Abstract
The mammalian colonic epithelium carries out a number of different transporting activities simultaneously, of which more than one is increased following activation with a single agonist. These separate activities can be quantified by solving a set of equations describing these activities, provided some of the dependent variables can be eliminated. Using variations in the experimental conditions, blocking drugs and comparing wild type tissues with those from transgenic animals this has been achieved for electrogenic ion transporting activity of the mouse colon.
Basal activity and that following activation with forskolin was measured by short circuit current in isolated mouse colonic epithelia from normal and cystic fibrosis (CF) mice.
Using amiloride it is shown that CF colons show increased electrogenic sodium absorption compared to wild type tissues. CF mice had elevated plasma aldosterone, which may be responsible for part or all of the increased sodium absorbtion in CF colons.
The derived values for electrogenic chloride secretion and for electrogenic potassium secretion were increased by 13 and 3 fold respectively by forskolin, compared to basal state values for these processes.
The loop diuretic, frusemide, completely inhibited electrogenic potassium secretion, but apparently only partially inhibited electrogenic chloride secretion. However, use of bicarbonate-free solutions and acetazolamide reduced the frusemide-resistant current, suggesting that electrogenic bicarbonate secretion accounts for the frusemide-resistant current.
It is argued that the use of tissues from transgenic animals is an important adjunct to pharmacological analysis, especially where effects in tissues result in the activation of more than one sort of response.
Keywords: Electrogenic epithelial transport, potassium secretion, sodium absorption, chloride secretion, bicarbonate secretion, cystic fibrosis, CFTR, forskolin, loop diuretics
Introduction
The epithelium lining the alimentary canal is responsible for a large number of processes designed to move ions, solutes and water into or out of the gut lumen. Of great significance in these transporting activities is the asymmetric nature of the epithelial cells, vectorial transport depending on the differential distribution of channels, pumps and carriers between the apical and basolateral membranes. In this study transporting activities in the mouse distal colon are investigated, but only those which are electrogenic. In particular, stimulation of electrogenic transport by cyclic AMP will be the focus of the study, transporting activity being measured by the short circuit current (SCC) technique. The cyclic nucleotide has a number of known targets in epithelia, as follows; apically located chloride channels (Li et al., 1988), namely the cystic fibrosis transmembrane conductance regulator (CFTR), basolaterally located NaK2Cl cotransporter (Haas et al., 1993) and basolaterally located cyclic AMP sensitive K+-channels, namely the KvLQT1 K+-channel sensitive to the chromanol, 293B (Lohrmann et al., 1995; Loussouarn et al., 1997). Thus the actions at the two membranes work in concert to promote anion secretion, chloride concentration increases within the cell to a level above its electrochemical equilibrium and exits through CFTR channels in the apical membrane, anion secretion being maintained by apical membrane hyperpolarization caused by K+-channel activation. Ca2+-raising secretagogues, by contrast, produce only a transient anion secretory response dependent on activation of basolateral Ca2+-sensitive K+-channels without increasing the probability of CFTR channel opening (Dharmasathaphorn & Pandol, 1986; Cuthbert et al., 1994). Cyclic AMP not only increases anion secretion but K+ ions entering via the cotransporter may leave via the apical membrane, as well as re-equilibrate across the basolateral membrane. Thus inhibition of the cotransporter with loop diuretics will block both chloride and potassium secretion. However, loop diuretics rarely cause complete inhibition of the responses to forskolin in chloride secreting epithelia, a point noted by many, but commented on by few (MacVinish et al., 1998). Finally, in the colon, Na+ can enter through the apical membrane and exit the basolateral membrane using basolateral Na-K-ATPase. Here we have used pharmacological methods as the basis for producing a formal analysis of basal and cyclic AMP stimulated electrogenic transporting activity in the mouse distal colon.
Methods
All experiments were performed on the distal colonic mucosa of mice. Animals were killed by CO2 narcosis and the terminal colon removed into Krebs Henseleit Solution (KHS). The colon was opened immediately, cleaned and the muscle layers dissected away under microscopic control. The most terminal part of the colon was mounted in an Ussing chamber, window area 20 mm2, and the tissue short circuited with series resistance compensation, exactly as described recently, elsewhere (Teather & Cuthbert, 1997). Only one preparation was taken from each mouse, so that any variation in the responses can not be due to variation in the part of the colon from which the preparations were derived. Two types of mice were used as follows; wild type mice which were littermates of cystic fibrosis (CF) mice, either Cftrtm1Cam (Ratcliff et al., 1993) or Cftrtm2Cam (Colledge et al., 1995). Previous studies have not revealed any differences between colonic epithelia of the CF null mice (Cftrtm1Cam) and those with the ΔF508 mutation (Cftrtm2Cam) (Colledge et al., 1995). Similarly, the wild type mice were a mixture of homo- and heterozygotes, and again no differences in the behaviour of the epithelium of the colon has been demonstrated (Ratcliff et al., 1993; Cuthbert et al., 1995). All litters were initially fed on a low fibre diet to avoid meconium ileus and the wild type transferred to normal chow once genotyping was completed. Animals were used as soon as possible after genotyping.
Three experimental paradigms were used as follows.
Protocol 1. After the SCC had stabilized, amiloride (100 μM) was applied to the apical surface to block all electrogenic sodium absorption. Ten minutes later forskolin (10 μM) was applied in both the apical and basolateral bathing solutions and finally, after a further 10 min, frusemide (1 mM) was added to the basolateral bathing fluid and the SCC recorded for a further period of 10 min. The following parameters were measured from all SCC records and tabulated before analysis; basal SCC, ΔSCC caused by amiloride, ΔSCC caused by forskolin, ΔSCC caused by frusemide and SCC after frusemide.
Protocol 2. The design was identical to Protocol 1 except that addition of forskolin was omitted. Protocols 1 and 2 were applied to both wild type and CF colonic epithelia.
Protocol 3. This experimental paradigm was applied only to wild type colonic epithelia, and was identical to Protocol 1 except the experiments were carried out in bicarbonate-free solution.
Krebs Henseleit Solution (KHS) was used in Protocols 1 and 2 and had the following composition (in mM); NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4, 1.2, KH2PO4, 1.2, NaHCO3 25 and glucose 11.1. This solution had a pH of 7.4 when bubbled with 95%O2/5%CO2 at 37°C. Bicarbonate-free solution had the same composition, except that NaCl was increased to 137 mM and NaHCO3 was omitted. This solution was buffered with HEPES buffer, 10 mM, pH 7.4 and the solution bubbled with pure O2. An unpaired Student's t-test was used to make comparisons between sets of data, a P value less than 0.05 was considered significant.
Plasma aldosterone concentration was measured for both CF and wild type mice by radioimmunoassay, after extraction from plasma with dichloromethane. Blood was collected from the thoracic cavity after severing major veins into heparinized syringes. The samples were immediately centrifuged to remove cells and the plasma stored at −20°C. Samples from eight wild type and eight CF animals were collected. On average 150 μl of plasma was obtained from each mouse, which was insufficient volume for the assay. Consequently the samples from wild type and CF mice were separately pooled. Sufficient plasma was obtained to allow three separate estimations in CF samples and five separate estimations in wild type samples of aldosterone, each measurement being made in triplicate.
Results
Typical SCC records from colonic epithelia subject to the regimens of Protocols 1 and 2 are illustrated in Figure 1. The differences between wild type and CF epithelia are particularly evident with the Protocol 1 procedure, where the SCC increases after forskolin in the former, yet falls after the same treatment in CF tissues. With Protocol 2 the SCC changes due to frusemide are rather small when applied without prior addition of forskolin. By pooling the data from all the Protocol 1 studies, a number of significant differences were revealed, as illustrated in Figure 2. Highly significant differences were seen between the responses of wild type and CF epithelia in response to both forskolin and frusemide. Also, the responses to amiloride were significantly greater in CF colons, even though the basal SCC was larger in wild type colons.
Figure 1.
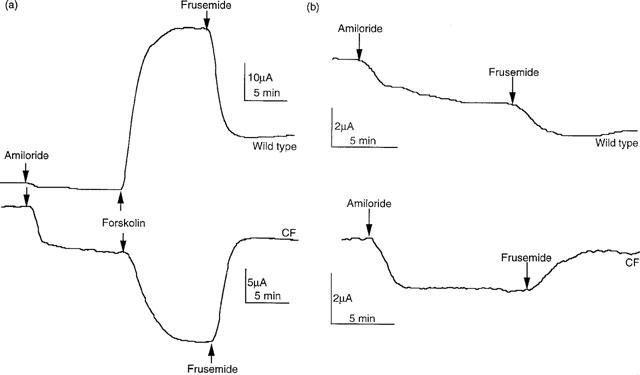
The effects of sequential administration of amiloride (100 μM, apical) and frusemide (1 mM, basolateral), with (a) or without (b) forskolin (10 μM, both sides) given between, in wild type and CF colonic epithelia. Specimen SCC traces for epithelia of 20 mm2 are shown. Note when forskolin is not added (b) the changes caused by frusemide are small. In this situation frusemide increased or decreased the SCC in wild type resulting in only a small mean change, whereas in CF epithelia an overall significant increase in current was found (see Figure 4a).
Figure 2.
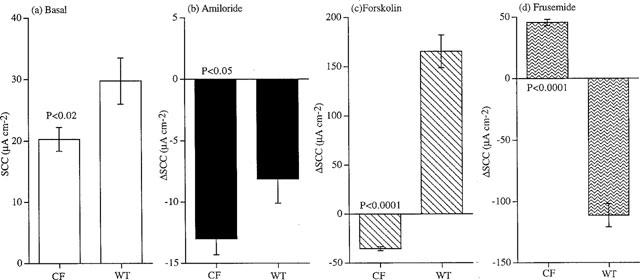
Cumulative data from 22 wild type colonic epithelia and 42 CF epithelia. Shown are the values (means±s.e.mean) for the basal SCC (a) and changes in SCC caused by the sequential addition of amiloride (100 μM, apical) (b), forskolin (10 μM, both sides) (c) and frusemide (1 mM, basolateral) (d), all as μA cm−2. P values are shown and were obtained with Student's t-test, comparing wild type with CF preparations.
For the formal analysis of the responses to Protocols 1 and 2 it is more convenient to deal with the actual SCC values, rather than changes in SCC. The data of Figure 2 were replotted as SCC in Figure 3 where the responses to amiloride, forskolin and frusemide are shown as step functions. Peak responses were used to construct Figure 3 and no allowance was made for any decline of the response during the 10 min of exposure to the drugs. The final SCC value remaining after frusemide is therefore a consequence of additions and subtractions of peak responses taking the original basal current as the starting point. Therefore the actual SCC remaining after frusemide is not exactly that found by calculation. The predicted value for the residual SCC after exposure to forskolin and then frusemide in CF colons (CF SCCforskolin/frusemide) was 17.5±4.0 μA cm−2 whereas the actual value was 18.8±2.3 μA cm−2. Similarly the predicted value in wild type colons (SCCforskolin/frusemide) was 75.6±19.0 μA cm−2 while the actual value was 61.0±6.7 μA cm−2.
Figure 3.
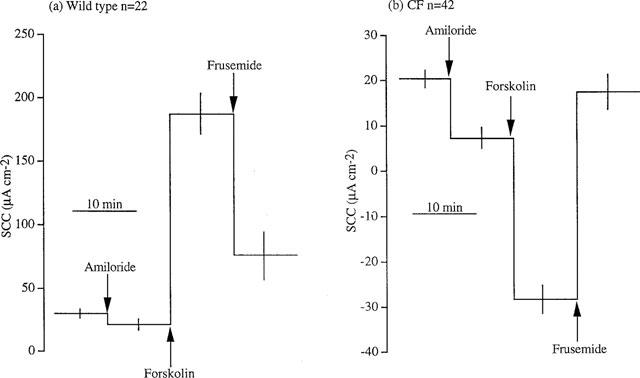
The total data from Figure 2 presented as actual SCC values. The mean values of the currents and their standard errors are given. Peak values have been used and no allowance made for any decline in the response during 10 min before the next drug was added.
It is notable that frusemide failed to reverse completely the SCC increase caused by forskolin in wild type colons (Figure 3a) but completely reversed the forskolin effect in the CF colon (Figure 3b). Using Protocol 2, i.e. no addition of forskolin, frusemide had little effect on SCC in unstimulated colons (Figure 4a). Addition of acetazolamide after frusemide in wild type colons increased the inhibition of the forskolin response from 68 to 75% (Table 1). The data shows that the response to frusemide increases to 168.4±9.9 μA cm−2 from 108.1±14.1 μA cm−2 when bicarbonate-free solution is used. However when wild type colons were mounted in HCO3−-free KHS the responses to forskolin were greater, but not significantly so, than those in KHS. Therefore, derived values were calculated to allow for the differences in the response to forskolin and analysis showed that inhibition by frusemide was significantly increased by bicarbonate removal. Inhibition of forskolin responses in bicarbonate-free solution were increased from 87% after frusemide to 91% by addition of acetazolamide (Figure 4b). Examples of responses in the two conditions are given in Figure 5.
Figure 4.
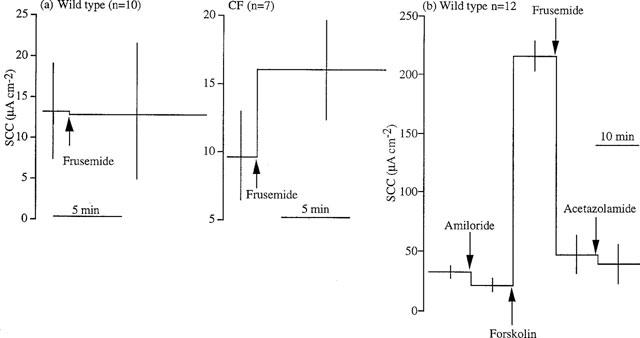
(a) Effects of frusemide (1 mM, basolateral) addition to wild type and CF colons, without addition of forskolin and in the presence of amiloride. (b) Effects on SCC by the sequential addition of amiloride (100 μM, apical), forskolin (10 μM, both sides) and frusemide (1 mM, basolateral) in 12 wild type colonic epithelia bathed in HCO−3-free medium. Means±s.e.mean are given in both (a) and (b).
Table 1.
Effect of frusemide and acetazolamide on forskolin responses in normal and bicarbonate free conditions
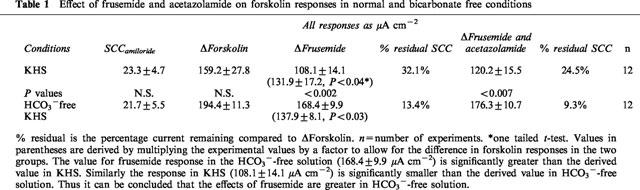
Figure 5.
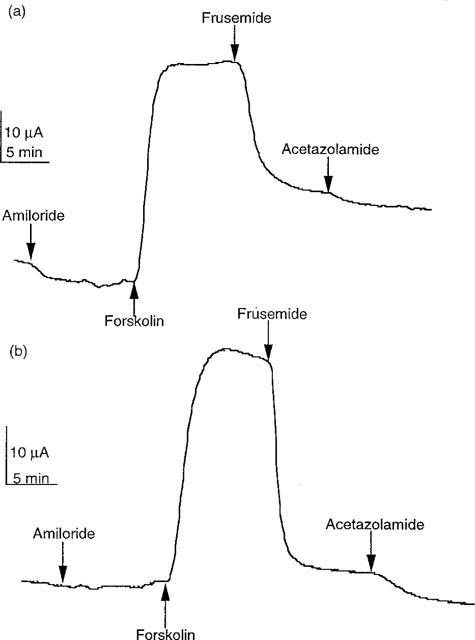
SCC records from two separate colonic epithelia, one bathed in KHS (a) and the other in HCO−3-free KHS (b) Each was subject to the sequential addition of amiloride (100 μM, apically), forskolin (10 μM, both sides), frusemide (1 mM, basolaterally) and acetazolamide (100 μM, both sides). Both tissues had an area of 20 mm2.
Plasma aldosterone measurements gave values of 224±19 pmol l−1 (five measurements) in wild type and 1086±23 pmol l−1 in CF (three measurements) each made in triplicate.
Theory
Assuming that SCC represents the electrogenic transport of chloride, potassium, sodium and other unknown ions the following formalism can be developed; SCCamiloride=x+y+z, where SCCamiloride is the SCC after amiloride, x is basal electrogenic chloride secretion, y is basal electrogenic potassium secretion and z is electrogenic transport of unknown species. In CF epithelia, chloride secretion can not occur because of the absence of CFTR and the relevant equation for SCC after amiloride is CF SCCamiloride=y+z. Forskolin stimulates chloride and potassium secretion, and assuming z is unaffected the relevant equations are SCCforskolin=x+Δx+y+Δy+z and CF SCCforskolin=y+Δy+z. In Protocols 1 and 2, and with either wild type or CF epithelia the SCC remaining at the end of the experiments, after frusemide had been added, is z, whether or not frusemide was added in the absence of forskolin(SCCfrusemide and CF SCCfrusemide) or after forskolin (SCCforskolin/frusemide and CF SCCforskolin/frusemide). It follows therefore that:
The values, taken from Figures 3 and 4a were:
SCCamiloride =21.7±4.3 μA cm−2
CF SCCamiloride=7.3±2.3 μA cm−2
SCCforskolin=186.9±16.5 μA cm−2
CF SCCforskolin=−28.3± 3.1μA cm−2
SCCfrusemide=12.7±8.3 μA cm−2
CFSCCfrusemide =16.1±3.6 μA cm−2
SCCforskolin/frusemide=75.6±19.0 μA cm−2
CF SCCforskolin/frusemide=17.5±4.0 μA cm−2.
This data gives values of the various transport parameters as follows:
Δx=200.9±17.5 μA cm−2, from (1)
≈ Δy=−35.6±5.0 μA cm−2, from (2)
x=14.4±4.9 cm−2, from (3)
y=−8.8±4.3 cm−2, from (4).
Four estimates of z can be derived from (5), they are respectively 12.7±8.3, 16.1±3.6, 75.6±10.0 and 17.5±4.0 μA cm−2. The first, second and fourth values are not significantly different from one another, while all three are significantly smaller than the third value (P<0.0001). These derived values are close to the residual SCCs at the end of the experiments after frusemide had been applied, which were, respectively 11.4±3.2, 15.9±3.1, 61.0±6.7 and 18.8±2.3 μA cm−2. Again the value for the residual current remaining after forskolin and frusemide in wild type colons is discrepant from the rest of the values for z. This point was commented upon in the Introduction, and is clarified by experiments using Protocol 3.
Discussion
The opportunity to collect a large body of data for CF colons arose from other studies on CF mice where the responses were used to check the genotyping from DNA tail clip analysis. Comparison with data for wild type colons showed that the amiloride sensitive SCC was significantly larger in the CF tissues. Aldosterone is known to increase electrogenic sodium transport in the mouse colon (Grubb & Boucher, 1997) and measurement of plasma aldosterone showed the level was greater in CF mice. Therefore the increase in sodium absorption in CF colons maybe due to the increase in hormone levels. In an earlier study (Cuthbert et al., 1994) we failed to record any increase in amiloride sensitive sodium absorption in CF colons compared to wild type with numbers approaching those in this study. However in that instance two or three colonic preparations were taken from each mouse, whereas here only a single preparation was taken from from each animal. As electrogenic sodium absorption occurs in only the most terminal part of the colon we have shown a difference in this study by using strictly comparable tissues. Amiloride sensitive sodium channels, (ENaC) are also upregulated by the lack of CFTR (Stutts et al., 1997; Briel et al., 1998). Whether part of the increase in CF colons is due to this effect can not be ascertained from this study, but it should be noted that the increase in sodium absorption in CF colons is modest compared to that found in airway epithelia (MacVinish et al., 1997) which are apparently insensitive to aldosterone (Knowles et al., 1985). In the airways sodium absorption and chloride secretory activities are expressed in most cells. As CFTR is produced only in the secretory crypts of the colon and sodium absorption occurs in the surface cells (Trezise et al., 1997), it is probably only the cells near to the mouth of the crypts that can absorb sodium ions before all CFTR function is lost. Thus only a relatively small proportion of the total epithelium could be modified by loss of CFTR.
Throughout this investigation forskolin has been used to stimulate adenylate cyclase. The SCC increase in wild type colons caused by forskolin (165.3±16.5 μA cm−2) underestimates the actual chloride secretory response by the SCC change caused by forskolin in CF colons (35.6±2.1 μA cm−2). The values of basal chloride and potassium secretion are small, and of opposite polarity (14.4±4.9 and −8.8±4.3 μA cm−2 respectively), therefore frusemide added in the absence of prior forskolin addition is not expected to produce much effect. In wild type colons frusemide caused a SCC change of −0.5±5.9 μA cm−2 (95% confidence limits of 12.8 to −13.7 μA cm−2), while in CF colons, where chloride secretion does not occur, it is predictable that frusemide would cause a SCC increase. Experimentally the value was 6.5±1.7 μA cm−2 (95% confidence limits of 2.4–10.5 μA cm−2). Forskolin increased the anion secretory current by 13 fold from the basal value, while potassium secretion was only increased 3 fold.
Three estimates of z gave values of 9–18 μA cm−2, which were not significantly different from each other, while in wild type colons there was a large residual current after forskolin and frusemide. This large value of z is not predicted by the formal treatment given above and needs to be addressed. First, in wild type colons treated with both drugs the situation is competitive at the level of the cotransporter. Incomplete inhibition might explain the lack of complete reversal. However, a similar discrepancy would exist in CF colons but this was not apparent and the hypothesis is rejected. Secondly, other electrogenic transporting activities may be present in wild type colons, which are not inhibited by frusemide, but which are dependent on CFTR. It is known that the CFTR channel is permeable to Cl− and HCO3−, but with a preference for Cl− (Kunzelmann et al., 1991; Poulsen et al., 1994). However in the absence of Cl− the colon can demonstrate electrogenic HCO3− secretion (Feldman et al., 1988) and others have demonstrated bicarbonate secretion in the mouse intestine (Hogan et al., 1997; Seidler et al., 1997). Clearly then HCO3−-secretion is not possible in CF colons because of the absence of CFTR, and neither do bicarbonate ions enter the cell on the cotransporter. However, when the cotransporter is inhibited with frusemide the ratio of intracellular Cl−/HCO3− will fall.
Throughout this study we have used supramaximally effective concentrations of drugs so that the maximal effect is achieved quickly and is easily discernible, For example, lower concentrations of frusemide produce the same maximal effect but take much longer to reach equilibrium. Acetazolamide only removed a small amount of the residual SCC after forskolin and frusemide in wild type colons, indicating that HCO3− transport is not dependent on the hydration of CO2, particularly as HCO3− can enter cells using a Cl−/HCO3− exchanger. Removal of HCO3− from the bathing fluid together with the addition of acetazolamide, after frusemide, was able to remove virtually all the current generated by forskolin (Table 1). The increase in the SCC response to forskolin in bicarbonate-free conditions, although this just failed to reach significance, may result from the removal of competition from the less permeable HCO3−. This data can not be introduced into the formal analysis in a numerical way because it only refers to a sample of 12 experiments and the experimental conditions were different (HCO3−-free KHS), however it does seem as if a large part of the residual current in wild type colons after forskolin and frusemide is due to bicarbonate secretion. In unstimulated colons the residual current after frusemide is small compared to the residual value in stimulated colons from which it may be concluded that forskolin also stimulates bicarbonate transport. However this is not necessarily so. An alternative way to measure ion transport under SCC conditions is to measure the net flux of ions. When this is done the net flux of chloride is often larger than expected from integration of the SCC response (Cuthbert & Margolius, 1982; Cuthbert et al., 1983). Here we have shown that the SCC response to forskolin underestimates the chloride secretory response due to the concomitant potassium secretory current. However, it would not be expected, if a large part of the anion secretory response was due to bicarbonate, that net flux would exceed the SCC. In this investigation it is shown that when bicarbonate is removed the chloride secretory response to forskolin is maintained, indicating that bicarbonate secretion is not an obligatory part of the response. It appears therefore that addition of frusemide to a forskolin stimulated colonic epithelium actually alters the experimental conditions to one which is essentially chloride free, as no chloride can enter the cell on the cotransporter, although entrance by other routes is possible. In this situation bicarbonate is free to leave the cell without competition from chloride via apical CFTR. The consequence is that it appears that frusemide is incapable of completely inhibiting chloride secretion when the bathing solution contains bicarbonate. Small residual currents, termed z, (∼15 μA cm−2) are not accounted for in this study, but account for only 5–10% of the maximal current after forskolin stimulation. In summary, we have used a formal pharmacological analysis to quantify electrogenic transport of sodium, potassium, chloride and bicarbonate ions in the rat colon epithelium stimulated by forskolin.
In biological situations where a system shows more than one type of response to a stimulus then such systems can be analysed in a formal way if the equations describing complex actions can be solved by appealing to the data. To this end we have eliminated some part of the response using specific blocking protocols (amiloride, frusemide, bicarbonate-free), have compared the stimulated (forskolin) with the resting condition and have compared normal with transgenic tissues to analyse the transporting activity of the mouse colon. The increasing availability of transgenics in which some component of the response mechanism is ‘knocked out' is likely to become an important method in understanding both function and drug action.
Acknowledgments
We thank Dr Frances Short of the Department of Chemical Pathology, St. Mary's Hospital, London for carrying out the assay for aldosterone. We are grateful for support from the Cystic Fibrosis Trust and the Medical Research Council, also to Dr W.H. Colledge and Professor M. J. Evans for the CF colons.
Abbreviations
- CF SCC
short circuit current in CF
- CF SCCamiloride
SCC remaining after amiloride in CF
- CF SCCforskolin
SCC remaining after amiloride and forskolin in CF
- CF SCCforskolin/frusemide
SCC remaining after amiloride, forskolin then frusemide in CF
- CF SCCfrusemide
SCC remaining after amiloride and frusemide in CF
- KHS
Krebs Henseleit Solution
- SCC
short circuit current
- SCCamiloride
SCC remaining after amiloride
- SCCforskolin
SCC remaining after amiloride and forskolin
- SCCforskolin/frusemide
SCC remaining after amiloride, forskolin then frusemide
- SCCfrusemide
SCC remaining after amiloride and frusemide
References
- BRIEL M., GREGER R., KUNZELMANN K. Cl− transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J. Physiol. 1998;508:825–836. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLEDGE W.H., ABELLA B.S., SOUTHERN K.W., RATCLIFF R., JIANG C., CHENG S.H., MacVINISH L.J., ANDERSON J.R., CUTHBERT A.W., EVANS M.J. Generation and characterisation of a ΔF508 cystic fibrosis mouse model. Nature Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- CUTHBERT A.W., HALSTEAD J., RATCLIFF R., COLLEDGE W.W., EVANS M.J. The genetic advantage hypothesis in cystic fibrosis heterozygotes: a murine study. J. Physiol. 1995;482:449–454. doi: 10.1113/jphysiol.1995.sp020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTHBERT A.W., MacVINISH L.J., HICKMAN M.E., RATCLIFF R., COLLEDGE W.H., EVANS M.J. Ion-transporting activity in the murine colonic epithelium of normal animals and animals with cystic fibrosis. Pflugers Arch. 1994;428:508–515. doi: 10.1007/BF00374572. [DOI] [PubMed] [Google Scholar]
- CUTHBERT A.W., MARGOLIUS H.S. Kinins stimulate net chloride secretion by the rat colon. Br. J. Pharmacol. 1982;75:587–598. doi: 10.1111/j.1476-5381.1982.tb09178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTHBERT A.W., McLAUGHLIN P., COOMBS R.R.A. Immediate hypersensitivity reaction to β-lactoglobulin in the epithelium lining the colon of guinea pigs fed cows' milk. Int. Archs. Allergy Appl. Immun. 1983;72:34–40. doi: 10.1159/000234837. [DOI] [PubMed] [Google Scholar]
- DHARMASATHAPHORN K., PANDOL S.J. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J. Clin. Invest. 1986;77:348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMAN G.M., BERMAN S.F., STEPHENSON R.L. Bicarbonate secretion in rat distal colon in vitro: a measurement technique. Am. J. Physiol. 1988;254:C383–C390. doi: 10.1152/ajpcell.1988.254.3.C383. [DOI] [PubMed] [Google Scholar]
- GRUBB B.R., BOUCHER R.C. Enhanced colonic Na+ absorption in cystic fibrosis mice versus normal mice. Am J. Physiol. 1997;272:G393–G400. doi: 10.1152/ajpgi.1997.272.2.G393. [DOI] [PubMed] [Google Scholar]
- HAAS M., McBRAYER D.G., YANKASKAS J.R. Dual mechanisms for Na-K-Cl cotransport regulation in airway epithelial cells. Am. J. Physiol. 1993;264:C189–C200. doi: 10.1152/ajpcell.1993.264.1.C189. [DOI] [PubMed] [Google Scholar]
- HOGAN D.L., CROMBIE D.L., ISENBERG J.I., SVENDSEN P., SCHAFFALITZKY DE MUKADELL O.B., AINSWORTH M.A. CFTR mediates cAMP and Ca2+ activated duodenal HCO−3 secretion. Am. J. Physiol. 1997;272:G872–G878. doi: 10.1152/ajpgi.1997.272.4.G872. [DOI] [PubMed] [Google Scholar]
- KNOWLES M.R., GATZY J.T., BOUCHER R.C. Aldosterone metabolism and transepithelial potential difference in normal and cystic fibrosis subjects. Paediatric Res. 1985;19:676–679. doi: 10.1203/00006450-198507000-00008. [DOI] [PubMed] [Google Scholar]
- KUNZELMANN K., GERLACH L., FROBE U., GREGER R. Bicarbonate permeability of epithelial chloride channels. Pflugers Arch. 1991;417:616–621. doi: 10.1007/BF00372960. [DOI] [PubMed] [Google Scholar]
- LI M., McCANN J.D., LIEDTKE C.M., NAIRN A.C., GREENGARD P., WELSH M.J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988;331:358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- LOHRMANN E., BURHOFF I., NITCHKE R.B., LANG H.J., MANIA D., ENGLERT H.C., HROPOT M., WARTH R., ROHM W., BLEICH M., GREGER R. A new class of inhibitors of cAMP-mediated Cl− secretion in rabbit colon, acting by the reduction of cAMP-activated K+ conductance. Pflugers Arch. 1995;429:517–530. doi: 10.1007/BF00704157. [DOI] [PubMed] [Google Scholar]
- LOUSSOUARN G., CHARPENTIER F., MOHAMMAD-PANAH R., KUNZELMANN K., BARÓ I., ESCANDE D. KvLQT1 potassium channel but not IsK is the molecular target for trans-6-cyano-4-(N-ethylsulfonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane. Mol. Pharmacol. 1997;52:1131–1136. doi: 10.1124/mol.52.6.1131. [DOI] [PubMed] [Google Scholar]
- MacVINISH L.J., GODDARD C., COLLEDGE W.H., HIGGINS C.F., EVANS M.J., CUTHBERT A.W. Normalisation of ion transport in murine cystic fibrosis nasal epithelium using gene transfer. Am. J. Physiol. 1997;273:C734–C740. doi: 10.1152/ajpcell.1997.273.2.C734. [DOI] [PubMed] [Google Scholar]
- MacVINISH L.J., HICKMAN M.E., MUFTI D.A.H., DURRINGTON H.J., CUTHBERT A.W. Importance of basolateral potassium channels in maintaining chloride secretion in murine nasal and colonic epithelia. J. Physiol. 1998;510:237–247. doi: 10.1111/j.1469-7793.1998.237bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULSEN J.H., FISCHER H., ILLEK B., MACHEN T.E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATCLIFF R., EVANS M.J., CUTHBERT A.W., MacVINISH L.J., FOSTER D., ANDERSON J.R., COLLEDGE W.H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nature Genet. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- SEIDLER U., BLUMENSTEIN L., KRETZ A., VIELLARD-BARON D., ROSSMANN H., COLLEDGE W.H., EVANS M., RATCLIFF R., GREGOR M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO−3 secretion. J. Physiol. 1997;505:411–423. doi: 10.1111/j.1469-7793.1997.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUTTS M.J., ROSSIER B.C., BOUCHER R.C. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J. Biol. Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- TEATHER S., CUTHBERT A.W. Induction of bradykinin B1 receptors in rat colonic epithelium. Br. J. Pharmacol. 1997;121:1005–1011. doi: 10.1038/sj.bjp.0701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREZISE A.E.O., RATCLIFF R., HAWKINS T.E., EVANS M.J., FREEMAN T.C., ROMANO P., HIGGINS C.F., COLLEDGE W.H. Co-ordinate regulation of the cystic fibrosis and multidrug resistance genes in cystic fibrosis knockout mice. Hum. Mol. Genet. 1997;6:527–537. doi: 10.1093/hmg/6.4.527. [DOI] [PubMed] [Google Scholar]


