Introduction
Endothelin (ET) is a potent vasoconstrictor peptide that is generated via unique processing of a low activity precursor, big ET-1 by endothelin converting enzymes (ECEs). ET has a physiological role in the maintenance of basal tone in humans (Haynes & Webb, 1994; Haynes, 1995; Haynes et al., 1995), but may also have a role in the pathophysiology of cardiovascular diseases, including atherosclerosis, coronary vasospasm and congestive heart disease (Kurihara et al., 1989; Bacon et al., 1996; Cohn, 1996). The ET system is therefore a potential therapeutic target in the effective management of these diseases. There are two current strategies being pursued to attenuate adverse haemodynamic effects and the migration and proliferation of vascular smooth muscle cells by ET. These include the use of antagonists to receptors that mediate responses to ET and the use of selective inhibitors to ECE. The pathways involved in big ET-1 processing and ET transport are only now being elucidated and these findings will be useful in predicting the characteristics of inhibitors that best suit inhibition of ECE. It will be important to determine whether ECE is expressed on the cell surface and/or intracellularly to decide whether inhibitors are required to penetrate the plasma membrane. This article reviews the secretory pathways involved in ET transport and the subcellular processing of big ET-1 by ECE.
ECEs are membrane-bound proteases with structural homology to neutral endopeptidase 24.11 (NEP) and Kell blood group protein (for review, see Opgenorth et al., 1992; Turner, 1993; Turner & Murphy, 1996). The cDNA sequences of two converting enzymes, ECE-1 and ECE-2 have been reported (Schmidt et al., 1994; Emoto & Yanagisawa, 1995; Shimada et al., 1995; Valdenaire et al., 1995; Yorimitsu et al., 1995). The enzymes, which have 59% overall homology, are membrane bound phosphoramidon-sensitive metalloproteases with specificity for big ET-1. ECE-1 appears to be the predominant endothelin converting enzyme in humans (Emoto & Yanagisawa, 1995). Two isoforms of ECE-1 (ECE-1α and ECE-1β) are encoded by a single gene and differ only in their cytoplasmic N-terminal domains. Studies on a soluble construct of ECE-1 (Korth et al., 1997), and molecular modelling experiments (Sansom et al., 1995) reveal that the putative extracellular domain contains the catalytic site for ECE activity. A third converting enzyme, ECE-3 has recently been purified from bovine iris microsomes (Hasegawa et al., 1998) and this enzyme has specificity for big ET-3. With the exception of studies exploiting selective antisera, the precise identity of the enzyme catalyzing conversion of big ET in tissues in functional studies is not yet known and therefore is referred to as ECE activity.
ECE expression in the human vasculature
ECE is widely expressed in human blood vessel endothelium, including the cerebral, pulmonary, coronary, splanchnic, renal, forearm and adrenal vasculature (Ahlborg et al., 1994; Haynes, 1995; Hemsén et al., 1995; Plumpton et al., 1995; Saleh et al., 1997; Davenport et al., 1998a; Herman et al., 1998; Russell et al., 1998a,1998b). ECE has also been identified in endocardial cells lining the ventricles of the heart and in endothelial cells of foetal vasculature including umbilical veins and arteries (Moldovan et al., 1996; Davenport et al., 1998a; Russell et al., 1998c). The pattern of ECE-like immunoreactive staining in human blood vessels corresponds to the distribution of ET and big ET (Bacon et al., 1996; Saleh et al., 1997; Davenport et al., 1998a; Russell et al., 1998d). Interestingly, infusion of ET-1 into the dorsal hand vein produced marked vasoconstriction whereas infusion of big ET-1 had no effect (Haynes et al., 1995), thus raising the possibility that endogenous big ET-1 is only processed by an intracellular enzyme in this vessel.
In contrast to the high level of ECE that is expressed in human endothelial cells, only low to moderate levels of expression have been detected in adjacent intimal and medial vascular smooth muscle cells (Davenport et al., 1998a). Human umbilical vein smooth muscle cells synthesize and secrete immunoreactive ET-1 and ET-3 (Yu & Davenport, 1995), indicating a possible physiological relevance of the smooth muscle converting enzyme.
Evidence for expression of an intracellular ECE
ECE expression is high in endothelial cells and processing of big ET-1 to ET-1 has been attributed to activity of a converting enzyme that is located on the plasma membrane and within intracellular compartments (Harrison et al., 1993; Xu et al., 1994; Corder et al., 1995). Some studies indicate that ECE is predominantly expressed or has main activity as an ectoenzyme (Harrison et al., 1993; Waxman et al., 1994; Corder et al., 1995; Takahashi et al., 1995; Barnes et al., 1996), and therefore acts mainly in a post-secretory processing role. When a homogenate prepared from a human endothelial hybrid cell line, EAHY 926, was sub-fractionated on a sucrose gradient the majority of ECE activity (60%) was associated with a plasma membrane enriched fraction (Waxman et al., 1994). In contrast, other studies have suggested that ECE is either primarily expressed or has predominant activity within intracellular compartments (Gui et al., 1993; Xu et al., 1994; Davenport et al., 1998b; Russell et al., 1998c). Immunocytochemical studies revealed only a low level of ECE-like immunoreactivity on the plasma membrane of cultured HUVECs and intense immunoreactive staining within intracellular organelles, determined by scanning electron microscopy, confocal laser scanning microscopy and immuno-electron microscopy (Russell et al., 1998c). Biochemical studies showed predominant ECE activity in intracellular compartments by comparing big ET-1 processing in permeabilized and non-permeabilized HUVECs (Davenport et al., 1998b).
Pathways involved in peptide transport
Proteins are transported from the endoplasmic reticulum to the plasma membrane in human endothelial cells via two distinct secretory pathways; the constitutive pathway involving continuous release and the regulated pathway involving stimulated release. The constitutive secretory pathway is modulated at the level of mRNA transcription and has been proposed as the mechanism for ET release from porcine endothelial cells (Yanagisawa et al., 1988). Although involvement of the regulated pathway in ET secretion was dismissed, based in part on a perceived lack of storage granules in endothelial cells (Yanagisawa et al., 1988), early morphological studies had successfully identified endothelial specific storage granules called Weibel-Palade bodies (Weibel & Palade, 1964).
Basal ET release from endothelial cells is via the constitutive secretory pathway
Secretory and plasmalemmal proteins, proteoglycans and lysosomal enzymes follow a common pathway through the endoplasmic reticulum and Golgi complex (Farquhar, 1985). The proteins are continuously shuttled from the trans-Golgi network to the cell surface in secretory vesicles via the constitutive secretory pathway. ET-like immunoreactivity has been detected in bovine aortic and human coronary artery endothelial cell secretory vesicles, indicating involvement of the constitutive pathway in peptide transport (Harrison et al., 1993; 1995; Russell et al., 1998d). Indeed, the constitutive pathway may be involved in peptide processing since ECE and big ET-like immunoreactive staining was found to be co-localized in endothelial secretory vesicles (Barnes et al., 1998). In vivo evidence suggest that endogenous synthesis, transport and release of ET contribute to maintenance of vascular tone in humans. Forearm vasodilatation, measured by increased blood flow, was observed when either phosphoramidon or the ETA selective antagonist BQ123 was infused into the brachial artery of healthy subjects (Haynes & Webb, 1994).
Evidence for a role of the regulated secretory pathway in ET transport
Weibel-Palade bodies store vasoactive compounds including histamine, von Willebrand factor, P-selectin and calcitonin gene-related peptide (Fujimoto et al., 1982; Wagner et al., 1982; McEver et al., 1989; Doi et al., 1995; Ozaka et al., 1997). These granules are also a repository for ET in rat (Doi et al., 1996; Ozaka et al., 1997), rabbit (Sakamoto et al., 1993), and human endothelial cells (Hamasaki et al., 1995; Russell et al., 1998d). Endothelial storage granules that are distinct from Weibel-Palade bodies were identified as storage sites for tissue-type plasminogen activator (Emeis et al., 1997). However, these granules are unlikely to be involved in storage of ET since, unlike ET, tissue-type plasminogen activator does not co-localize with von Willebrand factor. Similarly, the soluble protein multimerin which was identified in round to rod-shaped, dense core granules resembling Weibel-Palade bodies did not co-localize with the Weibel-Palade body proteins von Willebrand factor or P-selectin (Hayward et al., 1998).
Peptides and proteins may contain signalling motifs that serve as important determinants for differential packaging at the trans-Golgi network. For example, the cytoplasmic and transmembrane domains of the integral membrane glycoprotein, P-selectin enhance the efficiency of endothelial storage graunule targeting (Fleming et al., 1998). Factor VIII is a coagulation protein that does not appear to possess signalling motifs for mobilization to the regulated pathway. However, when Factor VIII was co-transfected with von Willebrand factor in AtT-20 cells it displayed altered intracellular trafficking from a constitutive to a regulated secretory pathway (Rosenberg et al., 1998). This suggests that proteins containing signalling motifs, such as von Willebrand factor, may chaperone other proteins that lack such structural determinants. It is not known whether big ET-1 contains signalling motifs that confer granular targeting. The localization of big ET-1 like immunoreactivity in Weibel-Palade bodies (Russell et al., 1998c) and secretory vesicles (Harrison et al., 1995) suggest that the peptide has no sorting domain. Mobilization of big ET-1 into secretory vesicles and granules may involve passive bulk flow in which the amount of peptide entering each pathway is determined by internal volume of the vesicle or granule and the number of the compartments formed per unit time (Kelly, 1985).
We have recently proposed that endothelial cell storage granules are an important site in the processing of big ET-1 to the mature peptide (Russell et al., 1998c,1998d). Antisera raised against big ET-1 and the isoforms of ECE-1 (ECE-1α and ECE-1β) were found to co-localize with von Willebrand factor in round to rod-shaped structures located beneath the plasma membrane (Figure 1). ECE activity that was sensitive to phosphoramidon and the ECE-1 selective inhibitor, PD159790 (Ahn et al., 1998) but insensitive to thiorphan was identified in subcellular sucrose fractions of HUVECs prepared by gradient centrifugation (unpublished findings). 5′-Nucleotidase activity, a marker for the plasma membrane, was identified in several fractions containing ECE activity, consistent with expression of ECE on the cell surface. However, high density fractions that contained only low levels of 5′-nucleotidase activity contained ECE activity and the storage granule glycoprotein, von Willebrand factor thus presenting the possibility that an active converting enzyme is expressed in the endothelial granules.
Figure 1.

Microscopy of permeabilized human umbilical vein endothelial cells immunolabelled with antisera raised against ECE-1. Cells labelled with the ECE-1 antibody and a secondary fluoresceinated goat anti-rabbit antibody showed positive immunofluorescence staining over the perinuclear region (PN) and Weibel-Palade bodies (WP) (A). Only a moderate level of immunoreactive staining was detected over the plasma membrane (PM). Cells incubated with preimmune serum showed negligible staining (B). Cells were double labelled with antisera to ECE-1 (C) and von Willebrand factor (D). Electronic overlay of images to ECE-1 and von Willebrand factor (E) revealed co-localization in Weibel-Palade bodies (yellow). Scale bar=70 μm (A, B) and 40 μm (C–E).
Several in vivo findings indicate that physiological or pathophysiological stimuli can lead to release of ET via the regulated secretory pathway. For example, high plasma ET levels were measured in a patient during the early phase of treatment for accidental hypothermia induced by cold water immersion (Yoshitomi et al., 1998). This type of stimulus has been proposed to mediate release of ET from endothelial cell storage granules. When healthy volunteers were subjected to a cold pressor test in which the forearm was immersed in ice water, an increase in venous plasma ET concentration was detected within 2 min (Fyhrquist et al., 1990). The detection of ET was too rapid to indicate de novo synthesis of the peptide and suggests release from intracellular stores.
Other mechanical and chemical stimuli mediate degranulation of Weibel-Palade bodies. Mechanical stretch applied to cultured bovine aortic endothelial cells produced rapid release of ET into the culture medium (⩽20 min) (Macarthur et al., 1994). The calcium ionophore A23187, which elevates [Ca2+]i, also mediated release of von Willebrand factor (Loesberg et al., 1983; Sporn et al., 1989) and ET (Russell et al., 1998c) from cultured HUVECs. Phorbol 12-myristate 13-acetate mediates degranulation by activation of protein kinase C and it is speculated that other as yet uncharacterized signal-transduction mechanisms may be important for full induction of the regulated secretory pathway by agonists such as histamine and thrombin (Carew et al., 1992).
Physiological and pathophysiological implications of dual transport pathways for ET
Identification of a dual secretory pathway for release of ET-1 is a recent and novel finding, not previously reported for a vasoactive peptide (Figure 2). Dual transport pathways have been described for other proteins, including von Willebrand factor and tissue-type plasminogen activator in endothelial cells and adrenocorticotropic hormone (ACTH) in pituitary tumor cells (Gumbiner & Kelly, 1982; Sporn et al., 1986; Mayadas et al., 1989; Emeis et al., 1997). ACTH precursor and all multimeric forms of von Willebrand factor are released constitutively whilst only mature ACTH and von Willebrand factor are released following an appropriate stimulus (Gumbiner & Kelly, 1982; Sporn et al., 1986; Mayadas et al., 1989).
Figure 2.
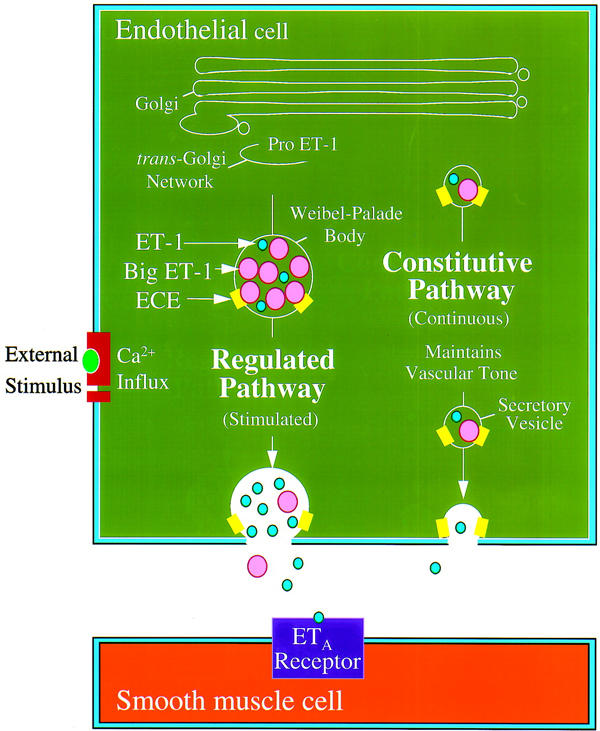
Schematic model of ET-1 transport in the human coronary artery. In this model, it is proposed that two distinct exocytic pathways are involved in the transport of ET-1 to the cell surface. ET-1 is stored in Weibel-Palade bodies with other vasoactive compounds and is released at the cell surface following an appropriate stimulus. ET-1 is also sorted into secretory vesicles and continuously released by a cyclic AMP independent constitutive pathway. It is proposed that a small amount of ET-1 is released luminally from this pathway, binding to ETB receptors on the endothelial cells to indirectly release vasodilators. However, most ET-1 is released (>80%) from the abluminal surface where it can activate the vasoconstrictor ETA receptors that predominate on human vascular smooth muscle cells. It is hypothesized that this continuous release via the constitutive pathway contributes to the maintenance of normal physiological tone.
Several studies have examined the polarity of secretion of proteins from endothelial cells. Whereas constitutive release of von Willebrand factor from HUVECs is reportedly nonpolarized, 90% of the glycoprotein secreted by the regulated pathway is toward the basolateral membrane (Sporn et al., 1989). Other studies show that the constitutive pathway may be polarized with the majority of ET-1 produced in HUVECs and porcine cerebral microvessel endothelia released from the basolateral membrane (Yoshimoto et al., 1991; Wagner et al., 1992). Directional secretion of ET-1 to the smooth muscle layer is consistent with the hypothesis that the peptide modulates vasomotor tone through local paracrine rather than humoral systemic effects. An additional autocrine effect is involved in ET-1 mediated vasodilatation. Activation of endothelial ETB receptors by ET-1 mediates synthesis of nitric oxide which in turn stimulates guanylate cyclase in the smooth muscle leading to relaxation.
Under normal physiological conditions endothelin and nitric oxide are constitutively released by the endothelium and provide a balance between vasoconstrictor and vasodilator activity. However, vascular injury can compromise endothelial cell integrity and cause reduced nitric oxide synthesis and over production of ET-1. Release of ET and von Willebrand factor (Ewenstein et al., 1987) via the regulated secretory pathway may provide an initial haemostatic response to vascular endothelial cell damage.
Novel ECE inhibitors
Intracellular ECE activity amounts to 85% of total activity in endothelial cells (Davenport et al., 1998b) which leads us to propose that an effective therapeutic inhibitor of the enzyme must first penetrate the plasma membrane. Although phosphoramidon is a valuable tool that enables examination of ECE activity, the compound is non-selective, efficiently blocking NEP activity. ECE and NEP activity can be differentiated by comparing the effects of phosphoramidon with the NEP selective inhibitor, thiorphan.
CGS 26303 and CGS 31447 are non-peptide ECE inhibitors that have IC50 values of 1.1 μM and 17 nM, respectively for ECE-1 inhibition (De Lombaert et al., 1994; 1997). However, neither compound is selective for ECE-1 with IC50 values of 0.9 and 4.8 nM, respectively, for inhibition of NEP. In a rabbit model of subarachnoid haemorrhage, administration of CGS 26303 both prevented and reversed cerebral vasospasm (Kwan et al., 1997), thus indicating the therapeutic potential of ECE inhibitors. SCH 54470 is an orally active triple inhibitor of ECE, NEP and the angiotensin converting enzyme, with IC50 values of 80, 90 and 2.5 nM, respectively (Vemulapalli et al., 1997). This inhibitor reduced ischaemia induced ET release in isolated perfused guinea-pig lungs, indicating inhibition of endogenous converting enzyme activity. The therapeutic benefit of non-selective inhibitors of ECE and NEP awaits further investigation. Whilst inhibition of NEP may be beneficial in reducing degradation of atrial natriuretic peptide, an endogenous vasodilator, its inhibition has been shown to cause vasoconstriction of human resistance vessels in vivo (Ferro et al., 1998). This latter effect is presumably a result of decreased ET degradation since NEP is highly efficient at cleaving the mature peptide at Asp18-Ile19 to produce inactive fragments (Sokolovsky et al., 1990).
A number of ECE inhibitors that are derived from natural products have been reported with varying degrees of selectivity for ECE (Table 1). WS75624A and WS75624B are ECE inhibitors isolated from the fermentation broth of Saccharothrix sp. (Tsurumi et al., 1995a). The compounds have similar inhibitory characteristics against selected metalloproteases with 30–40 fold selectivity for ECE (IC50=0.03 μg ml−1) over collagenase (IC50=1.0 μg ml−1) and NEP (IC50=1.25 μg ml−1). WS79089B, isolated from the culture broth of Streptosporangium roseum (Tsurumi et al., 1995b), has a higher reported selectivity for ECE (IC50=0.14 μM) over collagenase and NEP (no inhibition at 50 μM). The sodium salt of this compound, FR901533 was effective in inhibiting the pressor effect of big ET-1 when administered intravenously in rats (Tsurumi et al., 1995b). This compound was also protective against the development of right ventricular overload and medial thickening of pulmonary arteries in rats with monocrotaline-induced pulmonary hypertension (Takahashi et al., 1998).
Table 1.
Selected endothelin converting enzyme inhibitors
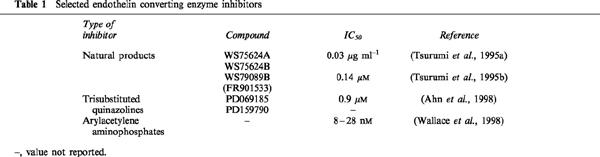
A series of arylacetylene-containing compounds also display selectivity for the inhibition of ECE over NEP (Wallace et al., 1998). An arylacetylene amino phosphonate dipeptide was found to inhibit ECE and NEP with IC50 values of 28 nM and 6.3 μM, respectively (225 fold selectivity for ECE over NEP). Greater selectivity (725 fold) was obtained with a tripeptide derivative, with IC50 values of 8 nM and 5.8 μM for the inhibition of ECE and NEP, respectively. Pretreatment of rats with these compounds inhibited increased mean arterial pressure produced by intravenous bolus injection of big ET-1. PD069185, a trisubstituted quinazoline, is the first ECE-1 selective inhibitor to be reported (IC50=0.9 μM). This compound has no effect on ECE-2 at a concentration of 100 μM and only marginal effects on NEP, stromelysin, gelatinase A, collegenase, interleukin-1β converting enzyme and thrombin at 100–300 μM (Ahn et al., 1998). Replacement of the −CCl3 group of PD069185 with −CF3 (PD159790) increased solubility without affecting selectivity. In biochemical experiments, 100 μM PD159790 effectively abolished conversion of big ET-1 to ET-1 in HUVEC cultures (Russell et al., 1998e). Although PD069185 was slightly more potent than PD159790 in inhibiting ET-1 production by cocultures of CHO/ECE-1 and CHO/prepro-ET-1 cells (EC50=3.8 and 11.5 μM, respectively), the latter compound was less toxic (Ahn et al., 1998). The cellular toxicity concentration for PD069185 was TC50=56 μM whereas no toxicity was observed at concentrations up to 100 μM for PD159790.
Do other ECEs exist?
Although ECE-1 appears to be the predominant endothelin converting enzyme in mammalian tissues, recent studies indicate that other ECEs may also have a physiological role in big ET processing. Future studies will need to identify converting enzymes involved in processing of the big ET isoforms and to determine their cell surface or intracellular localization.
Recently, Schweizer et al. (1997) proposed three isoforms of ECE-1 encoded by the same gene. The isoform designated as ECE-1a has an identical sequence to ECE-1β (Valdenaire et al., 1995; Schweizer et al., 1997). The isoforms designated ECE-1b and ECE-1c have an identical sequence to ECE-1α except that the N-terminus is predicted to be extended by an additional 17 and 1 amino acids, respectively in each isoform. The functional importance of the different isoforms is presently unclear since all three enzymes expressed in CHO cells have similar kinetic rate constants for processing of big ET precursors to the corresponding mature peptides. However, ELISAs using antisera directed to the N-terminus of ECE-1α/ECE-c indicate that this isoform predominates in human tissue compared with ECE-1β/ECE-1a (Mockridge et al., 1998).
Targeted null mutation in the mouse ECE-1 gene produces embryos that exhibit marked craniofacial and cardiac defects as well as an absence of epidermal melanocytes and enteric neurons of the distal gut (Yanagisawa et al., 1998). Surprisingly, high levels of mature ET peptide were detectable in the homozygous ECE-1 knockout embryos, leading the authors to speculate that a further non-ECE-1 protease is expressed, for example ECE-2 (Yanagisawa et al., 1998). It has been postulated that ECE-2, which has optimal activity at pH=5.5, may have a role in processing big ET-1 in the trans-Golgi network where the vesicular fluid is acidified (Emoto & Yanagisawa, 1995). It remains to be determined whether converting enzymes with acidic pH optimum, such as ECE-2 have a pathogenic role in diseases in which cellular pH is reduced, for example ischaemic heart disease. Hearts subjected to global ischaemia show lactate accumulation with concomitant intracellular acidosis (intracellular pH=5.8; Docherty et al., 1997), and a correlation between myocardial ischaemia and increased plasma levels of ET is now well established (Tonnessen et al., 1993; Cohn, 1996).
ET-3 has been detected in human plasma but the efficiency of cloned ECE-1 to convert big ET-3 to the mature peptide is usually low or not detectable. Several findings have indicated the possible existence of another converting enzyme in neuronal cells. These cells lack detectable levels of ECE-1 mRNA (Xu et al., 1994), but produce ET-3 (Shinmi et al., 1989; Fuxe et al., 1991) suggesting the existence of a big ET-3 specific converting enzyme. Interestingly, a novel endothelin converting enzyme (ECE-3) was purified by SDS–PAGE from bovine iris microsomes that has specificity for big ET-3 (Hasegawa et al., 1998). This enzyme contrasts to ECE-1 and ECE-2, both of which have specificity for big ET-1 and big ET-2, and may potentially modulate ET-3 production in the nerve terminals.
ECE expressed on endothelial cells may also differ to ECE expressed on vascular smooth muscle cells. Whilst endothelial ECE has been found to have specificity for big ET-1 (Plumpton et al., 1996; Davenport et al., 1998b), smooth muscle ECE is capable of processing big ET-1, big ET-2 and big ET-3 (Tsukahara et al., 1993; Maguire et al., 1997; Davenport et al., 1998b). The physiological role of this and other novel putative converting enzymes on regulation of ET production remains to be determined.
In conclusion, evidence is emerging within endothelial cells for the continuous release of ET via the constitutive pathway for maintenance of normal vascular tone. In addition, further release of ET, possibly in high concentrations via the regulated pathway, may be involved in responses to vascular injury. Intracellular ECEs present within the secretory pathways are therefore potential therapeutic targets for diseases in which ET has been implicated.
Acknowledgments
This work was supported by grants from the British Heart Foundation, Isaac Newton Trust, and the Royal Society.
Abbreviations
- ECE
endothelin converting enzyme
- ET
endothelin
- HUVEC
human umbilical vein endothelial cell
- NEP
neutral endopeptidase
References
- AHLBORG G., OTTOSSON-SEEBERGER A., HEMSÉN A., LUNDBERG J.M. Big ET-1 infusion in man causes renal ET-1 release, renal and splanchnic vasoconstriction, and increased mean arterial blood pressure. Cardiovasc. Res. 1994;28:1559–1563. doi: 10.1093/cvr/28.10.1559. [DOI] [PubMed] [Google Scholar]
- AHN K., SISNEROS A.M., HERMAN S.B., PAN S.M., HUPE D., LEE C., NIKAM S., CHENG X.-M., DOHERTY A.M., SCHROEDER R.L., HALEEN S.J., KAW S., EMOTO N., YANAGISAWA M. Novel selective quinazoline inhibitors of endothelin converting enzyme-1. Biochem. Biophys. Res. Commun. 1998;243:184–190. doi: 10.1006/bbrc.1998.8081. [DOI] [PubMed] [Google Scholar]
- BACON C.R., CARY N.R.B., DAVENPORT A.P. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ. Res. 1996;79:794–801. doi: 10.1161/01.res.79.4.794. [DOI] [PubMed] [Google Scholar]
- BARNES K., BROWN C., TURNER A.J. Endothelin-converting enzyme. Ultrstructural localization and its recycling from the cell surface. Hypertension. 1998;31:3–9. doi: 10.1161/01.hyp.31.1.3. [DOI] [PubMed] [Google Scholar]
- BARNES K., SHIMADA K., TAKAHASHI M., TANZAWA K., TURNER A.J. Metallopeptidase inhibitors induce an up-regulation of endothelin-converting enzyme levels and its redistribution from the plasma membrane to an intracellular compartment. J. Cell. Sci. 1996;109:919–928. doi: 10.1242/jcs.109.5.919. [DOI] [PubMed] [Google Scholar]
- CAREW M.A., PALEOLOG E.M., PEARSON J.D. The roles of protein kinase C and intracellular Ca2+ in the secretion of von Willebrand factor from human vascular endothelial cells. Biochem. J. 1992;286:631–635. doi: 10.1042/bj2860631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN J.N. Is there a role for endothelin in the natural history of heart failure. Circulation. 1996;94:604–606. doi: 10.1161/01.cir.94.4.604. [DOI] [PubMed] [Google Scholar]
- CORDER R., KHAN N., HARRISON V.J. A simple method for isolating human endothelin converting enzyme free from contamination by neutral endopeptidase 24.11. Biochem. Biophys. Res. Commun. 1995;207:355–362. doi: 10.1006/bbrc.1995.1195. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P., KUC R.E., MOCKRIDGE J.W. Endothelin-converting enzyme in the human vasculature: evidence for differential conversion of big endothelin-3 by endothelial and smooth-muscle cells. J. Card. Pharmacol. 1998b;31:S1–S3. doi: 10.1097/00005344-199800001-00002. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P., KUC R.E., PLUMPTON C., MOCKRIDGE J.W., BARKER P.J., HUSKISSON N.S. Endothelin-converting enzyme in human tissues. Histochem. J. 1998a;30:359–374. [Google Scholar]
- DE LOMBAERT S., GHAI R.D., JENG A.Y., TRAPANI A.J., WEBB R.L. Pharmacological profile of a non-peptidic dual inhibitor of neutral endopeptidase 24.11 and endothelin-converting enzyme. Biochem. Biophys. Res. Commun. 1994;204:407–412. doi: 10.1006/bbrc.1994.2473. [DOI] [PubMed] [Google Scholar]
- DE LOMBAERT S., STAMFORD L.B., BLANCHARD L., TAN J., HOYER D., DIEFENBACHER C.G., WEI D.C., WALLACE E.M., MOSKAL M.A., SAVAGE P., JENG A.Y. Potent non-peptidic dual inhibitors of endothelin-converting enzyme and neutral endopeptidase 24.11. Bioorg. Med. Chem. Lett. 1997;7:1059–1064. [Google Scholar]
- DOCHERTY J.C., GUNTER H.E., KUZIO B., SHOEMAKER L., YANG L.J., DESLAURIERS R. Effects of cromakalim and glibenclamide on myocardial high energy phosphates and intracellular pH during ischemia-reperfusion: P-31 NMR studies. J. Mol. Cell. Cardiol. 1997;29:1665–1673. doi: 10.1006/jmcc.1997.0404. [DOI] [PubMed] [Google Scholar]
- DOI Y., OZAKA T., FUKUSHIGE H., FURUKAWA H., YOSHIZUKA M., FUJIMOTO S. Increase in number of Weibel-Palade bodies and endothelin-1 release from endothelial cells in the cadmium-treated rat thoracic aorta. Virchows Arch. 1996;428:367–373. doi: 10.1007/BF00202203. [DOI] [PubMed] [Google Scholar]
- DOI Y., OZAKA T., KATSUKI M., FUKUSHIGE H., TOYAMA E., KANAZAWA Y., ARASHIDANI K., FUJIMOTO S. Histamine release from Weibel-Palade bodies of toad aortas induced by endothlin-1 and sarafotoxin-S6b. Anat. Rec. 1995;242:374–382. doi: 10.1002/ar.1092420310. [DOI] [PubMed] [Google Scholar]
- EMEIS J.J., VAN DEN EIJNDEN-SCHRAUWEN Y., VAN DEN HOOGEN C.M., DE PRIESTER W., WESTMUCKETT A., LUPU F. An endothelial storage granule for tissue-type plasminogen activator. J. Cell. Biol. 1997;139:245–256. doi: 10.1083/jcb.139.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMOTO N., YANAGISAWA M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J. Biol. Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- EWENSTEIN B.M., WARHOL M.J., HANDIN R.I., POBER J.S. Comparison of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J. Cell. Biol. 1987;104:1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M.G. Progress in unraveling pathways of Golgi traffic. Annu. Rev. Cell. Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- FERRO C.J., SPRATT J.C., HAYNES W.G., WEBB D.J. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97:2323–2330. doi: 10.1161/01.cir.97.23.2323. [DOI] [PubMed] [Google Scholar]
- FLEMING J.C., BERGER G., GUICHARD J., CRAMER E.M., WAGNER D.D. The transmembrane domain enhances granular targeting of P-selectin. Eur. J. Cell. Biol. 1998;75:331–343. doi: 10.1016/s0171-9335(98)80066-6. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO S., YAMAMOTO K., ARASHIDANI K., HAYABUCHUCHI I., YOSHIZUKA M., NOMIYAMA T. Endothelial specific granules in the umbilical veins of the postnatal rabbit. Cell. Tissue Res. 1982;227:509–518. doi: 10.1007/BF00204781. [DOI] [PubMed] [Google Scholar]
- FUXE K., TINNER B., STAINES W., HEMSEN A., HERSH L., LUNDBERG J.M. Demonstration and nature of endothelin-3-like immunoreactivity in somatostatin and choline acetyltransferase-immunoreactive nerve-cells of the neostratum of the rat. Neurosc. Lett. 1991;123:107–111. doi: 10.1016/0304-3940(91)90169-t. [DOI] [PubMed] [Google Scholar]
- FYHRQUIST F., SAIJONMAA O., METSÄRINNE K., TIKKANEN I., ROSENLÖF K., TIKKANEN T. Raised plasma endothelin-1 concentration following cold pressor test. Biochem. Biophys. Res. Commun. 1990;169:217–221. doi: 10.1016/0006-291x(90)91456-3. [DOI] [PubMed] [Google Scholar]
- GUI G., XU D., EMOTO N., YANAGISAWA M. Intracellular localization of membrane-bound endothelin-converting enzyme from rat lung. J. Cardiovasc. Pharmacol. 1993;22:S53–S56. doi: 10.1097/00005344-199322008-00016. [DOI] [PubMed] [Google Scholar]
- GUMBINER B., KELLY R.B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982;28:51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- HAMASAKI K., DOI Y., SAKAMOTO Y., KASHIMURA M., FUJIMOTO S. The contraction of small arteries in the perimetrium by presurgical medication with the luteinizing hormone-releasing hormone agonist to patients with leiomyomas: an electron and immunoelectron microscopy. Fukuoka Acta Med. 1995;86:389–397. [PubMed] [Google Scholar]
- HARRISON V.J., BARNES K., TURNER A.J., WOOD E., CORDER R., VANE J.R. Identification of endothelin 1 and big endothelin 1 in secretory vesicles isolated from bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6344–6348. doi: 10.1073/pnas.92.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON V.J., CORDER R., ÄNGGÅRD E.E., VANE J.R. Evidence for vesicles that transport endothelin-1 in bovine aortic endothelial cells. J. Cardiovasc. Pharmacol. 1993;22:S57–S60. doi: 10.1097/00005344-199322008-00017. [DOI] [PubMed] [Google Scholar]
- HASEGAWA H., HIKI K., SAWAMURA T., AOYAMA T., OKAMOTO Y., MIWA S., SHIMOHAMA S., KIMURA J., MASAKI T. Purification of a novel endothelin-converting enzyme specific for big endothelin-3. FEBS. Lett. 1998;428:304–308. doi: 10.1016/s0014-5793(98)00554-7. [DOI] [PubMed] [Google Scholar]
- HAYNES W.G. Endothelins as regulators of vascular tone in man. Clin. Sci. 1995;88:509–517. doi: 10.1042/cs0880509. [DOI] [PubMed] [Google Scholar]
- HAYNES W.G., FERRO C.E., WEBB D.J. Physiologic role of endothelin in maintenance of vascular tone in humans. J. Cardiovasc. Pharmacol. 1995;26:S183–S185. [PubMed] [Google Scholar]
- HAYNES W.G., WEBB D.J. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- HAYWARD C.P.M., CRAMER E.M., SONG Z., ZHENG S., FUNG R., MASSÉ J.-M., STEAD R.H., PODOR T.J. Studies of multimerin in human endothelial cells. Blood. 1998;91:1304–1317. [PubMed] [Google Scholar]
- HEMSÉN A., AHLBORG G., OTTOSSON-SEEBERGER A., LUNDBERG J.M. Metabolism of big endothelin-1 (1-38) and (22-38) in the human circulation in relation to production of endothelin-1 (1-21) Reg. Peptides. 1995;55:287–297. doi: 10.1016/0167-0115(94)00119-i. [DOI] [PubMed] [Google Scholar]
- HERMAN W.H., EMANCIPATOR S.N., RHOTEN R.L.P., SIMONSON M.S. Vascular and glomerular expression of endothelin-1 in normal human kidney. Am. J. Physiol. 1998;44:F8–F17. doi: 10.1152/ajprenal.1998.275.1.F8. [DOI] [PubMed] [Google Scholar]
- KELLY R.B. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- KORTH P., EGIDY G., PARNOT C., LEMOULLEC J.-M., CORVOL P., PINET F. Construction, expression and characterization of a soluble form of human endothelin-converting-enzyme-1. FEBS. Lett. 1997;417:365–370. doi: 10.1016/s0014-5793(97)01323-9. [DOI] [PubMed] [Google Scholar]
- KURIHARA H., YOSHIZUMI M., SUGIYAMA T., YAMAOKI K., NAGAI R., TAKAKU F., SATOH H., INUI J., YANAGISAWA M., MASAKI T., YAZAKI Y. The possible role of endothelin-1 in the pathogenesis of coronary vasospasm. J. Cardiovasc. Pharmacol. 1989;13:S132–S137. doi: 10.1097/00005344-198900135-00033. [DOI] [PubMed] [Google Scholar]
- KWAN A.L., BAVBEK M., JENG A.Y., MANIARA W., TOYODA T., LAPPE R.W., KASSELL N.F., LEE K.S. Prevention and reversal of cerebral vasospasm by an endothelin-converting enzyme inhibitor, CGS 26303, in an experimental model of subarachnoid hemorrhage. J. Neurosurg. 1997;87:281–286. doi: 10.3171/jns.1997.87.2.0281. [DOI] [PubMed] [Google Scholar]
- LOESBERG C., GONSALVES M.D., ZANDBERGEN J., WILLEMS C., VAN AKEN W.G., STEL H.V., VAN MOURIK J.A., DE GROOT P.G. The effect of calcium on the secretion of factor VIII-related antigen by cultured human endothelial cells. Biochim. Biophys. Acta. 1983;763:160–168. doi: 10.1016/0167-4889(83)90039-3. [DOI] [PubMed] [Google Scholar]
- MACARTHUR H., WARNER T.D., WOOD E.G., CORDER R., VANE J.R. Endothelin-1 release from endothelial cells in culture is elevated both acutely and chronically by short periods of mechanical stretch. Biochem. Biophys. Res. Commun. 1994;200:395–400. doi: 10.1006/bbrc.1994.1462. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., JOHNSON C.M., MOCKRIDGE J.W., DAVENPORT A.P. Endothelin converting enzyme (ECE) activity in human vascular smooth muscle. Br. J. Pharmacol. 1997;122:1647–1654. doi: 10.1038/sj.bjp.0701564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYADAS T., WAGNER D.D., SIMPSON P.J. von Willebrand factor biosynthesis and partitioning between constitutive and regulated pathways of secretion after thrombin stimulation. Blood. 1989;73:706–711. [PubMed] [Google Scholar]
- MCEVER R.P., BECKSTEAD J.H., MOORE K.L., MARSHALL-CARLSON L., BAINTON D.F. GMP-140, a platelet α-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J. Clin. Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOCKRIDGE J.W., KUC R.E., HUSKISSON N.S., BARKER P.J., DAVENPORT A.P. Characterization of site-directed antisera against endothelin-converting enzymes (ECE-1α and ECE-1β) J. Cardiovasc. Pharmacol. 1998;31:S35–S37. doi: 10.1097/00005344-199800001-00012. [DOI] [PubMed] [Google Scholar]
- MOLDOVAN F., BENANNI H., FIET J., CUSSENOT O., DUMAS J., DARBORD C., SOLIMAN H.R. Establishment of permanent human endothelial-cells achieved by transfection with SV40 large T-antigen that retain typical phenotypical and functional-characteristics. In Vitro Cell. Dev. Biol. 1996;32:16–23. doi: 10.1007/BF02722989. [DOI] [PubMed] [Google Scholar]
- OPGENORTH T.J., WU-WONG J.R., SHIOSAKI K. Endothelin-converting enzymes. FASEB. J. 1992;6:2653–2659. doi: 10.1096/fasebj.6.9.1612289. [DOI] [PubMed] [Google Scholar]
- OZAKA T., DOI Y., KAYASHIMA K., FUJIMOTO S. Weibel-Palade bodies as a storage site of calcitonin gene-related peptide and endothelin-1 in blood vessels of the rat carotid body. Anat. Rec. 1997;247:388–394. doi: 10.1002/(SICI)1097-0185(199703)247:3<388::AID-AR10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- PLUMPTON C., ASHBY M.J., KUC R.E., O'REILLY G., DAVENPORT A.P. Expression of endothelin peptides and mRNA in the human heart. Clin. Sci. 1996;90:37–46. doi: 10.1042/cs0900037. [DOI] [PubMed] [Google Scholar]
- PLUMPTON C., HAYNES W.G., WEBB D.J., DAVENPORT A.P. Phosphoramidon inhibition of the in vivo conversion of big endothelin-1 to endothelin-1 in the human forearm. Br. J. Pharmacol. 1995;116:1821–1828. doi: 10.1111/j.1476-5381.1995.tb16669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG J.B., FOSTER P.A., KAUFMAN R.J., VOKAC E.A., MOUSSALLI M., KRONER P.A., MONTGOMERY R.R. Intracellular trafficking of factor VIII to von Willebrand factor storage grranules. J. Clin. Invest. 1998;101:613–624. doi: 10.1172/JCI1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL F.D., COPPELL A.L., DAVENPORT A.P. In vitro enzymatic processing of radiolabelled big ET-1 in human kidney. Biochem. Pharmacol. 1998a;55:697–701. doi: 10.1016/s0006-2952(97)00515-7. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., SKEPPER J.N., DAVENPORT A.P. Endothelin peptide and converting enzymes in human endothelium. J. Cardiovasc. Pharmacol. 1998b;31:S19–S21. doi: 10.1097/00005344-199800001-00008. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., SKEPPER J.N., DAVENPORT A.P. Human endothelial cell storage granules. A novel intracellular site for isoforms of the endothelin-converting enzyme. Circ. Res. 1998c;83:314–321. doi: 10.1161/01.res.83.3.314. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., SKEPPER J.N., DAVENPORT A.P. Evidence using immunoelectron microscopy for regulated and constitutive pathways in the transport and release of endothelin. J. Cardiovasc. Pharmacol. 1998d;31:424–430. doi: 10.1097/00005344-199803000-00014. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., SKEPPER J.N., CHENG X.M., AHN K., DAVENPORT A.P. Evidence for an intracellular endothelin converting enzyme in human endothelial cells. Nuanyn Schmiedebergs Arch. Pharmacol. 1998e;358:R710. [Google Scholar]
- SAKAMOTO Y., DOI Y., OHSATO K., FUJIMOTO S. Immunoelectron microscopy on the localization of endothelin in the umbilical vein of perinatal rabbits. Anat. Rec. 1993;237:482–488. doi: 10.1002/ar.1092370407. [DOI] [PubMed] [Google Scholar]
- SALEH D., FURUKAWA K., TSAO M.S., MAGHAZACHI A., CORRIN B., YANAGISAWA M., BARNES P.J., GIAID A. Elevated expression of endothelin-1 and endothelin-converting enzyme-1 in idiopathic pulmonary fibrosis: possible involvement of proinflammatory cytokines. Am. J. Resp. Cell. Mol. Biol. 1997;16:187–193. doi: 10.1165/ajrcmb.16.2.9032126. [DOI] [PubMed] [Google Scholar]
- SANSOM C.E., HOANG V.M., TURNER A.J. Molecular modeling of the active site of endotehlin-converting enzyme. J. Card. Pharmacol. 1995;26:S75–S77. [PubMed] [Google Scholar]
- SCHMIDT M., KRÖGER B., JACOB E., SEULBERGER H., SUBKOWSKI T., OTTER R., MEYER T., SCHMALZING G., HILLEN H. Molecular characterization of human and bovine endothelin converting enzyme (ECE-1) FEBS. Lett. 1994;356:238–243. doi: 10.1016/0014-5793(94)01277-6. [DOI] [PubMed] [Google Scholar]
- SCHWEIZER A., VALDENAIRE O., NELBÖCK P., DEUSCHLE U., DUMAS MILNE EDWARDS J.-B., STUMPF J.G., LÖFFLER B.-M. Human endothelin-converting enzyme (ECE-1): three isoforms with distinct subcellular localizations. Biochem. J. 1997;328:871–877. doi: 10.1042/bj3280871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMADA K., MATSUSHITA Y., WAKABAYASHI K., TAKAHASHI M., MATSUBARA A., IIJIMA Y., TANZAWA K. Cloning and functional expression of human endothelin-converting enzyme cDNA. Biochem. Biophys. Res. Commun. 1995;207:807–812. doi: 10.1006/bbrc.1995.1258. [DOI] [PubMed] [Google Scholar]
- SHINMI O., KIMURA S., SAWAMURA T., SUGITA Y., YOSHIZAWA T., UCHIYAMA Y., YANAGISAWA M., GOTO K., MASAKI T., KANAZAWA I. Endothelin-3 is a novel neuropeptide: isolation and sequence determination of endothelin-1 and endothelin-3 in porcine brain. Biochem. Biophys. Res. Commun. 1989;164:587–593. doi: 10.1016/0006-291x(89)91760-9. [DOI] [PubMed] [Google Scholar]
- SOKOLOVSKY M., GALRON R., KLOOG Y., BDOLAH A., INDIG F.E., BLUMBERG S., FLEMINGER G. Endothelins are more sensitive than sarafotoxins to neutral endopeptidase: possible physiological significance. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4702–4706. doi: 10.1073/pnas.87.12.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPORN L.A., MARDER V.J., WAGNER D.D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46:185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- SPORN L.A., MARDER V.J., WAGNER D.D. Differing polarity of the constitutive and regulated pathways for von Willebrand factor in endothelial cells. J. Cell. Biol. 1989;108:1283–1289. doi: 10.1083/jcb.108.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI M., FUKUDA K., SHIMADA K., BARNES K., TURNER A.J., IKEDA M., KOIKE H., YAMAMOTO Y., TANZAWA K. Localization of rat endothelin-converting enzyme to vascular endothelial cells and some secretory cells. Biochem. J. 1995;311:657–665. doi: 10.1042/bj3110657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI T., KANDA T., INOUE M., SUMINO H., KOBAYASHI I., IWAMOTO A., NAGAI R. Endothelin converting enzyme inhibitor protects development of right ventricular overload and medial thickening of pulmonary arteries in rats with monocrotaline-induced pulmonary hypertension. Life Sci. 1998;63:PL137–PL143. doi: 10.1016/s0024-3205(98)00347-6. [DOI] [PubMed] [Google Scholar]
- TONNESSEN T., NAESS P.A., KIRKEBOEN K.A., OFFSTAD J., ILEBEKK A., CHRISTENSEN G. Release of endothelin from the porcine heart after short-term coronary-artery occlusion. Cardiovasc. Res. 1993;27:1482–1485. doi: 10.1093/cvr/27.8.1482. [DOI] [PubMed] [Google Scholar]
- TSUKAHARA Y., MATSUMURA Y., KUNINOBU K., KOJIMA T., TAKAOKA M., MORIMOTO S. Phosphoramidon-sensitive endothelin converting enzyme in cultured vascular smooth muscle cells converts big endothelin-3 to endothelin-3. Life Sci. 1993;53:465–471. doi: 10.1016/0024-3205(93)90697-2. [DOI] [PubMed] [Google Scholar]
- TSURUMI Y., FUJIE K., NISHIKAWA M., KIYOTO S., OKUHARA M. Biological and pharmacological properties of highly selective new endothelin converting enzyme inhibitor WS79089B isolated from Streptosporangium roseum No. 79089. J. Antibiotics. 1995b;48:169–174. doi: 10.7164/antibiotics.48.169. [DOI] [PubMed] [Google Scholar]
- TSURUMI Y., UEDA H., HAYASHI K., TAKASE S., NISHIKAWA M., KIYOTO S., OKUHARA M. WS75624 A and B, new endothelin converting enzyme inhibitors isolated from Saccharothrix sp. No. 75624. J. Antibiotics. 1995a;48:1066–1072. doi: 10.7164/antibiotics.48.1066. [DOI] [PubMed] [Google Scholar]
- TURNER A.J. Endothelin-converting enzymes and other families of metallo-endopeptidases. Biochem. Soc. Transactions. 1993;21:697–701. doi: 10.1042/bst0210697. [DOI] [PubMed] [Google Scholar]
- TURNER A.J., MURPHY L.J. Molecular Pharmacology of endothelin converting enzymes. Biochem. Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- VALDENAIRE O., ROHRBACHER E., MATTEI M.-G. Organization of the gene encoding the human endothelin-converting enzyme (ECE-1) J. Biol. Chem. 1995;270:29794–29798. doi: 10.1074/jbc.270.50.29794. [DOI] [PubMed] [Google Scholar]
- VEMULAPALLI S., CHINTALA M., STAMFORD A., WATKINS R., CHIU P., SYBERTZ E., FAWZI A.B. Renal effects of SCH 54470: a triple inhibitor of ECE, ACE, and NEP. Cardiovasc. Drug Rev. 1997;15:260–272. [Google Scholar]
- WAGNER D.D., OLMSTED J.B., MARDER V.J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J. Cell. Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER O.F., CHRIST G., WOJTA J., VIERHAPPER H., PARZER S., NOWOTNY P.J., SCHNEIDER B., WALDHÄUSL W., BINDER B.R. Polar secretion of endothelin-1 by cultured endothelial cells. J. Biol. Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- WALLACE E.M., MOLITERNI J.A., MOSKAL M.A., NEUBERT A.D., MARCOPULOS N., STAMFORD L.B., TRAPANI A.J., SAVAGE P., CHOU M., JENG A.Y. Design and synthesis of potent, selective inhibitors of endothelin-converting enzyme. J. Med. Chem. 1998;41:1513–1523. doi: 10.1021/jm970787c. [DOI] [PubMed] [Google Scholar]
- WAXMAN L., DOSHI K.P., GAUL S.L., WANG S., BEDNAR R.A., STERN A.M. Identification and characterization of endothelin converting activity from EAHY 926 cells: evidence for the physiologically relevant human enzyme. Arch. Biochem. Biophys. 1994;308:240–253. doi: 10.1006/abbi.1994.1034. [DOI] [PubMed] [Google Scholar]
- WEIBEL E.R., PALADE G.E. New cytoplasmic components in arterial endothelia. J. Cell. Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU D., EMOTO N., GIAID A., SLAUGHTER C., KAW S., DEWIT D., YANAGISAWA M. ECE-1: A membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA H., YANAGISAWA M., KAPUR R.P., RICHARDSON J.A., WILLIAMS S.C., CLOUTHIER D.E., DE WIT D., EMOTO N., HAMMER R.E. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- YORIMITSU K., MOROI K., INAGAKI N., SAITO T., MASUDA Y., MASAKI T., SEINO S., KIMURA S. Cloning and sequencing of a human endothelin converting enzyme in renal adenocarcinoma (ACHN) cells producing endothelin-2. Biochem. Biophys. Res. Commun. 1995;208:721–727. doi: 10.1006/bbrc.1995.1397. [DOI] [PubMed] [Google Scholar]
- YOSHIMOTO S., ISHIZAKI Y., SASAKI T., MUROTA S. Effect of carbon dioxide and oxygen on endothelin production by cultured porcine cerebral endothelial cells. Stroke. 1991;22:378–383. doi: 10.1161/01.str.22.3.378. [DOI] [PubMed] [Google Scholar]
- YOSHITOMI Y., KOJIMA S., OGI M., KURAMOCHI M. Acute renal failure in accidental hypothermia of cold water immersion. Am. J. Kid. Diseases. 1998;31:856–859. doi: 10.1016/s0272-6386(98)70057-5. [DOI] [PubMed] [Google Scholar]
- YU J.C.M., DAVENPORT A.P. Secretion of endothelin-1 and endothelin-3 by human cultured vascular smooth muscle cells. Br. J. Pharmacol. 1995;114:551–557. doi: 10.1111/j.1476-5381.1995.tb13262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


