Abstract
The effect of mibefradil (Ro 40-5967), an inhibitor of T-type Ca2+ current (ICa(T)), on myoblast fusion and on several voltage-gated currents expressed by fusion-competent myoblasts was examined.
At a concentration of 5 μM, mibefradil decreases myoblast fusion by 57%. At this concentration, the peak amplitudes of ICa(T) and L-type Ca2+ current (ICa(L)) measured in fusion-competent myoblasts are reduced by 95 and 80%, respectively. The IC50 of mibefradil for ICa(T) and ICa(L) are 0.7 and 2 μM, respectively.
At low concentrations, mibefradil increased the amplitude of ICa(L) with respect to control.
Mibefradil blocked three voltage-gated K+ currents expressed by human fusion-competent myoblasts: a delayed rectifier K+ current, an ether-à-go-go K+ current, and an inward rectifier K+ current, with a respective IC50 of 0.3, 0.7 and 5.6 μM.
It is concluded that mibefradil can interfere with myoblast fusion, a mechanism fundamental to muscle growth and repair, and that the interpretation of the effect of mibefradil in a given system should take into account the action of this drug on ionic currents other than Ca2+ currents.
Keywords: Myoblast, potassium current, mibefradil, myoblast fusion
Introduction
Mibefradil is a pharmacological agent accepted as a rather selective inhibitor of Ca(T) channels (Ertel & Ertel, 1997). This agent has recently been used in the therapy of some cardiovascular diseases (Clozel et al., 1997) before being withdrawn from the market in June 1998 due to its interactions with other drugs (Po & Zhang, 1998).
Recently, we have proposed that a low-threshold transient Ca2+ current (ICa(T)) of fusion-competent myoblasts might contribute to an inward flux of Ca2+ ions (Liu et al., 1998) and thereby play a role in myoblast fusion, a process essential to skeletal muscle development and repair. For this reason, we examined the effect of mibefradil on myoblast fusion, expecting it to be reduced if ICa(T) was involved. We found that mibefradil markedly reduces myoblast fusion. However, we also found that, in addition to inhibiting ICa(T), mibefradil very efficiently blocks several types of voltage-gated K+ channels, as shall be described here.
Methods
Dissociation and cell cultures
Samples of human skeletal muscle were obtained during corrective orthopaedic surgery of young patients (9 months to 17 years old) without any known neuromuscular disease in accordance with the guidelines of the ethical committee of the University Hospital of Geneva (written informed consent was obtained from patients or their legal guardians). Myoblasts (Baroffio et al., 1993) and fusion-competent myoblasts (Krause et al., 1995) were prepared as described.
Electrophysiology
Whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) was used as in Bernheim et al. (1996). Leak current subtraction procedures are discussed in the Figure legends. (i) Potassium currents. Extracellular solution (mM): NMG-Cl (100), KCl (5), MgCl2 (3), HEPES (5), NaOH (50), acetic acid (50), and glucose (8). The pH was adjusted to 7.3 with NMG. Intracellular (pipette) solution (mM): KCl (110), NaCl (5), MgCl2 (1), HEPES (5), BAPTA (20), and glucose (5). The pH was adjusted to 7.3 with KOH. (ii) Currents through calcium channels. Extracellular solution (mM): BaCl2 (10), TEA-Cl (90), tetrodotoxin (10 μM), NMG-Cl (50), KCl (5), MgCl2 (2), HEPES (5), and glucose (8). The pH was adjusted to 7.3 with NMG. Intracellular (pipette) solution (mM): KCl (145), MgCl2 (2), HEPES (10), BAPTA (1), Mg-ATP (3), and glucose (5). The pH was adjusted to 7.3 with KOH.
Fusion index
The fusion index is defined as the number of nuclei in myotubes divided by the total number of nuclei counted. Cultures were fixed 5 min with ice-cold 100% methanol, and stained with haematoxylin. Nuclei were counted in 20 randomly chosen microscope fields in two separate cultures. One microscope field usually contains between 100 and 150 nuclei. In t-tests, n refers to the number of microscope fields counted.
Statistics
All data are expressed as means±s.e.mean.
Results
Effect of mibefradil on myoblast fusion
Human myoblasts can be maintained in an undifferentiated state for several weeks, as long as they are exposed to a growth medium (Baroffio et al., 1993; Ham et al., 1989). Alternatively, myoblast fusion can be rapidly induced when the proliferation medium is replaced with differentiation medium (StClair et al., 1992), and maximum fusion is usually reached within 48 h.
In preliminary experiments, we examined the effect on fusion of a concentration of mibefradil known to block a large fraction of ICa(T) (5 μM; Clozel et al., 1997). Figure 1 illustrates the dramatic effect 5 μM mibefradil has on myoblast fusion. In Figure 1A, it can be seen that after 30 h of exposure to the differentiation medium containing the drug, the fusion index was reduced by 43% with respect to control. Eight hours later, when fusion was nearly maximal in the control cultures (Baroffio et al., 1996), the fusion index in sister cultures exposed to mibefradil was still at the same low level and was reduced by 57% with respect to control. The photomicrographs in Figure 1B illustrate the difference between a control culture (top) and its sister culture exposed to mibefradil (bottom). In the presence of the drug, although the cells look perfectly healthy, there are very few multinucleated myotubes, with the vast majority of the cells remaining as mononucleated myoblasts.
Figure 1.
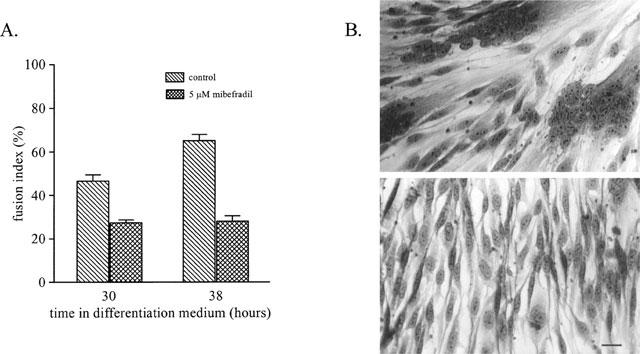
Mibefradil reduces myoblast fusion in primary culture. (A) Myoblast fusion was induced by exposing myoblasts to differentiation medium and the fusion index was evaluated after 30 and 38 h, in control conditions and in sister cultures exposed to 5 μM mibefradil. Mibefradil decreased myoblast fusion significantly (P<0.0001) at the two time points evaluated. (B) Upper photograph: culture fixed after 38 h in differentiation medium (control condition) and stained with haematoxylin. Lower photograph: sister culture after 38 h in differentiation medium containing 5 μM mibefradil. Scale bar is 25 μm.
Effect of mibefradil on Ca2+ currents
The sensitivity of voltage-gated Ca2+ channels to mibefradil was examined in fusion-competent myoblasts. Fusion-competent myoblasts are obtained by plating myoblasts at a very low density in differentiation medium. Under these conditions, the cells are induced to differentiate and thus begin to express Ca2+ and K+ currents (Bernheim et al., 1996; Liu et al., 1998), but they are prevented from fusing due to lack of neighbouring cells (Krause et al., 1995). In the differentiation medium, not all myoblasts necessarily express the same currents at a given time. This is due to the fact that (1) only about 70% of the myoblasts are going to fuse (Baroffio et al., 1993), (2) cells are not strictly synchronized, and (3) the currents appear in a sequential order with some currents being probably expressed just before fusion. Regarding Ca2+ currents, Figure 2A (inset) shows the inward current activated by a depolarization in a myoblast expressing exclusively ICa(T). This current was recorded in the presence of 10 mM Ba2+ in the extracellular solution. Also, in order to eliminate any contribution of a voltage-gated sodium current (Hamann et al., 1994), tetrodotoxin (10 μM) was added to the bath solution which contained no sodium ions. It can be seen that the inward current activates rapidly and then quickly inactivates within a few tens of ms. The addition of mibefradil (2 μM) markedly decreased the inward current (the effect was reversible; data not shown). Figure 2A shows the current-voltage relationship of ICa(T), together with that recorded in the presence of 2 μM mibefradil. The current suppressed by mibefradil activates at potentials more depolarized than −60 mV. This current is also blocked efficiently by amiloride and Ni2+ (data not shown). These data all consistently indicate that the inward current is ICa(T).
Figure 2.
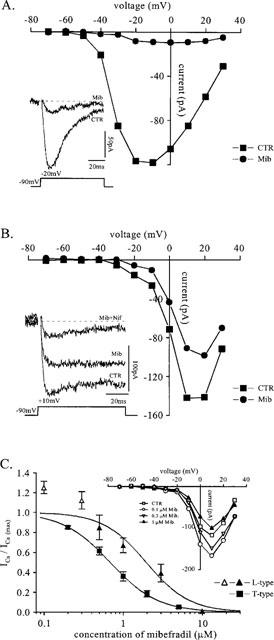
Effect of mibefradil on Ca2+ currents of fusion-competent myoblasts. (A) Recording from a fusion-competent myoblast (membrane capacitance 42 pF) exposed 3 days to differentiation medium. The cell, superfused with a solution containing 10 mM Ba2+ (see Methods), was held at −90 mV and stepped for 80 ms to a series of potentials between −70 and +30 mV. Leak current was estimated by adding 1 mM cadmium at the end of the experiment and was subtracted. Peak Ba2+ currents (current through T-type Ca2+ channels) were plotted as a function of the voltage during a step in the absence and in the presence of 2 μM mibefradil. Inset: Ba2+ current recorded during a step to −20 mV with and without 2 μM mibefradil. (B) Fusion-competent myoblast (membrane capacitance 10 pF) exposed 3 days to differentiation medium. Steady-state Ba2+ currents (current through mainly L-type Ca2+ channels) measured at the end of a 80 ms step and plotted as a function of the voltage during the step, in the absence and in the presence of 2 μM mibefradil. Leak current was estimated as in (A) and subtracted. Inset: Ba2+ current recorded during a step to +10 mV in control, in 2 μM mibefradil, and 3 μM nifedipine in addition to mibefradil. (C) Peak Ba2+ currents (current through T-type Ca2+ channels) recorded during a step to −20 mV and normalized to their amplitude measured in control solution are plotted as a function of mibefradil concentration. The line is a Hill equation (IC50=0.68 μM, Hill coefficient=1.4, n=5). All recordings were from fusion-competent myoblasts cultured 3 days in differentiation medium. A similar experiment was performed with the current through L-type calcium channels. Normalized steady-state Ba2+ currents measured at the end of the 80 ms voltage step to +10 mV were plotted as a function of mibefradil concentration and fitted with a Hill equation (IC50=2 μM, Hill coefficient=1.6, n=4 or more); the open triangles represent Ba2+ currents potentiated by low concentrations of mibefradil (n=8). Inset: Effect of low concentrations of mibefradil on current through L-type calcium channels. Steady state Ba2+ currents (leak subtracted) were measured at the end of the 80 ms step and plotted as a function of the voltage during the step for different mibefradil concentrations.
In Figure 2B, similar data are shown for a myoblast expressing ICa(L) in addition to ICa(T). Inset, it can be seen that the same concentration of mibefradil (2 μM) was less efficient in blocking ICa(L) than ICa(T). The addition of 3 μM nifedipine, a blocker of ICa(L), markedly reduced the current remaining in the presence of mibefradil, indicating that this steady inward current was indeed mainly ICa(L). Comparison of the current-voltage relationship of Figure 2B in the absence and in the presence of mibefradil with that of Figure 2A confirms that ICa(L) is less efficiently inhibited by mibefradil than ICa(T) at all voltages.
Figure 2C illustrates the degree of inhibition of ICa(T) and ICa(L) at different mibefradil concentrations. The IC50 of mibefradil for ICa(T) is 0.7 μM. For ICa(L), as few fusion-competent myoblasts express this current, the inhibition by mibefradil was tested both on fusion-competent myoblasts (n=3) and in freshly formed myotubes (n=5). The two sets of data were pooled as there was no difference between them. Interestingly, the effect of mibefradil on ICa(L) of fusion-competent myoblasts and freshly-formed myotubes is bimodal. At 0.1 μM, there is an increase in the amplitude of the inward current by 25% with respect to the control level. At higher concentrations, however, inhibition takes place. The IC50 for ICa(L), taking into account exclusively the filled triangles, is near 2 μM.
Figure 2C indicates that the concentration of mibefradil (5 μM) used to inhibit fusion in Figure 1 would have blocked 95% of ICa(T) and 80% of ICa(L). As we observed previously that ICa(L) is not required for fusion of primary cultures of human myoblasts (Bernheim et al., 1996), the effect of mibefradil on myoblast fusion would suggest that a functional ICa(T) is important in the fusion process. This, however, only applies if the effect of mibefradil is restricted to Ca2+ currents. Indeed, we recently demonstrated that a block of an inward rectifier K+ current (IK(IR)), which contributes to the final hyperpolarization of myoblasts to −65 mV, could nearly totally inhibit myoblast fusion (Liu et al., 1998). Therefore, it was important to demonstrate that mibefradil is not affecting IK(IR), or any of the other K+ currents, which also contribute to the hyperpolarization of myoblasts, i.e. IK(NI), a non-inactivating K+ current (Bernheim et al., 1996), which we know now is the human ether-à-go-go K+ channel (Ih-eag, Bijlenga et al., 1998; Occhiodoro et al., 1998), and IK(DR), a delayed rectifier K+ current (Widmer et al., 1995).
Effect of mibefradil on K+ currents
Figure 3A illustrates the effect of mibefradil on IK(DR). The cell represented had no Ih-eag, which occurs in only a small fraction of fusion-competent myoblasts (Bernheim et al., 1996), so that IK(DR) could be studied in isolation. The inset shows the marked reduction of the outward current when the myoblast was exposed to 3 μM mibefradil. The blocking effect occurred at all voltages, as attested by the current-voltage relationship in Figure 3A.
Figure 3.
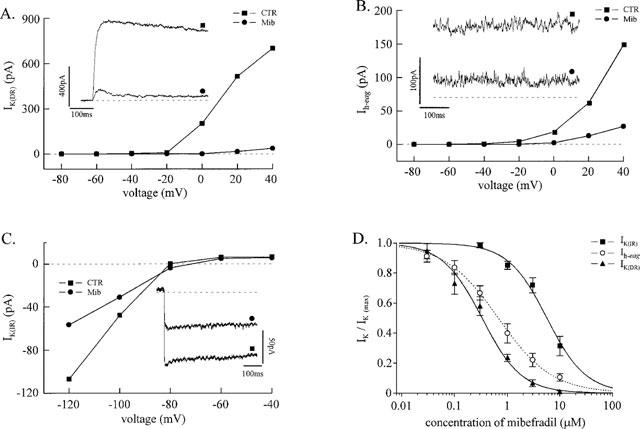
Mibefradil inhibits different types of K+ currents of fusion-competent myoblasts. All experiments were done with fusion-competent myoblasts exposed to differentiation medium for 3 days. (A) A myoblast (membrane capacitance 18 pF) was held steadily at −80 mV and stepped for 500 ms to a series of voltages between −80 and +40 mV. The outward current obtained at the end of the step was plotted as a function of the voltage during a step in the absence and in the presence of 3 μM mibefradil. Leak current was obtained by linear extrapolation from the current-voltage relationship between −80 and −40 mV and subtracted. This cell had no Ih-eag, so that the outward current represents exclusively IK(DR). Currents recorded from the same cell (during a step to +40 mV) without and with 3 μM mibefradil are shown. (B) Another fusion-competent myoblast (membrane capacitance 17 pF) was held steadily at +40 mV for 3 min, and stepped for 500 ms to a series of voltages between −80 and +40 mV. Symbols represent the amplitude of Ih-eag recorded in the absence and in the presence of 3 μM mibefradil. Leak current was obtained as in (A) and subtracted. Steady-state currents at +40 mV in the absence and in the presence of 3 μM mibefradil are shown (same cell). The dotted line indicates the leak at +40 mV. (C) A fusion-competent myoblast (membrane capacitance 26 pF) expressing IK(IR) was held steadily at −60 mV and stepped to a series of voltages between −120 and −40 mV for 500 ms. Leak subtracted IK(IR) currents measured in the absence and in the presence of 10 μM mibefradil were plotted against the voltage during the step. Leak current was obtained by adding 500 μM Ba2+ to the bath solution. Inward K+ currents recorded from the same cell at −120 mV in the absence and in the presence of 10 μM mibefradil are shown. (D) IK(DR), Ih-eag, and IK(IR) normalized to their respective control values are plotted as a function of mibefradil concentration. The three data sets were fitted with a Hill equation.
In Figure 3B, it can be seen that mibefradil is also very efficient at inhibiting another outward K+ current, Ih-eag. As Ih-eag does not inactivate with time, the inset illustrates the steady current recorded at +40 mV in the absence and in the presence of 3 μM mibefradil. As for IK(DR), the block of Ih-eag by mibefradil occurred at all voltages.
At a higher concentration range, mibefradil can also block IK(IR). Figure 3C shows that IK(IR) is reduced by 50% by mibefradil at a concentration of 10 μM. The complete dose-response curve for five cells is illustrated in Figure 3D. The IC50 of mibefradil for IK(IR) is 5.6 μM.
Figure 3D also illustrates the effect of various mibefradil concentrations on IK(DR) and Ih-eag. The IC50 of mibefradil for IK(DR) and Ih-eag are 0.3 and 0.7 μM, respectively, i.e. very similar to that of ICa(T) in human fusion-competent myoblasts.
Discussion
This study on the effect of mibefradil on human myoblasts was initiated in order to evaluate the role of ICa(T) in myoblast fusion. Unfortunately, despite its strong inhibitory effect on fusion, mibefradil did not allow us to assess the importance of ICa(T) in the fusion process. However, an interesting observation emerged from this study: mibefradil inhibits several types of voltage-gated K+ currents including an inward rectifier, and is as efficient an inhibitor of the outward K+ currents as of ICa(T).
Selectivity of mibefradil on T-type Ca2+ current
In venous smooth muscle myocytes and heart cells, mibefradil has a selectivity for ICa(T) over ICa(L) in an excess of 10 (Ertel & Ertel, 1997; Mishra & Hermsmeyer, 1994). In neurons, however, the situation is different. In embryonic rat spinal cord motoneurones, all inward Ca2+ currents were blocked in the same concentration range with an IC50 of 1.4 μM (Viana et al., 1997). In myoblasts, the IC50 of mibefradil for ICa(L) is in the same range (2 μM) as the IC50 for rat motoneurones, although, the IC50 for ICa(T) is three times smaller (0.7 μM). Hence, in skeletal muscle myoblasts, the selectivity of mibefradil for ICa(T) is halfway between that of neurones and smooth muscle cells. It is possible that the differences in results on various cell types with respect to selectivity for ICa(T) reflects the existence of different types of Ca(T) channels in these cells. A Ca(T) channel has recently been cloned (α1G; Perez-Reyes et al., 1998), and it appears that there exists more than one type of Ca(T) channel (α1H; Cribbs et al., 1998). In any case, the difference in mibefradil IC50 for low- and high-voltage activated Ca2+ channels in skeletal muscle myoblasts is not sufficiently large to allow a very specific inhibition of ICa(T) versus ICa(L).
Enhancement of ICa(L) by low concentrations of mibefradil
Low concentrations of mibefradil significantly and very reproducibly enhanced ICa(L) in human fusion-competent myoblasts. An increase of ICa(L) by low concentrations of mibefradil has also been described in Chinese hamster ovary (CHO) cells co-transfected with the smooth muscle L-type α1Cb subunit and an L-type β subunit. This enhancement of ICa(L) by mibefradil was, however, only observed in a subpopulation of transfected CHO cells, and was not present when CHO cells were transfected with the α1Cb subunit alone (Welling et al., 1995). At higher concentrations, an inhibitory effect of mibefradil was, on the other hand, always observed. It has been hypothesized that this dual action could be caused by the existence of two separate binding sites for mibefradil (Welling et al., 1995). We did not further explore the underlying mechanism but if this should occur in cell types that are the pharmacological target of this type of agent, an increase in Ca2+ influx through depolarized membranes containing Ca(L) channels could occur at certain mibefradil concentrations.
Effect of mibefradil on K+ currents
There are several indications that mibefradil may act on channels other than Ca2+ channels. For example, mibefradil inhibits Ca2+-activated Cl− currents and volume-sensitive Cl− currents in endothelial cells with an IC50 in the range of 5 μM (Nilius et al., 1997). However, in these cells, mibefradil at concentrations up to 30 μM did not affect an inwardly rectifying K+ channel (Kir1.0 or Kir4.0 families; Voets et al., 1996; Nilius et al., 1997), and we have not noticed any report of an effect of this drug on outward K+ currents. Therefore, it was unexpected to observe that, when compared to ICa(T), mibefradil could block outward K+ currents with equal efficiency (Ih-eag) or even more efficiently (IK(DR)). Interestingly, these two outward currents belong to different superfamilies, as IK(DR) is a member of the Shaker superfamily, and Ih-eag is the human ether-à-go-go K+ channel (Bijlenga et al., 1998; Occhiodoro et al., 1998). If this effect of mibefradil on K+ channels occurs in other cell types, effects on action potential duration, resting membrane potential or even rhythmic activity should be expected. Regarding IK(IR) in human myoblasts (Kir2.0 family; Liu et al., 1998), the sensitivity to mibefradil is clearly present but is more comparable to that of ICa(L), than to that of ICa(T) or of the outward K+ currents of the same preparation.
Effect of mibefradil on fusion
There is a marked effect of mibefradil on myoblast fusion. However, at the concentration used in the experiment illustrated in Figure 1, more than 90% of ICa(T), IK(DR) and Ih-eag and approximately 50% of IK(IR) would have been blocked. Our experiences with caesium ions on myoblast fusion indicated that blocking 50% of IK(IR) reduced fusion by approximately 25%. Therefore, the block of IK(IR) by mibefradil cannot alone explain the 57% inhibition of fusion seen with 5 μM mibefradil. At present, we cannot distinguish whether there is an isolated or combined contribution of ICa(T), IK(DR) or Ih-eag to the residual 32% inhibition in fusion by mibefradil. It is worth noting however that a high concentration of this drug interferes with a mechanism essential to muscle growth and repair.
Acknowledgments
We thank Dr E.A. Entel for his comments on the manuscript, M. Berti and P. Brawand for their excellent technical assistance on cell cultures, and Dr A. Kaelin for providing the muscle biopsies. Mibefradil was the kind gift of Dr E.A. Ertel (Hoffmann-LaRoche, Switzerland). This work was supported by grants from the Fonds National Suisse pour la Recherche Scientifique (No. 31-054177.98 and 31-46893.96), the Foundation Suisse pour la Recherche sur les Maladies Musculaires, and the Sir Jules Thorn Overseas Trust.
Abbreviations
- ICa(L)
L-type Ca2+ current
- ICa(T)
T-type Ca2+ current
- Ih-eag
Human ether-à-go-go K+ current
- IK(DR)
Delayed rectifier K+ current
- IK(IR)
Inward rectifier K+ current
- IK(NI)
Non-inactivating K+ current
References
- BAROFFIO A., AUBRY J.P., KAELIN A., KRAUSE R.M., HAMANN M., BADER C.R. Purification of human muscle satellite cells by flow cytometry. Muscle Nerve. 1993;16:498–505. doi: 10.1002/mus.880160511. [DOI] [PubMed] [Google Scholar]
- BAROFFIO A., HAMANN M., BERNHEIM L., BOCHATON-PIALLAT M.L., GABBIANI G., BADER C.R. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. doi: 10.1046/j.1432-0436.1996.6010047.x. [DOI] [PubMed] [Google Scholar]
- BERNHEIM L., LIU J.-H., HAMANN M., HAENGGELI C.A., FISCHER-LOUGHEED J., BADER C.R. Contribution of a non-inactivating potassium current to the resting potential of fusion-competent human myoblasts. J. Physiol. 1996;493:129–141. doi: 10.1113/jphysiol.1996.sp021369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIJLENGA P., OCCHIODORO T., LIU J.-H., BADER C.R., BERNHEIM L., FISCHER-LOUGHEED J. An ether-à-go-go K+ current h-eag, contributes to the hyperpolarization of human fusion-competent myoblasts. J. Physiol. 1998;512:317–323. doi: 10.1111/j.1469-7793.1998.317be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOZEL J.P., ERTEL E.A., ERTEL S.I. Discovery and main pharmacological properties of mibefradil (Ro 40-5967), the first selective T-type calcium channel blocker. J. Hypertens. Suppl. 1997;15:S17–S25. doi: 10.1097/00004872-199715055-00004. [DOI] [PubMed] [Google Scholar]
- CRIBBS L.L., LEE J.H., YANG J., SATIN J., ZHANG Y., DAUD A., BARCLAY J., WILLIAMSON M.P., FOX M., REES M., PEREZ-REYES E. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- ERTEL S.I., ERTEL E.A. Low-voltage-activated T-type Ca2+ channels. Trends. Pharmacol. Sci. 1997;18:37–42. doi: 10.1016/s0165-6147(96)01021-8. [DOI] [PubMed] [Google Scholar]
- HAM R.G., STCLAIR J.A., BLAU H.M., WEBSTER C.Serum-free media for growth and differentiation of human muscle satellite cells Cellular and Molecular Biology of Muscle Development 1989New York: Liss; 905–914.Kedes, L.E & Stockdale, F.E., eds [Google Scholar]
- HAMANN M., WIDMER H., BAROFFIO A., AUBRY J.P., KRAUSE R.M., KAELIN A., BADER C.R. Sodium and potassium currents in freshly isolated and in proliferating human muscle satellite cells. J. Physiol. 1994;475:305–317. doi: 10.1113/jphysiol.1994.sp020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. Eur. J. Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- KRAUSE R.M., HAMANN M., BADER C.R., LIU J.-H., BAROFFIO A., BERNHEIM L. Activation of nicotinic acetylcholine receptors increases the rate of fusion of cultured human myoblasts. J. Physiol. 1995;489:779–790. doi: 10.1113/jphysiol.1995.sp021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J.-H., BIJLENGA P., FISCHER-LOUGHEED J., OCCHIODORO T., KAELIN A., BADER C.R., BERNHEIM L. Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J. Physiol. 1998;510:467–476. doi: 10.1111/j.1469-7793.1998.467bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISHRA S.K., HERMSMEYER K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ. Res. 1994;75:144–148. doi: 10.1161/01.res.75.1.144. [DOI] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., KAMOUCHI M., VIANA F., VOETS T., DROOGMANS G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca(2+)- and volume-activated Cl− channels in macrovascular endothelial cells. Br. J. Pharmacol. 1997;121:547–555. doi: 10.1038/sj.bjp.0701140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCCHIODORO T., BERNHEIM L., LIU J.-H., BIJLENGA P., SINNREICH M., BADER C.R., FISCHER-LOUGHEED J. Cloning of a human ether-à-go-go potassium channel expressed in myoblasts at the onset of fusion. FEBS Lett. 1998;434:177–182. doi: 10.1016/s0014-5793(98)00973-9. [DOI] [PubMed] [Google Scholar]
- PEREZ-REYES E., CRIBBS L.L., DAUD A., LACERDA A.E., BARCLAY J., WILLIAMSON M.P., FOX M., REES M., LEE J.H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- PO A.L., ZHANG W.Y. What lessons can be learnt from withdrawal of mibefradil from the market. Lancet. 1998;351:1829–1830. doi: 10.1016/s0140-6736(05)78800-0. [DOI] [PubMed] [Google Scholar]
- STCLAIR J.A., MEYER S., DEMAREST S.D., HAM R.G. Improved medium with EGF and BSA for differentiated human skeletal muscle cells. Muscle Nerve. 1992;15:774–779. doi: 10.1002/mus.880150705. [DOI] [PubMed] [Google Scholar]
- VIANA F., VAN DEN BOSCH L., MISSIAEN L., VANDENBERGHE W., DROOGMANS G., NILIUS B., ROBBERECHT W. Mibefradil (Ro 40-5967) blocks multiple types of voltage-gated calcium channels in cultured rat spinal motoneurones. Cell Calcium. 1997;22:299–311. doi: 10.1016/s0143-4160(97)90068-3. [DOI] [PubMed] [Google Scholar]
- VOETS T., DROOGMANS G., NILIUS B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J. Physiol. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLING A., LACINOVA L., DONATIN K., LUDWIG A., BOSSE E., FLOCKERZI V., HOFMANN F. Expression of the L-type calcium channel with two different beta subunits and its modulation by Ro 40-5967. Pflügers Arch. 1995;429:400–411. doi: 10.1007/BF00374156. [DOI] [PubMed] [Google Scholar]
- WIDMER H., HAMANN M., BAROFFIO A., BIJLENGA P., BADER C.R. Voltage-dependent potassium current precedes fusion of human muscle satellite cells (Myoblasts) J. Cell. Physiol. 1995;162:52–63. doi: 10.1002/jcp.1041620108. [DOI] [PubMed] [Google Scholar]


