Abstract
Peroxynitrite is a strong oxidant that results from reaction between NO and superoxide. It has been recently proposed that peroxynitrite plays a pathogenetic role in inflammatory processes. Here we have investigated the therapeutic efficacy of raxofelast, a new hydrophilic vitamin E-like antioxidant agent, in rats subjected to carrageenan-induced pleurisy.
In vivo treatment with raxofelast (5, 10, 20 mg kg−1 intraperitoneally 5 min before carrageenan) prevented in a dose dependent manner carrageenan-induced pleural exudation and polymorphonuclear migration in rats subjected to carrageenan-induced pleurisy. Lung myeloperoxidase (MPO) activity and malondialdehyde (MDA) levels, as well as histological organ injury were significantly reduced by raxofelast.
Immunohistochemical analysis for nitrotyrosine, a footprint of peroxynitrite, revealed a positive staining in lungs from carrageenan-treated rats. No positive nitrotyrosine staining was found in the lungs of the carrageenan-treated rats, which received raxofelast (20 mg kg−1) treatment.
Furthermore, in vivo raxofelast (5, 10, 20 mg kg−1) treatment significantly reduced peroxynitrite formation as measured by the oxidation of the fluorescent dihydrorhodamine 123, prevented the appearance of DNA damage, the decrease in mitochondrial respiration and partially restored the cellular level of NAD+ in ex vivo macrophages harvested from the pleural cavity of rats subjected to carrageenan-induced pleurisy.
In conclusion, our study demonstrates that raxofelast, a new hydrophilic vitamin E-like antioxidant agent, exerts multiple protective effects in carrageenan-induced acute inflammation.
Keywords: Raxofelast, peroxynitrite, carrageenan, free radicals, inflammation
Introduction
The role of oxyradical formation in various forms of inflammation is well established. Recent data demonstrate that the expression of the inducible isoform of nitric oxide (NO) synthase also plays important pathogenetic roles in various models of inflammation (Moncada et al., 1991; Nathan 1992; Cuzzocrea et al., 1998a). The systemic inflammatory response is also associated with the production of oxygen-derived free radicals (Youn et al., 1991; McCord, 1993), and there is now substantial evidence that much of the cytotoxicity is due to a concerted action of oxygen- and nitrogen-derived free radicals and oxidants. Peroxynitrite, a cytotoxic oxidant species formed from the reaction of NO and superoxide (Beckman et al., 1990) may mediate part of the oxidative injury associated with simultaneous production of NO and oxyradicals. The biological activity and decomposition of peroxynitrite is very much dependent on the cellular or chemical environment (presence of proteins, thiols, glucose, the ratio of NO and superoxide, carbon dioxide levels and other factors), and these factors influence its toxic potential (Beckman et al., 1990; Villa et al., 1994; Rubbo et al., 1994; Pryor & Squadrito, 1995). Peroxynitrite formation has been demonstrated in various inflammatory disorders (Halliwell, 1995; Salvemini et al., 1996a,1996b; Cuzzocrea et al., 1997b,1997c;1998a) and in circulatory shock (Wiseman et al., 1994; Szabò, 1996).
Using the experimental model described here, previous work has demonstrated the anti-inflammatory potential of various therapeutic approaches aimed at the inhibition of NO synthesis and peroxynitrite formation (Tracey et al., 1995; Cuzzocrea et al., 1997c; 1998a). In our studies we utilized raxofelast (IRFI 016; 5-acetyloxy-2,3-dihydro-4,6,7-trimethyl-2-benzofuranacetic acid; Figure 1) a new hydrophilic vitamin E-like antioxidant agent (Campo et al., 1997). It was selected from a series of new compounds (Ceccarelli et al., 1993) designed with the aim of maximizing the antioxidant potency of phenols chemically related to α-tocopherol (vitamin E). The antioxidant activity of raxofelast has been convincingly demonstrated in several in vitro studies (Mattioli et al., 1991) and in various models of ischaemia-reperfusion injury (Campo et al., 1992; 1994). The purpose of the present study was to investigate the protective effect of raxofelast against the cellular energetic failure and the development of inflammation in rats treated with carrageenan.
Figure 1.
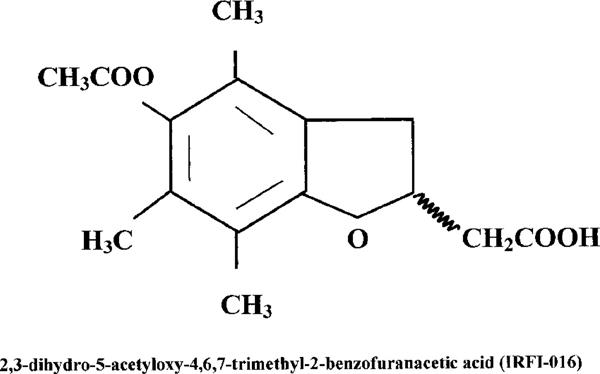
Chemical structure of IRFI 016 (raxofelast).
Methods
Experimental groups
In the treated group of animals, raxofelast, was given intraperitoneally (i.p.) 5 min before carrageenan (5, 10, 20 mg kg−1) (carrageenan+raxofelast group). In a vehicle-treated group of rats, vehicle (saline) was given instead of raxofelast (carageenan group). In separate groups of rats, surgery was performed in its every aspect identical to the one in the carrageenan group, except that carrageenan was not injected (control group). In an additional group of animals, control surgery was combined with the administration of raxofelast (dose as above) (control+raxofelast).
Carrageenan-induced pleurisy
Rats were lightly anaesthetized under isoflurane and submitted to a skin incision at the level of the left sixth intercostal space. The underlying muscles were dissected and 0.2 ml saline alone or containing 1% λ-carrageenan were injected into the pleural cavity. The skin incision was closed with a suture and the animals were allowed to recover. At 4 h after the injection of carrageenan, the animals were sacrificed under CO2 vapour. The chest was carefully opened and the pleural cavity washed with 2 ml of saline solution with heparin (5 u ml−1) and indomethacin (10 μg ml−1). The exudate and washing were removed by aspiration and the total volume measured. Exudates contaminated with blood were discarded. The results were calculated by subtracting the volume injected (2 ml) from the total volume recovered. Leucocytes in the exudate were suspended in phosphate buffer saline and counted with an optical microscope using a Burker's chamber after vital Trypan Blue stain.
Cell culture
Resident pleural cells macrophages were collected 4 h after the carrageenan injection from rats treated with or without raxofelast (Cuzzocrea et al., 1998b). The cells (1×106 ml−1), being mainly macrophages (approximately 70%) were cultured in DMEM medium, supplemented with M-glutamine (3.5 mM), penicillin (50 u ml−1, streptomycin (50 μg ml−1) and heparin sodium (10 u ml−1) in 12-well 2 h and allowing cells to adhere at 37°C in a humidified 5% CO2 incubator. Nonadherent cells were removed by rinsing the plates three times with 5% dextrose water. After removing nonadherent cells (approximately 10%), adherent macrophages were scraped from the measurement of DNA strand breaks and cellular NAD+. Mitochondrial respiration and peroxynitrite formation were measured in the adherent cells in the subsequent 1 h period.
Measurement of peroxynitrite-induced oxidation of dihydrorhodamine 123
The formation of peroxynitrite was measured by the peroxynitrite-dependent oxidation of dihydrorhodamine 123 to rhodamine 123, as previously described (Cuzzocrea et al., 1998b). Cells were rinsed with phosphate-buffered saline and then medium was replaced with phosphate-buffered saline containing 5 μM dihydrorhodamine 123. After a 60 min incubation at 37°C, the fluorescence of rhodamine 123 was measured using a fluorimeter at an excitation wavelength of 500 nm, emission wavelength of 536 nm (slit widths 2.5 and 3.0 nm, respectively). Thus this method is an indirect measurement of peroxynitrite production because also other oxidant species can induce oxidation of dihydrorhodamine 123.
Measurement of mitochondrial respiration
Cell respiration was assessed by the mitochondrial-dependent reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan (Zingarelli et al., 1996). Cells in 96-well plates were incubated at 37°C with MTT (0.2 mg ml−1) for 1 h. Culture medium was removed by aspiration and the cells were solubilized in DMSO (100 μl). The extent of reduction of MTT to formazan within cells was quantitated by the measurement of OD550. As previously discussed (Darley-Usmar & Halliwell, 1996), the measurement of reduction of MTT appears to be mainly by the mitochondrial complexes I and II, it also may involve NADH- and NADPH-dependent energetic processes that occur outside the mitochondrial inner membrane. Thus, this method cannot be used to separate the effect of free radicals, oxidants or other factors on the individual enzymes in the mitochondrial respiratory chain, but is useful to monitor changes in the general energetic status of the cells (Darley-Usmar & Halliwell, 1996).
Determination of DNA single-strand breaks
The formation of DNA strand breaks in double-stranded DNA was determined by the alkaline unwinding methods as previously described (Zingarelli et al., 1996; Schraufstatter et al., 1986). Cells in 12-well plates were scraped into 0.2 ml of solution A buffer (myoinositol 250 mM, NaH2PO3 10 mM, pH 7.2.). The cell lysate was then transferred into plastic tubes designated T (maximum fluorescence), P (fluorescence in sample used to estimate extent of DNA unwinding), or B (background fluorescence). To each tube, 0.2 ml of solution B (alkaline lysis solution: NaOH 10 mM, urea 9 M, ethylenediamineteraacetic acid 2.5 mM, sodium dodecyl sulphate 0.1%) was added and incubated at 4°C for 10 min to allow cell lysis and chromatin disruption. 0.1 ml each of solutions C (0.45 volume solution B in 0.2 N NaOH) and D (0.4 volume solution B in 0.2 N NaOH) were then added to the P and B tubes. 0.1 ml of solution E (neutralizing solution: glucose 1 M, mercaptoethanol 14 mM) was added to the T tubes before solutions C and D were added. From this point incubations were carried out in the dark. A 30 min incubation period at 0 °C was then allowed during which the alkali diffused into the viscous lysate. Since the neutralizing solution, solution E, was added to the T tubes before addition of the alkaline solutions C and D, the DNA in the T tubes was never exposed to a denaturing pH. At the end of the 30 min incubation, the contents of the B tubes were sonicated for 30 s to ensure rapid denaturation of DNA in the alkaline solution. All tubes were then incubated at 15°C for 10 min. Denaturation was stopped by chilling to 0°C and adding 0.4 ml of solution E to the P and B tubes. 1.5 ml of solution F (ethidium bromide 6.7 μg ml−1 in 13.3 mM NaOH) was added to all the tubes and fluorescence (excitation: 520 nm, emission: 590 nm) was measured by a fluorimeter. Under the conditions used, in which ethidium bromide binds preferentially to double stranded DNA, the percentage of double stranded DNA (D) may be determined using the equation: % D=100×[F(P)−F(B)]/[F(T)−F(B)]; where F(P) is the fluorescence of the sample, F(B) the background fluorescence, i.e. fluorescence due to all cell components other than double stranded DNA, and F(T) the maximum fluorescence.
Measurement of cellular NAD+ levels
Cells in 12-well plates were extracted in 0.25 ml of 0.5 N HC104 scraped, neutralized with 3 M KOH, and centrifuged for 2 min at 10,000×g. The supernatant was assayed for NAD+ using a modification of the colorimetric method (Heller et al., 1995) in which NADH produced by enzymatic cycling with alcohol dehydrogenase, reduces MTT to formazan through the intermediation of phenazine methosulphate. The rate of MTT reduction is proportional to the concentration of the co-enzyme. The reaction mixture contained 10 μl of a solution of 2.5 mg ml−1 MTT, 20 μl of a solution of 4 mg ml−1 phenazine methosulphate, 10 μl of a solution of 0.6 mg ml−1 alcohol dehydrogenase (300 u mg−1), and 190 μl of 0.065 M glycyl-glycine buffer, pH 7.4, that contained 0.1 M nicotinamide and 0.5 M ethanol. The mixture was warmed to 37°C for 10 min, and the reaction was started by the addition of 20 μl of the sample. The rate of increase in absorbance was read immediately after the addition of NAD+ samples and after 10- and 20-min incubation at 37°C against blank at 560 nm in the ELISA microplate reader (SLT-Labinstruments Salzburg, Austria).
Light microscopy
Lung biopsies were taken 4 h after the induction of pleurisy by carrageenan injection. The tissue slices were fixed in Dietric solution (14.25% ethanol, 1.85% formaldehyde, 1% acetic acid) for 1 week at room temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, U.S.A.). Section (thickness 7 μm) were deparaffinized with xylene, stained with trichromic Van Gieson and observed in Dialux 22 Leitz microscope.
Immunohistochemical localization of nitrotyrosine
Tyrosine nitration, a specific ‘footprint' of peroxynitrite formation, was detected as previously described (Cuzzocrea et al., 1997b) in lung sections by immunohistochemistry. Tissues were fixed in 10% buffered formalin and 8 μm sections were prepared from paraffin embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% H2O2 in 60% methanol for 30 min. The sections were permeabilized with 0.1% Triton X-100 in phosphate buffered saline for 20 min. Non-specific adsorption was minimized by incubating the section in 2% normal goat serum in phosphate buffered saline for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with avidin and biotin (DBA, Milan, Italy). The sections were then incubated overnight with 1 : 1000 dilution of primary anti-nitrotyrosine antibody (DBA, Milan, Italy) or with control solutions. Controls included buffer alone or non specific purified rabbit IgG. Specific labelling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex (DBA, Milan, Italy).
Myeloperoxidase activity
Myeloperoxidase (MPO) activity, an index of polymorphonuclear leukocyte (PMN) accumulation, was determined as previously described (Mullane et al., 1988). At the specified time following the intrapleural injection of carrageenan lung tissues, were obtained and weighed. Each piece of tissue was homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20,000×g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured spectrophotometrically at 650 nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 μmol of peroxide min−1 at 37°C and was expressed in milliunits per gram weight of wet tissue.
Malondialdehyde (MDA) measurement
Malondialdehyde (MDA) levels in the lung tissue were determined as an index of lipid peroxidation, as described by Okhawa et al. (1979). Lung tissue, collected at the specified time, were homogenized in 1.15% KCl solution. An aliquot (100 μl) of the homogenate was added to a reaction mixture containing 200 μl of 8.1% SDS, 1500 μl of 20% acetic acid (pH 3.5), 1500 μl of 0.8% thiobarbituric acid and 700 μl distilled water. Samples were then boiled for 1 h at 95°C and centrifuged at 3000×g for 10 min. The absorbance of the supernatant was measured by spectrophotometry at 650 nm.
Materials
Raxofelast was supplied by Biomedica Foscama, Ferentino (FR), Italy. Cell culture medium, heparin and foetal calf serum were obtained from Sigma (Milan, Italy). Biotin blocking kit, biotin-conjugated goat anti-rabbit IgG, primary anti-nitrotyrosine antibody and avidin-biotin peroxidase complex were obtained from DBA (Milan, Italy). All other reagents and compounds used were obtained from Sigma Chemical Company (Sigma, Milan, Italy).
Data analysis
All values in the figures and text are expressed as mean±standard error (s.e.m.) of the mean of n observations. For the in vitro studies, data represent the number of wells studied (6–9 wells from 2–3 independent experiments). For the in vivo studies n represents the number of animals studied. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments performed on different experimental days. The results were analysed by one-way ANOVA followed by a post-hoc Bonferroni test. A P-value less than 0.05 was considered significant.
Results
Effects of raxofelast in carrageenan-induced pleurisy
All carrageenan-injected rats developed an acute pleurisy, producing 1.8±0.15 ml of turbid exudate (Figure 2A). Trypan blue stain revealed 77±6.4×106 rat−1 PMNs in comparison to control rat (2.4±0.8×106 rat−1) (Figure 2B). Control animals demonstrated no abnormalities in the pleural cavity or fluid. The degree of peritoneal exudation and polymorphonuclear migration were significantly reduced in rats with raxofelast (Figure 2A,B). Raxofelast treatment did not cause significant changes in these parameters in control rats (Figure 2A,B).
Figure 2.
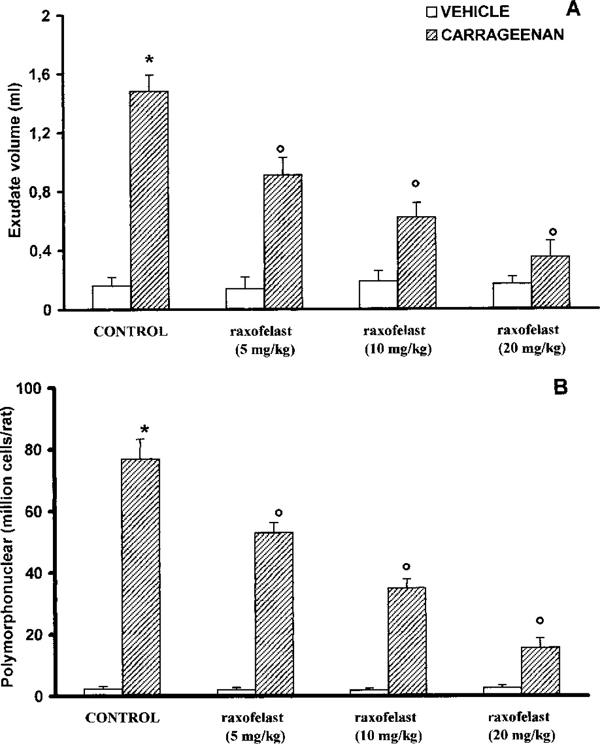
Volume exudate (A) and polymorphonuclear accumulation (B) in pleural cavity at 4 h after carrageenan injection. Raxofelast (5, 10, 20 mg kg−1) treatment significantly reduced in a dose dependent manner pleural exudation and leukocyte migration. Data are means±s.e.mean of ten rats for each group. *P<0.01 versus control; °P<0.01 versus carrageenan.
Lungs obtained from carrageenan-treated rats were examined for the measurement of MPO activity, the latter being indicative of neutrophil infiltration, and for MDA levels, in order to estimate lipid peroxidation. As shown in Figure 3A, B, MPO activity and MDA levels (7.2±6.4 mu 100 mg−1 wet tissue and 286±4.4 μM mg−1 wet tissue, respectively) were significantly (P<0.01) increased in the lung from carrageenan-treated rats when compared to control rats (16±3.5 mu 100 mg−1 wet tissue; 133±6.8 μM mg−1 wet tissue respectively.) MPO activity and MDA levels were significantly (P<0.01) reduced in a dose dependent manner by raxofelast treatment (Figure 3A,B).
Figure 3.
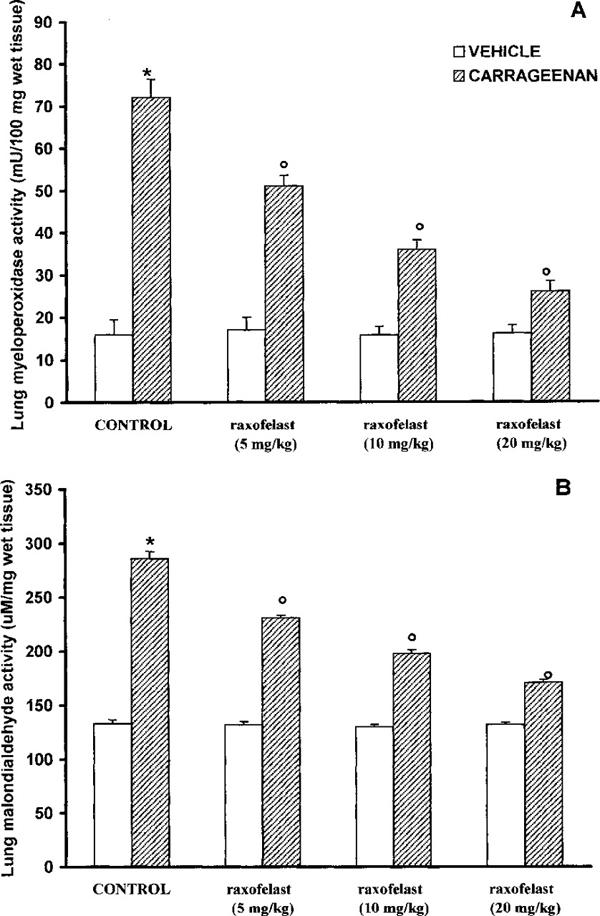
Myeloperoxidase (MPO) (A) and malondialdehyde (MDA) (B) in the lungs of carrageenan-treated rats. MPO activity and MDA levels were significantly increased in the lungs of the carrageenan-treated rats in comparison to control rats raxofelast (5, 10, 20 mg kg−1) significantly reduced the carrageenan-induced increase in MPO activity and MDA levels. Values are means±s.e.mean of ten rats for each group. *P<0.01 versus control; °P<0.01 versus carrageenan.
Histological examination of lung section showed inflammatory infiltration by neutrophil, lymphocytes and plasma cells extravasation (Figure 4B). Raxofelast treatment reduced histological organ injury (Figure 4C).
Figure 4.
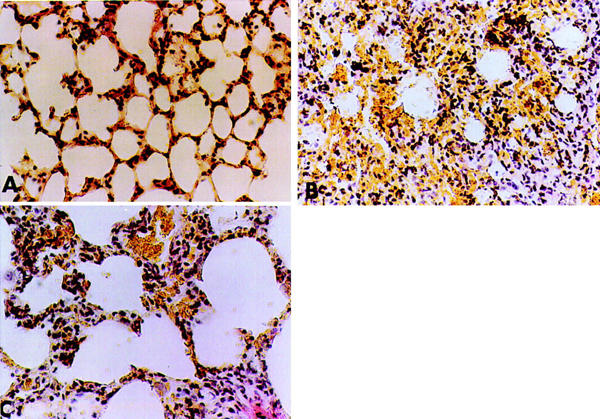
Representative lung sections from (A) control rats demonstrating the normal alveolar architecture. Lung sections from a carrageenan-treated rat (B) demonstrate inflammatory infiltration by neutrophil, lymphocytes and plasma. Lung sections from a carrageenan-treated rat that received raxofelast (20 mg kg−1) (C) demonstrate reduced inflammatory infiltration. Original magnification: ×62.5. Figure is representative of at least three experiments performed on different experimental days.
Lung sections were also analysed for the presence of nitrotyrosine, a footprint of peroxynitrite. Immunohistochemical analysis, using a specific anti-nitrotyrosine antibody, revealed a positive staining in lungs from carrageenan-treated rats, which was primarily localized in alveolar macrophages and in airway epithelial cells (Figure 5B). No positive nitrotyrosine staining was found in the lungs of the carrageenan-treated rats when they were treated with raxofelast (Figure 5C).
Figure 5.
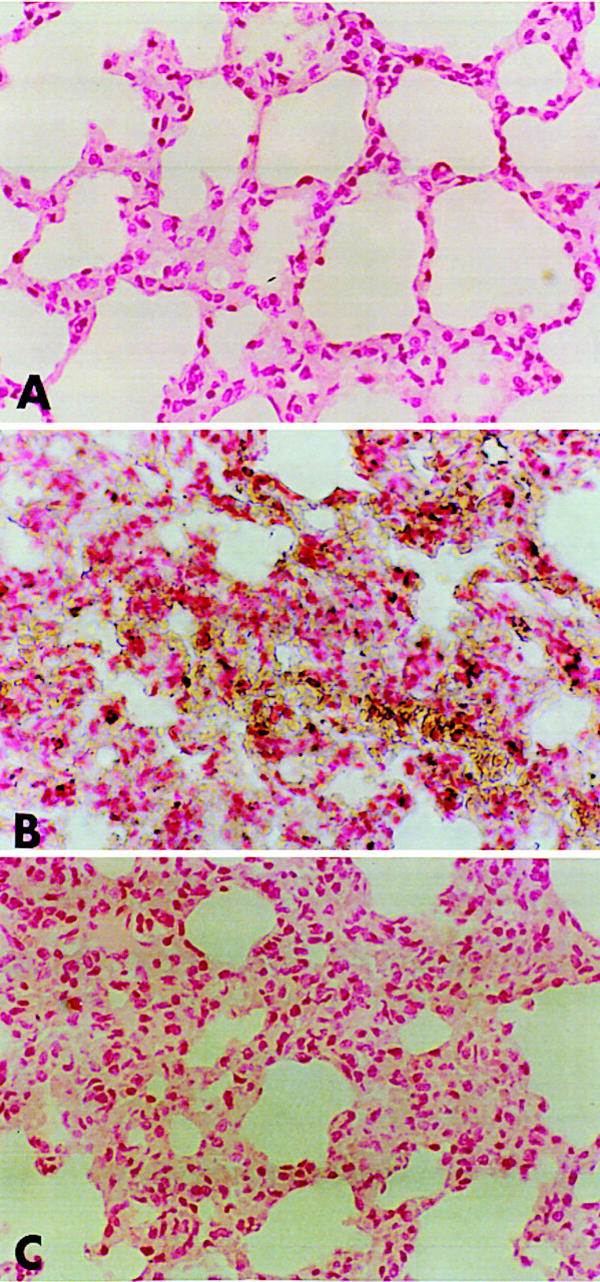
Immunohistochemical localization of nitrotyrosine in the rat lung. Staining was absent in control tissue (A). Four hours following carrageenan injection, nitrotyrosine immunoreactivity was localized mainly to macrophages and some epithelial cells (B). There is a marked reduction in the immunostaining in the lungs of carrageenan-treated rats when rats were pretreated with raxofelast (20 mg kg−1) (C). Original magnification: ×125. Figure is representative of at least three experiments performed on different experimental days.
Raxofelast protects against the cellular energetic failure
In pleural macrophages obtained from carrageenan-treated rats, a significant peroxynitrite production was detectable (66±4 pmoles min−1 cells; Figure 6A). A marked increase in DNA strand breakage was also observed after carrageenan-induced pleurisy (Figure 6B). Carregeenan-mediated disruption of cellular energetic pool was evidenced by a significant decrease in mitochondrial respiration and intracellular concentration of NAD+ (Figure 7A,B). The in vivo treatment of the animals with raxofelast significantly inhibited in a dose dependent manner dihydrorhodamine 123 oxidation, prevented the carrageenan-induced DNA single strand breakage (Figure 6A,B), significantly inhibited the decrease in cellular respiration and partially restored the depletion of intracellular levels of NAD+ (Figure 7A,B).
Figure 6.
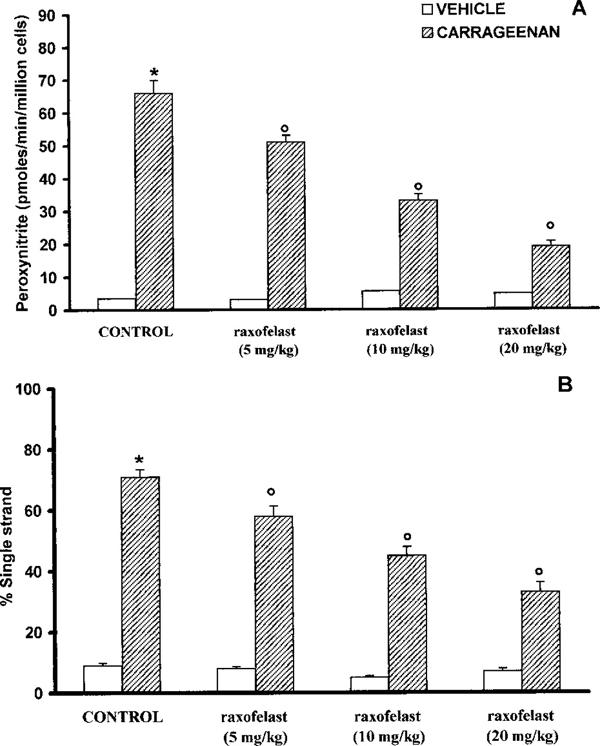
Peroxynitrite formation (A) and DNA single strand breakage development in pleural macrophages harvested from control and carrageenan-treated rats. Four hours after carrageenan injection a significant peroxynitrite production and a marked increase in DNA strand breakage was observed. Raxofelast significantly (5, 10, 20 mg kg−1) inhibited in a dose dependent manner dihydrorhodamine 123 oxidation and prevented the carrageenan-induced DNA single strand breakage. Data represent the number of wells studied (6–9 wells from 2–3 independent experiments). *P<0.01 versus macrophages from control rats; °P<0.01 represents a significant inhibitory effect of raxofelast.
Figure 7.
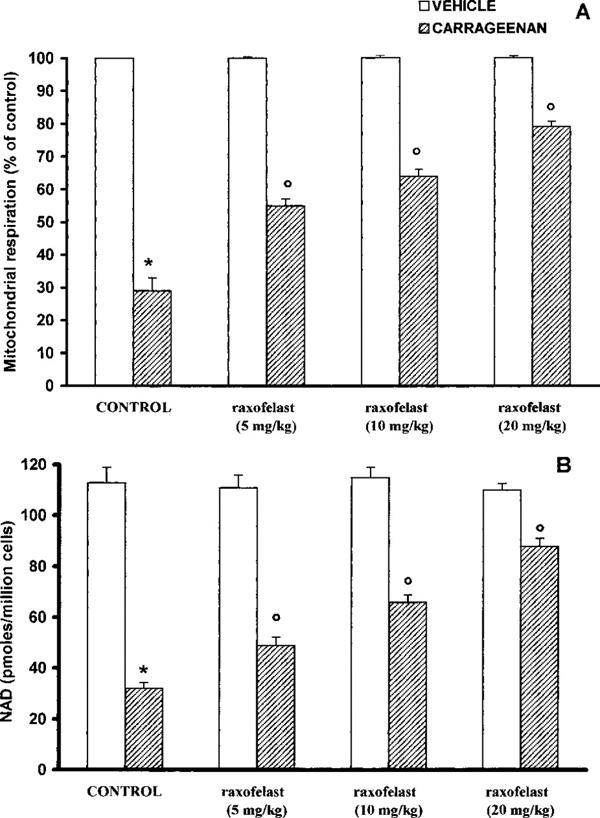
Reduction of mitochondrial respiration (A) and cellular levels of NAD+ (B) in macrophages from control and carrageenan-treated rats. Four hours after carregeenan administration the disruption of cellular energetic pool was evidenced by a significant decrease in mitochondrial respiration and intracellular concentration of NAD+. Raxofelast (5, 10, 20 mg kg−1) significantly inhibited in a dose dependent manner the decrease in cellular respiration and partially restored the depletion of intracellular levels of NAD+. Data represent the number of wells studied (6–9 wells from 2–3 independent experiments). *P<0.01 versus macrophages from control rats; °P<0.01 represents protective effects of raxofelast.
Discussion
Inflammatory process is invariably characterized by a production of prostaglandins, leukotrienes, histamine, bradykinin, platelet-activing factor (PAF) and interleukin I (IL-1), by a release of chemicals from tissues and migrating cells (Vane & Botting, 1987; Tomlinson et al., 1994). Furthermore, one possibility that has been frequently discussed is that production of reactive oxidants such as hydrogen peroxide, superoxide and hydroxyl radical at site of inflammation contributes to tissue damage (Oh-Ishi et al., 1989; Dawson et al., 1991; Peskar et al., 1991; Da Motta et al., 1994; Salvemini et al., 1996a,1996b; Cuzzocrea et al., 1997c; 1998a). Pharmacological inhibitors of NOS have been shown to reduce the development of carrageenan-induced inflammation and support a role of NO in this model of inflammation (Tracey et al., 1995; Wei et al., 1995; Salvemini et al., 1996a,1996b; Cuzzocrea et al., 1997c; 1998a). More recent studies have shown the formation of peroxynitrite in carrageenan-induced inflammation (Salvemini et al., 1996a,1996b; Cuzzocrea et al., 1997c; 1998a,1998b). Using nitrotyrosine immunohistochemistry, this study has confirmed the production of peroxynitrite in the lung of rats subjected to carrageenan-induced pleurisy.
The biological activity and decomposition of peroxynitrite is very much dependent on the cellular or chemical environment (presence of proteins, thiols, glucose, the ratio of NO and superoxide, carbon dioxide levels and other factors), and these factors influence its toxic potential (Beckman et al., 1990; Villa et al., 1994; Rubbo et al., 1994; Pryor & Squadrito, 1996).
In the present study we found that (1) raxofelast reduces the development of carrageenan-induced pleurisy (2) raxofelast reduces morphological injury and neutrophil infiltration in carrageenan-induced inflammation; and (3) raxofelast reduces nitrotyrosine immunostaining, an indicator of peroxynitrite formation in inflammation.
It could be speculated that the pharmacological effects of raxofelast may be dependent upon a combination of: (1) a scavenging action toward oxygen radicals, which results in the prevention of peroxynitrite formation with consequent protection against the development of peroxynitrite-induced cellular energetic failure, (2) a reduced neutrophil recruitment into the inflammatory site, which may represent an important additional mechanism for the observed anti-inflammatory action. The reduced neutrophil infiltration into the inflamed tissue may be related to a prevention of endothelial oxidant injury by raxofelast and to a preservation of endothelial barrier function. Therefore, the relative contribution of the raxofelast's multiple modes of action observed in the current study to be determined in further studies.
Recent studies have proposed that a novel pathway, involving the nuclear enzyme poly (ADP-ribose) synthetase (PARS) play an important role in inflammation (Szabò et al., 1997a; 1998; Cuzzocrea et al., 1998b,1998c). It is well known that this pathway plays an important role in various forms of shock (Szabò et al., 1997b) and reperfusion injury (Thiemermann et al., 1997; Zingarelli et al., 1997; Cuzzocrea et al., 1997b). Raxofelast has been shown to display potent protective effects against oxidative damage in in vitro studies (Mattioli et al., 1991) and in various models of oxyradical-mediated ischaemia-reperfusion injury (Campo et al., 1992; 1994). The protection by raxofelast against the development of DNA single strand breakage and the partially restoration of intracellular NAD+ depletion (as shown in Figures 6A and 7B), may thus be related to a decreased peroxynitrite formation, which may thus lead to a prevention of the activation of PARS in inflammation.
Taken together, the results of the present study coupled with recent data by several groups support the view that raxofelast can exert protective effects on cellular injury. In conclusion raxofelast, an α-tocopherol analogue with free radical scavenging properties, reduces the inflammatory response in rats subjected to carrageenan-induced pleurisy. It may therefore have potential therapeutic effects.
Acknowledgments
We gratefully acknowledge Biomedica Foscama Research Centre, Ferentino, (FR), Italy for the generous supply of raxofelast. We also thank Fabio Giuffrè and Carmelo La Spada for their excellent technical assistance during this study, Mrs Caterina Cutrona for secretarial assistance and Miss Valentina Malvagni for editorial assistance with the manuscript.
Abbreviations
- ecNOS
constitutive endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- MPO
myeloperoxidase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PARS
poly (ADP-ribose) synthetase
- PMN
polymorphonuclear cells
References
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPO G.M., SQUADRITO F., IOCULANO M., AVENOSO A., ZINGARELLI B., CALANDRA S., SCURI R., SAITTA A., CAPUTI A.P. IRFI 016 a new radical scavenger limits ischemic damage following coronary artery occlusion in rats. Res. Commun. Chem. Pathol. Pharmacol. 1992;76:287–303. [PubMed] [Google Scholar]
- CAMPO G.M., SQUADRITO F., IOCULANO M., POLLICINO A.M., RIZZO A., CALAPAI G., CALANDRA S., SCURI R., CAPUTI A.P. Protective effect of IRFI 016, a new antioxidant agent, in myocardial damage following coronary artery occlusion and reperfusion in rats. Pharmacol. 1994;48:157–166. doi: 10.1159/000139175. [DOI] [PubMed] [Google Scholar]
- CAMPO G.M., GECCARELLI S., SQUADRITO F., ALTAVILLA D., DORIGOTTI L., CAPUTI A.P. Raxofelast (IRFI 016): a new hydrophilic vitamin E-like antioxidant agent. Cardiovasc. Drug Rev. 1997;15:157–1173. [Google Scholar]
- CECCARELLI S., DE VELLIS P., SCURI R., ZANARELLA S. Synthesis of novel 2-substituted-5-oxycoumarans via a direct route to 2,3-dihydro-5-hydroxy-2-benzofuranacetic acids. J. Heterocycl. Chem. 1993;30:679–690. [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., CALAPAI G., NAVA F., CAPUTI A.P. Zymosan-activated plasma induces paw oedema by nitric oxide and prostaglandin production. Life Sci. 1997a;60:215–220. doi: 10.1016/s0024-3205(96)00618-2. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., SZABÓ A., SALZMAN A.L., CAPUTI A.P., SZABÓ C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 1997b;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., GILARD E., HAKE P., SALZMAN A.L., SZABÓ C. Protective effect of melatonin in carrageenan-induced models of local inflammation. J. Pineal Res. 1997c;23:106–116. doi: 10.1111/j.1600-079x.1997.tb00342.x. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., GILARD E., HAKE P., SALZMAN A.L., SZABÓ C. Anti-inflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Rad. Biol. Med. 1998a;24:450–459. doi: 10.1016/s0891-5849(97)00280-3. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., CAPUTI A.P., ZINGARELLI B. Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology. 1998b;93:96–101. doi: 10.1046/j.1365-2567.1998.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., GILARD E., HAKE P., SALZMAN A.L., SZABÓ C. Protective effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthase in carrageenan-induced models of local inflammation. Eur. J. Pharmacol. 1998c;342:67–76. doi: 10.1016/s0014-2999(97)01417-9. [DOI] [PubMed] [Google Scholar]
- DA MOTTA J.I., CUNHA F.Q., VARGAFTIG B.B., FERREIRA S.H. Drug modulation of antigen-induced paw oedema in guinea-pigs: effects of lipopolysaccharide, tumour necrosis factor and leucocyte depletion. Br. J. Pharmacol. 1994;112:111–116. doi: 10.1111/j.1476-5381.1994.tb13038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARLEY-USMAR V., HALLIWELL B. Blood radicals. Reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharmaceut. Res. 1996;13:649–655. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- DAWSON J., SEDGWICK A.D., EDWARDS J.C., LEES P. A comparative study of the cellular, exudative and histological responses to carrageenan, dextran and zymosan in the mouse. Int. J. Tissue React. 1991;13:171–185. [PubMed] [Google Scholar]
- HALLIWELL B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann. Rheumat. Dis. 1995;54:505–510. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER B., WANG Z.Q., WAGNER E.F., RADONS J., BURKLE A., FEHSEL K., BURKART V., KOLB H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J. Biol. Chem. 1995;270:11176. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- MATTOLI S., MEZZETTI M., ALLEGRA L., BRAGA P.C. Dihydro-hydroxy-trimethyl-benzofuranyl acetic acid inhibits lipid peroxydation and lysosomal enzyme release in bronchial epithelial cells and macrophages. Ital. J. Chest Dis. 1991;45:78–80. [Google Scholar]
- MCCORD J. Oxygen-derived free radicals. New Horizons. 1993;1:70–76. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, Pathophysiology and Pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MULLANE K.M., WESTLIN W., KRAEMER R. Activated neutrophils release mediators that may contribute to myocardial injury and dysfunction associated with ischemia and reperfusion. Ann. N.Y. Acad. Sci. U.S.A. 1988;524:103–121. doi: 10.1111/j.1749-6632.1988.tb38534.x. [DOI] [PubMed] [Google Scholar]
- NATHAN C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- OH-ISHI S., HAYASHI I., HAYASHI M., YAMAKI K., UTSUNOMIYA I. Pharmacological demonstration of inflammatory mediators using experimental inflammatory models: rat pleurisy induced by carrageenin and phorbol myristate acetate. Dermatologica. 1989;179 suppl 1:68–71. doi: 10.1159/000248453. [DOI] [PubMed] [Google Scholar]
- PESKAR B.M., TRAUTMANN M., NOWAK P., PESKAR B.A. Release of 15-hydroxy-5,8,11,13-eicosatetraenoic acid and cysteinyl-leukotrienes in carrageenin-induced inflammation: effect of non-steroidal anti-inflammatory drugs. Agents Actions. 1991;33:240–246. doi: 10.1007/BF01986569. [DOI] [PubMed] [Google Scholar]
- PRYOR W., SQUADRITO G. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995;268:L699–L772. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- RUBBO H., RADI R., TRUJILLO M., TELLERI R., KALYANARAMAN B., BARNES S., KIRK M., FREEMAN B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidised lipid derivatives. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996a;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., BOURDON D.M., STERN M.K., CURRIE M.G., MANNING P.T. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 1996b;303:217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., HINSHAW D.B., HYSLOP P.A., SPRAGG R.G., COCHRANE C.G. Oxidant injury of cells: DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J. Clin. Invest. 1986;77:1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C. The role of peroxynitrite in the pathophysiology of shock, inflammation and ischemia-reperfusion injury. Shock. 1996;6:79–87. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., LIM L.H.K., CUZZOCREA S., GETTING S.J., ZINGARELLI B., FLOWER R.J., SALZMAN A.L., PERRETTI M. Inhibition of poly (ADP-ribose) synthetase exerts anti-inflammatory effects and inhibits neutrophil recruitment. J. Exp. Med. 1997a;186:1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., CUZZOCREA S., ZINGARELLI B., O'CONNOR M., SALZMAN A.L. Endothelial dysfunction in endotoxic shock: importance of the activation of poly (ADP ribose synthetase (PARS) by peroxynitrite. J. Clin. Invest. 1997b;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., VIRÀG L., CUZZOCREA S., SCOTT G.S., HAKE P., O'CONNOR M., ZINGARELI B., MA Y., HIRSCH R., BOIOVIN G.P., SALZMAN A.L., KUN E. Protection against peroxynitrite-induced fibroblast injury and arthritis development by inhibition of poly (ADP-Ribose) synthetase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3867–3872. doi: 10.1073/pnas.95.7.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIEMERMANN C., BOWES J., MYNNY F.P., VANE J.R. Inhibition of the activity of poly(ADP ribose) synthase reduces ischaemia-reperfusion injury in the heart and skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLINSON A., APPLETON I., MOORE A.R., GILROY D.W., WILLIS D., MITCHELL J.A., WILLOUGHBY Cyclo-oxygenase and nitric oxide isoforms in rat carrageenin-induced pleurisy. Br. J. Pharmacol. 1994;113:693–698. doi: 10.1111/j.1476-5381.1994.tb17048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRACEY W.R., NAKANE M., KUK J., BUDZIK G., KLINGHOFER V., HARRIS R., CARTER G. The nitric oxide synthase inhibitor, L-NG-monomethylarginine, reduces carrageenan-induced pleurisy in the rat. J. Pharmacol. Exp. Ther. 1995;273:1295–1299. [PubMed] [Google Scholar]
- VANE J., BLOTTING R. Inflammation and the mechanism of action of antiinflammatory drugs. FASEB J. 1987;1:89–96. [PubMed] [Google Scholar]
- VILLA L.M., SALAS E., DARLEY-USMAR M., RADOMSKI M.W., MONCADA S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI X.Q., CHARLES I.G., SMITH A., URE J., FENG G.J., HUANG F.P., XU D., MULLER W., MONCADA S., LIEW F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- WIZEMANN T., GARDNER C., LASKIN J., QUINONES S., DURHAM S., GOLLER N., OHNISHI T., LASKIN D. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J. Leukoc. Biol. 1994;56:759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- YOUN Y.K., LALONDE C., DEMLING R. Use of antioxidant therapy in shock and trauma. Circ. Shock. 1991;35:245–249. [PubMed] [Google Scholar]
- ZINGARELLI B., CUZZOCREA S., ZSENGELLER Z., SALZMAN A.L., SZABÓ C. Protection against myocardial ischemia and reperfusion injury by 3-aminobenzamide, an inhibitor of Poly (ADP-Ribose) synthetase. Cardiovasc. Res. 1997;36:205–215. doi: 10.1016/s0008-6363(97)00137-5. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., O'CONNOR M., WONG H., SALZMAN A.L., SZABÓ C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipolysaccharide. J. Immunol. 1996;156:350–358. [PubMed] [Google Scholar]


