Abstract
The aim of our study is to clarify the relationship between expression pattern of P2X receptors and the cell type of male adult rat (Wistar) dorsal root ganglion (DRG) neurons. We identified the nociceptive cells of acutely dissociated DRG neurons from adult rats type using capsaicin sensitivity.
Two types of ATP-activated currents, one with fast, the other with slow desensitization, were found under voltage-clamp conditions. In addition, cells with fast but not slow desensitization responded to capsaicin, indicating that there was a relationship between current kinetics and capsaicin-sensitivity.
Both types of neurons were responsive to ATP and α, β methylene-ATP (α,βmeATP). The concentration of α,βmeATP producing half-maximal activation (EC50) of neurons with fast desensitization was less (11 μM) than that of neurons with slow desensitization (63 μM), while the Hill coefficients were similar. Suramin and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium (PPADS) antagonized α,βmeATP-induced currents in both types of neurons.
In situ hybridization revealed that small cells of the DRG predominantly expressed mRNAs of P2X3 and medium-sized cells expressed mRNAs of P2X2 and P2X3. In contrast, both of mRNAs were not detected in large cells of the DRG.
These results suggest that capsaicin-sensitive, small-sized DRG neurons expressed mainly the homomeric P2X3 subunit and that capsaicin-insensitive, medium-sized DRG neurons expressed the heteromultimeric receptor with P2X2 and P2X3.
Keywords: Dorsal root ganglia, P2X, ATP, capsaicin, patch-clamp, in situ hybridization, pain
Introduction
Extracellular ATP opens ligand-gated cation channels (P2X receptors) in neuronal preparations (Suprenant et al., 1995). In sensory neurons, the properties of ATP-gated cation channels have been extensively studied using electrophysiological approaches since the early 1980's (Jahr & Jessell, 1983; Krishtal et al., 1983; 1988; Bean, 1990). Recently, the P2X receptors have been cloned into seven subunits (P2X1–P2X7) (Soto et al., 1997). One of these, the P2X3 receptor subunit, was cloned from a rat DRG cDNA library (Lewis et al., 1995; Chen et al., 1995) and expressed in capsaicin-sensitive, small-sized DRG neurons (Chen et al., 1995), suggesting that ATP might be involved in the transduction of pain via P2X receptors.
ATP-evoked currents in heterologously expressed P2X3 receptor showed rapid desensitization, whereas P2X2 receptor showed slow desensitization under voltage-clamp conditions. The sensitivity of P2X receptors to the ATP analogue, α,β-methylene ATP (α,βmeATP) is also different from homomeric P2X receptors. α,βmeATP can evoke a rapidly desensitizing current in homomeric P2X3 receptors but evokes no response in homomeric P2X2 receptors. Although two different P2X subtypes among the P2X1–P2X4 subunits were coexpressed in human embryonic kidney (HEK) 293 cells, only a combination of the P2X2 and P2X3 subtypes resulted in functional ligand-gated channels. This heteromer of P2X2 and P2X3 (P2X2+3) showed distinct functional properties from homomeric P2X2 or P2X3 channels as regards agonist sensitivity, desensitization kinetics and Ca2+ influx (Lewis et al., 1995; Ueno et al., 1998; Virginio et al., 1998). These properties of P2X2+3 were close to those observed in nodose ganglion neurons (Lewis et al., 1995). In fact, based on the early electrophysiological studies, ATP evoked non-desensitizing currents in sensory neurons (Krishtal et al., 1988; Bean et al., 1990; Khakh et al., 1995). Thus, the majority of native ATP-gated channels in sensory neurons were thought to be the P2X2+3. However, controversial data were reported using cultured DRG neurons from neonatal rats and a part of the nociceptors in trigeminal ganglion neurons, indicating that ATP and α,βmeATP evoked rapidly desensitizing currents (Robertson et al., 1996; Cook et al., 1997a,1997b). These current properties were similar to those recorded in homomeric P2X3 receptors. Additionally, nociceptors in trigeminal ganglion showed two types of currents with respect to the desensitizing kinetics (Cook et al., 1997a,1997b). These data suggest that there are several types of neurons with different expression of P2X subunits. However, there is no report which clearly demonstrate the relationship between current properties and cell characterization in DRG neurons.
In the present study using acutely dissociated DRG neurons from adult rats, capsaicin was used to discriminate between nociceptive and non-nociceptive DRG neurons under voltage-clamp conditions. We observed two types of the ATP-evoked responses in DRG neurons and clarified the relationship between the properties of ATP-evoked responses and the cell types according to cell size and responsiveness to capsaicin. Furthermore, the differential distribution of mRNAs for P2X2 and P2X3 in the DRG was confirmed using in situ hybridization histochemistry. These findings revealed that the character of ATP-activated responses in DRG neurons was dependent on the cell-type, and provided the first evidence that the P2X3 and P2X2+3 receptors can function in a subset of nociceptive and non-nociceptive cells, respectively, in the DRG.
Methods
DRG neuron isolation
Wistar rats (8-weeks-old) were decapitated under ether anaesthesia and the DRG were removed from the L4-6 segments. The DRG were treated first with 20 unit ml−1 papain (Worshington Biochemical Co. Freehold, NJ, U.S.A.) dissolved in Tyrode's solution for 10 min at 37°C. The tissue was then treated with 4 mg ml−1 collagenase typeII (CLS2; Worshington Biochemical Co.) and 2.5 unit ml−1 Dispase (Calbiochem, La Jolla, CA, U.S.A.) dissolved in Tyrode's solution for 60 min at 37°C. At the end of this treatment, the enzyme solution was removed and the cells were then mechanically dissociated by trituration through a pasteur pipette. Cells were plated on 35 mm polystyrene dishes for physiological experiments.
Electrical recording
Recordings were made using the conventional whole cell patch-clamp method (Hamill et al., 1981). All experiments were performed at room temperature (21–23°C). The pipette solution contained (in mM): CsCl 140, MgCl2 2, EGTA 5, HEPES 10, pH adjusted to 7.2 with CsOH. The pipette resistance was 2 to 5 MΩ. Series resistance (2–8 MΩ) and cell capacitance (12 to 80 pF) were compensated up to 80%. Currents were filtered at 300 Hz with a 8-pole Bessel filter (Frequency Devices, Haverhill, MA, U.S.A.) and measured with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, U.S.A.). Data were then sampled at 1 kHz and stored on-line with a 486 PC using pClamp (Axon Instruments). Drugs were dissolved in an external solution of the following composition (in mM): NaCl 150, KCl 5, CaCl2 2, MgCl2 1, D-glucose 10, HEPES 10, pH adjusted to 7.4 with NaOH. The solutions of drugs were applied with a polyethylene Y-tube (equilibration time <20 ms) (Ueno et al., 1997). The bath was continuously perfused with normal external solution from a separate perfusion line, and the solution was removed from the bath with a Leiden aspirator (Medical Systems, Greenvale, NY, U.S.A.). Concentration-effect curves were fitted with Hill equation:
where I is the current elicited by the ATP concentration X,Imax is the fitted maximal current, EC50 is the concentration producing a half maximal response, and n is the Hill coefficient.
In situ hybridization
The following antisense oligonucleotides were used as probes for in situ hybridization, and these were complementary to nucleotide residues 2400–2444 of the rat P2X2 cDNA (Y09910) and 1202–1246 of the rat P2X3 cDNA (X91167); 5′-ttatggctgtagagcttgtttttgttcatgaacgttaacaaaatc-3′ for P2X2 and 5′-caaacttcctggctttgtagtgatcagcccctttgaggaaattga-3′ for P2X3. These oligonucleotides were labelled with 35S-dATP using terminal deoxyribonucleotidyl transferase (BRL, Gaithersburg, MD, U.S.A.) at a specific activity of 0.5×109 d.p.m. μg−1 DNA. Male Wistar rats, weighing approximately 200 g, were used. Under pentobarbitone anaesthesia at a lethal dose, the DRG were freshly removed and frozen in powdered dry ice. Cryostat sections, 20 μm in thickness, were prepared and mounted on glass slides precoated with 3-aminopropyltriethoxysilane. They were fixed with 4% paraformaldehyde for 10 min and acetylated for 10 min with 0.25% acetic anhydride in 0.1 M triethanolamine-HCl (pH 8.0). The sections were prehybridized for 1 h in a buffer containing 50% formamide 0.1 M Tris-HCl (pH 7.5), 4×SSC (1×SCC; 150 mM NaCl and 15 mM sodium citrate), 0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.02% bovine serum albumin, 0.6 M NaCl, 0.25% sodium dodecyl sulphate (SDS), 200 μg ml−1 tRNA, 1 mM EDTA, and 10% dextran sulphate. Hybridization was performed at 42°C for 10 h in the prehybridization buffer supplemented with 10,000 c.p.m. μl−1 of 33P-labelled oligonucleotide probes. The slides were washed at room temperature for 20 min in 2×SSC containing 0.1% sarkosyl and twice at 55°C for 40 min in 0.1×SSC containing 0.1% sarkosyl. The sections were dipped in Kodak NTB2 nuclear track emulsion and exposed for 2 months.
Drugs
Drugs used were ATP (Sigma), α,βmeATP (Sigma), capsaicin, suramin (Wako Pure Chemistry, Osaka, Japan) and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium (PPADS) (RBI, Natick, MA, U.S.A.). The pH of the solutions containing ATP or α,βmeATP was readjusted to 7.4 with NaOH.
Statistics
The reported probabilities for significant differences were obtained using the paired Student's t-test.
Results
There were two types of ATP-activated currents in dissociated DRG neurons and they showed rapid or slow desensitization kinetics. We found a mutual relevance between the properties of the desensitization kinetics and the capsaicin sensitivity. In Figure 1a and b, the typical current responses to ATP, α,βmeATP and capsaicin are shown in these two subpopulations of DRG neurons. DRG neurons with rapidly desensitizing currents were also responsive to capsaicin, while neurons with slow desensitizing ones were not. In our experiments, a cell was classified as capsaicin-sensitive neuron if there was an inward current in response to 3 μM capsaicin. Capsaicin (3 μM) evoked a rapid activation followed by a very slowly desensitizing inward current, and the value of the peak amplitude was −786±148 pA (mean±s.e.mean, n=12). Capsaicin-sensitive neurons tested were small and medium size in diameter (21–37 μm). ATP produced the rapidly desensitizing currents in about 70% of the capsaicin-sensitive neurons tested (12/17). Capsaicin-insensitive neurons usually possessed medium and large size in diameter (29–63 μm). About 48% of capsaicin-insensitive neurons tested (10/21) responded to both ATP and α,βmeATP and these cells showed medium size in diameter (29–45 μm). Especially, all large-sized cells (>48 μm) had no sensitivity to ATP and α,βmeATP (Figure 1c). The application of ATP in capsaicin-insensitive neurons evoked a rapid activation followed by a sustained inward current. With respect to desensitization kinetics, in capsaicin-sensitive neurons, the rapid desensitization of the α,βmeATP-induced current (100 μM) was well fitted to two exponential components, while in capsaicin-insensitive ones, it was well fitted to one exponential component. There were significant differences in the cell size and time constant for desensitization (τd) between the capsaicin-sensitive and capsaicin-insensitive neurons, but not in the peak amplitude induced by each agonist (Table 1). Capsaicin-sensitive neurons were also small in diameter, suggesting that these cells were nociceptive neurons. α,βmeATP-induced currents (10 μM) in both types of neurons were completely inhibited by suramin (30 μM) (n=3 for each type of neurons) or PPADS (10 μM) (n=3 for each type of neurons).
Figure 1.

Responses of cells to ATP (10 μM), α,βmeATP (10 μM) and capsaicin (3 μM). (a) Representative current traces for capsaicin-sensitive neurons. (b) Representative current traces for capsaicin-insensitive neurons. (c) Representative current traces for large-sized (>50 μm) neurons. Data in a, b and c were obtained from a single cell, respectively. The application of agonists is indicated by bars above each record. Cells were voltage-clamped to −50 mV.
Table 1.
Properties of agonists-induced responses in capsaicin-sensitive and -insensitive cells

When α,βmeATP (30 μM) was applied twice with an interval of 5 min, the current responses of capsaicin-sensitive and capsaicin-insensitive neurons reacted in a different manner. The α,βmeATP-evoked currents in capsaicin-sensitive neurons dramatically ran down at the second application of agonist (Figure 2a). In contrast, the α,βmeATP-evoked currents in capsaicin-insensitive neurons retained its responsiveness to repeated applications of α,βmeATP (Figure 2b).
Figure 2.

Effects of repeated applications of α,βmeATP. Typical current responses to α,βmeATP (30 μM) in capsaicin-sensitive (a) or -insensitive (b) neurons at every 5 min. Horizontal solid bars show the application of 30 μM α,\βmeATP.
We examined the concentration-response relationship for ATP activation in capsaicin-sensitive and capsaicin-insensitive DRG neurons (Figure 3). The capsaicin-sensitive neurons showed an EC50 value of 5.6 μM and a Hill coefficient of 1.0. For the capsaicin-insensitive neurons, the EC50 value and Hill coefficient were 23.7 and 1.0, respectively.
Figure 3.

ATP concentration-response relationship for capsaicin-sensitive and -insensitive DRG neurons. (a) The currents in response to concentrations of ATP from 1 μM to 1 mM are shown for capsaicin-sensitive (upper panel) and capsaicin-insensitive (lower panel) neurons. (b) Concentration-response curves for ATP-elicited currents are plotted. The peak current elicited by ATP application was measured and this response was normalized to the response of the same cell to 10 μM α,βmeATP for capsaicin-sensitive neurons (○; mean±s.e.mean for data from four to six cells) or to 100 μM ATP for capsaicin-insensitive neurons (□; mean±s.e.mean for data from four to six cells). The mean normalized response was calculated for each concentration applied.
We also examined the concentration-response relationship for α,βmeATP activation for both types of DRG neurons since α,βmeATP is a relatively selective agonist for P2X receptors consisting of the P2X3 subunit. The capsaicin-sensitive neurons showed an EC50 value of 10 μM and a Hill coefficient of 0.9. The capsaicin-insensitive neurons showed an EC50 value of 66 μM and a Hill coefficient of 1.0 (Figure 4).
Figure 4.

α,βmeATP concentration-response relationship for capsaicin-sensitive and -insensitive DRG neurons. (a) The currents in response to concentrations of α,βmeATP from 1 μM to 1 mM are shown for capsaicin-sensitive (upper panel) and capsaicin-insensitive (lower panel) neurons. (b) Concentration-response curves for α,βmeATP-elicited currents are plotted. The peak current elicited by the α,βmeATP applications was measured and this response was normalized to the response of the same cell to 10 μM α,βmeATP for capsaicin-sensitive neurons (•; mean±s.e.mean for data from four to nine cells) or to 100 μM α,βmeATP for capsaicin-insensitive neurons (▪; mean±s.e.mean for data from four to seven cells). The mean normalized response was calculated for each concentration applied. The smooth lines show the values predicted by the Hill equation (1).
In situ hybridization analysis using antisense oligonucleotide probes specific for either P2X2 or P2X3 mRNA exhibited consistent labellings in the neuronal somata of the DRG. The specificity of hybridization was confirmed by the disappearance of the signals when an excess dose of the corresponding cold probes was added into the hybridization fluid. DRG are composed of neurons showing various sizes, which are largely classified into small cells less than 25 μm, large cells greater than 35 μm, and medium-sized cells around 30 μm in diameter. Small cells were labelled only for P2X3 mRNA and medium-sized cells were labelled both for P2X2 and P2X3 mRNAs. The intense labelling for P2X3 mRNA was found in both small cells and medium-sized cells, but not in the large cells. On the other hand, signals for P2X2 mRNA were localized exclusively in medium-sized cells.
Discussion
In the present study, we used acutely dissociated DRG neurons from adult rats and recorded the responses via P2X receptors under voltage-clamp conditions. Thus, functional receptors that are expressed on somata in DRG neurons were detected. About 70% of the capsaicin-sensitive neurons tested were responsive to both ATP and α,βmeATP. Furthermore, these agonists evoked rapidly desensitizing currents in this type of neuron. Capsaicin selectively activates nociceptors and causes burning pain and neurogenic inflammation in vivo (Simone et al., 1989). The effects of capsaicin are largely restricted to subpopulation of DRG neurons with small cell bodies in vitro (Holzer, 1991). These are presumably nociceptive neurons with unmyelinated (C) fibres or some thinly myelinated (Aδ) fibres. In addition, capsaicin-activated cation channels have been cloned recently and an in situ hybridization study has demonstrated that these vanilloid receptors are located in small-sized DRG neurons (Caterina et al., 1997). The sizes of the capsaicin-sensitive neurons in our preparation were also consistent with these results. Therefore, in the present study, the ATP- or α,βmeATP-evoked responses in the capsaicin-sensitive neurons can be assumed to the expression pattern of P2X receptors in nociceptors.
The ATP-activated current in the capsaicin-sensitive neurons rapidly desensitized. Although the homomeric expression of P2X1 subunit also showed rapidly desensitizing current, the desensitization of the P2X1 subunit can be fitted to a single exponential decay (Collo et al., 1996). In contrast, the desensitization kinetics of the P2X3 subunit were bi-exponential (Lewis et al., 1995). In the present study, the currents elicited by α,βmeATP in the capsaicin-sensitive neurons exhibited a bi-exponential decay. Additionally, the concentration-response curve for this type of neuron was similar to that showing P2X3 expression alone (Lewis et al., 1995; Ueno et al., 1998), suggesting that homomeric P2X3 receptors mainly express in capsaicin-sensitive neurons.
What do P2X subunits contribute to the ATP-activated response in capsaicin-insensitive DRG neurons? The ATP-activated currents in capsaicin-insensitive neurons were the slowly desensitizing type. Especially, the medium-sized cells of the capsaicin-insensitive neurons responded to applications of ATP and α,βmeATP, and the currents evoked by these agonists (10 μM) were completely blocked by suramin and PPADS at a concentration of 30 μM. The α,βmeATP-evoked currents (up to 30 μM) in the capsaicin-insensitive neurons showed sustained current kinetics in the continuous presence of the agonist. On the basis of the characterization of heterologously expressed P2X receptors, these current kinetics and pharmacological properties are in agreement with the coexpression of the P2X2 and P2X3 subunits (Ueno et al., 1998). Although homomeric P2X2 receptor is insensitive to α,βmeATP, P2X2+3 showed slowly desensitizing current in response to both ATP and α,βmeATP (Lewis et al., 1995; Ueno et al., 1998). However, because in situ hybridization data demonstrated the six mRNAs of the P2X receptors (P2X1–P2X6) in DRG (Collo et al., 1996), other subunits might be involved in the ATP- or α,βmeATP-evoked responses in capsaicin-insensitive neurons. The P2X4 subunit was detected as one of the major subunits expressed in DRG (Lewis et al., 1995). The expression studies of the homomeric P2X4 subunit revealed pharmacological properties distinctly different from the P2X2 and P2X3 subunits. The EC50 value for ATP activation was around 10 μM, and α,βmeATP produced less than 10% of the ATP-activated current in the P2X4 subunit. The current evoked by ATP was almost insensitive to the P2X antagonists, suramin and PPADS. The inhibitory concentration of 50% (IC50) values of these antagonists ranged from >100 μM to >500 μM (Bo et al., 1995; Buell et al., 1996; Seguela et al., 1996; Soto et al., 1996). In contrast, our results were not consistent with the homogenous expression of P2X4. Therefore, the involvement of the P2X4 subunit in the slowly desensitizing current of the capsaicin-insensitive neurons is less extensive than that of other subunits. Consequently, it is most likely the P2X2+3 that is mainly expressed in the capsaicin-insensitive neurons. This type of DRG neuron showed a larger diameter than that of neurons with rapidly desensitizing current. The cell size ranged from 28–48 μm. DRG cells of this size largely consisted of neurons with myelinated A-fibre (Harper & Lawson, 1985). In the present experiments, diameter of dissociated cells seems to be a little larger than that of corresponding cell subpopulation observed in in situ hybridization. The difference is probably due to the fact that dissociated cells tends to enlarge the size by effects of dissociation damage and that diameter of cross-section does not always show the maximum value.
The current kinetics evoked by α,βmeATP and the sensitivity to the P2X antagonist in the capsaicin-insensitive neurons were consistent with the properties of P2X2+3, whereas the concentration-response relationship for α,βmeATP was not identical with data observed in P2X2+3 and nodose ganglion neurons (Lewis et al., 1995). The value of the EC50 for α,βmeATP in the capsaicin-insensitive neurons was about ten times larger. The coexpression of P2X2 and P2X3 subunits could make several subsets of heteromeric receptors with unknown stoichiometries. Indeed, the blocking effect of trinitrophenyl-ATP on nodose ganglion neurons and P2X2+3 suggested the existence of heteromeric channels having various combinations of the P2X2 and P2X3 subunits (Thomas et al., 1998). In addition, our heterologous expression study using the C6BU-1 cell line provided a larger EC50 value for α,βmeATP than that observed when P2X2 and P2X3 subunits were coexpressed (Ueno et al., 1998). However, we can not exclude the possibility that other subunits such as P2X1 and P2X4 affect the concentration-response relationship for agonists.
Recently, specific antisera to P2X2 and P2X3 have been developed (Vulchanova et al., 1996; 1997). Using these antisera, immunohistochemical analysis has clarified the differential distribution of these immunoreactivities in the cell bodies of DRG neurons. The existence of these signals was restricted to a specific subpopulation of the DRG. The P2X3 immunoreactivity was detected predominantly in small neurons (Vulchanova et al., 1997). As was the case with our in situ hybridization analysis, the P2X3 signals were also extensively labelled in small-sized neurons. These results provide supporting evidence that the homomeric P2X3 receptor is expressed in nociceptive neurons with unmyelinated fibres. As for the distribution of P2X2, the overall intensity for P2X2 immunoreactivity in the DRG was stronger than that of P2X3 (Vulchanova et al., 1997). In our in situ hybridization analysis, the P2X2 signal was not detected in small-sized neurons. Electrophysiological data also supported that the P2X2 receptor functions in medium-sized neurons. Taken together the present results, P2X2 is expressed in neurons with myelinated fibres, which are not related to nociception. The expression pattern of P2X receptors was different in each subpopulation of DRG neurons. However, the physiological significance of this differential expression of P2X receptors in the DRG was not clarified. It will be necessary to perform further investigations using behavioural approaches in vivo.
Two types of ATP-activated current were observed in acutely dissociated DRG neurons under the voltage clamp condition. The morphological differences and capsaicin sensitivity between two subpopulations provide an indication that capsaicin-sensitive DRG neurons expressed mainly homomeric P2X3 subtype and capsaicin-insensitive neurons expressed heteromultimeric complex of P2X2 and P2X3 subtypes.
Figure 5.
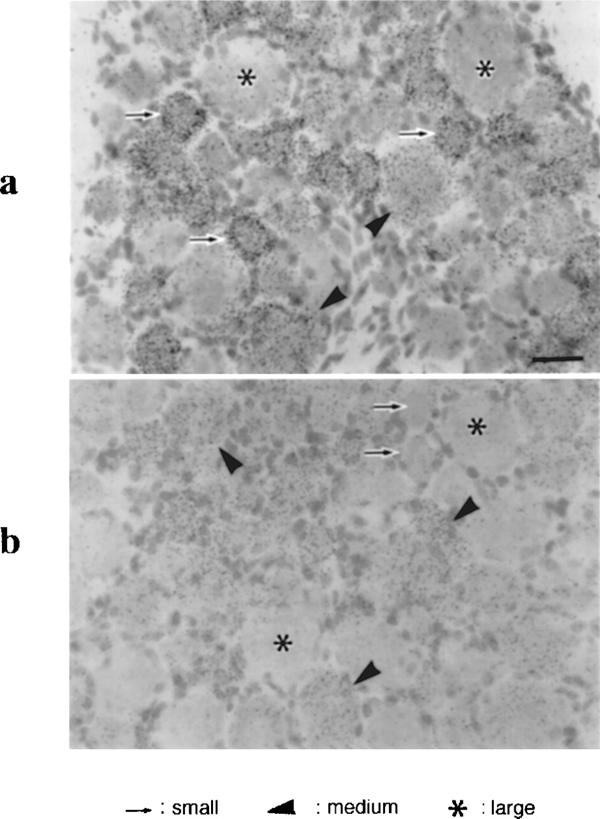
Bright-field micrographs showing signals for the P2X3 (a) and P2X2 (b) mRNA. In the dorsal root ganglion, intense signals of P2X3 are seen in small (arrows) and medium-sized ganglion cells (arrowheads), but not in large cells (asterisks) which are more than 40 μm in diameter. Significant signals for P2X2 were found in medium-sized cell bodies (arrowheads), but not in small (arrows) and large cell bodies (asterisks).
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (No. 08457416) from the Ministry of Education, Science and Culture of Japan and Domestic research Fellowship from Japan Science and Technology Corporation.
Abbreviations
- α,βmeATP
α,β-methylene ATP
- DRG
dorsal root ganglia
- HEK
human embryonic kidney
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium
References
- BEAN B.P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J. Neurosci. 1990;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BO X., ZHANG Y., NASSAR M., BURNSTOCK G., SCHOEPFER R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- BUELL G., LEWIS C., COLLO G., NORTH R.A., SUPRENANT A. An antagonist-insensitive P-2x receptor expressed in epithelial and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHEN C.C., AKOPIAN A.N., SIVILOTTI L., COLQUHOUN D., BURNSTOCK G., WOOD J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK S.P., MCCLESKEY E.W. Desensitization, recovery and Ca2+-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacol. 1997a;36:1303–1308. doi: 10.1016/s0028-3908(97)00132-9. [DOI] [PubMed] [Google Scholar]
- COOK S.P., VULCHANOVA L., HARGREAVES K.M., ELDE R., MCCLESKEY E.W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997b;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARPER A.A., LAWSON S.N. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J. Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- JAHR C.E., JESSELL T.M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304:730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., HUMPHREY P.P., SUPRENANT A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. J. Physiol. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHTAL O.A., MARCHENKO S.M., OBUKHOV A.G., VOLKOVA T.M. Receptors for ATP in rat sensory neurones: the structure-function relationship for ligands. Br. J. Pharmacol. 1988;95:1057–1062. doi: 10.1111/j.1476-5381.1988.tb11739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHTAL O.A., MARCHENKO S.M., PIDOPLICHKO V.I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci. Lett. 1983;35:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- LEWIS C., NEIDHART S., HOLY C., NORTH R.A., BUELL G., SUPRENANT A. Coexpression of P2X(2) and P2X(3) receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- ROBERTSON S.J., RAE M.G., ROWAN E.G., KENNEDY C. Characterization of a P2X-purinoceptor in cultured neurons of the rat dorsal root ganglia. Br. J. Pharmacol. 1996;118:951–956. doi: 10.1111/j.1476-5381.1996.tb15491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGUELA P., HAGHIGHI A., SOGHOMONIAN J.J., COOPER E. A novel neuronal P2x ATP receptor ion channel with widespread distribution in the brain. J. Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMONE D.A., BAUMANN T.K., LAMOTTE R.H. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- SOTO F., GARCIA-GUZMAN M., GOMEZ-HERNANDEZ J.M., HOLLMANN M., KARSCHIN C., STUHMER W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOTO F., GARCIA-GUZMAN M., STUHMER W. Cloned ligand-gated channels activated by extracellular ATP (P2X receptors) J. Memb. Biol. 1997;160:91–100. doi: 10.1007/s002329900298. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., BUELL G., NORTH R.A. P2X receptors bring new structure to ligand-gated ion channels. Trends. Neurosci. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- THOMAS S., VIRGINIO C., NORTH R.A., SURPRENANT A. The antagonist trinitrophenyl-ATP reveals co-existence of distinct P2X receptor channels in rat nodose neurons. J. Physiol. 1998;509:411–417. doi: 10.1111/j.1469-7793.1998.411bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UENO S., BRACAMONTES J., ZORUMSKI C., WEISS D.S., STEINBACH J.H. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J. Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UENO S., KOIZUMI S., INOUE E. Characterization of Ca2+ influx through recombinant P2X receptor in C6BU-1 cells. Br. J. Pharmacol. 1998;124:1484–1490. doi: 10.1038/sj.bjp.0701963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRGINIO C., NORTH R.A., SURPRENANT A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in nodose neurones. J. Physiol. (Lond.) 1998;510:27–35. doi: 10.1111/j.1469-7793.1998.027bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., WANG J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULCHANOVA L., REIDL M.S., SHUSTER S.J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacol. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]


