Abstract
This study sought to evaluate whether the effects of acute and long-term treatment with 17-β-estradiol on the vasomotor responses in rat aortic rings are mediated through the same mechanism.
Ovariectomized rats were treated daily with either 17-β-estradiol-3-benzoate (100 μg kg−1) or vehicle for 1 week.
The effect of long-term 17-β-estradiol treatment on the responses to cumulative doses of phenylephrine, 5-HT, calcium, potassium and 17-β-estradiol was determined in aortic rings. In the same rings, the effect of acute exposure to 17-β-estradiol (5 and 10 μM) on the dose response curves for phenylephrine, 5-HT, calcium, potassium and acetylcholine were estimated. The measurements were made in rings with and without intact endothelium. The tone-related basal release of nitric oxide (NO) was measured in rings with intact endothelium.
Long-term 17-β-estradiol treatment reduced the maximum developed contraction to all contracting agents studied. This effect was abolished in endothelium denuded vessels. Acute 17-β-estradiol treatment also reduced maximal contraction. This effect, however, was independent of the endothelium.
Long-term 17-β-estradiol treatment significantly increased the ability of the rings to dilate in response to acetylcholine whereas acute exposure to 17-β-estradiol had no effect. The tone-related release of NO was significantly increased after long-term exposure to 17-β-estradiol.
In conclusion, this study indicate that the acute and long-term effects of 17-β-estradiol in the rat aorta are mediated through different mechanisms. The long-term effect is mediated through the endothelium most likely by increasing NO release. In contrast, the acute effect of 17-β-estradiol seems to be through an effect on the vascular smooth muscle cells.
Keywords: 17-β-Estradiol, acute, aorta, calcium, long-term, nitric oxide, rat, vasomotor response
Introduction
Cardiovascular disease is more prevalent in men and post-menopausal women than in pre-menopausal women or post-menopausal women treated with oestrogen replacement therapy (Sullivan et al., 1988; Barret-Connor & Bush, 1991; Stampfer et al., 1991). Observations which suggest a cardioprotective effect of oestrogen. Although randomized clinical trials are yet to come, the protective action of oestrogen has been demonstrated in numerous animal studies (Kushwaha & Hazzard 1981; Adams et al., 1990; Holm et al., 1995; Sulistiyani et al., 1995).
The cardioprotective effect of oestrogen has been explained by its ability to lower plasma cholesterol (Walsh et al., 1991). However, oestrogen also has a direct effect on the arterial wall (Bush et al., 1987; Holm et al., 1997). Long-term treatment of cholesterol-fed monkeys with 17-β-estradiol has been shown to augment the endothelium dependent vasodilatory response to acetylcholine, an effect which also has been illustrated in humans (Williams et al., 1990; Herrington et al., 1994; Collins et al., 1995). This effect has also been demonstrated after acute administration of 17-β-estradiol (<20 min) (Williams et al., 1992). These findings are of particular interest as several studies have demonstrated a relationship between endothelium-dependent vasodilatation and the development of atherosclerosis in both animals and humans (Ludmer et al., 1986; Gilligan et al., 1994; Reis et al., 1994; Collins et al., 1995). Considering these observations, it has been hypothesized that an impaired endothelium-dependent vasodilatation is a predictor for the development of atherosclerosis (Hayashi et al., 1995). Accordingly, drugs which are able to prevent this reduction could potentially prevent the development of atherosclerosis (Hayashi et al., 1997).
The function of the endothelium is easily studied in an isolated vessel preparation and this method has been used extensively to study the effects of acute and long-term treatment of 17-β-estradiol on the vasomotor responses of different vessels (Vargas et al., 1989; Miller & Vanhoutte 1990; Jiang et al., 1992; Paredes-Carbajal et al., 1995). However, the effects of the two treatments have never been directly compared. This in spite of the fact that the effect seen after acute exposure to 17-β-estradiol in vitro have been used to explain the cardioprotective role of long-term 17-β-estradiol treatment (Collins et al., 1993; Han et al., 1995; Kitazawa et al., 1997). One obvious difference between the two types of 17-β-estradiol treatments are the concentration need to initiate the effects seen. While the plasma concentration needed to mediate the long-term effects of 17-β-estradiol is within the nanomolar range, it requires concentrations that are approximately 1000 times higher to initiate the acute effect seen in vitro. Some authors, however, have suggested that the dose question regarding the concentration is more of a technical nature as the actual concentration achieved in the tissue in vivo might be higher than the circulating plasma level (Collins et al., 1993; Kitazawa et al., 1997). This effect is explained partially by the lipophilic nature of 17-β-estradiol and the ability of vascular smooth muscle cells to synthesize 17-β-estradiol (Bayard et al., 1995). A similar phenomenon is known to occur with the calcium channel blocker nifedipine (Opie & Singh, 1987) where a higher concentration in vitro than in vivo is necessary to elicit vascular effects. Furthermore, one of the limitations of the previous studies has been that only very few agents which affect the vasomotor responses have been studied. This questions whether the effect seen is specific for the individual agent or it is of a more general nature.
The current study therefore, examines whether the effects of acute and long-term 17-β-estradiol treatment on the vasomotor responses of the rat aorta by using a variety of agents with different mechanisms of action. Additionally, the interaction between acute and long-term 17-β-estradiol treatment is examined.
Methods
Animals
Sixty-two sexually mature, 8-weeks-old, female Sprague-Dawley rats (200–220 g) from Møllegaards Breeding Centre (Ll. Skedsved, Denmark) were individually housed under controlled conditions with food and water ad libitum. In all rats, bilateral ovariectomy was performed through an incision on the back under halothane anaesthesia. After surgery, buprenorphine (0.015 mg rat−1, s.c.) was administered to reduce postsurgical pain. A 6–7 day recovery period was allowed prior to the initiation of treatment. The animals were divided into two experimental groups treated daily with either 17-β-estradiol-3-benzoate (100 μg kg−1, s.c.) or vehicle for seven days. This treatment was defined as long-term treatment. All the experiments were performed in accordance with the Danish legislation on use of animals in research.
Aortic ring preparation
On the day of experiment, the rats were euthanized by cervical dislocation. The thoracic aorta was immediately isolated and cut into four rings (3 mm long). Each ring was suspended in an organ bath (5 ml) between two parallel stainless steel hooks. One hook was fixed, while the other was connected to a force transducer for the measurement of isometric tension. The organ baths contained a Krebs solution at 37°C, bubbled with 95% O2 and 5% CO2. The composition of the Krebs solution was as follows (mM): NaCl 121.5; KCl 4.7; CaCl2 2.5; MgSO4 1.2; KH2PO4 1.2, NaCHO3 25.0 and glucose 11.1. In Krebs solutions with high concentrations of potassium, the potassium was exchanged with sodium on a molar basis. The rings were initially stretched until a basal tension of 2.0 g and allowed to equilibrate for at least 45 min. At a basal tension of 2.0 g the rings were found to develop maximal active tension to stimulation with a contracting agent.
Bioassay
Each experiment began with the repeated contraction of the rings with 80 nM phenylephrine until the responses were reproducible. The responses were defined as reproducible when the fluctuation around the mean value of three consecutive responses was less than±10%. Rings from the two long-term treatment groups were always studied in parallel. In the first part of the study, a set of rings with functionally intact endothelium were studied. The presence of functional endothelium was verified at the beginning of each experiment by the ability of acetylcholine (10 μM) to relax a precontracted vessel (Furchgott & Zawadzki, 1980). Vessels that were able to relax less than 20% were excluded from the study. In the second part of the study rings denuded from the endothelium were investigated. The rings were excluded from this part of the study if they were able to relax to acetylcholine. Except for the acetylcholine dose-response curve and the basal release of nitric oxide (NO) which for obvious reasons was not determined in the endothelium denuded arteries the following dose response curves were determined in both parts of the study. Each ring was exposed to only one contracting or dilating agent.
The influence of 17-β-estradiol on the dose response curves for phenylephrine, 5-HT and potassium
The effect of long-term 17-β-estradiol treatment on cumulative dose response curves for either phenylephrine, 5-HT or potassium was determined in the concentration range of 10−9–10−5 M, 10−8–10−3 M and 5–126 mM, respectively. Following this determination, the rings were washed in Krebs solution and allowed to relax. The effect of acute 17-β-estradiol treatment on the dose response curves was then evaluated by adding 5 μM of 17-β-estradiol to the bathing solution 30 min prior to obtaining a second series of dose response curves. This procedure was repeated with 10 μM 17-β-estradiol. Pilot studies had demonstrated that the dose response curves were reproducible over the time of the experiments.
The influence of 17-β-estradiol on the dose-response curve for calcium
The effect of 17-β-estradiol on the dose response curve for calcium was determined after removal of the extracellular calcium. Twenty minutes prior to the determination, the extracellular calcium was removed by adding a calcium-free Krebs solution containing 100 μM EDTA to the organ bath. Complete removal of the extracellular calcium was tested by demonstrating that no contraction occurred in response to a calcium-free Krebs solution containing potassium (100 mM). A cumulative dose response curve for calcium between 1 μM and 7.5 mM was determined. Although maximum contraction was not reached at 7.5 mM calcium, it was not possible to increase the concentration of calcium further since the calcium-sensitivity of the tissue was found to decrease after exposure to higher concentrations of calcium. The influence of acute exposure to 17-β-estradiol was investigated as described above.
The influence of 17-β-estradiol on the dose response curve for acetylcholine
A cumulative dose response curve for acetylcholine (10−8–10−5 M) was determined on rings precontracted with phenylephrine (10 μM) in order to investigate the influence of long-term 17-β-estradiol treatment on endothelium dependent vasodilatation. The influence of acute 17-β-estradiol treatment was investigated as described above. A time control was included in these experiments.
Basal release of NO
The effect of long term 17-β-estradiol treatment on the basal release of NO was quantified by measuring the contraction developed to 100 μM Nω-nitro-L-arginine methyl ester (L-NAME) on precontracted rings. The rings were precontracted with phenylephrine to 30% of the contraction developed to 126 mM potassium.
The direct vasodilating effect of 17-β-estradiol
To determine if the vasodilating effect of 17-β-estradiol was affected by long-term 17-β-estradiol treatment, a cumulative dose response curve for 17-β-estradiol (10−8–10−3 M) was obtained in precontracted rings. The rings were precontracted with a dose of phenylephrine that was equivalent to 50% of the contraction developed to potassium (126 mM)
Evaluation of oestrogen exposure in vivo
To evaluate the in vivo exposure to 17-β-estradiol, blood samples from 17-β-estradiol-3-benzoate on vehicle treated animals (n=6) were taken and the plasma level of 17-β-estradiol was determined by Medi-Lab (Copenhagen, Denmark). Furthermore, the uteri from these animals were dissected free and weighed.
Data analysis
For each ring, the response to the highest concentration of the contracting agent was defined as the maximum contraction (100%), this also applies to the dose response curves for calcium. The EC50-values were calculated by Excel (Microsoft®) by fitting an exponential curve to the data. The effects of the various agents on the vascular reactivity were compared by a two way analysis of variance. A P-value below 0.05 was taken as significant.
Drugs
Acetylcholine, L-phenylephrine, 5-HT.kreatininsulphate, Nω-nitro-L-arginine methyl ester and water-soluble 17-β-estradiol (from Sigma Chemicals) was dissolved in isotonic glucose solution (278 mM). 17-β-estradiol-3-benzoate (Sigma) was suspended in saline and propylene glycol (Fluka) 1 : 1. All other chemicals used were obtained from Merck.
Results
Acute and long-term effects of 17-β-estradiol treatment on contraction in rings with intact endothelium
Long-term treatment with 17-β-estradiol significantly (P=0.002, n=24) decreased the maximal developed tension to all the contracting agents studied by 13–17%, as compared to the control (Figure 1). The sensitivity (calculated as the concentration of agonist causing a half-maximal response (EC50-value)) to neither phenylephrine, 5-HT nor calcium was affected (Table 1, Figure 1). The sensitivity to potassium, however, was decreased by 20% as compared to the control group (P=0.01, n=6) (Table 1).
Figure 1.
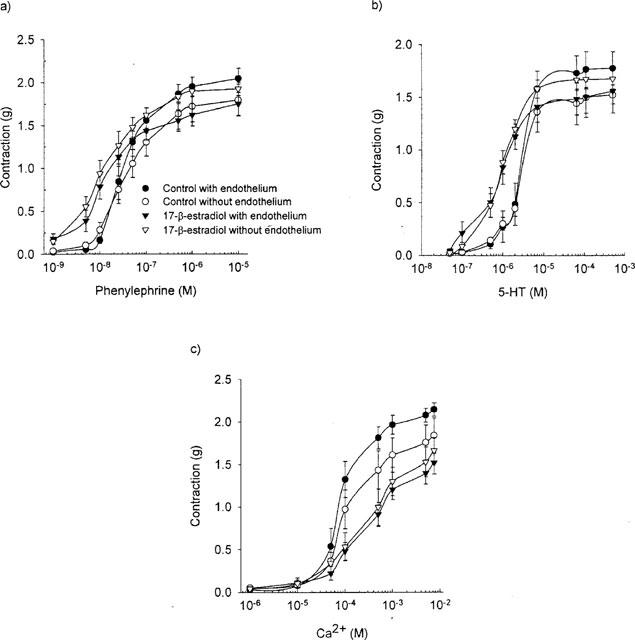
The effect of long-term exposure to 17-β-estradiol-3-benzoate (100 (μg day) kg−1) on the dose response curve for (a) phenylephrine (b) 5-HT and (c) calcium in endothelium intact and endothelium denuded rings.
Table 1.
−Log (EC50) to different contracting agents in rings with intact endothelium
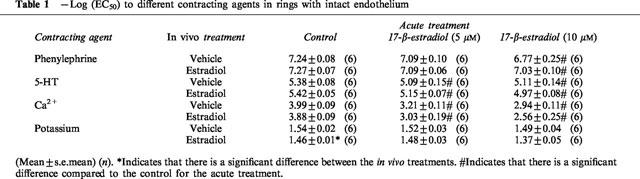
As in the case of long-term exposure to 17-β-estradiol, acute exposure to 17-β-estradiol also decreased the maximal developed tension to all contracting agents investigated compared to the control situation (Figure 2), although it only reached statistical significance after exposure to 10 μM. It was, however, not possible to estimate the maximal contraction developed to calcium after acute exposure to 17-β-estradiol (see Methods and Figure 2c). The sensitivity to both phenylephrine and 5-HT was reduced after acute exposure to 17-β-estradiol although it only reached statistical significance after exposure to 10 μM in the case of phenylephrine. Acute exposure to 17-β-estradiol caused an almost parallel right-ward shift of the dose response curve for calcium (Figure 2c). Under the assumption that the same maximal contraction could be reached as in the control rings, the sensitivity to calcium in the presence of 17-β-estradiol was calculated. Under these circumstances the sensitivity to calcium was statistically significant and reduced both in the presence of 5 and 10 μM 17-β-estradiol. The sensitivity to potassium was not affected by acute exposure to 17-β-estradiol.
Figure 2.
The effect of acute exposure to 17-β-estradiol on the dose response curve for (a) phenylephrine (b) 5-HT (c) calcium and (d) potassium in endothelium intact rings exposed to vehicle for seven days.
There was no interaction between the effects of long-term and acute exposure to 17-β-estradiol on either the maximum contraction or the sensitivity i.e. the effect of acute exposure to 17-β-estradiol was not dependent on the long-term treatment.
Acute and long-term effects of 17-β-estradiol treatment on dilation
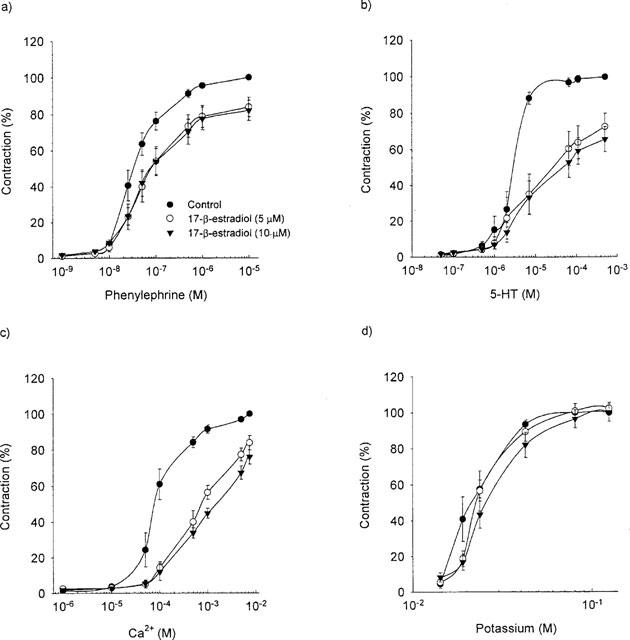
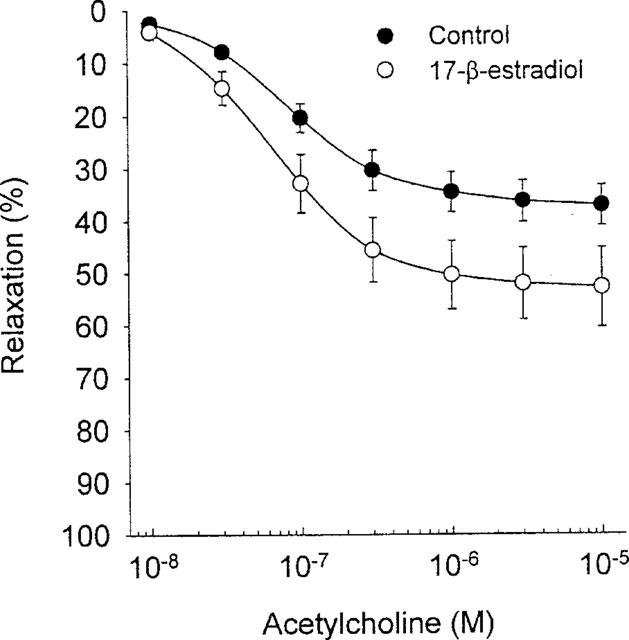
Long-term treatment with 17-β-estradiol significantly increased the maximum relaxation to acetylcholine by 10% (P=0.038; n=9) without affecting the sensitivity (Figure 3). Acute treatment with 17-β-estradiol, however, had no effect on either the maximum contraction or the sensitivity to acetylcholine (data not shown).
Figure 3.
The long-term effect of 17-β-estradiol-3-benzoate (100 (μg day) kg−1) or vehicle on the dose response curve for acetylcholine.
17-β-estradiol dose dependently dilated the rings with a maximum relaxation of 65–68% and an EC50-value of 16–22 μM. This effect was not affected by long-term 17-β-estradiol treatment (Figure 4).
Figure 4.
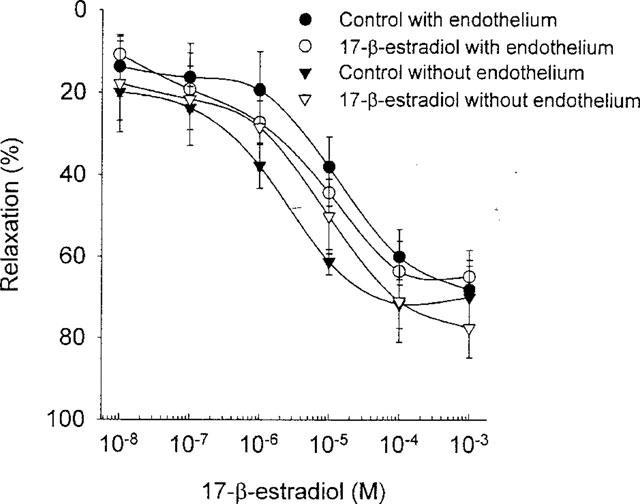
The effect of long-term exposure to 17-β-estradiol-3-benzoate (100 (μg day) kg−1) on the dose response curve 17-β-estradiol in endothelium intact and endothelium denuded rings.
Tone related NO-release
Long-term 17-β-estradiol treatment increased the basal release of NO measured as contraction to L-NAME from 40% in the control rings to 57% in the rings treated with 17-β-estradiol (P=0.034; n=7).
Effect of endothelium denudation on contraction after acute and long-term 17-β-estradiol treatment
In a separate set of rings, the endothelium was denuded. In these rings an effect of the long-term 17-β-estradiol treatment was observed on the maximal contraction to any of the contracting agent studied. (P=0.28, n=26–32, Figure 1). The long-term 17-β-estradiol treatment did not affect the EC50-value to any of the contracting agents. (Table 2). However, independent of the long-term treatment with 17-β-estradiol the rings denuded of endothelium were 2–6 times more sensitive to phenylephrine and 5-HT than the rings with functional endothelium (compare Tables 1 and 2). The acute 17-β-estradiol treatment was independent of the presence of the endothelium (Figure 5). The same was true for the direct vasodilatory effect of 17-β-estradiol (Figure 4).
Table 2.
−Log (EC50) to different contracting agents in rings with denuded endothelium
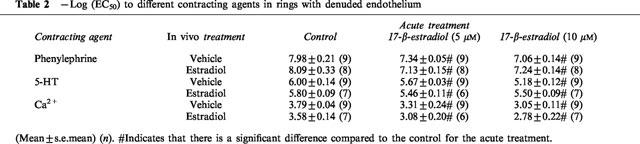
Figure 5.
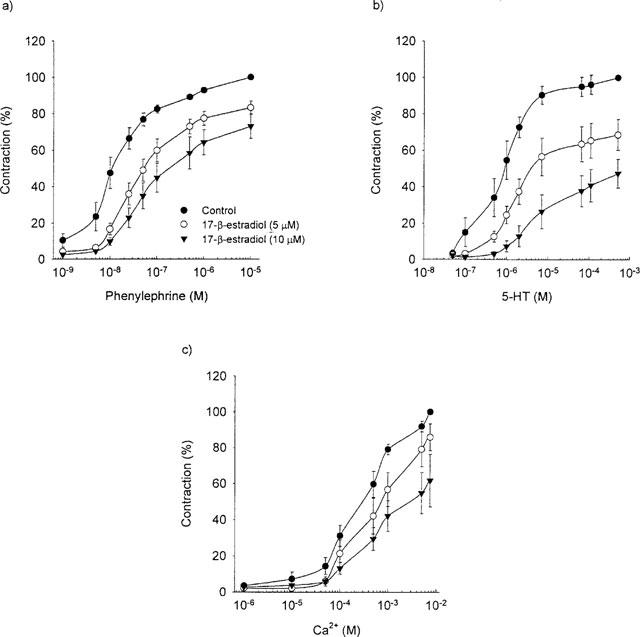
The effect of acute exposure to 17-β-estradiol on the dose response curve for (a) phenylephrine, (b) 5-HT and (c) calcium in endothelium denuded rings exposed to vehicle for seven days.
Level of in vivo exposure to 17-β-estradiol
The plasma levels of 17-β-estradiol in the samples taken from treated animals were ≈amp;100 pg ml−1 (0.4 nM) whereas the concentration in the vehicle treated animals were below the detection level of 20 pg ml−1. The normal plasma level of 17-β-estradiol in rats that have been reported in the literature varies from 20–360 pg ml−1 for non-pregnant rats (Wellman et al., 1996; Huang et al., 1997). The physiological effect of the treatment was detected by an increase in the uterus weight from 133±5 mg to 549±26 mg (P<0.05, n=6).
Discussion
To the best of our knowledge, this is the first study to demonstrate that the effects of acute and long-term 17-β-estradiol treatment on vasomotor responses may not be mediated through the same mechanisms. While the acute effect was independent of the presence of a functional endothelium, the effect of long-term treatment was abolished after the endothelium was removed. In addition, this study demonstrates that the acute effect of 17-β-estradiol treatment is unaffected by long-term treatment.
Long-term effects of 17-β-estradiol
The maximum developed contraction to phenylephrine was significantly decreased after long-term 17-β-estradiol treatment in this study, an effect which has previously been demonstrated both in vivo and in vitro (Ezimokhai et al., 1994; Williams et al., 1994; Paredes-Carbajal et al., 1995). It has been proposed that long-term 17-β-estradiol treatment affects the α-adrenergic receptor density and/or affinity (Bento & Moraes, 1992; Collucci et al., 1982; Gisclard et al., 1988; Larson et al., 1984). However, the effect observed in this study seems more general in nature since the maximum developed contraction to two different receptor ligands (α1-adrenoceptor and 5-HT) and the contraction mediated by passive depolarization of the vascular smooth muscle was affected. This is supported by the work of Honda et al. (1996) and Vedernikov et al. (1997) who also found that the maximum developed contraction to potassium was affected by long-term 17-β-estradiol treatment. Thus, the long-term effect of 17-β-estradiol cannot be explained solely by an effect on receptor density and/or receptor affinity.
Another mechanism suggested to explain the long-term effect of 17-β-estradiol on the vasomotor responses is via processes in the endothelium. The endothelium produces and releases various vasoactive substances that modulate the response of the underlying vascular smooth muscle cells (Furchgott & Zawadzki, 1980; Furchgott & Vanhoutte, 1989; Vita et al., 1990). One of the mediators has been identified as NO that is released under agonist stimulation (Palmer et al., 1987). There is, however, also evidence for a continuous release of NO both in vivo and in vitro (Griffith et al., 1984; Rees et al., 1989). Indeed, we found a dose dependent relaxation to acetylcholine in rings with intact endothelium and evidence for a basal release of NO. Both these effects were increased after long-term 17-β-estradiol treatment. It therefore seems possible that one of the mechanisms by which long-term 17-β-estradiol treatment can depress the contraction is by increasing the release of NO from the endothelial cells.
To investigate the role of the endothelium in the long-term effect of 17-β-estradiol, we included a set of experiments in which the endothelium was removed. Removal of the endothelium abolished the effect of the long-term 17-β-estradiol treatment, indicating that the site of action of long-term 17-β-estradiol treatment is associated with the endothelium, a finding which has also been previously reported by others (Maddox et al., 1987; Paredes-Carbajal et al., 1995; Wellman et al., 1996; Meyer et al., 1997). These findings are supported by reports studying various effects of 17-β-estradiol on the endothelial NO synthases all showing that a treatment period of approximately 24 h is necessary for the effect to become apparent (Weiner et al., 1994; Hayashi et al., 1995; Hishikawa et al., 1995; Magness et al., 1997; Wingrove & Stevenson, 1997).
Our findings therefore, suggest that long-term 17-β-estradiol treatment affects the vasomotor responses through increasing the release of endothelium dependent NO. This process, in turn, decreases the ability of the aorta to contract to different contracting agents and increases the dilation to acetylcholine. The endothelium, however, also produces various other vasoactive substances which is affected by estrogen treatment e.g. prostaglandins and endothelin-1 (Myers et al., 1996; Wingrove & Stevenson, 1997). However, it was not the scope of these paper to investigate a particular mechanism by which long-term 17-β-estradiol affects vascular reactivity but only to elucidate whether it is reasonable to assume that the mechanism by which acute and long-term treatment with 17-β-estradiol is identical.
Acute effects of 17-β-estradiol
In this study we demonstrated a direct dilatory effect of 17-β-estradiol, an effect which was observed within 5 min after the application of the compound to the vessel. Based on this finding and previous reports (Jiang et al., 1991; Mügge et al., 1993; Chester et al., 1995) it seems unlikely that this effect is mediated through the classical nuclear/cytostolic oestrogen receptors, although both the classical oestrogen receptor and specific binding site for steroidal sex hormones in cell membranes have been described (Pietras & Sezego, 1979; Karas et al., 1994; Losordo et al., 1994). Also, to support this hypothesis various studies have shown that the acute effects of 17-β-estradiol was unaffected by inhibiting the protein synthesis (Wellman et al., 1996; Kitazawa et al., 1997). Furthermore, the effects have been demonstrated to be readily reversible, which is incompatible with an effect on gene activation (Kitazawa et al., 1997) and the binding of various oestrogenic compounds to the nuclear oestrogen receptor do not reflect their ability to inhibit evoked contraction (Kitazawa et al., 1997). On the other hand, the effects of the acute 17-β-estradiol exposure do not seem to be due to an unspecific action of 17-β-estradiol on the cell membrane since it does not affect cell membrane resting potential and 17-β-estradiol and 17-α-estradiol have the same physio-chemical properties but not the same inhibitory effect on contraction (Vargas et al., 1989; Thomas et al., 1995).
It has been hypothesized by several authors that 17-β-estradiol is a calcium-channel antagonist (Stice et al., 1987; Jiang et al., 1991; 1992; Collins et al., 1993; Salas et al., 1994). Our acute 17-β-estradiol treatment caused an almost parallel right-ward shift in the dose response curve for calcium under depolarization condition, supporting this hypothesis. Inhibition of calcium-influx could explain the endothelium independent inhibition of the contraction seen after acute 17-β-estradiol treatment in this study. Indeed, this is supported by the observations of Vargas et al. (1989) who found that the inhibitory effect of 17-β-estradiol on the dose response curve for phenylephrine was completely abolished under calcium-free conditions. Also, it has been reported that the inhibitory effect of 17-β-estradiol on the contractile response to U46619 was weaker under calcium-free conditions (Han et al., 1995). Work by Han et al. (1995) has shown that 17-β-estradiol relaxes arteries by inhibiting calcium influx without affecting the calcium sensitivity of the contractile proteins or the resting tone of the arteries whereas patch-clamp studies have further revealed that 17-β-estradiol inhibits the voltage operated calcium-current (L-type) in vascular smooth muscle cells. Findings which are in harmony with the parallel shift of the calcium dose response curve under depolarization condition (Jiang et al., 1992; Zhang et al., 1994). All findings explain the parallel right-ward shift of the calcium-dose response curve after acute 17-β-estradiol treatment and the endothelium independent inhibition of the contraction to various agents seen after acute 17-β-estradiol treatment.
Even though the hypothesis of 17-β-estradiol being a calcium-antagonist seems to explain most of the effects of acute 17-β-estradiol treatment, it does not explain the direct vasodilatory effect of 17-β-estradiol. Indeed, the vasodilatory effect of 17-β-estradiol has been demonstrated to be independent of the presence of extracellular calcium (Babai et al., 1995). However, neither does this effect seem to be mediated through an increase in NO release, since it was found to be endothelium independent. In contrast to the effect of long-term 17-β-estradiol treatment where most of the observed effects seems to be explained by an increase in NO synthesis and release, this mechanism does not explain any of the effects seen after acute 17-β-estradiol treatment, since we did not observe any effect on the NO system after acute 17-β-estradiol treatment. Taken together, these results demonstrate that the effect of 17-β-estradiol on NO production is only seen after long-term treatment.
Another possible mechanism of action for 17-β-estradiol could be through inhibition of tyrosine kinases in the vascular smooth muscle cell. Tyrosine kinases play an important role in the development of contraction mediated by different agonists, an effect which is accomplished within minutes (Laniyonu et al., 1994; Jinsi & Deth 1995; Toma et al., 1995; Yousif et al., 1997). Indeed, many of the so called phyto-oestrogens, compounds with oestrogenic activity isolated from plant material, are known tyrosine kinase inhibitors (e.g. genistein and tyrophostine). Also, both 17-β-estradiol and tyrosine kinase inhibitors has been described to modulate arterial contractility in vivo as well as in vitro (Williams et al., 1994; Paredes-Carbajal et al., 1995; Jinsi & Deth 1995; Sauro et al., 1996) an effect which has been shown to be independent of extracellular calcium (Abebe & Agrawal, 1995). The direct vasodilating effect of 17-β-estradiol could therefore be mediated via an inhibition of the tyrosine kinases in the vascular smooth muscle cell, however, this theory remains speculative at the moment.
In conclusion, this study demonstrates that the acute and long-term effect of 17-β-estradiol in the rat aorta is probably mediated through different mechanisms. Furthermore, this study suggests that the long-term effect is mediated through the endothelium most likely by increasing NO release. The acute effect seems to be mediated through an effect on the calcium homeostasis in the vascular smooth muscle cells independent of the endothelium. Certainly other mechanisms can be involved in both effects.
Acknowledgments
We are extremely grateful for the critical review of the manuscript by Dr Ronda Stavisky and for the excellent technical assistance from Anne Lund.
Abbreviations
- EC50-value
concentration of agonist causing a half-maximal response
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
References
- ABEBE W., AGRAWAL D.K. Role of tyrosine kinases in norepinephrine-induced contraction of vascular smooth muscle. J. Cardiovas. Pharmacol. 1995;26:153–159. doi: 10.1097/00005344-199507000-00024. [DOI] [PubMed] [Google Scholar]
- ADAMS M.R., KAPLAN J.R., MANUCK S.B., KORITNIK D.R., PARKS J.S., WOLF M.S., CLARKSON T.B. Inhibition of coronary artery atherosclerosis by 17-β-estradiol in ovariectomized monkeys. Athersclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- BABAI H., EVANS T., IRVING G., McCURRIE J.R. Calcium-independence of the relaxant effect of oestrogens on isolated rat aorta. Br. J. Pharmacol. 1995;116:402. [Google Scholar]
- BARRET-CONNER E., BUSH T.L. Estrogen and coronary heart diseases in women. JAMA. 1991;265:1861–1867. [PubMed] [Google Scholar]
- BAYARD F., CLAMENS S., MEGGETTO F., BLAES N., DELSOL G., FAYE J.C. Estrogen synthesis, estrogen metabolism, and functional estrogen receptors in rat arterial smooth muscle cells in culture. Endocrinology. 1995;136:1523–1529. doi: 10.1210/endo.136.4.7895662. [DOI] [PubMed] [Google Scholar]
- BENTO A.C., DE MORAES S. Effects of estrogen pretreatment of the spare alpha 1-adrenoceptors and the slow and fast components of the contractile response of the isolated female rat aorta. Gen. Pharmacol. 1992;23:565–570. doi: 10.1016/0306-3623(92)90129-8. [DOI] [PubMed] [Google Scholar]
- BUSH T.L., BARRET-CONNOR E., COWAN L.D., CRIQUI M.H., WALLACE R.B., SUCHINDRAN C.M., TYROLER H.A., RIFKIND B.M. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- CHESTER A.H., JIANG C., BORLAND J.A., YACOUB M.H., COLLINS P. Oestrogen relaxes human epicardial coronary arteries through non-endothelium-dependent mechanisms. Coron. Artery. Dis. 1995;6:417–422. doi: 10.1097/00019501-199505000-00009. [DOI] [PubMed] [Google Scholar]
- COLLINS P., ROSANO G.M., JIANG C., LINDSAY D., SARREL P.M., POOLE-WILSON P.A. Cardiovascular protection by oestrogen–a calcium antagonist effect. Lancet. 1993;341:1264–1265. doi: 10.1016/0140-6736(93)91158-i. [DOI] [PubMed] [Google Scholar]
- COLLINS P., ROSANO G.M., SARREL P.M., ULRICH L., ADAMOPOULOS S., BEALE C.M., MCNEILL J.G., POOLE-WILSON P.A. 17-beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- COLLUCCI W.S., GIMBRONE M.A., MACLAUGHILN M.K., HALPERN W., ALEXANDER R.W. Increased vascular catecholamine sensitivity and α-adrenergic receptor affinity in female and estrogen treated male rats. Circ. Res. 1982;50:805–811. doi: 10.1161/01.res.50.6.805. [DOI] [PubMed] [Google Scholar]
- EZIMOKHAI M., ALOAMAKA C.P., CHERIAN T., MORRISON J. The role of extracellular calcium in pregnancy-induced attenuation of Phenylephrine contraction in rat aorta with functional endothelium. J. Comp. Physiol. Biochem. Sys. Enviro. Physiol. 1994;164:81–87. doi: 10.1007/BF00714575. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., VANHOUTTE P.M. Endothelium-derived relaxation and contracting factors. FASEB J. 1989;3:2007–20018. [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., QUYYUMI A.A., CANNON R.O. Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- GISCLARD V., MILLER V.M., VANHOUTTE P.M. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J. Pharmacol. Exp. Ther. 1988;244:19–22. [PubMed] [Google Scholar]
- GRIFFITH J.C., HENDERSON A.H., HUGHES D., LEWIS M.J. Isolated perfused coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J. Physiol. 1984;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN S.Z., KARAKI H., OUCHI Y., AKISHITA M., ORIMO H. 17 beta-Estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation. 1995;91:2619–2626. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- HAYASHI T., YAMADA K., ESAKI T., KUZUYA M., SATAKE S., ISHIKAWA T., HIDAKA H., IGUCHI A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem. Biophys. Res. Com. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- HAYASHI T., YAMADA K., ESAKI T., MUTOH E., IGUCHI A. Effect of estrogen on isoforms of nitric oxide synthase: possible mechanism of anti-atherosclerotic effect of estrogen. Gerontology. 1997;43:124–134. doi: 10.1159/000213883. [DOI] [PubMed] [Google Scholar]
- HERRINGTON D.M., BRADEN G.A., WILLIAMS J.K., MORGAN T.M. Endothelial-dependent coronary vasomotor responsiveness in postmenopausal women with and without estrogen replacement therapy. Am. J. Cardiol. 1994;73:951–952. doi: 10.1016/0002-9149(94)90136-8. [DOI] [PubMed] [Google Scholar]
- HISHIKAWA K., NAKAKI T., MARUMO T., SUZUKI H., KATO R., SARUTA T. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett. 1995;360:291–293. doi: 10.1016/0014-5793(95)00124-r. [DOI] [PubMed] [Google Scholar]
- HOLM P., ANDERSEN H.O., NORDESTGAARD B.G., HANSEN B.F., KJELDSEN K., STENDER S. Effect of oestrogen replacement therapy on development of experimental arteriosclerosis: a study in transplanted and balloon-injured rabbit aortas. Atherosclerosis. 1995;115:191–200. doi: 10.1016/0021-9150(94)05513-i. [DOI] [PubMed] [Google Scholar]
- HOLM P., KORSGAARD N., SHALMI M., ANDERSEN H.L., HOUGAARD P., SKOUBY S.O., STENDER S. Significant reduction of the anti-atherogenic effect of estrogen by long-term inhibition of nitric oxide synthesis in cholesterol-clamped rabbits. J. Clin. Invest. 1997;100:821–828. doi: 10.1172/JCI119597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONDA H., KANEKO H., KONDO M., KOGO H. Comparison of endothelium-derived relaxing factor activity between nonpregnant and pregnant rats. Comp. Biochem. Physiol. Pharmacol. Toxicol. Endocrinol. 1996;114:193–196. doi: 10.1016/0742-8413(96)00040-0. [DOI] [PubMed] [Google Scholar]
- HUANG A., SUN D., KOLLER A., KALEY G. Gender difference in the myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NO. Am. J. Physiol. 1997;272:H1804–H1809. doi: 10.1152/ajpheart.1997.272.4.H1804. [DOI] [PubMed] [Google Scholar]
- JIANG C.W., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br. J. Pharmacol. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG C.W., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Progesterone induces endothelium-independent relaxation of rabbit coronary artery in vitro. Eur. J. Pharmacol. 1992;211:163–167. doi: 10.1016/0014-2999(92)90524-8. [DOI] [PubMed] [Google Scholar]
- JINSI A., DETH R.C. Alpha 2-adrenoceptor-mediated vasoconstriction requires a tyrosine kinase. Eur. J. Pharmacol. 1995;277:29–34. doi: 10.1016/0014-2999(95)00053-n. [DOI] [PubMed] [Google Scholar]
- KARAS R.H., PATTERSON B.L., MENDELSOHN M.E. Human vascular smooth-muscle cells contain functional estrogen-receptor. Circulation. 1994;89:1943–1950. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- KITAZAWA T., HAMADA E., KITAZAWA K., GAZNABI A.K. Non-genomic mechanism of 17 beta-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J. Physiol. 1997;499:497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSHWAHA R.S., HAZZARD W.R. Exogenous estrogens attenuate dietary hypercholestrolemia and atherosclerosis in the rabbit. Metabolism. 1981;30:359–366. doi: 10.1016/0026-0495(81)90116-5. [DOI] [PubMed] [Google Scholar]
- LANIYONU A.A., SAIFEDDINE M., YANG S.G., HOLLENBERG M.D. Tyrosine kinase inhibitors and the contractile action of G-protein- linked vascular agonists. Can. J. Physiol. Pharmacol. 1994;72:1075–1085. doi: 10.1139/y94-150. [DOI] [PubMed] [Google Scholar]
- LARSON B., ANDERSON K.E., BATRA S., MAITTIASSON A., SJOGREN C. Effects of estradiol on norepinephrine-induced contraction, alpha-adrenocepto number and norepinephrine-content in the female rabbit aorta. J. Pharmacol. Exp. Ther. 1984;215:615–618. [PubMed] [Google Scholar]
- LOSORDO D.W., KEARNEY M., KIM E.A., JEKANOWSKI J., ISNER J.M. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89:1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- LUDMER P.L., SELWYN A.P., SHOOK T.L., WAYNE R.R., MUDGE G.H., ALEXANDER R.W., GANZ P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. New Eng. J. Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- MADDOX Y.T., FALCON J.G., RIDINGER M., CUNARD C.M., RAMWELL P.W. Endothelium-dependent gender differences in the response of the rat aorta. J. Pharmacol. Exp. Ther. 1987;240:392–395. [PubMed] [Google Scholar]
- MAGNESS R.R., SHAW C.E., PHERNETTON T.M., ZHENG J., BIRD I.M. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am. J. Physiol. 1997;272:H1730–H1740. doi: 10.1152/ajpheart.1997.272.4.H1730. [DOI] [PubMed] [Google Scholar]
- MEYER M.C., CUMMINGS K., OSOL G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. Am. J. Physiol. 1997;272:H2264–H2270. doi: 10.1152/ajpheart.1997.272.5.H2264. [DOI] [PubMed] [Google Scholar]
- MILLER V.M., VANHOUTTE P.M. 17 beta-Estradiol augments endothelium-dependent contractions to arachidonic acid in rabbit aorta. Am. J. Physiol. 1990;258:R1502–R1507. doi: 10.1152/ajpregu.1990.258.6.R1502. [DOI] [PubMed] [Google Scholar]
- MUGGE A., RIEDEL M., BARTON M., KUHN M., LICHTLEN P.R. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardio. Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- MYERS S.I., TURNAGE R.H., BARTULA L., KALLEY B., MENG Y. Estrogen increases male rat aortic endothelial cell (RAEC) PGI2 release. Prostaglandin. Leukou. Ess. Fat. Acid. 1996;54:403–409. doi: 10.1016/s0952-3278(96)90023-x. [DOI] [PubMed] [Google Scholar]
- OPEI L.H., SINGH B.N.Calcium channel antagonists (slow channel blockers) Drugs for the heart 1987Orlando: Grune and Stratton; 34–55.2nd ed. ed. Opei, H.L., pp [Google Scholar]
- PALMER R.M.J., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PAREDES-CARBAJAL M.C., JUAREZ-OROPEZA M.A., ORTIZ-MENDOZA C.M., MASCHER D. Effects of acute and chronic estrogenic treatment on vasomotor responses of aortic rings from ovariectomized rats. Life Sci. 1995;57:473–486. doi: 10.1016/0024-3205(95)00281-a. [DOI] [PubMed] [Google Scholar]
- PIETRAS R.J., SEZEGO C.M. Estrogen receptors in uterine plasma membrane. J. Sterod. Biochem. Mol. Biol. 1979;195:730–736. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., MONCADA S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIS S.E., GOLTH S.T., BLUMNETHAL R.S., RESAR J.R., ZACUR H.A., GERSTENBLITH G., BRINKER J.A. Ethyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postemenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- SALAS E., LOPEZ M.G., VILLARROYA M., SANCHEZ-GARCIA P., DE PASCUAL R., DIXON W.R., GARCIA A.G. Endothelium-independent relaxation by 17-alpha-estradiol of pig coronary arteries. Eur. J. Pharmacol. 1994;258:47–55. doi: 10.1016/0014-2999(94)90056-6. [DOI] [PubMed] [Google Scholar]
- SAURO M.D., SUDAKOW R., BURNS S. In vivo effects of angiotensin II on vascular smooth muscle contraction and blood pressure are mediated through a protein tyrosine-kinase- dependent mechanism. J. Pharmacol. Exp. Ther. 1996;277:1744–1750. [PubMed] [Google Scholar]
- STAMFER M.J., COLDITZ G.A., WILLEET W.C., MANSON J.E., ROSNER B., SEIZER F.E., HENNEKENS C.H. Postmenopausal estrogen therapy and cardiovascular diseases. Ten year follow-up from the Nurses' Health Study. N. Engl. J. Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- STICE S.L., FORD S.P., ROSAZZA J.P., VAN ORDEN D.E. Role of 4-hydroxylated estradiol in reducing Ca2+ uptake of uterine arterial smooth muscle cells through potential-sensitive channels. Biol. Reprod. 1987;36:361–368. doi: 10.1095/biolreprod36.2.361. [DOI] [PubMed] [Google Scholar]
- SULISTIYANI S.J., ADELMAN J.S., CHANDRASEKARAN A., RAYO J., STCLAIR R.W. Effects of 17-α-dihydroequilin sulfate, a conjugated equine estrogen and ethylestradiol on atherosclerosis in the rabbit. Atherioscler. Thromb. Vasc. Biol. 1995;15:837–846. doi: 10.1161/01.atv.15.7.837. [DOI] [PubMed] [Google Scholar]
- SULLIVAN J.M., VANDER ZWAAG R., LEMP G.F., HUGHES J.P., MADDOCK V., KROETZ F.W., RAMANATHAN K.B., MIRVIS D.M. Postmenopausal estrogen use and coronary atherosclerosis. Ann. Intern. Med. 1988;108:358–363. doi: 10.7326/0003-4819-108-3-358. [DOI] [PubMed] [Google Scholar]
- THOMAS G., ITO K., ZIKIC E., BHATTI T., HAN C., RAMWELL P.W. Specific inhibition of the contraction of the rat aorta by estradiol 17 beta. J. Pharmacol. Exp. Ther. 1995;273:1544–1550. [PubMed] [Google Scholar]
- TOMA C., JENSEN P.E., PRIETO D., HUGHES A., MULVANY M.J., AALKJAER C. Effects of tyrosine kinase inhibitors on the contractility of rat mesenteric resistance arteries. Br. J. Pharmacol. 1995;114:1266–1272. doi: 10.1111/j.1476-5381.1995.tb13342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGAS R., THOMAS G., WROBLEWSKA B., RAMWELL P.W. Differential effects of 17 alpha and 17 beta estradiol on PGF2 alpha mediated contraction of the porcine coronary artery. Adv. Prostaglandin. Thromboxane. Leuko. Res. 1989;19:277–280. [PubMed] [Google Scholar]
- VEDERNIKOV Y.P., LIAO Q.P., JAIN V., SAADE G.R., CHWALISZ K., GARFIELD R.E. Effect of chronic treatment with 17 beta-estradiol and progesterone on endothelium-dependent and endothelium-independent relaxation in isolated aortic rings from ovariectomized rats. Am. J. Obst. Gynecol. 1997;176:603–608. doi: 10.1016/s0002-9378(97)70555-6. [DOI] [PubMed] [Google Scholar]
- VITA J.A., TREASURE C.B., NABEL E.G. Coronary vasomotor response to acetylcholine relates to risk factors for coronary arteries. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- WALSH B.W., SHIFF I., ROSNER B., BREENBERG L., RAVNIKAR V., SACKS F.M. Effects of postmenopausal estrogen replacement therapy on the concentration and metabolism of plasma lipoproteins. N. Engl. Med. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- WEINER C.P., LIZASOAIN I., BAYLIS S.A., KNOWLES R.G., CHARLES I.G., MONCADA S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Pro. Nat. Acad. Sci. U.S.A. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLMAN G.C., BONEV A.D., NELSON M.T., BRAYDEN J.E. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Cir. Res. 1996;79:1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.K., ADAMS M.R., HERRINGTON D.M., CLARKSON T.B. Acute administration of estrogen and vascular responses of atherosclerotic coronary arteries. J. Am. Col. Cardiol. 1992;20:452–457. doi: 10.1016/0735-1097(92)90116-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.K., ADAMS M.R., KLOPFENSTEIN H.S. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.K., KIM Y.D., ADAMS M.R., CHEN M-F., MYERS A.K., RAMWELL P.W. Effects of estrogen on cardiovascular responses of premenopausal monkeys. J. Pharmacol. Exp. Ther. 1994;271:671–676. [PubMed] [Google Scholar]
- WINGROVE C.S., STEVENSON J.C. 17-β-estradiol inhibits stimulated endothelin release in human vascular endothelial cells. Eur. J. Endocrinol. 1997;137:205–208. doi: 10.1530/eje.0.1370205. [DOI] [PubMed] [Google Scholar]
- YOUSIF M.H., ORIOWO M.A., WILLIAMS K.I. Evidence for tyrosine kinase involvement in noradrenaline-induced vasoconstriction of the rabbit perfused ovarian vascular bed. Br. J. Pharmacol. 1997;120:190. [Google Scholar]
- ZHANG F., RAM J.L., STANDLEY P.R., SOWERS J.R. 17-β-oestradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am. J. Physiol. 1994;266:C975–C980. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]


