SIELER D., ILLES P. Inhibition by ethanol of excitatory amino acid receptors in rat locus coeruleus neurons in vitro. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:299–308. doi: 10.1007/pl00005171. [DOI] [PubMed] [Google Scholar]
- PAQUET M., TREMBLAY M., SOGHOMONIAN J.-J., SMITH Y. AMPA and NMDA glutamate receptor subunits in midbrain dopaminergic neurons in the squirrel monkey: an immunohistochemical and in situ hybridization study. J. Neurosci. 1997;17:1377–1396. doi: 10.1523/JNEUROSCI.17-04-01377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSONS C.G., GRUNER R., ROZENTAL J., MILLAR J., LODGE D. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memenatine (1-amino-3,5-dimethyladamantan) Neuropharmacology. 1993;32:1337–1350. doi: 10.1016/0028-3908(93)90029-3. [DOI] [PubMed] [Google Scholar]
- PEOPLES R.W., WEIGHT F.F. Ethanol inhibition of N-methyl-D-aspartate-activated ion current in rat hippocampal neurons is not competitive with glycine. Brain. Res. 1992;571:342–344. doi: 10.1016/0006-8993(92)90674-x. [DOI] [PubMed] [Google Scholar]
- POELCHEN W., NIEBER K., ILLES P. Tolerance to inhibition by ethanol to N-methyl-D-aspartate-induced depolarization in rat locus coeruleus neurons in vitro. Eur. J. Pharmacol. 1997;332:267–271. doi: 10.1016/s0014-2999(97)01113-8. [DOI] [PubMed] [Google Scholar]
- ROHRBACHER J., KRIEGLSTEIN K, , HONERKAMP S, , LEWEN A., MISGELD U. 5,7-Dihydroxytryptamine uptake discriminates living serotonergic cells from dopaminergic cells in rat midbrain culture. Neurosci. Lett. 1995;199:207–210. doi: 10.1016/0304-3940(95)12060-h. [DOI] [PubMed] [Google Scholar]
- SCHEIBLER P., MÜLLER D., ILLES P., ALLGAIER C. NMDA receptor function and mRNA subunit expression in rat cultured mesencephalic neurones. Eur. J. Neurosci. 1998;10 suppl 10:130. doi: 10.1038/sj.bjp.0702284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOEPFER R., MONYER H., SOMMER B., WISDEN W., SPRENGEL R., KUNER T., LOMELI H., HERB A., KÖHLER M., BURNASHEV N., GÜNTHER W., RUPPERSBERG P., SEEBURG P. Molecular biology of glutamate receptors. Prog. Neurobiol. 1992;42:353–357. doi: 10.1016/0301-0082(94)90076-0. [DOI] [PubMed] [Google Scholar]
- SHIMODA K., SAUVE Y., MARINI A., SCHWARTZ J.P., COMMISSIONG J.W. A high percentage yield of tyrosinehydroxylase-positive cells from rat E14 mesencephalic cell culture. Brain Res. 1992;586:319–331. doi: 10.1016/0006-8993(92)91642-r. [DOI] [PubMed] [Google Scholar]
- SUCHER N.J., AWOBULUYI M., CHOI Y.-B., LIPTON S.A. NMDA receptors: from genes to channels. Trends Pharmacol. Sci. 1996;17:348–355. [PubMed] [Google Scholar]
- TRUBE G., MALHERBE P. Interaction between ifenprodil-like antagonists and polyamines at cloned NMDA receptors. Eur. J. Neurosci. 1998;10 suppl 10:131. [Google Scholar]
- WILLIAMS K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- WOODWARD J.J. A comparison of the effects of ethanol and the competitive glycine antagonist 7-chlorokynurenic acid on N-methyl-D-aspartic acid-induced neurotransmitter release from rat hippocampal slices. J. Neurochem. 1994;62:987–991. doi: 10.1046/j.1471-4159.1994.62030987.x. [DOI] [PubMed] [Google Scholar]
- WOODWARD J.J., GONZALES R.A. Ethanol inhibition of N-methyl-D-aspartate-stimulated endogenous dopamine release from rat striatal slices: reversal by glycine. J. Neurochem. 1990;54:712–715. doi: 10.1111/j.1471-4159.1990.tb01931.x. [DOI] [PubMed] [Google Scholar]
- ZHONG J., CARROZZA D.P., WILLIAMS K., PRITCHETT D.B., MOLINOFF P.B. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J. Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- ZUKIN R.S., BENNETT M.V.L. Alternatively spliced isoforms of the NMDAR1 receptor subunit. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]


