Abstract
Several thrombin cellular effects are dependent upon stimulation of proteinase activated receptor-1 (PAR-1) localized over the cellular surface. Following activation by thrombin, a new N-terminus peptide is unmasked on PAR-1 receptor, which functions as a tethered ligand for the receptor itself. Synthetic peptides called thrombin receptor activating peptides (TRAPs), corresponding to the N-terminus residue unmasked, reproduce several thrombin cellular effects, but are devoid of catalytic activity. We have evaluated the bronchial response to intravenous administration of human α-thrombin or a thrombin receptor activating peptide (TRAP-9) in anaesthetized, artificially ventilated guinea-pigs.
Intravenous injection of thrombin (100 u kg−1) caused bronchoconstriction that was recapitulated by injection of TRAP-9 (1 mg kg−1). Animal pretreatment with the thrombin inhibitor Hirulog™ (10 mg kg−1 i.v.) prevented thrombin-induced bronchoconstriction, but did not affect bronchoconstriction induced by TRAP-9. Both agents did not induce bronchoconstriction when injected intravenously to rats.
The bronchoconstrictor effect of thrombin and TRAP-9 was subjected to tolerance; however, in animals desensitized to thrombin effect, TRAP-9 was still capable of inducing bronchoconstriction, but not vice versa.
Depleting animals of circulating platelets prevented bronchoconstriction induced by both thrombin and TRAP-9.
Bronchoconstriction was paralleled by a biphasic change in arterial blood pressure, characterized by a hypotensive phase followed by a hypertensive phase. Thrombin-induced hypotension was not subject to tolerance and was inhibited by Hirulog™; conversely, hypertension was subject to tolerance and was not inhibited by Hirulog™. Hypotension and hypertension induced by TRAP-9 were neither subject to tolerance nor inhibited by Hirulog™.
Our results indicate that thrombin causes bronchoconstriction in guinea-pigs through a mechanism that requires proteolytic activation of its receptor and the exposure of the tethered ligand peptide. Platelet activation might be triggered by the thrombin effect.
Keywords: Thrombin, thrombin receptor activating peptide, proteinase activated receptor, Hirulog™, bronchoconstriction, lung
Introduction
It is known that following tissue damage, extrinsic coagulation pathway becomes activated and thrombin is formed by its precursor, prothrombin (Schiffrin, 1994; Cicala & Cirino, 1998). Much attention has recently been focused on the cellular responses triggered by thrombin, highlighting a role for this enzyme in cell activation and inflammation. Both in vitro and in vivo studies have demonstrated that thrombin is chemotactic for monocytes (Bar Shavit et al., 1983) and neutrophils (Bizios et al., 1986; Drake & Issekutz, 1992); this activity might be mediated by high affinity receptors demonstrated to be present on both macrophages and neutrophils (Kudhal et al., 1991; Sonne, 1988). Thrombin increases IL-1- and TNFα-induced neutrophil chemotaxis (Drake et al., 1992), it causes fibroblast and smooth muscle cell proliferation (Panettieri et al., 1995), stimulates endothelial cells to produce PGI2 (Weksler et al., 1978); all these effects contribute to inflammation and tissue repair processes independently of haemostatic mechanisms.
Thrombin-like proteinases have been found in bronchoalveolar lavage fluids obtained from active immunized experimental animals (Linssen et al., 1991). The role of coagulation cascade activation and fibrin deposition during lung inflammation has been investigated; it has been shown that asthmatic subjects present alterations in coagulation parameters and an increase of platelet reactivity (Idell et al., 1989; Gresele et al., 1993; Fuchs-Buder et al., 1996). However, due to the lack of appropriate tools, such as specific inhibitors, the role of thrombin in bronchial asthma has never been addressed.
It is now clear that thrombin produces many of its biological activities through stimulation of proteinase activated receptor-1 (PAR-1) that is localized over the surface of several cells. PAR-1 is a seven transmembrane-domain receptor, demonstrated to be cleaved by thrombin between Arginine41 and Serine42 residues, unmasking a new N-terminus peptide (14 aminoacids) which functions as a tethered ligand for the receptor itself (Vu et al., 1991; Coughlin et al., 1992). It has been shown that synthetic peptides, ranging between 5 and 14 aminoacids, called thrombin receptor activating peptides (TRAPs), corresponding to the N-terminus residue unmasked, are capable of reproducing several cellular effects of thrombin, but are devoid of the thrombin catalytic activity (Chao et al., 1992; Garcia et al., 1993; Hoffman & Church, 1993; Glusa & Paintz, 1994; Glusa et al., 1996). This finding has led to expand the knowledge of the role of thrombin in inflammation, indicating that some cellular events mediated by thrombin are independent of fibrin formation, since they can be reproduced by TRAPs.
Here, we have analysed comparatively the bronchial response to thrombin and TRAP-9 (a PAR-1 agonist peptide) in anaesthetized, artificially ventilated guinea-pigs, to investigate whether thrombin had any effect on in vivo bronchial smooth muscle contractility through activation of its receptor PAR-1 and the exposition of the tethered ligand peptide.
Preliminary results have been presented to the British Pharmacological Society Meeting in Bristol, in July 1997.
Methods
Measurement of bronchoconstriction and blood pressure
Guinea-pigs (Charles River, 400–500 g) were anaesthetized with sodium pentobarbitone (40 mg kg−1 i.p.) and Hypnorm™ (0.5 ml kg−1 i.m.), placed supine on an operating table, a cannula was inserted into the trachea and were artificially ventilated with a constant volume, by a respiration pump (Ugo Basile, Varese, Italy; rate 60 breaths min−1, 1 ml air per 100 g body weight), connected to a bronchospasm transducer (Ugo Basile, Varese, Italy). Spontaneous breathing was abolished by administration of pancuronium (2 mg kg−1 i.v.). Both cervical vagi nerves were transected at the level of the neck. The right jugular vein was cannulated for drug administration; the left carotid artery was cannulated with a cannula containing heparinized saline (5 u ml−1) and connected to a pressure transducer for a continuous monitoring of arterial blood pressure. After surgery, a single dose of histamine (10 μg kg−1 i.v.) was administered to evaluate animal responsiveness to a bronchoconstrictor agent. Thrombin (50 and 100 u kg−1, corresponding to 0.034 and 0.07 mg kg−1 respectively), TRAP-9 (0.1, 0.3 or 1 mg kg−1) or the control peptide (1 mg kg−1) were then administered intravenously each 20 min for three consecutive times.
Different groups of animals were administered the thrombin inhibitor Hirulog™ (10 mg kg−1) i.v., 30 min before giving thrombin (100 u kg−1 i.v.) or TRAP-9 (1 mg kg−1 i.v.). Experiments were also performed on rats using the above protocol described for guinea-pigs, thrombin (10, 30 or 50 u kg−1) or TRAP-9 (1 mg kg−1) were intravenously administered and the bronchial response was evaluated.
Cross-desensitization study
To evaluate if there was cross-desensitization to the effects of thrombin and TRAP-9, different groups of animals were treated with TRAP-9 (1 mg kg−1 i.v.) after three administrations of thrombin (100 u kg−1 i.v.) or, conversely, they were treated with thrombin (100 u kg−1 i.v.) after three administrations of TRAP-9 (1 mg kg−1 i.v.).
Bronchoalveolar lavage
At the end of the experiment, three bronchoalveolar lavages, with 5 ml of saline each, through a cannula inserted into the trachea, were performed. The saline was left in contact for 1 min and then aspirated with a syringe, placed in a graduated tube and then centrifuged at 200×g for 15 min. The supernatant was discarded and the pellet was suspended in 1 ml of saline.
Leucocyte count
Total leucocyte count was performed by diluting cells with Turk's solution (0.01% w/v crystal violet and 3% v/v acetic acid) and counted by an optical microscope. Differential cell analysis was performed on air-dried smears of cell suspension and counted by optical microscopy, under oil immersion.
Platelet depletion
Thrombocytopenia was induced by treating guinea-pigs with a polyclonal rabbit antiplatelet serum intravenously, prior to administration of either thrombin (100 u kg−1 i.v.) or TRAP-9 (1 mg kg−1 i.v.). The dose and the lag-time chosen were previously determined by dosing animals with 0.1, 0.3, 0.5 and 1 ml (i.v.) of the polyclonal antiplatelet serum, at time interval ranging between 1 and 20 h. A volume of 250 μl gave 3 h later a reduction in platelet count from 500.4±113.5×103 μl−1 to 49.5±9.30×103 μl−1 (n=5, P<0.01). Platelet count was performed on 100 μl of blood samples, withdrawn from the carotid artery, by using a hemocytometer, Cell Dyn 610 (Sequoia Turner).
Histology
In another set of experiments, after receiving three administrations of thrombin (100 u kg−1 i.v.) or TRAP-9 (1 mg kg−1 i.v.) guinea-pigs were killed and the lungs removed and placed in formalin (sol. 10% v/v). Section of lungs (10 μm) were embedded in paraffin and stained with ematoxylin eosin and the lung damage evaluated by optical microscopy.
Statistical analysis
Data are expressed as means±s.e.mean and analysed with a computerized statistical package (Tallarida & Murray, 1987). Bronchoconstriction is expressed as percentage of bronchoconstriction relative to the maximum percentage (100%). Maximum bronchoconstriction was simulated by clamping the air piping upstream the tracheal cannula thereby diverting all pumped air to the transducer. Results are analysed with one way analysis of variance (ANOVA), followed by Bonferroni's test for multiple comparisons, or by one sample or unpaired two tailed Student's t-test when appropriate. A value of P<0.05 was taken as significant.
Drugs
TRAP-9 (SFLLRNPND), the control TRAP peptide (SFLLANPND) and Hirulog™ (Biogen, Cambridge, MA, U.S.A.) were prepared as previously described (Chao et al., 1992; Maraganore et al., 1990). Human α-thrombin and heparin were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Polyclonal rabbit antiplatelet serum was a kind gift of Prof C.P. Page (King's College, London, U.K.).
Results
Thrombin- and TRAP-9-induced bronchoconstriction
Intravenous administration to guinea-pigs of human α-thrombin at the dose of 50 u kg−1 caused a small, not significant, bronchoconstriction (9.70±4.0%; P>0.05 n=4, one sample t-test), while at the dose of 100 u kg−1 (Figures 1a and 2a) caused a bronchoconstriction of 33.14±8.61% (P<0.01, one sample t-test; n=7) that was reduced to 12.41±6.11% (P<0.01, Bonferroni's test versus first administration; n=7) at the second administration and to 4.3±1.70% (P<0.01, Bonferroni's test versus first administration; n=7) at the third administration. Intravenous administration of TRAP-9 at the dose of 0.1 mg kg−1 did not cause bronchoconstriction, at the dose of 0.3 mg kg−1 there was a trend towards bronchoconstriction (2.67±1.76%; P>0.05 n=3, one sample t-test). Injection of TRAP-9 at the dose of 1 mg kg−1 (Figures 1b and 2c) induced a bronchoconstriction of 30.8±11.42% (P<0.05, one sample t-test; n=7) which was reduced to 18.43±8.18% (N.S. versus first administration; n=7) at the second and to 4.76±1.61% (P<0.01 versus first administration; n=7) at the third administration clearly mimicking thrombin profile. Doses of 1 mg kg−1 for TRAP-9 and 100 u kg−1 for thrombin were selected for all successive experiments. The control peptide did not show any bronchoconstrictor effect up to 1 mg kg−1.
Figure 1.
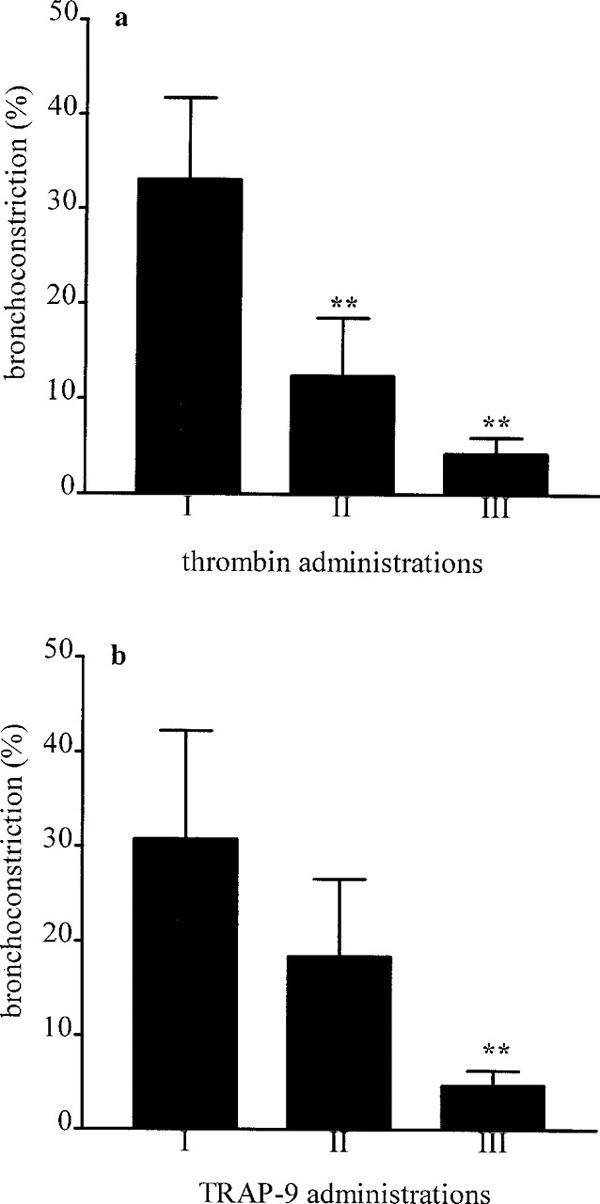
Bronchoconstriction induced by thrombin (a) and TRAP-9 (b). Thrombin (100 u kg−1 i.v.) or TRAP-9 (1 mg kg−1 i.v.) were injected for three consecutive times each 20 min. I, first administration; II, second administration; III, third administration. Each bar represents the mean of the response obtained from seven different animals. **P<0.01 versus first administration (Bonferroni's test).
Figure 2.
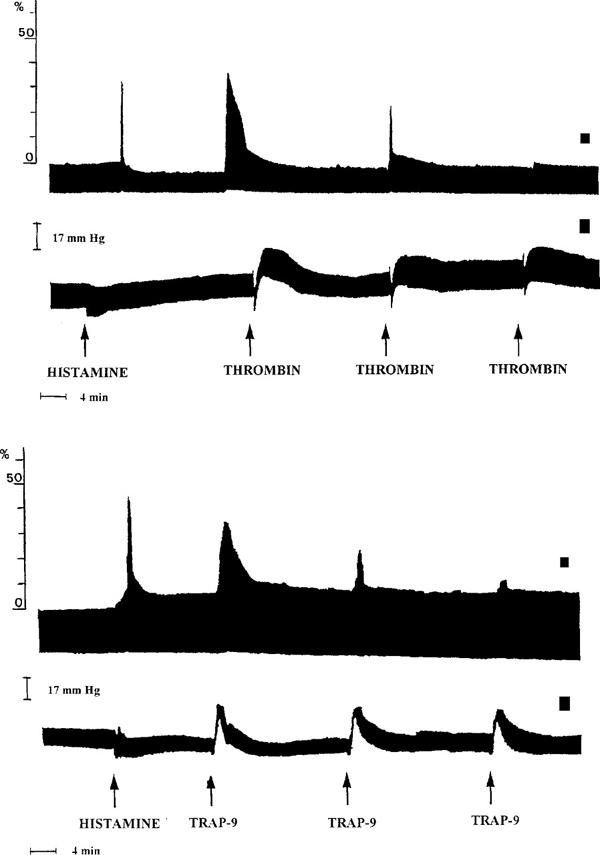
Typical traces representing thrombin- and TRAP-9-induced bronchoconstriction (a and c) and changes in arterial blood pressure (b and d). Thrombin (100 u kg−1) and TRAP-9 (1 mg kg−1) were administered for three consecutive times each 20 min, bronchoconstriction and arterial blood pressure were evaluated. Histamine (10 μg kg−1 i.v.) was previously administered to check animal responsiveness to a bronchoconstrictory agent.
In a cross-desensitization study we found that animals no more responsive to thrombin exhibited bronchoconstriction following TRAP-9 administration; in contrast, animals no more responsive to TRAP-9 were also insensitive to thrombin administration (data not shown).
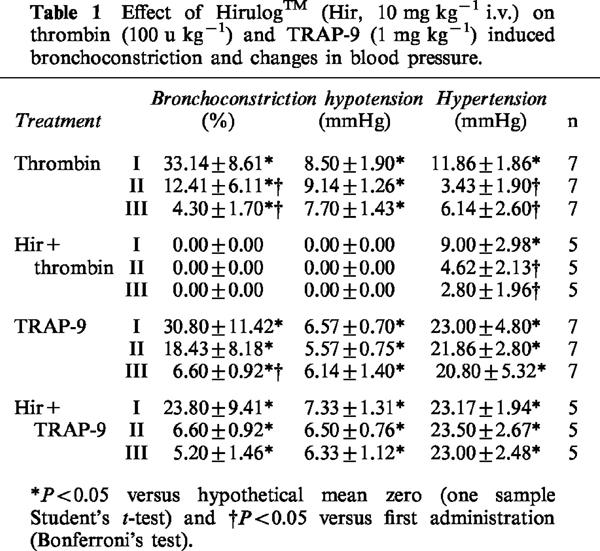
Pretreatment of animals with Hirulog™ (10 mg kg−1 i.v., 30 min before) abolished thrombin-induced bronchoconstriction, but did not change TRAP-9-induced bronchoconstriction (Table 1).
Table 1.
Effect of Hirulog™ (Hir, 10 mg kg−1 i.v.) on thrombin (100 u kg−1) and TRAP-9 (1 mg kg−1) induced bronchoconstriction and changes in blood pressure.
Neither thrombin (10, 30 or 50 u kg−1) nor TRAP-9 (1 mg kg−1) caused bronchoconstriction when administered intravenously to rats (data not shown).
Arterial blood pressure
Intravenous administration of thrombin to guinea-pigs at the dose of 50 and 100 u kg−1 caused a fast fall in blood pressure of 9.25±1.75 mmHg (n=4) and of 8.5±1.9 mmHg (n=7) respectively that was not subjected to desensitization. This decrease in blood pressure was followed by an increase in blood pressure that was of 10.50±0.87 mmHg (n=4) and of 11.86±1.86 mmHg (n=7) for 50 and 100 u kg−1 of thrombin, respectively. TRAP-9 given i.v., at the dose of 0.1, 0.3 or 1 mg kg−1 caused a similar pattern with a fast fall in blood pressure, followed by an increase. The hypotension for the doses of 0.1, 0.3 and 1 mg kg−1 was of 2.7±1.60 mmHg (n=4), 5.7±1.45 mmHg (n=3) and 6.57±0.7 mmHg (n=7) respectively and was not subjected to desensitization. The hypertension that followed was of 10.25±1.44 mmHg (n=4), 14.33±4.05 mmHg (n=3) and 23.00±4.8 mmHg (n=7) respectively, and was repeated unchanged at the second and at the third administration, for all three doses used, while, for thrombin, it was subjected to desensitization. The control peptide was completely inactive. Hirulog™, a stoichiometric inhibitor of thrombin, abolished thrombin-induced hypotension, without affecting the following increase in blood pressure. Conversely, Hirulog™ did not affect TRAP-9-induced changes in arterial blood pressure (Table 1).
Bronchoalveolar lavage
Total leucocyte count in bronchoalveolar lavage fluids (BALFs) obtained from animals injected either with thrombin (100 u kg−1 i.v.) or TRAP-9 (1 mg kg−1 i.v) was not significantly different from that obtained from control animals (control, 6.03±0.63×106, n=9; thrombin, 8.19±0.78×106, n=6; TRAP-9, 7.45±0.99×106, n=6; P>0.05).
Platelet depletion
Platelet depletion of guinea-pigs by a polyclonal rabbit antiplatelet serum prevented thrombin (100 u kg−1 i.v.) and TRAP-9 (1 mg kg−1 i.v) induced bronchoconstriction.
Histological analysis
Histological analysis of lung sections showed an extensive lung damage in animals injected with either α-thrombin (100 u kg−1 i.v.) or TRAP-9 (1 mg kg−1 i.v.); both bronchoconstriction and vasoconstriction were also evident (Figure 3).
Figure 3.
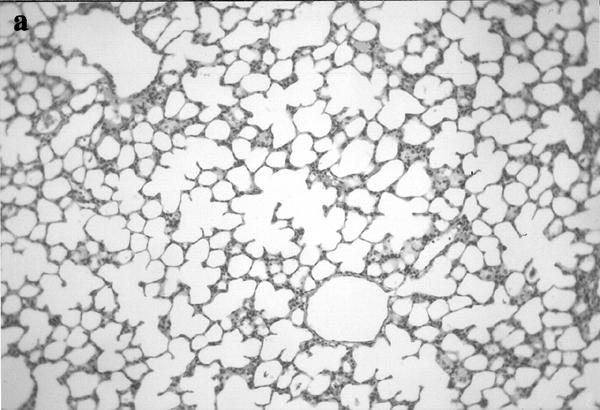
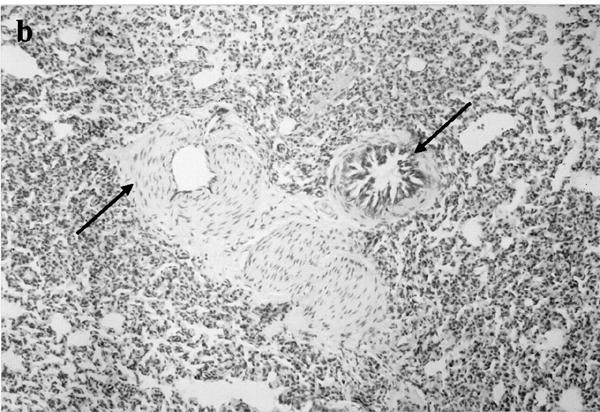
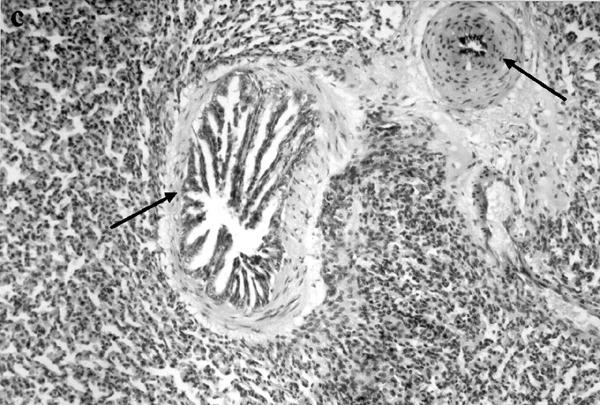
Histological analysis of lungs obtained from control animals (a; ×250), and thrombin (b; ×125) or TRAP-9 (c; ×250) injected animals. In b and c it is evident a strong bronchoconstriction (arrows) and vasoconstriction (arrows).
Discussion
Intravenous administration of thrombin to guinea-pigs caused bronchoconstriction and this effect was reproduced by intravenous injection of TRAP-9. When animals were pretreated with Hirulog™, a stoichiometric thrombin inhibitor that binds the catalytic site of thrombin, thrombin-induced bronchoconstriction was abolished, while TRAP-9-induced bronchoconstriction was unchanged. This finding indicates that thrombin-induced bronchoconstriction requires thrombin catalytic activity and is likely mediated by the tethered ligand peptide exposed on the PAR-1 receptor following proteolytic activation. However, this mechanism cannot explain the tolerance to the bronchoconstrictor effect of both thrombin and TRAP-9, implying that the bronchoconstrictor effect is not dependent upon receptor activation only. Indeed, if bronchoconstriction had been dependent exclusively upon a receptor mechanism, desensitization should have occurred only to thrombin effect, due to receptor consumption, leaving unaffected TRAP-9 response due to its ability to stimulate the receptor directly.
Involvement of inflammatory cells was ruled out by the finding that in bronchial lavage there was no difference in leucocyte count between control and thrombin or TRAP-9-treated guinea-pigs. Similarly, mast cell degranulation, that we have previously shown to be involved in thrombin and TRAP-induced edema (Cirino et al., 1996), appeared not to be implicated since by histological analysis there was no evidence of degranulated mast cells in the tissue. Following this experimental evidence, we sought to investigate platelet involvement. It is widely documented that platelets behave as inflammatory cells, being able, under stimulation, to synthesize and secrete several mediators, among which are powerful bronchoconstrictor agents, such as histamine, serotonin, platelet activating factor (PAF) and arachidonic acid metabolites (Page, 1989). In vitro, both thrombin and TRAP-9 promote platelet activation and degranulation, the latter being able to stimulate platelets from primates and guinea-pigs, but not from other animal species (Kinlough-Rathbone et al., 1993; Connolly et al., 1994). In vivo, both agents cause 111In-labelled platelet accumulation in the pulmonary vasculature of guinea-pigs (Chiu et al., 1997). In thrombocytopenic animals both thrombin and TRAP-9 did not cause bronchoconstriction, implying that platelet activation might be the trigger of the bronchoconstrictor effect observed in our experimental conditions. This hypothesis is further supported by the inability of both agents to cause bronchoconstriction when injected intravenously to rats, whose platelets lack the PAR-1 receptor.
Platelet degranulation by thrombin and TRAP-9 could also account for tolerance to bronchoconstrictor effect observed. Indeed, a similar effect has been observed for intravenous administration to guinea-pigs of PAF, which induces a platelet-dependent bronchoconstriction (Vargaftig et al., 1980). However, the finding that TRAP-9 was still able to cause bronchoconstriction in guinea-pigs desensitized to thrombin effect strongly suggests that an additional mechanism, besides platelet degranulation, occurs. Interestingly, similar results have been obtained in vitro, on guinea-pig aortic preparations, where a thrombin receptor agonist peptide (TRAP 42-55) was able to cause relaxation of the tissue desensitized to thrombin action (Muramutsu et al., 1992; Yang et al., 1992). These differences have been attributed to differences of receptor dynamics depending on whether the receptor is activated by a free ligand or a tethered ligand. Furthermore, it is also possible that in addition to platelet stimulation the effect of thrombin and TRAP-9 is due to a direct action on bronchial smooth muscle, as has been observed, in vitro, using lung parenchymal smooth muscle (Mandhane et al., 1995).
The biphasic change in arterial blood pressure characterized by a rapid drop, followed by an equally rapid increase in blood pressure resembles the in vitro effect.
Thrombin effect on vascular tissues in vitro has been widely studied and it has been shown that thrombin induces in dog isolated coronary arteries, as well as in other vascular tissues, a slowly developing contraction characterized by an initial relaxation (Glusa & Paintz, 1994; Tesfamariam, 1994). Thrombin-induced hypotension was prevented by Hirulog™ pretreatment indicating that proteolytically active thrombin is essential for this effect. However, there was no tolerance to the effect observed, implying that even though receptor activation is necessary for the action, no receptor consumption occurs, or other mechanisms are involved in the hypotensive effect. Conversely, thrombin-induced hypertension was not affected by Hirulog™ pretreatment, but it was subject to tolerance suggesting that proteolytically active thrombin is not a requisite.
Lung histological analysis clearly showed for both thrombin and TRAP-9 not only an extensive lung damage characterized by alveolar atelectasia, but bronchial obstruction and also a pronounced vessel constriction. However, it is now clear that more than one PAR receptor is present in cells (Nystedt et al., 1994; Ishihara et al., 1997). In vitro studies have shown that thrombin and TRAPs causes smooth muscle contraction or relaxation depending on the vascular tissue considered (Muramutsu et al., 1992; Simonet et al., 1992; Yang et al., 1992; Tesfamariam, 1994), these variabilities of thrombin effects among tissues are probably linked to a different tissue distribution of PAR receptors. Thus, the different tolerance to the effects of thrombin and TRAP-9 observed might be dependent upon a different affinity of the two agents for different receptors. In addition, it could be that another region of the receptor is stimulated by thrombin or another receptor not proteolytically activated is involved. Thus, in the interpretation of the in vivo effects of thrombin and TRAPs, there could be a possible interaction of circulating thrombin with at least three different receptors, even if they have a different affinity.
In conclusion, our results show that thrombin causes bronchoconstriction in guinea-pig through a mechanism that involves the proteolytic activation of PAR-1 receptor. Thrombin effect is triggered by platelet activation but, besides this, other mechanisms might be involved, such as the stimulation of PAR-1 receptor on smooth muscle. This is to our knowledge the first study showing an effect of thrombin on the lung functionality in vivo, implying activation of, at least, PAR-1 receptor. Molecules able to antagonize the effects of TRAP on PAR-1 receptor could be useful in controlling bronchial asthma without interfering with haemostatic mechanisms.
Acknowledgments
This paper is dedicated to the memory of Prof Stuart Stone, a brilliant scientist and a good friend.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- PAR-1
proteinase activated receptor 1
- TRAP
thrombin receptor activating peptide
References
- BAR-SHAVIT R., KAHN A., WILNER G.D. Monocyte chemiotaxis: Stimulation by specific exosite region in thrombin. Science. 1983;220:728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- BIZIOS R., LAI L., FENTON J.W., II, MALIK A.B. Thrombin-induced chemiotaxis and aggregation of neutrophils. J. Cell. Physiol. 1986;128:485–490. doi: 10.1002/jcp.1041280318. [DOI] [PubMed] [Google Scholar]
- CHAO B.H., KALKUNTE S., MARAGANORE J.M., STONE S.R. Essential groups in synthetic agonist peptides for activation of the platelet thrombin receptor. Biochemistry. 1992;31:6175–6178. doi: 10.1021/bi00142a001. [DOI] [PubMed] [Google Scholar]
- CHIU P.J.S., TETZLOFF G.G., FOSTER C., CHINTALA M., SYBERTZ E.J. Characterization of in vitro and in vivo platelet response to thrombin and thrombin receptor-activating peptides in guinea pigs. Eur. J. Pharmacol. 1997;321:129–135. doi: 10.1016/s0014-2999(96)00931-4. [DOI] [PubMed] [Google Scholar]
- CICALA C., CIRINO G. Linkage between inflammation and coagulation: an update on the molecular basis of the crosstalk. Life Sci. 1998;62:1817–1824. doi: 10.1016/s0024-3205(97)01167-3. [DOI] [PubMed] [Google Scholar]
- CIRINO G., CICALA C., BUCCI M., SORRENTINO L., MARAGANORE J., STONE S.R. Thrombin functions as an inflammatory mediator through activation of its receptor. J. Exp. Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOLLY T.M., CONDRA C., FENG D., COOK J., STRANIERI M.T., REILLY C.F., NUTT R.F., GOULD R.J. Species variability in platelet and other cellular responsiveness to thrombin receptor-derived peptides. Thromb. Haemost. 1994;72:627–633. [PubMed] [Google Scholar]
- COUGHLIN S.R., VU T.H., HUNG D.T., WHEATON V. Characterization of a functional thrombin receptor. J. Clin. Invest. 1992;89:351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE W.T., ISSEKUTZ A.C. A role for α-thrombin in polymorphonuclear leukocyte recruitment during inflammation. Sem. Thromb. Hemost. 1992;18:333–340. doi: 10.1055/s-2007-1002572. [DOI] [PubMed] [Google Scholar]
- DRAKE W.T., LOPES N.N., FENTON J.W., II, ISSEKUTZ A.C. Thrombin enhancement of interleukin-1 and tumor necrosis factor-α induced polymorphonuclear leukocyte migration. Lab. Invest. 1992;67:617–627. [PubMed] [Google Scholar]
- FUCHS-BUDER T., DE MOERLOOSE P., RICOU B., REBER G., VIFIAN C., NICOD L., ROMAND J.A., SUTER P.M. Time course of procoagulant activity and D dimer in bronchoalveolar fluid of patients at risk for or with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1996;153:163–167. doi: 10.1164/ajrccm.153.1.8542111. [DOI] [PubMed] [Google Scholar]
- GARCIA J.G.N., PATTERSON C., BAHLER C., ASCHNER J., HART C.M., ENGLISH D. Thrombin receptor activating peptides induce Ca2+ mobilization, barrier dysfunction, prostaglandin synthesis and platelet-derived growth factor mRNA expression in cultured endothelium. J. Cell. Physiol. 1993;156:541–549. doi: 10.1002/jcp.1041560313. [DOI] [PubMed] [Google Scholar]
- GLUSA E., PAINTZ M. Relaxant and contractile responses of porcine pulmonary arteries to a thrombin receptor activating peptide (TRAP) Arch. Pharmacol. 1994;349:431–436. doi: 10.1007/BF00170891. [DOI] [PubMed] [Google Scholar]
- GLUSA E., PAINTZ M., BRETSCHNEIDER E. Relaxant and contractile responses of porcine pulmonary arteries to thrombin and thrombin receptor activating peptides. Sem. Thromb. Hemost. 1996;22:261–265. doi: 10.1055/s-2007-999017. [DOI] [PubMed] [Google Scholar]
- GRESELE P., DOTTORINI M., SELLI M.L., IANNACCI L., CANINO S., TODISCO T., ROMANO S., CROOK P., PAGE C.P., NENCI G.G. Altered platelet function associated with the bronchial hyperresponsiveness accompanying nocturnal asthma. J. Allergy Clin. Immunol. 1993;91:894–902. doi: 10.1016/0091-6749(93)90347-i. [DOI] [PubMed] [Google Scholar]
- HOFFMAN M., CHURCH F.C. Response of blood leukocytes to thrombin receptor peptides. J. Leu. Biol. 1993;54:145–151. doi: 10.1002/jlb.54.2.145. [DOI] [PubMed] [Google Scholar]
- IDELL S., JAMES K.K., GILLIES C., FAIR D.S., THRALL R.S. Abnormalities of pathways of fibrin turnover in lung lavage of rats with oleic acid and bleomycin-induced lung injury support alveolar fibrin deposition. Am. J. Pathol. 1989;135:387–399. [PMC free article] [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KINLOUGH-RATHBONE R.L., PERRY D.W., GUCCIONE A., RAND M.L., PACKMAN M.A. Degranulation of human platelets by the thrombin receptor peptide SFLLRN: comparison with degranulation by thrombin. Thromb. Haemost. 1993;70:1019–1023. [PubMed] [Google Scholar]
- KUDHAL K., FISKER S., SONNE O. A thrombin receptor in resident rat peritoneal macrophages. Exp. Cell. Res. 1991;193:45–53. doi: 10.1016/0014-4827(91)90536-4. [DOI] [PubMed] [Google Scholar]
- LINSSEN M., LEMMERMANN C.H., LIPPONER L., WILHELMS O.H. Late phase increase of thrombin-like proteinase, protein, leukocytes in bronchoalveolar lavage (BAL) fluid and chemiluminescenze of BAL after ovalbumin aerosol inhalation by actively immunized rats. Int. Arch. Allergy Appl. Immunol. 1991;94:284–287. doi: 10.1159/000235385. [DOI] [PubMed] [Google Scholar]
- MANDHANE P., SAIFEDDINE M., GREEN F.H.I., HOLLENBERG M.D. Contractile actions of thrombin receptor-derived polypeptides in rat and guinea pig lung parenchymal smooth muscle. Proc. West. Pharmacol. 1995;38:93–96. [PubMed] [Google Scholar]
- MARAGANORE J.M., BOURDON P., JABLONSKI J., RAMACHANDRAN K.L., FENTON J.W., II Design and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombin. Biochemistry. 1990;29:7095–7101. doi: 10.1021/bi00482a021. [DOI] [PubMed] [Google Scholar]
- MURAMUTSU I., LANIYONU A., MOORE J.G., HOLLENBERG M.D. Vascular actions of thrombin receptor peptide. Can. J. Physiol. Pharmacol. 1992;70:996–1003. doi: 10.1139/y92-137. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential novel proteinase activated receptor 2. Proc. Natl. Acad. Sci. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE C.P. Platelets as inflammatory cells. Immunopharmacology. 1989;17:51–59. doi: 10.1016/0162-3109(89)90008-8. [DOI] [PubMed] [Google Scholar]
- PANETTIERI R.A., JR, HALL I.P., MAKI C.S., MURRAY R.K. Alpha-Thrombin increases cytosolic calcium and induces human airway smooth muscle cell proliferation. Am. J. Respir. Cell. Mol. Biol. 1995;13:205–216. doi: 10.1165/ajrcmb.13.2.7626288. [DOI] [PubMed] [Google Scholar]
- SCHIFFRIN E.L. The endothelium and control of blood vessel function in health and disease. Clin. Invest. Med. 1994;17:602–620. [PubMed] [Google Scholar]
- SIMONET S., BONHOMME E., LAUBIE M., THURIEAU C., FAUCHERE J.L., VERBEUREN T.J. Venous and arterial endothelial cells respond differently to thrombin and its endogenous receptor agonist. Eur. J. Pharmacol. 1992;216:135–137. doi: 10.1016/0014-2999(92)90222-p. [DOI] [PubMed] [Google Scholar]
- SONNE O. The specific binding of thrombin to human polymorphonuclear leucocytes. Scand. J. Clin. Lab. Invest. 1988;48:831–838. doi: 10.3109/00365518809088768. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Springer Verlag: New York; 1987. Manual of pharmacologic calculations with computer programs; p. 297. [Google Scholar]
- TESFAMARIAM B. Distinct receptors and signaling pathways in α-thrombin- and thrombin receptor peptide-induced vascular contractions. Circ. Res. 1994;74:930–936. doi: 10.1161/01.res.74.5.930. [DOI] [PubMed] [Google Scholar]
- VARGAFTIG B.B., LEFORT J., CHIGNARD M., BENVENISTE J. Platelet activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur. J. Pharmacol. 1980;65:185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WEKSLER B.B., LEY C.W., JAFFE E.F. Stimulation of endothelial cell PGI2 production by thrombin, trypsin and ionophore A23187. J. Clin. Invest. 1978;62:923–938. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG S.G., LANIYONU A., SAIFFEDINE M., MOORE G.J., HOLLENBERG M.D. Actions of thrombin and thrombin receptor peptide analogues in gastric and aortic smooth muscle: development of bioassays for structure-activity studies. Life Sci. 1992;51:1325–1332. doi: 10.1016/0024-3205(92)90631-x. [DOI] [PubMed] [Google Scholar]


