Abstract
The modulatory effects of extracellular H+ and Zn2+ were tested against ATP-responses at rat P2X4 (rP2X4) receptors expressed in Xenopus oocytes under voltage-clamp conditions.
ATP (0.1–100 μM, at pH 7.5), evoked inward currents via rP2X4 receptors (EC50 value, 4.1±0.98 μM; nH, 1.2±0.1). ATP potency was reduced 2 fold, at pH 6.5, without altering maximal activity. ATP potency was reduced by a further 4 fold, at pH 5.5, and the maximal activity of ATP was also reduced. Alkaline conditions (pH 8.0) had no effect on ATP-responses.
Zn2+ (100 nM–10 μM) potentiated ATP-responses at the rP2X4 receptor by 2 fold, whereas higher concentrations (30 μM–1 mM) inhibited ATP-responses. Zn2+ potentiation was due to an increase in ATP potency, whereas its inhibitory action was due to a reduction in ATP efficacy.
Zn2+ modulation of ATP-responses was pH-dependent. At pH 6.5, the bell-shaped curve for Zn2+ was shifted to the right by 1 log unit. At pH 5.5, Zn2+ potentiation was abolished and its inhibitory effect reduced considerably.
Suramin (50 μM) also potentiated ATP-responses at rP2X4 receptors. Neither H+ (pH 6.5 and 5.5), Zn2+ (10–100 μM) or a combination of both failed to reveal an inhibitory action of suramin at rP2X4 receptors.
In conclusion, H+ and Zn2+ exerted opposite effects on the rP2X4 receptor by lowering and raising agonist potency, respectively. H+ (⩾3 μM) and Zn2+ (⩾30 μM) also reduces agonist efficacy by lowering the number of rP2X4 receptors available for activation. The striking differences between the modulatory actions of H+ and Zn2+ at rP2X4 and rP2X2 receptors are discussed.
Keywords: Extracellular pH, zinc, ATP, P2X receptor, Xenopus oocyte
Introduction
Adenosine 5′-triphosphate (ATP) can act as a fast excitatory transmitter at neuronal P2X receptors in the central, peripheral and enteric nervous systems (Edwards et al., 1992; Evans et al., 1992; Silinsky & Gerzanich, 1993; Galligan & Bertrand, 1994; Sperlagh et al., 1995; Bardoni et al., 1997; Nieber et al., 1997). So far, seven P2X receptor subunits (P2X1–7) have been identified (North & Barnard, 1997), although the recently-cloned human P2XM subunit may possibly represent the eighth member (Urano et al., 1997). Apart from P2X7, transcripts for other P2X subunits have been localized in neuronal tissues.
The P2X4 receptor subunit is concentrated in mammalian nervous systems and, along with P2X2 and P2X6, represent the more common P2X subunits found in adult neural tissues (Bo et al., 1995; Buell et al., 1996; Collo et al., 1996; Séguéla et al., 1996; Soto et al., 1996; Wang et al., 1996; Dhulipala et al., 1998; Lê et al., 1998). Homomeric P2X4 receptors are characterized by a low sensitivity to P2 receptor antagonists, PPADS (pyridoxal-α5-phosphate-6-azophenyl-2′,4′-disulphonic acid) and suramin. The blocking activity of PPADS and suramin is greater at human P2X4 (hP2X4) than the rat homologue (rP2X4), yet still lower than at most other human and rat P2X receptor subtypes (Garcia-Guzman et al., 1997). The recombinant rP2X6 receptor, however, is also insensitive to PPADS and suramin (Collo et al., 1996).
Previously, we have shown that the activity of agonists and antagonists at one neuronal P2X receptor subtype, rP2X2, is exceedingly sensitive to changes to extracellular pH (King et al., 1996, 1997). The concentration-response (C/R) curve for ATP (and other agonists) was shifted leftwards under acidic conditions and rightwards under alkaline conditions, without changing the maximal activity of the agonist. Even small changes in pH (⩾0.03 pH units) significantly altered the amplitude of ATP-responses at rP2X2 (Wildman et al., 1997). Additionally, the blocking activity of suramin was greatly enhanced at rP2X2 under acidic conditions and declined under alkaline conditions (King et al., 1997). Extracellular zinc (Zn2+) also potentiated agonist and antagonist activity at rP2X2 receptors (Brake et al., 1994; Nakazawa & Ohno, 1996, 1997; Wildman et al., 1998). However, Zn2+ modulation of agonist activity at rP2X2 receptors is more complex than the corresponding H+ modulation, since the former shows time-dependency and converts to inhibition after prolonged exposure while the latter is constant, time-independent and can overcome Zn2+ inhibition (Wildman et al., 1998).
ATP-responses at rat and human P2X4 receptors are also affected by extracellular pH (Stoop et al., 1997; Clarke et al., 1998), but in a different way to rP2X2 receptors. For these P2X4 homologues, acidic and alkaline conditions respectively reduced and enhanced ATP-responses although the precise actions on the potency and efficacy of ATP remain to be determined. It has also been reported that ATP activity is potentiated by Zn2+ at rat and human P2X4 receptors (Séguéla et al., 1996; Soto et al., 1996; Garcia-Guzman et al., 1997; Nakazawa & Ohno, 1997) although there is no information on how ATP potency and efficacy is altered. Additionally, it remains to be shown if Zn2+-potentiation of agonist activity is time-dependent, as for P2X2, and how Zn2+ and H+ interact at P2X4 receptors. In the present study, therefore, we describe the separate effects of extracellular pH and Zn2+ and their joint interaction on both agonist and antagonist activity at a neuronal P2X receptor subunit, the rP2X4 subtype. The striking differences between the modulatory effects of H+ and Zn2+ at rP2X4 and rP2X2 receptors are discussed.
Methods
Oocyte preparation
Xenopus laevis frogs were anaesthetized in Tricaine (0.2% w/v), killed by decapitation, and ovarian lobes surgically removed. Oocytes (stages V and VI) were defolliculated by a 2-step process involving collagenase treatment (Type IA, 2 mg ml−1 in a Ca2+-free Ringer's solution, for 2–3 h) followed by stripping away the follicular layer with fine forceps. Defolliculated oocytes were stored in Barth's solution (pH 7.5, at 4°C) containing (mM): NaCl, 110; KCl, 1; NaHCO3, 2.4; Tris HCl, 7.5; Ca(NO3)2, 0.33; CaCl2, 0.41; MgSO4, 0.82; gentamycin sulphate, 50 μg l−1. Defolliculated oocytes were injected cytosolically with rat P2X4 cRNA (40 nl, 1 μg ml−1), incubated for 48 h at 18°C in Barth's solution then kept at 4°C for up to 12 days until used in electrophysiological experiments.
Electrophysiology
ATP-activated membrane currents (IATP) (Vh=−60 to −90 mV) were recorded from cRNA-injected oocytes using a twin-electrode voltage-clamp amplifier (Axoclamp 2B). The voltage-recording and current-recording microelectrodes (1–5 MΩ tip resistance) were filled with 3.0 M KCl. Oocytes were superfused with Ringer's solution (5 ml min−1, at 18°C) containing (mM): NaCl, 110; KCl, 2.5; HEPES, 5; BaCl2, 1.8, adjusted to pH 7.5. Where stated, the pH of the bathing solution was adjusted using either 1.0 N HCl or 1.0 N NaOH to achieve the desired level. Electrophysiological data were stored on a computer using a MP100 WSW interface (Biopac Systems Inc.) and analysed using the software package Acknowledge III (Biopac).
Solutions
All solutions were nominally Ca2+-free to avoid the activation of a Ca2+-dependent Cl− current (ICl,Ca) in oocytes (Bo et al., 1995). ATP was prepared in a Ca2+-free Ringer's solution (concentrations as stated in the text) and superfused by a gravity-feed continuous flow system which allowed rapid addition and washout. ATP was added for 120 s or until the current reached a peak, then washed out for a period of 15 min. Data were normalized to the maximum current (Imax) evoked by ATP at pH 7.5 for agonist concentration-response (C/R) relationships studied at all pH levels. The agonist concentration required to evoke 50% of the maximum response (EC50) was taken from Hill plots, constructed using the formula log(I/Imax−I) where I is the current evoked by each concentration of ATP. High concentrations of ATP (300 μM–3 mM) can activate an inward Na+-current (INa) in a small proportion of defolliculated oocytes and this current is inhibited by UTP (300 μM) (Kupitz & Atlas, 1993). To avoid such endogenous currents, UTP (300 μM) was added to the superfusate in experiments (mainly at pH 5.5) where it was necessary to use high concentrations of ATP (>300 μM). UTP (300 μM) had no effect on ATP potency at rP2X4 receptors at pH 7.5 (EC50 values: 3.4±1.0 μM vs 4.0±1.3 μM, paired data, n=3).
The effects of extracellular zinc were investigated on agonist activity in two ways. First, Zn2+ was added to ATP solutions and C/R curves for ATP were constructed (data normalized to the maximal ATP-response at pH 7.5). Pre-incubation with Zn2+ for 15 min prior to adding ATP solutions had the same effect on agonist responses as did the simultaneous application of Zn2+ and ATP. Second, C/R curves for Zn2+ were constructed using a submaximal concentration of ATP (EC20) (data normalized to responses to the respective EC20 concentration for ATP at pH 8.0, 7.5, 6.5 and 5.5).
Statistics
Data are presented as means±s.e.mean of four sets of data from different oocyte batches. Significant differences were determined by either unpaired Student's t-test or one-way analysis of variance (ANOVA) followed by Dunnett's test, using commercially available software (Instat v2.05a, GraphPad).
Drugs
All common salts and reagents were AnalaR grade (Aldrich Chemicals, U.K.). Adenosine 5′-triphosphate disodium salt (ATP), uridine 5′-triphosphate sodium salt (UTP) and zinc chloride were purchased from Sigma Chemical Co. (Poole, Dorset, U.K.). Suramin was a gift from Bayer plc (Newbury, Berkshire, U.K.).
Results
Effect of extracellular pH on IATP
At pH 7.5, ATP (100 nM–100 μM) evoked inward membrane currents in defolliculated oocytes expressing rP2X4 receptors (EC50 value, 4.1±0.98 μM; Hill co-efficient (nH), 1.2±0.1, n=4). At pH 8.0, there was no significant change in ATP potency or maximal activity (EC50 value, 2.8±0.6 μM; nH, 1.1±0.1; n=4) (see Figure 1). Acidification of the superfusate significantly reduced ATP potency (P<0.01) (pH 6.5: EC50 value, 8.4±1.2 μM; nH, 1.0±0.1, n=5; pH 5.5: EC50 value, 31.7±4.9 μM; nH, 1.0±0.1, n=6). The efficacy of ATP was diminished only at pH 5.5 (37±4% of maximal ATP-responses at pH 7.5) (see Figure 1). The modulatory effects of H+ (at either pH 6.5 or 5.5) were reversed after readjusting the superfusate to pH 7.5. Water-injected (control) defolliculated oocytes failed to respond to ATP (100 μM).
Figure 1.

Extracellular pH modulates ATP activity at rP2X4 receptor. (A) Whole-cell currents activated by ATP (10 μM) at four levels of extracellular pH (pHe) (8.0, 7.5, 6.5, 5.5). All records from the same oocyte (Vh=−90 mV). (B) Concentration/response (C/R) curves for ATP (30 nM–1 mM) at the same four levels of pHe. Whole-cell currents to ATP (IATP) were normalized to the maximal ATP-response at pH 7.5. Data points are means±s.e.mean, n=4.
Zn2+ potentiation of IATP
Zn2+ (0.1–10 μM) potentiated membrane currents to ATP (3 μM) at rP2X4 receptors by approximately 2 fold (EC50 value, 1.29±0.2 μM, n=4) (Figure 2A and B). This potentiating effect was not sustained at higher concentrations (30 μM–1 mM), at which point Zn2+ caused an inhibition of ATP-responses (Figure 2A and B). Where Zn2+ (0.1 μM–1 mM) was applied 15 min prior to the addition of ATP, the concentration-dependent potentiating and inhibitory activities of Zn2+ remained unaltered (Figure 2B). The potentiating and inhibitory effects of Zn2+ were reversed after washout.
Figure 2.

Zn2+ modulates ATP activity at rP2X4 receptor. (A) Whole-cell currents to ATP (3 μM) and modulation of agonist activity by Zn2+ (1–300 μM) added to the superfusate (pHe, 7.5). All records from the same oocyte (Vh=−90 mV). (B) Concentration/response (C/R) curves for Zn2+ modulation of whole cell currents at rP2X4 ion-channel activated by three micromolar ATP (at pH 7.5). Zn2+ was added to the superfusate and applied either simultaneously with ATP or 15 min prior to, and during, application of ATP. (C) Effect of extracellular pH (pHe) on the modulatory actions of Zn2+ on ATP-responses at rP2X4 receptor. ATP was applied at a concentration equivalent to the EC20 value at four levels pHe (8.0, 7.5, 6.5, 5.5). Data points are means±s.e.mean, n=4.
Zn2+ modulation of ATP-responses was affected by acidifying the extracellular solution. While alkaline conditions (pH 8.0) had no significant effect on Zn2+ modulation of ATP-responses, acidification (pH 6.5) displaced the bell-shaped Zn2+ curve to the right by 1 log unit without diminishing the extent of Zn2+ potentiation (Figure 2C). At pH 5.5, Zn2+ failed to potentiate ATP-responses and the inhibitory action of Zn2+ was also reduced (Figure 2C).
Effect of Zn2+ on concentration dependence of IATP
The effects of Zn2+ on the potency and efficacy of ATP at rP2X4 was studied in detail over a range of pH 7.5–5.5. Zn2+ was applied at two concentrations at each pH level, the first Zn2+ concentration giving maximal potentiation of ATP-responses (pH 7.5, 10 μM; pH 6.5, 100 μM; pH 5.5, 10 μM) and a second concentration causing a significant inhibition of ATP-responses (pH 7.5, 100 μM; pH 6.5, 1000 μM; pH 5.5, 100 μM).
At pH 7.5, the potency of ATP (10 nM–100 μM) was increased significantly (P<0.01) in the presence of Zn2+ (10 μM) (EC50 values, 1.0±0.3 μM vs 4.1±0.98 μM, n=4). ATP potency was not significantly different at a higher level of Zn2+ (100 μM) (EC50 value, 1.3±0.3 μM, n=4), but ATP efficacy (i.e., maximal activity) was reduced considerably (59±7% of control) (Figure 3A). Hill co-efficients for ATP curves were similar in the absence and presence of Zn2+ (nH: 0 μM, 1.2±0.1; 10 μM, 1.0±0.1; 100 μM, 1.0±0.1).
Figure 3.
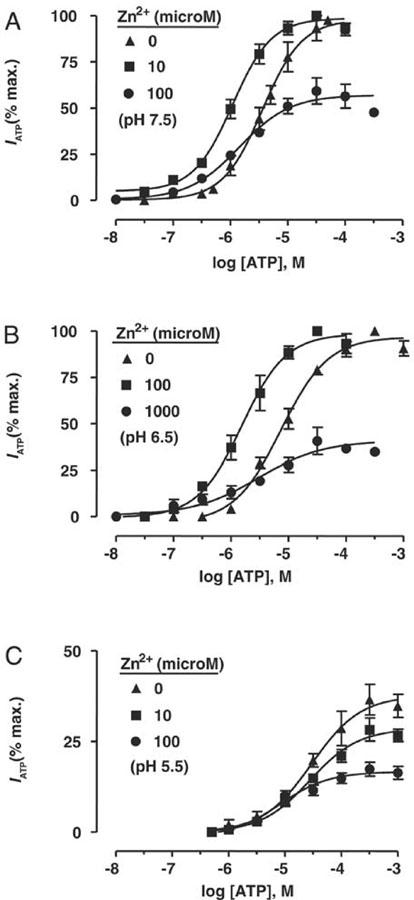
Interaction of H+ and Zn2+ on ATP activity at rP2X4 receptor. Concentration/response (C/R) curves for ATP at three levels of extracellular pH (in A, pH 7.5; in B, pH 6.5; in C, pH 5.5), in the absence then presence of concentrations of Zn2+ ions that caused potentiation and inhibition of ATP-responses. Zn2+ potentiation was caused by an increase in ATP potency, displacing C/R curves to the left. Zn2+ inhibition was due to a decrease in ATP efficacy, without altering agonist potency. Data points are means±s.e.mean, n=4.
Similar effects were seen at pH 6.5. ATP potency was increased significantly (P<0.01) in the presence of Zn2+ (100 μM) (EC50 values: 1.9±0.5 μM vs 8.4±1.2 μM, n=4), whereas ATP potency was not enhanced further by a higher concentration of Zn2+ (1000 μM) (EC50 2.1±1.3 μM, n=4) although agonist efficacy was reduced considerably (43±5% of control) (Figure 3B). Hill co-efficients for ATP curves were similar in the absence and presence of Zn2+ (nH: 0 μM, 1.0±0.1; 100 μM, 1.0±0.1; 1000 μM, 0.9±0.1).
At pH 5.5 (and 300 μM UTP present: see Methods), there was no significant change in ATP potency in the presence of Zn2+ (10 and 100 μM) (EC50 values: 0 μM, 31.7±4.9 μM; 10 μM, 32.6±9.2 μM; 100 μM, 24.9±3.9 μM, n=5). The efficacy of ATP, although reduced considerably at pH 5.5, was decreased further by Zn2+ (peak activity wrt maximal ATP activity at pH 7.5: 0 μM, 37±4%; 10 μM, 27±4%; 100 μM, 17±2%) (Figure 3C). Hill co-efficients for ATP curves were similar in the absence and presence of Zn2+ (nH: 0 μM, 1.0±0.1; 100 μM, 0.8±0.2; 1 μM, 0.9±0.1).
Effect of H+ and Zn2+ on suramin blockade
The P2 receptor antagonist, suramin (50 μM), failed to inhibit ATP-responses at rP2X4 receptors at pH 7.5. Instead, suramin caused a modest potentiation of ATP-activated inward currents (Figure 4A). The extent of this potentiation (149±11%, n=3) was not significantly altered at pH 6.5 (148±4%, n=3) and pH 5.5 (143±13%, n=3). In the presence of Zn2+ (10 μM) which, of itself, potentiated ATP-responses, suramin (50 μM) failed either to potentiate further or inhibit ATP-activated currents (Figure 4B). This apparent Zn2+ antagonism of suramin activity was observed at pH 7.5 and 6.5, but not at 5.5. At this lowest pH level, Zn2+ failed to potentiate ATP-responses and also failed to reduce suramin potentiation of ATP-responses. These results suggest that potentiating actions of Zn2+ and suramin are not additive at pH 7.5 and pH 6.5. Also, suramin potentiation may involve a mechanism different from Zn2+ potentiation, since only the former can occur at pH 5.5.
Figure 4.

Suramin activity at rP2X4 receptor. (A) Histograms of ATP activity in the absence and presence of suramin (50 μM), and 20 min after washout of suramin. The agonist was applied at the respective EC50 value at pH 7.5, 6.5 and 5.5, and control responses were taken as 1 (activity index=1). (B) Histograms of ATP activity in the absence and presence of Zn2+ ions, further addition of suramin (50 μM), and 20 min after washout of suramin and Zn2+ ions. Zn2+ was applied at 10 μM (pH 7.5 and 5.5) and 100 μM (pH 6.5), these concentrations causing maximal potentiation of ATP-responses at the above pH levels.
Discussion
In the present study, ATP-activated inward currents at the rP2X4 receptor were found to be sensitive to changes in extracellular pH. Acidification of the bathing medium progressively shifted the ATP C/R curve to the right and decreased agonist potency by as much as 8 fold, whereas alkaline changes shifted the C/R curve marginally to the left. Similar phenomena were observed for rP2X4 receptors expressed in either HEK293 cells (Stoop et al., 1997) or Xenopus oocytes (Clarke et al., 1998). We have extended these observations by calculating EC50 values and showing that H+ (at pH 5.5) also reduces the efficacy of ATP. At pH 5.5, H+ appears to decrease the number of rP2X4 channels available for agonist activation, in a manner comparable to a non-competitive antagonist, although the effects of H+ were reversed by washout. Can levels as low as pH 5.5 be reached in vivo? Localized acidosis has been reported following bone fracture (pH 4.7), during ischaemia (pH 5.7), inflammation (pH 5.4), during epileptic seizures and injuries related to CNS degenerative changes (DeSalles et al., 1987; Chesler, 1990; Steen et al., 1992; Ransom & Philbin, 1992). It is also evident that acidic shifts occur transiently during CNS neurotransmission (Yanovsky et al., 1995).
The above findings at rP2X4 receptors differed radically from the effects of H+ at rP2X2 receptors (King et al., 1996, 1997), where acidic changes to the bathing solution enhanced ATP potency without affecting the efficacy of the agonist. It was further noted that rP2X2 receptors were more sensitive than rP2X4 receptors to small changes in extracellular pH, as confirmed by Stoop et al., (1997). At both rP2X4 and rP2X2 receptors, however, the change in amplitude of ATP-activated currents was immediate when changing extracellular pH, did not alter with time and was reversed immediately on washout. The speed with which H+ exerts its action on these two P2X subtypes suggests H+ ions act at extracellular site, but there is little structural information to implicate specific (and strategic) amino acid residues. It appears that histidine residues in the extracellular loop of the rP2X2 subunits can be discounted (Stoop et al., 1997; King et al., 1997).
The software programme, Bound and Determined (BAD), calculates the fractional ratios of ATP species for a given amount of ATP (Brookes & Storey, 1992). We have used this programme beforehand, when studying H+ modulation of ATP activity at P2X2 receptors, to try to determine the ATP species most likely to activate the rP2X2 subunit (King et al., 1996). We have repeated such analysis for rP2X4, comparing EC50 values at the four pHe levels tested against the calculated fractional ratios of ATP species present (data not shown). The fractional amount of free ATP (ATP4−) remained constant (about 30%) for the respective EC50 values for ATP over the range of pH 8.0–6.5, but fell sharply (to 8%) at pH 5.5. Thus, it is unlikely that ATP4− alone stimulated the rP2X4 receptor. Of the other ATP species present, the fractional amounts of HATP and BaHATP increased while NaATP, KATP and BaATP decreased with progressive acidification of the superfusate. Such changes in the relative amounts of ATP species could not explain the observed changes in ATP potency over the range of pH 8.0–5.5. As concluded in an earlier paper on rP2X2 receptors (King et al., 1996), it is more likely that receptor protonation rather than agonist protonation accounts for the change in ATP potency at rP2X4 receptors.
Other investigators have already shown that extracellular Zn2+ can potentiate ATP-responses at rat and human P2X4 receptors (Séguéla et al., 1996; Soto et al., 1996; Garcia-Guzman et al., 1997; Nakazawa & Ohno 1997). Here, we demonstrated the concentration-dependence of this effect and also confirmed that actions of Zn2+ were reversed on washout. Additionally, we found that high concentrations of Zn2+ can exert an inhibitory effect on ATP activity. A bell-shaped C/R relationship also has been observed for the actions of Zn2+ on ATP-activated currents in rat sympathetic neurons (Cloues et al., 1993), at which rP2X2 and rP2X4 transcripts have been localized (Collo et al., 1996). From studying the effects of both potentiating and inhibitory concentrations of Zn2+ on the C/R curve for ATP, it appears that inhibition by Zn2+ was due to decrease in agonist efficacy and not a decrease in ATP potency. Thus, high concentrations of Zn2+ can reduce the number of rP2X4 receptors available for agonist activation in a manner comparable to a non-competitive antagonist. Since Zn2+ further reduced the efficacy of ATP at pH 5.5, it appears that the ability of Zn2+ and H+ to reduce the number of available rP2X4 receptors were additive. The locus for the inhibitory actions of Zn2+ and H+ has not been determined. However, these inhibitory actions raise an interesting issue. Since Zn2+ and H+ are found in synaptic vesicles (Johnson & Scarpa, 1976; Assaf & Chung, 1984) and probably are released along with ATP during central neurotransmission, these modulators at the right concentrations might exert a physiological antagonism of rP2X4 receptors which, otherwise, are insensitive to known P2 receptor antagonists.
Extracellular Zn2+ acted differently at rP2X4 and rP2X2 receptors. Both the potentiating and inhibitory actions of Zn2+ at rP2X4 were dependent on concentration and independent of time, while the potentiating effect of Zn2+ at rP2X2 is dependent on time and, irrespective of concentration, eventually replaced by inhibition (Wildman et al., 1998). Additionally, the maximal Zn2+ potentiation of ATP-responses is markedly less pronounced at rP2X4 (2 fold) than rP2X2 receptors (15 fold) at pH 7.5 (see Figure 5), and the respective EC50 values for Zn2+ are somewhat dissimilar (rP2X4, 1.29±0.2 μM; rP2X2, 6.1±1.2 μM). Furthermore, the time-dependent inhibitory actions of Zn2+ at rP2X2 involve a reduction in ATP potency and efficacy, whereas inhibition at rP2X4 involves a reduction in ATP efficacy alone. These distinguishing features for Zn2+ modulation at rP2X2 and rP2X4 receptors might be useful criteria to determine the presence of either P2X2 or P2X4 subunits in native P2X receptors in neurons in the CNS and periphery. In the same vein, H+ potentiation of ATP-responses at native P2X receptors is now viewed as signatory for the presence of P2X2 subunits (Stoop et al., 1997).
Figure 5.
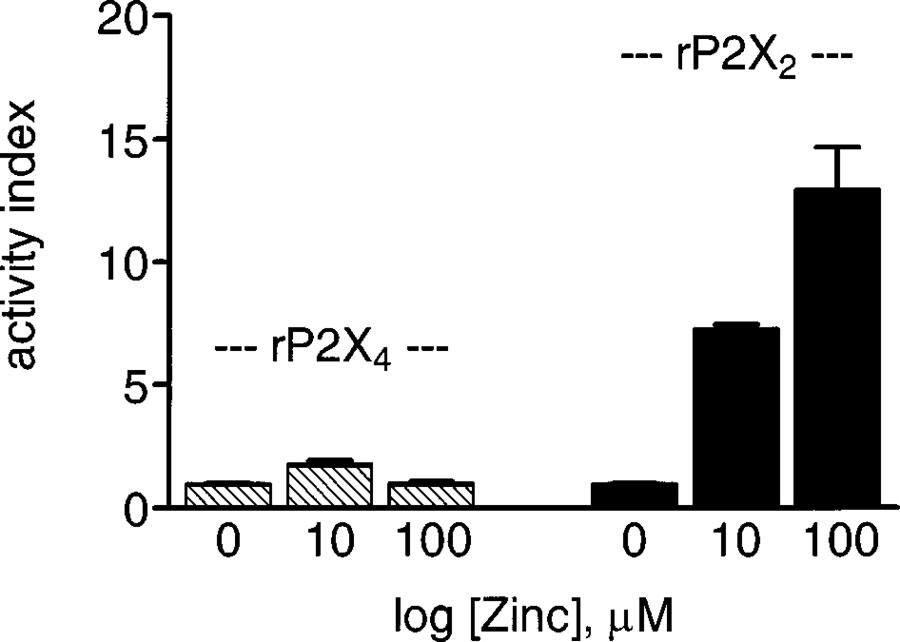
Comparison of Zn2+ modulation on ATP-responses at rP2X4 and rP2X2 receptors. Potentiating effects of Zn2+ (10 and 100 μM) on the ATP activity at rP2X4 receptor and P2X2 receptor, at pH 7.5. ATP was applied at a concentration just above threshold to give ∼5% of the maximal response at each P2X subtype, and these control responses were taken as 1 (activity index=1). Zn2+ had a more profound effect on ATP-responses at rP2X2 receptors at which Zn2+ maximally potentiated ATP-responses were increased 15 fold. In contrast, Zn2+ maximally potentiated ATP-responses at rP2X4 receptors by 2 fold only.
In agreement with earlier reports (Bo et al., 1995; Buell et al., 1996; Collo et al., 1996; Séguéla et al., 1996; Soto et al., 1996; Wang et al., 1996), suramin failed to inhibit ATP-responses at rP2X4 receptors. We attempted to uncover an inhibitory action by suramin by altering pH or adding Zn2+, or using both, on the basis that the blocking activity of suramin is greatly enhanced at rP2X2 at pH 5.5 (King et al., 1997) and in the presence of Zn2+ (Wildman et al., 1998). However, there was no evidence for an inhibitory action by suramin at rP2X4 under such modified conditions. In point of fact, H+ and Zn2+ appeared to be better inhibitors of ATP-responses at rP2X4 than any of the known P2 receptor antagonists.
In conclusion, extracellular pH and Zn2+ affect only agonist activity and not antagonist activity at rP2X4 receptors. H+ and Zn2+ exert opposing actions by decreasing and increasing agonist potency, yet both share a common feature of also lowering the efficacy of ATP. These actions are in sharp contrast to the effects of H+ and Zn2+ on agonist and antagonist activity at rP2X2 receptors. Although both rP2X4 and rP2X2 transcripts are found throughout central and peripheral nervous system, their differing activity profiles with H+ and Zn2+ suggest that P2X signalling can be altered in an opposite manner by these modulators. It has been reported for the enteric nervous system that P2X receptors show either a P2X2-like phenotype (Zhou & Galligan, 1996) or a P2X4-like phenotype (Barajas-Lopez et al., 1996), although neither H+ nor Zn2+ have been tested on ATP-responses in the ENS. If similar phenotypic subsets of endogenous P2X receptors occur elsewhere throughout the CNS and PNS, the modulatory properties of H+ and Zn2+ may have significant and selective actions on P2X signalling at discrete nuclei and ganglia, and provide the means to amplify or inhibit such signalling.
Acknowledgments
This work was supported by the British Heart Foundation and Roche Bioscience (Palo Alto, U.S.A.). We are grateful to Dr X. Bo and Dr R. Schoepfer for the gift of cDNA encoding the rat P2X4 receptor.
Abbreviations
- ATP
adenosine 5′-triphosphate
- EC50
agonist concentration producing 50% of the maximal response
- IATP
ATP-activated membrane current
- nH
Hill co-efficient
- pHe
extracellular pH
- PPADS
pyridoxal-α5-phosphate-6-azophenyl-2′,4′-disulphonic acid
- UTP
uridine 5′-triphosphate
- Vh
holding potential
References
- ASSAF S.Y., CHUNG S.H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- BARAJAS-LÓPEZ C., HUIZINGA J.D., COLLINS S.M., GERZANICH V., ESPINOZA-LUNA R., PERES L.A. P2X-purinoceptors of myenteric neurones from the guinea-pig ileum and their unusual pharmacological properties. Brit. J. Pharmacol. 1996;119:1541–1548. doi: 10.1111/j.1476-5381.1996.tb16070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARDONI R., GOLDSTEIN P.A., LEE C.J., GU J.G., MACDERMOTT A.B. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J. Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BO X., ZHANG Y., NASSAR M., BURNSTOCK G., SCHOEPFER R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- BRAKE A.J., WAGENBACH M.J., JULIUS D. New structural motif for ligand gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- BROOKS S.P.J., STOREY K.B. Bound and Determined: a computer program for making buffers of defined ion concentrations. Anal. Biochem. 1992;210:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- BUELL G., LEWIS C., COLLO G., NORTH R.A., SURPRENANT A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- CHESLER M. The regulation and modulation of pH in the nervous system. Prog. Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- CLARKE C.E., MEADOWS H.J., TOMLINSON W.J., CARPENTER D., SANGER G.J., BENHAM C.D. Extracellular acidification inhibits current flow through human P2X4 receptors expressed in Xenopus oocytes. J. Physiol. 1998;506:44P. [Google Scholar]
- CLOUES R., JONES S., BROWN D.A. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflügers Arch. 1993;424:152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESALLES A.A., KONTOS H.A., WARD J.B., MARMAROU A., BECKER D.P. Brain tissue pH in severely head injured patients: a report of 3 cases. Neurosurgery. 1987;20:297–301. doi: 10.1227/00006123-198702000-00017. [DOI] [PubMed] [Google Scholar]
- DHULIPALA P.D.K., WANG Y.X., KOTLIKOFF M.I. The human P2X4 receptor gene is alternatively spliced. Gene. 1998;207:259–266. doi: 10.1016/s0378-1119(97)00647-1. [DOI] [PubMed] [Google Scholar]
- EDWARDS F.A., GIBB A.J., COLQUHOUN D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., DERKACH V., SURPRENANT A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- GALLIGAN J.J., BERTRAND P.P. ATP mediates fast synaptic potentials in enteric neurons. J. Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-GUZMAN M., SOTO F., GOMEZ-HERNANDEZ J.M., LUND P., STÜHMER W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol. Pharmacol. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- JOHNSON R.G., SCARPA A. Internal pH of isolated chromaffin granules. J. Biol. Chem. 1976;251:2189–2191. [PubMed] [Google Scholar]
- KING B.F., WILDMAN S.S., ZIGANSHINA L.E., PINTOR J., BURNSTOCK G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Brit. J. Pharmacol. 1997;121:1445–1453. doi: 10.1038/sj.bjp.0701286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., ZIGANSHINA L.E., PINTOR J., BURNSTOCK G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Brit. J. Pharmacol. 1996;117:1317–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPITZ Y., ATLAS D. A putative ATP-activated Na+ channel involved in sperm-induced fertilization. Science. 1993;261:484–486. doi: 10.1126/science.8392753. [DOI] [PubMed] [Google Scholar]
- LÊ K.T., VILLENEUVE P., RAMJAUN A.R., MCPHERSON P.S., BEADET A., SÉGUÉLA P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neurosci. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- NAKAZAWA K., OHNO Y. Dopamine and 5-hydroxytryptamine selectively potentiate neuronal type ATP receptor channels. Eur. J. Pharmacol. 1996;296:119–122. doi: 10.1016/0014-2999(95)00774-1. [DOI] [PubMed] [Google Scholar]
- NAKAZAWA K., OHNO Y. Effects of neuroamines and divalent cations on cloned and mutated ATP-gated channels. Eur. J. Pharmacol. 1997;325:101–108. doi: 10.1016/s0014-2999(97)00107-6. [DOI] [PubMed] [Google Scholar]
- NIEBER K., POELCHEN W., ILLES P. Role of ATP in fast excitatory synaptic potentials in locus ceuruleus neurones of the rat. Brit. J. Pharmacol. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A., BARNARD E.A. Nucleotide receptors. Curr. Opin. Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- RANSOM B.R., PHILBIN D.M., JR Anoxia-induced extracellular ionic changes in the CNS white matter: the role of glial cells. Can. J. Physiol. Pharmacol. 1992;70:S181–S189. doi: 10.1139/y92-261. [DOI] [PubMed] [Google Scholar]
- SÉGUÉLA P., HAGHIGHI A., SOGHOMONIAN J., COOPER E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. J. Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M., GERZANICH V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J. Physiol. 1993;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOTO F., GARCIA-GUZMAN M., GOMEZ-HERNANDEZ J.M., HOLLMANN M., KARSCHIN C., STÜHMER W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERLAGH B., KITTEL A., LAJTHA A., VIZI E.S. ATP acts as fast neurotransmitter in rat habenula: neurochemical and enzyme cytochemical evidence. Neurosci. 1995;66:915–920. doi: 10.1016/0306-4522(94)00588-v. [DOI] [PubMed] [Google Scholar]
- STEEN K.H., REEH P.W., ANTON F., HANDWERKER H.O. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociception in rat skin, in vitro. J. Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOOP R., SURPRENANT A., NORTH R.A. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J. Neurophysiol. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- URANO T., NISHIMORI H., HAN H.J., FURUHATA T., KIMURA Y., NAKAMURA Y., TOKINO T. Cloning of P2XM, a novel human P2X receptor gene regulated by p53. Cancer Res. 1997;57:3281–3287. [PubMed] [Google Scholar]
- WANG C.Z., NAMBA N., GONOI T., INAGAKI N., SEINO S. Cloning and pharmacological characterization of a fourth P2X subtype widely expressed in brain and peripheral tissues including various endocrine tissues. Biochem. Biophys. Res. Comm. 1996;220:196–202. doi: 10.1006/bbrc.1996.0380. [DOI] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Potentiation of ATP-responses at a recombinant P2X2 receptor by neurotransmitters and related substances. Brit. J. Pharmacol. 1997;120:221–224. doi: 10.1038/sj.bjp.0700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Brit. J. Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOVSKY Y., REYMANN K., HAAS H.L. pH-dependent facilitation of synaptic transmission by histamine in the CA1 region of mouse hippocampus. Eur. J. Neurosci. 1995;7:2017–2020. doi: 10.1111/j.1460-9568.1995.tb00624.x. [DOI] [PubMed] [Google Scholar]
- ZHOU X., GALLIGAN J.J. P2X purinoceptor in cultured myenteric neurons of guinea-pig small intestine. J. Physiol. 1996;496:719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]


