Abstract
We have used the whole-cell patch clamp technique to study the effect of fluoxetine, a commonly used antidepressant drug, on the volume-regulated anion channel (VRAC) in calf pulmonary artery endothelial (CPAE) cells. We also examined its effects on other Cl− channels, i.e. the Ca2+-activated Cl− current (ICl,Ca) and the cystic fibrosis transmembrane conductance regulator (CFTR) to assess the specificity of this compound for VRAC.
At pH 7.4 fluoxetine induced a fast and reversible block of the volume-sensitive chloride current (ICl,swell), with a Ki value of 6.0±0.5 μM (n=6-9). The blocking efficiency increased with increasing extracellular pH (Ki=0.32±0.01 μM at pH 8.8, n=3-9), indicating that the blockade is mediated by the uncharged form of fluoxetine.
Fluoxetine inhibited Ca2+-activated Cl− currents, ICl,Ca, activated by loading CPAE cells via the patch pipette with 1000 nM free Ca2+ (Ki=10.7±1.6 μM at pH 7.4, n=3-5). The CFTR channel, transiently transfected in CPAE cells, was also inhibited with a Ki value of 26.9±9.4 μM at pH 7.4 (n=3).
This study describes for the first time the effects of fluoxetine on anion channels. Our data reveal a potent block of VRAC at fluoxetine concentrations close to plasma concentrations. The results suggest a hydrophobic interaction with high affinity between uncharged fluoxetine and volume-activated chloride channels. Ca2+-activated Cl− currents and CFTR are also blocked by fluoxetine, revealing a novel characteristic of the drug as a chloride channel modulator.
Keywords: Patch-clamp, volume-regulated anion channels, fluoxetine, Ca2+-activated Cl− currents, cystic fibrosis transmembrane conductance regulator, endothelium
Introduction
Fluoxetine (FLX, Prozac), a substituted propylamine (Figure 1) prescribed for the treatment of depression, is assumed to derive its antidepressant activity from its ability to block the reuptake of 5-hydroxytryptamine, while it exerts minimal effects on other neurotransmitter reuptake systems (Wong et al., 1995). However, several studies have been published which show that, in addition to this selective action, many other effector systems are modulated. So far, inhibitory effects of Prozac have been demonstrated on cation channels, such as Ca2+ channels (Stauderman et al., 1992), K+ channels (Farrugia, 1996; Rae et al., 1995; Tytgat et al., 1997) and Na+ channels (Pancrazio et al., 1998). However, to our knowledge, effects of FLX on anion channels have never been studied.
Figure 1.
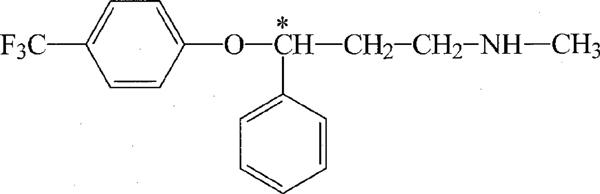
Chemical structure of fluoxetine.
In this study we have tested whether Prozac would also modulate volume regulated anion channels and the correspondent swelling-activated chloride current (ICl,swell). Many, if not all mammalian cells express volume-regulated anion channels (VRAC) which are important regulators of various cell functions such as cell volume, pH control, transport of osmolytes and the membrane potential (Nilius et al., 1996; 1997a; Okada, 1997; Strange et al., 1996; Kirk, 1997). Many inhibitors have been described, but most of them are also modulating other channels and a selective, high affinity VRAC blocker is still missing. Since FLX belongs to a family of propylamines with a −CF3 group, supporting the idea that these compounds comprise in general promising candidates for high selectivity VRAC blockers (Roy et al., 1998), it was interesting to study its actions on VRAC.
Furthermore, we wanted to examine whether the Ca2+-activated Cl− current (ICl,Ca) and the cystic fibrosis transmembrane conductance regulator (CFTR) were also affected by FLX. CFTR is a non-rectifying, 8–10 pS, protein kinase A activated chloride channel (Cliff et al., 1992), belonging to the family of ATP-binding cassette proteins (Hyde et al., 1990). Mutations in the gene for this channel cause cystic fibrosis. ICl,Ca is a strongly outwardly rectifying, 8 pS, calcium activated chloride current, described in various excitable and non excitable cells, including calf pulmonary artery endothelial (CPAE) cells (Nilius et al., 1997c).
We show here that FLX exerts a potent blocking effect on VRAC in endothelial cells at concentrations close to therapeutical concentrations. This finding may indicate that this channel is also a possible target of this drug. The Ca2+-activated Cl− current and CFTR are also affected by FLX.
Methods
Vector construction
We used the pCINeo/IRES-GFP plasmid (Trouet et al., 1997) for expressing wild type (WT) CFTR in CPAE. For insertion of WT CFTR the green fluorescent protein (GFP)-vector was cut with EcoRI, dephosphorylated and thereupon blunt-ended with T4 DNA-polymerase. The WT CFTR cDNA was obtained from a pcDNA/CFTR plasmid through SacI digestion. The obtained fragment was blunt-ended using T4 DNA-polymerase. Ligation was performed using standard procedures.
Cell culture and transfection
Cells from a cultured bovine pulmonary artery endothelial (CPAE) cell line (American Tissue Type Culture Collection, CCL 209) were used. The cells were grown in Dulbecco's modified Eagle's medium containing 20% foetal calf serum, 2 mM L-glutamine, 2 U ml−1 penicillin and 2 mg ml−1 streptomycin. Cultures were maintained at 37°C in a fully humidified atmosphere of 10% CO2 in air.
Cells were detached by exposure to 0.05% trypsin in a Ca2+- and Mg2+-free solution, reseeded on gelatin coated cover slips, and kept in culture for 2 to 4 days before use. For electrophysiological experiments, only non-confluent single endothelial cells were used.
CPAE cells do not express CFTR (Nilius et al., 1997e). Therefore, cells were transiently transfected with WT CFTR in the pCINeo/IRES-GFP vector (Trouet et al., 1997) using the same method as described previously (Kamouchi et al., 1997). In short, 150,000 cells were incubated with a transfection cocktail containing 3 μg DNA and 12 μl polycationic SuperFect Transfection Reagent (Qiagen, Hilden, Germany). Cells were then transferred to gelatin-coated cover slips 24 h after transfection and electrophysiological measurements were done during 2–4 days after transfection. Incorporation of WT CFTR in the bicistronic unit allows coupled expression of the channels and GFP. Transfected cells, positive for GFP, could be identified in the patch-clamp set up. GFP was excited at a wavelength between 450 and 490 nm and the emitted light was passed through a 520 nm long pass filter.
Solutions and drugs
The standard extracellular solution was a modified Krebs solution (Kr) containing (in mM): NaCl 150, KCl 6, MgCl2 1, CaCl2 1.5, glucose 10, N-(hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid) (HEPES) 10, titrated with NaOH to pH 7.4. The osmolarity, as measured with a vapour pressure osmometer (Wescor 5500, Schlag, Gladbach, Germany), was 320±5 mOsm.
ICl,swell
At the beginning of the patch-clamp recording, the Krebs solution was replaced by an isotonic-Cs+ solution (ISO, 320±5 mOsm) containing (in mM): NaCl 105, CsCl 6, CaCl2 1.5, MgCl2 1, D-mannitol 90, glucose 10, HEPES 10, adjusted to pH 7.4 with NaOH. Volume-sensitive Cl− currents were activated by exposing the cells to a 25% hypotonic extracellular solution (HTS, 240±5 mOsm), containing (in mM): NaCl 105, CsCl 6, CaCl2 1.5, MgCl2 1, glucose 10, HEPES 10 (HTS7.4 and HTS6) or Tris(hydroxymethyl)-aminomethane (TRIS) 10 (HTS8.8), adjusted to pH 7.4 (HTS7.4) or pH 6 (HTS6) with NaOH or titrated to pH 8.8 (HTS8.8) with HCl.
The standard pipette solution contained (in mM): CsCl 40, Cs-aspartate 100, MgCl2 1, CaCl2 1.93, ethylene glycol-O,O′-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA) 5, Na2 ATP 4, HEPES 10, adjusted to pH 7.2 with CsOH (290±5 mOsm).
The presence of Cs+ instead of K+ in the extra- and intracellular solutions blocked the inwardly rectifying K+ currents, which are present in CPAE cells (Voets et al., 1996a). To suppress the Ca2+-activated Cl− current, the free Ca2+ concentration in the pipette solution was buffered at 100 nM, which is below the threshold for activation of this current (Nilius et al., 1997c), but which is sufficient for full activation of ICl,swell during cell swelling in CPAE cells (Szücs et al., 1996).
ICl,Ca
Krebs solution was replaced by a slightly hypertonic Krebs-Cs+ solution (345±5 mOsm) containing (in mM): NaCl 150, CsCl 6, MgCl2 1, CaCl2 1.5, glucose 10, D-mannitol 25, HEPES 10, titrated with NaOH to pH 7.4. The slightly increased osmolarity prevented coactivation of VRAC. ICl,Ca was activated by loading CPAE cells via the patch pipette with 1000 nM free Ca2+ as described previously (Nilius et al., 1997c,1997d). The standard pipette solution contained (in mM): CsCl 40, Cs-aspartate 100, MgCl2 1, CaCl2 4.33, EGTA 5, Na2ATP 4, HEPES 10, adjusted to pH 7.2 with CsOH (290±5 mOsm).
CFTR
To eliminate K+ currents, Krebs solution was replaced by a Krebs-Cs+ solution (320±5 mOsm) containing (in mM): NaCl 150, CsCl 6, MgCl2 1, CaCl2 1.5, glucose 10, HEPES 10, titrated with NaOH to pH 7.4. The CFTR-channel was activated by a cocktail containing 100 μM 3-isobutyl-1-methylxanthine (IBMX) and 1 μM forskolin (both from Sigma-Aldrich Chemie) dissolved in the Krebs-Cs+ solution. The standard pipette solution contained (in mM): CsCl 40, Cs-aspartate 100, MgCl2 1, CaCl2 1.93, EGTA 5, Na2ATP 4, HEPES 10 adjusted to pH 7.2 with CsOH (290±5 mOsm).
Fluoxetine hydrochloride was purchased from Sigma Aldrich.
Current measurements and data analysis
Whole-cell membrane currents were measured in ruptured patches. All experiments were performed at room temperature (20–23°C). Currents were monitored with an EPC-7 patch clamp amplifier (List Electronic, Germany) and sampled at 2 ms intervals (1024 points per record, filtered at 200 Hz), unless otherwise mentioned.
ICl,swell
In most experiments we applied a ‘ramp' protocol, which consisted of: a step to −80 mV for 0.4 s, followed by a step to −150 mV for 0.1 s and a 1.3 s linear voltage ramp to +100 mV. This voltage protocol was repeated every 15 s from a holding potential of 0 mV. Current-voltage relations were constructed from the ramp current, and time courses were obtained by averaging the current in a small voltage window around +100 mV and −150 mV. In some experiments we used a ‘step' protocol consisting of 1.5 s voltage steps, applied every 10 s from a holding potential of −50 mV to test potentials from −100 to +100 mV with increments of 40 mV. Currents were sampled at 1 ms intervals.
ICl,Ca and CFTR
In all experiments we used a ‘step' protocol consisting of 1.5 s voltage steps, applied every 10 s from a holding potential of −20 mV to test potentials from −100 to +100 mV with increments of 20 mV. Currents were sampled at 1 ms intervals.
Data were analysed in Winascd (G. Droogmans) and in Origin (MicroCal Software, Inc.). Pooled data are given as the means±s.e.mean.
Results
Fluoxetine inhibits various Cl− channels
The CFTR-currents were activated by a cocktail containing 100 μM IBMX and 1 μM forskolin dissolved in the Krebs-Cs+ solution, as described in detail elsewhere (Cuppens et al., 1998). Figure 2a and b show CFTR-current traces in control conditions (a) and in the presence of 10 μM FLX (b), measured during the voltage step protocol, performed at pH 7.4. The corresponding current-voltage relations (Figure 2c) show that the CFTR currents are approximately equally inhibited at positive and negative potentials. The dose-inhibition curve at +100 mV was fitted to the equation
 |
Figure 2.

(a) CFTR-current traces in response to 1.5-s voltage steps to potentials from −100 to +100 mV (+20 mV increment), applied every 10 s from a holding potential of −20 mV. Sampling interval was 1 ms. The dashed line indicates zero current. Currents were measured in control conditions at pH 7.4. (b) Currents recorded in the presence of 10 μM FLX at pH 7.4 using the same protocol as in (a). (c) Current-voltage relations derived from the CFTR-current traces in (a) and in (b). Currents were measured at the end of the voltage steps. (d) ICl,Ca current traces in control conditions using the same protocol as in (a). (e) Currents recorded in the presence of 10 μM FLX at pH 7.4 using the same protocol as in (a). (f) Current-voltage relations derived from the ICl,Ca current traces in (d) and in (e). Currents were measured at the end of the voltage steps. (g) Current traces in response to 1.5-s voltage steps to potentials from −100 to +100 mV (+40 mV increment), applied every 10 s from a holding potential of −50 mV. Sampling interval was 1 ms. The dashed line indicates zero current. Currents were measured in control conditions at pH 7.4 (HTS7.4). (h) Currents recorded in the presence of 10 μM FLX at pH 7.4 using the same protocol as in (g). (i) Current-voltage relations derived from the VRAC current traces in (g) and in (h). Currents were measured at the end of the voltage steps.
in which C is the drug concentration, p the Hill coefficient and Ki the drug concentration needed for half maximal block. The value of Ki was 26.9±9.4 μM (n=3).
Ca2+-activated Cl− currents were activated by loading CPAE cells via the patch pippete with 1 μM free Ca2+ as described previously (Nilius et al., 1997d). Figure 2d and e show current traces in control conditions (d) and in the presence of 10 μM FLX (e), measured during the voltage step protocol, performed at pH 7.4. The corresponding current-voltage relations are shown in Figure 2f. The value of Ki (at +100 mV and pH 7.4) was 10.7±1.6 μM (n=3-5). Volume-activated chloride channels and the corresponding current, ICl,swell, were activated by replacing the isotonic solution (ISO) by the hypotonic solution (HTS), as described in detail elsewhere (Nilius et al., 1994a). Figure 2g and h show current traces in control conditions (g) and in the presence of 10 μM FLX (h), measured during the voltage step protocol, performed at pH 7.4. The corresponding current-voltage relations (Figure 2i) show that the VRAC currents are approximately equally inhibited at positive and negative potentials. ICl,swell and the Ca2+-activated Cl− current reverse at potentials more positive than the theoretical equilibrium potential for Cl− because of permeation of organic anions such as aspartate in the pipette (for a detailed discussion see Nilius et al., 1997a, 1997c). We will focus the following experiments on the interaction between FLX and VRAC.
Inhibition by fluoxetine of VRAC
Figure 3a shows a typical time course experiment in which two different concentrations of FLX (5 and 50 μM) were added to the external solution during a maintained superfusion with hypotonic solution (HTS7.4). FLX induced a fast and concentration-dependent block of ICl,swell, which was almost complete at 50 μM. This block was fully reversible and recovery of the current upon washout was fast. Current-voltage relationships (Figure 3b) are obtained from voltage ramps before and after addition of the different drug concentrations. Very similar results were obtained in analogous experiments (n⩾7).
Figure 3.

(a) Time course of activation of ICl,swell during superfusion with hypotonic solution (HTS) (following superfusion with Krebs solution (Kr) and isotonic-Cs+ solution (ISO)), reversible inhibition of the current with 5 and 50 μM of FLX and deactivation of the current after returning to isotonic-Cs+ solution. The dashed line indicates zero current. Data were obtained from ramp protocols by averaging the current in a small voltage window around +100 mV and −150 mV. (b) Current-voltage relations obtained from voltage ramps at the times indicated in (a).
At pH 7.4, FLX occurs mainly with one single positive charge, due to a secondary amine group with a pKa value of 9.5. In order to investigate whether its inhibitory effect on ICl,swell is mediated by the positively charged or by the neutral form, we performed experiments in which the pH of the HTS solution was increased to 8.8 (HTS8.8) or decreased to 6.0 (HTS6) during drug application. Short (1–2 min) changes in pH did not significantly affect the amplitude of currents measured in isotonic or hypotonic conditions, as described in detail elsewhere (Nilius et al., 1998). Figure 4a shows a typical time course experiment in which the pH of the external solution was changed during application of 5 μM FLX. Current-voltage relationships (Figure 4b) are obtained from voltage ramps before and after addition of 5 μM FLX at different pH values. Figure 4 shows that the inhibitory effect of 5 μM FLX on ICl,swell is increased at higher pH and almost absent at lower pH values. Very similar results were obtained in analogous experiments (n=8).
Figure 4.

(a) Time course of the current at +100 mV and −150 mV showing the effect of different pH values on the block of ICl,swell by 5 μM FLX. (b) Current-voltage relations were obtained from voltage ramps at the times indicated in (a).
From the experimental protocols shown in Figures 3 and 4, we have evaluated the concentration dependence of the inhibition of ICl,swell for FLX at two pH values (pH 7.4 and 8.8) and at +100 mV (Figure 5, filled squares for pH 7.4, filled circles for pH 8.8). The inhibition is expressed as the percentage reduction of the background corrected ICl,swell at +100 mV. The background current (current under isotonic conditions) was not affected by FLX, except at the highest concentrations. At pH 7.4, the estimated value of Ki was 6.0±0.5 μM, with a Hill coefficient of 1.04±0.09. However, the estimated value of Ki for pH 8.8 was 0.32±0.01 μM, with a Hill coefficient of 1.32±0.05. This more potent block at higher pH could be explained by assuming that only the uncharged form of FLX exerts a blocking effect. The uncharged concentrations can be calculated by the following equation:
 |
Figure 5.

Dose-response curve for inhibition of VRAC by FLX at two different pH values: pH 7.4 and 8.8. Each data point represents the average±s.e.mean of n cells as indicated. The filled line represents the best fit of the data to equation [1]. The open symbols represent the inhibition as a function of the effective concentration of the uncharged form as derived from the data points at pH 7.4 and 8.8 and calculated by equation [3]. The dashed line represents the best fit of the pooled data to equation (1).
In the case of FLX, [base] corresponds to the concentration of the uncharged drug and [acid] to the concentration of the charged drug in the solution. It can be calculated that at pH 6 only 0.03% of FLX is in its uncharged form, while at pH 7.4 and 8.8 the uncharged form amounts to 0.8 and 19.9% respectively. Hence, a pH shift from pH 7.4 to 8.8 increases the concentration of the uncharged form approximately 20 fold. The same factor of increase is found when we compare the Ki values obtained at pH 7.4 and 8.8. This confirms the assumption that the blocking effect of FLX on ICl,swell is exerted by the neutral form. The effective concentration of the uncharged form at each concentration of FLX ([FLX]) can be calculated by the following equation:
The open symbols in Figure 5 represent the inhibition as a function of the effective concentration of the uncharged form, as derived from the data points at pH 7.4 (open squares) and at pH 8.8 (open circles). It is obvious that the inhibition curves obtained at the two pH values are not significantly different if plotted as a function of the concentration of the uncharged form of FLX. The dashed line represents the best fit of the pooled data to equation [1]. The estimated Ki value for the non-ionised form of FLX was 50.1±2.86 nM, with a Hill coefficient of 1.17±0.08.
Discussion
The experimental results obtained in this study demonstrate a fast and concentration-dependent block of volume-regulated anion channels in endothelial cells by FLX. This blocking effect was not specific for VRAC, since Ca2+-activated Cl− channels and the cystic fibrosis transmembrane conductance regulator were also inhibited by the drug in a similar concentration range. These results could reveal a novel characteristic of the antidepressant drug FLX, sc. as a novel chloride channel modulator. In this context it is interesting to compare FLX with other chloride channel blockers. FLX is a voltage-independent chloride channel modulator. These differ in their sensitivity for blocking the channel (mM to μM range) and apparently there is no structural relationship. All compounds however seem to be relatively hydrophobic and contain aromatic parts, frequently phenolic structures. An amine function is often present and a −CF3 group is likely to increase the blocking sensitivity (Roy et al., 1998). Relying on the structure of FLX it is also fair to catalogue the drug in the group of the voltage-independent chloride channel blockers such as tamoxifen, quinine and quinidine, mibefradil, (Nilius et al., 1994b, 1997b; Voets et al., 1996b), for a review see (Nilius et al., 1997a).
The inhibitory effect of FLX on VRAC is markedly potentiated at higher pH values. The pronounced pH-effect is not due to an effect on the channel itself, since short (1–2 min) changes in pH do not significantly affect the amplitude of currents measured in isotonic or hypotonic conditions (Nilius et al., 1998). Our results strongly suggest that inhibition of VRAC is mediated by the uncharged form of FLX. A possible explanation could be that the block is due to an intracellular action following permeation of the uncharged form through the cell membrane, as has been proposed for chromones (Heinke et al., 1995). However, if uncharged FLX enters the cell, it would be ionized immediately (the internal solution has a pH of 7.2) and subsequent wash-out would be rather slow. Since this is in contradiction to our results, it is more likely that the high affinity block by the neutral FLX is due to hydrophobic interactions with the channel protein(s) within the membrane bilayer. The voltage-independent nature of the block supports this hypothesis. A similar mechanism has been described for the inhibitory action of quinine and quinidine on ICl,swell (Voets et al., 1996b). If we assume that only the non-ionized form of FLX is responsible for block of VRAC, the estimated Ki value is 50.1±2.9 nM as derived from the measurements at pH 7.4 and 8.8.
In previous studies it has been shown that FLX inhibits cation channels. In most of these studies, however, the concentration at which FLX was found to affect these channels was much higher than the anticipated plasma concentrations in patients being treated for depression (Rae et al., 1995; Tytgat et al., 1997). Therapeutic blood levels range from 0.2 to 3.4 μM (Altamura et al., 1994; Baumann, 1996; Preskorn et al., 1991) and brain concentrations seem to be significantly higher than their corresponding plasma concentrations (Altamura et al., 1994). The apparent brain concentration of FLX relative to plasma is 20 : 1 (Karson et al., 1993). This concentration range fits with the blocking range for all Cl− channels tested in this study. FLX might therefore affect Cl− activity in the brain at the normally used therapeutic concentrations. In addition to the block of Ca2+-activated Cl− channels and CFTR, our data reveal for the first time a potent block of volume regulated anion channels, VRAC, by Prozac with Ki values close to plasma concentrations. It has been shown that VRAC is an ubiquitously expressed channel with very similar properties in many cell types (Nilius et al., 1994c). The functional importance of this channel has been discussed in detail and comprises among others, effects on volume regulation, osmolyte transport and electrogenesis (Nilius et al., 1997a; Okada, 1997; Strange et al., 1996). Because of these multiple effects, it is not unlikely that modulation of VRAC contributes to the numerous and complicated mechanisms of action of the psychotropic drug. This might be especially important, inasmuch as VRAC has been described in neuronal and glial cells and endothelial cells of the blood-brain barrier, and is critically involved in volume regulation and maintaining the osmotic composition of the fluid compartments in the central nervous system (Strange, 1992).
Interestingly, VRAC is also involved in glutamate release during cerebral ischemia (Bausch & Roy, 1996), and its activation is tightly linked to the cellular energy metabolism, which may have significance for several human diseases such as stroke-like episodes (MELAS), encephalopathy, brain swelling and edema (Patel et al., 1998). Under all these circumstances, modulation of VRAC by Prozac may play a role in the overall action of this psychotropic drug. Therefore, brain Cl− channels may provide multifunctional targets of FLX. In conclusion, volume-activated chloride currents are efficiently blocked by the uncharged form of fluoxetine. Given that the uncharged fluoxetine exerts its inhibitory effect in the nanomolar range, it might provide a lead compound for the development of novel modulatory, high affinity compounds of volume-activated chloride currents. Ca2+-activated Cl− currents and CFTR are also blocked by fluoxetine, revealing a novel characteristic of the drug as a chloride channel modulator.
Acknowledgments
We thank Drs J. Eggermont and J. Tytgat for many helpful discussions, Prof R. Casteels for critical reading of the manuscript and J. Prenen for excellent technical support. The technical help of D. Hermans, A. Florizone and M. Crabbé is greatly acknowledged. The work has been supported by grants from the Federal Belgian State (Interuniversity Poles of Attraction Programme, Prime Ministers Office IUAP Nr.3P4/23) and Flemish Government (F.W.O. G.0237.95, C.O.F./96/22-A0659), and by the European Commission (BMH4-CT96-0602). We thank Anne Vankeerberghen (Centre for Human Genetics) for providing WT CFTR. Ch. M. is a Research Assistant of the Flemish Fund for Scientific Research (F.W.O.-Vlaanderen).
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- CPAE
calf pulmonary artery endothelial cells
- EGTA
ethylene glycol-O,O'-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid
- FLX
fluoxetine
- GFP
green fluorescent protein
- HEPES
N-(hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)
- HTS
hypotonic solution
- IBMX
3-isobutyl-1-methylxanthine
- ICl,Ca
Ca2+-activated Cl− current
- ICl,Swell
swelling activated chloride current
- ISO
isotonic solution
- Kr
Krebs solution
- TRIS
Tris(hydroxymethyl)aminomethane
- VRAC
volume-regulated anion channel
- WT
wild type
References
- ALTAMURA A.C., MORO A.R., PERCUDANI M. Clinical pharmacokinetics of fluoxetine. Clin. Pharmacokinet. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- BAUMANN P. Pharmacokinetic-pharmacodynamic relationship of the selective serotonin reuptake inhibitors. Clin. Pharmacokinet. 1996;31:444–469. doi: 10.2165/00003088-199631060-00004. [DOI] [PubMed] [Google Scholar]
- BAUSCH A.R., ROY G. Volume-sensitive chloride channels blocked by neuroprotective drugs in human glial cells (U-138MG) Glia. 1996;18:73–77. doi: 10.1002/(SICI)1098-1136(199609)18:1<73::AID-GLIA8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- CLIFF W.H., SCHOUMACHER R.A., FRIZZELL R.A. cAmp-activated Cl channels in Cftr-transfected cystic fibrosis pancreatic epithelial cells. Am. J. Physiol. 1992;262:C1154–C1160. doi: 10.1152/ajpcell.1992.262.5.C1154. [DOI] [PubMed] [Google Scholar]
- CUPPENS H., LIN W., JASPERS M., COSTES B., TENG H., VANKEERBERGHEN A., JORISSEN M., DROOGMANS G., REYNAERT I., GOOSSENS M., NILIUS B., CASSIMAN J.J. Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation. J. Clin. Invest. 1998;101:487–496. doi: 10.1172/JCI639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRUGIA G. Modulation of ionic currents in isolated canine and human jejunal circular smooth muscle cells by fluoxetine. Gastroenterology. 1996;110:1438–1445. doi: 10.1053/gast.1996.v110.pm8613049. [DOI] [PubMed] [Google Scholar]
- HEINKE S., SZÜCS G., NORRIS A., DROOGMANS G., NILIUS B. Inhibition of volume-activated chloride currents in endothelial cells by chromones. Br. J. Pharmacol. 1995;115:1393–1398. doi: 10.1111/j.1476-5381.1995.tb16629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYDE S.C., EMSLEY P., HARTSHORN M.J., MIMMACK M.M., GILEADI U., PEARCE S.R., GALLAGHER M.P., GILL D.R., HUBBARD R.E., HIGGINS C.F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- KAMOUCHI M., DROOGMANS G., NILIUS B. G-protein modulated K+ inward rectifier channels in macrovascular endothelial cells. J. Physiol. 1997;504:545–556. doi: 10.1111/j.1469-7793.1997.545bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARSON C.N., NEWTON J.E., LIVINGSTON R., JOLLY J.B., COOPER T.B., SPRIGG J., KOMOROSKI R.A. Human brain fluoxetine concentrations. J. Neuropsychiatry Clin. Neurosci. 1993;5:322–329. doi: 10.1176/jnp.5.3.322. [DOI] [PubMed] [Google Scholar]
- KIRK K. Swelling-activated organic Osmolyte Channels. J. Membr. Biol. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- NILIUS B., EGERMONT J., VOETS T., BUYSE G., MANOLOPOULOS V., DROOGMANS G. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol. 1997a;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- NILIUS B., EGGERMONT J., VOETS T., DROOGMANS G. Volume-activated Cl− channels. Gen. Pharmacol. 1996;27:1131–1140. doi: 10.1016/s0306-3623(96)00061-4. [DOI] [PubMed] [Google Scholar]
- NILIUS B., OIKE M., ZAHRADNIK I., DROOGMANS G. Activation of a Cl− current by hypotonic volume increase in human endothelial cells. Journal of General Physiology. 1994a;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., DROOGMANS G. Modulation of volume-regulated anion channels by extra- and intracellular pH. Pflügers Arch. 1998;436:742–748. doi: 10.1007/s004240050697. [DOI] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., KAMOUCHI M., VIANA F., VOETS T., DROOGMANS G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca2+- and volume-activated Cl− channels in macrovascular endothelial cells. Br. J. Pharmacol. 1997b;121:547–555. doi: 10.1038/sj.bjp.0701140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., SZÜCS G., WEI L., TANZI F., VOETS T., DROOGMANS G. Calcium-activated chloride channels in bovine pulmonary artery endothelial cells. J. Physiol. (Lond.) 1997c;497:95–107. doi: 10.1113/jphysiol.1997.sp021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., VOETS T., VAN DEN BREMT K., EGGERMONT J., DROOGMANS G. Kinetic and pharmacological properties of the calcium-activated chloride current in macrovascular endothelial cells. Cell Calcium. 1997d;22:53–63. doi: 10.1016/s0143-4160(97)90089-0. [DOI] [PubMed] [Google Scholar]
- NILIUS B., SEHRER J., DROOGMANS G. Permeation properties and modulation of volume-activated Cl−-currents in human endothelial cells. Br. J. Pharmacol. 1994b;112:1049–1056. doi: 10.1111/j.1476-5381.1994.tb13189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., SEHRER J., VIANA F., DE GREEF C., RAEYMAEKERS L., EGGERMONT J., DROOGMANS G. Volume-activated Cl- currents in different mammalian non-excitable cell types. Pflügers Arch. 1994c;428:364–371. doi: 10.1007/BF00724520. [DOI] [PubMed] [Google Scholar]
- NILIUS B., SZÜCS G., HEINKE S., VOETS T., DROOGMANS G. Multiple types of chloride channels in bovine pulmonary artery endothelial cells. Journal of Vascular Research. 1997e;34:220–228. doi: 10.1159/000159226. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- PANCRAZIO J.J., KAMATCHI G.L., ROSCOE A.K., LYNCH C. Inhibition of neuronal Na+ channels by antidepressant drugs. J. Pharmacol. Exp. Ther. 1998;284:208–214. [PubMed] [Google Scholar]
- PATEL A.J., LAURITZEN I., LAZDUNSKI M., HONORÉ E. Disruption of mitochondrial respiration inhibits volume-regulated anion channels and provoke neuronal cell swelling. J. NeuroSci. 1998;18:3117–3123. doi: 10.1523/JNEUROSCI.18-09-03117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESKORN S.H., SILKEY B., BEBER J., DOREY C. Antidepressant response and plasma concentrations of fluoxetine. Ann. Clin. Psychiatry. 1991;3:147–151. [Google Scholar]
- RAE J.L., RICH A., ZAMUDIO A.C., CANDIA O.A. Effect of Prozac on whole cell ionic currents in lens and corneal epithelia. Am. J. Physiol. 1995;269:C250–C256. doi: 10.1152/ajpcell.1995.269.1.C250. [DOI] [PubMed] [Google Scholar]
- ROY G., BERNATCHEZ G., SAUVÉ R. Halide and alkyl phenols block volume-sensitive chloride channels in human glial cells (U-138MG) J. Membr. Biol. 1998;162:191–200. doi: 10.1007/s002329900356. [DOI] [PubMed] [Google Scholar]
- STAUDERMAN K.A., GANDHI V.C., JONES D.J. Fluoxetine-induced inhibition of synaptosomal [3H]5-HT release: possible Ca2+-channel inhibition. Life Sci. 1992;50:2125–2138. doi: 10.1016/0024-3205(92)90579-e. [DOI] [PubMed] [Google Scholar]
- STRANGE K. Regulation of solute and water balance and cell volume in the central nervous system. J. Am. Soc. Nephrol. 1992;3:12–27. doi: 10.1681/ASN.V3112. [DOI] [PubMed] [Google Scholar]
- STRANGE K., EMMA F., JACKSON P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- SZÜCS G., HEINKE S., DROOGMANS G., NILIUS B. Activation of the volume-sensitive chloride current in vascular endothelial cells requires a permissive intracellular Ca2+ concentration. Pflügers Arch. 1996;431:467–469. doi: 10.1007/BF02207289. [DOI] [PubMed] [Google Scholar]
- TROUET D., NILIUS B., VOETS T., DROOGMANS G., EGGERMONT J. Use of a bicistronic GFP-expression vector to characterise ion channels after transfection in mammalian cells. Pflügers Arch. 1997;434:632–638. doi: 10.1007/s004240050445. [DOI] [PubMed] [Google Scholar]
- TYTGAT J., MAERTENS C., DAENENS P. Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (Kv1.1) Br. J. Pharmacol. 1997;122:1417–1424. doi: 10.1038/sj.bjp.0701545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOETS T., DROOGMANS G., NILIUS B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J. Physiol. (Lond.) 1996a;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOETS T., DROOGMANS G., NILIUS B. Potent block of volume-activated chloride currents in endothelial cells by the uncharged form of quinine and quinidine. Br. J. Pharmacol. 1996b;118:1869–1871. doi: 10.1111/j.1476-5381.1996.tb15616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG D.T., BYMASTER F.P., ENGLEMAN E.A. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411–414. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]


