Abstract
Excessive cyclo-oxygenase-2 (COX-2) induction may play a role in chronic neurological diseases characterized by inflammation and astrogliosis. We have previously identified an astroglial receptor for extracellular nucleotides, a P2Y receptor, whose stimulation leads to arachidonic acid (AA) release, followed, 3 days later, by morphological changes resembling reactive astrogliosis. Since COX-2 may be upregulated by AA metabolites, we assessed a possible role for COX-2 in P2Y receptor-mediated astrogliosis. A brief challenge of rat astrocytes with the ATP analogue α,β-methylene ATP (α,βmeATP) resulted, 24 h later, in significantly increased COX-2 expression. The selective COX-2 inhibitor NS-398 completely abolished α,βmeATP-induced astrocytic activation. Constitutive astroglial COX-1 or COX-2 did not play any role in purine-induced reactive astrogliosis. PGE2, a main metabolite of COX-2, also induced astrocytic activation. These data suggest that a P2Y receptor mediates reactive astrogliosis via induction of COX-2. Antagonists selective for this receptor may counteract excessive COX-2 activation in both acute and chronic neurological diseases.
Keywords: ATP, cyclo-oxygenase-2, inflammation, astrogliosis, P2Y receptors
Introduction
Recent reports have implicated cyclo-oxygenase-2 (COX-2) in a variety of neurological disorders, including chronic pain and inflammation (Dolan et al., 1998), acute (e.g., ischaemia; Ohtsuki et al., 1996) as well as chronic (e.g., Alzheimer's disease; Tocco et al., 1997) neurodegenerative diseases characterized by a marked inflammatory component and activation of astroglial cells (Ohtsuki et al., 1996; Blom et al., 1997; Tocco et al., 1997). A key role for the inducible enzyme in the pathogenesis of these disorders is also supported by the results of epidemiological studies showing that steroidal as well as non-steroidal anti- inflammatory drugs lower the risk of developing Alzheimer's disease (Breitner et al., 1994).
Extracellular ATP exerts its effects through P2 receptors: these are ligand-gated ion channels (P2X receptors) or G-protein-coupled receptors (P2Y receptors) (Abbracchio & Burnstock, 1994). In brain, ATP has been implicated both in fast neuro-neuronal communication via P2X receptors, and in regulation of several properties of astrocytes via P2Y receptors, some of which may be involved in mechanisms of neural injury following massive ATP release during trauma and ischaemia (Neary et al., 1996). We have previously shown that a brief (2 h) challenge of rat brain astrocytes with the relatively hydrolysis-resistant ATP analogues α,β-methylene ATP (α,βmeATP) and β,γ-methylene ATP (β,γmeATP) results, 3 days later, in marked concentration-dependent elongation of astrocytic processes which intensively stain for the astroglial marker GFAP (glial fibrillary acidic protein) (Bolego et al., 1997). This effect, which reproduces in vitro the astrocytic hypertrophy known to occur in in vivo reactive astrogliosis (Ridet et al., 1997) was abolished by pertussis toxin, suggesting the involvement of a P2Y receptor. Astrocytic activation by ATP analogues was not due to modulation of either phospholipase C or adenylyl-cyclase activity (Bolego et al., 1997), nor to changes of the intracellular Ca2+ concentrations (Centemeri et al., 1997), but to a rapid activation of phospholipase A2 and arachidonic acid (AA) release (Bolego et al., 1997). Arachidonic acid is a substrate for both constitutive COX-1 and mitogen-activated COX-2 (Wu, 1996). The involvement of COX-2-mediated production of proinflammatory cytokines and PGE2 by astrocytes in neurodegeneration has been suggested (Blom et al., 1997). A specific aim of the present study was therefore to evaluate a role for the inducible enzyme in P2Y receptor-mediated reactive astrogliosis.
Methods
Rat astrocytic cultures
Primary astrocytic cultures were established from rat corpus striatum of 7-day-old pups as previously described (Bolego et al., 1997). Cells were initially plated in serum-supplemented medium and after 24 h placed in chemically defined serum-free medium.
Treatment of cultures
Challenge with the various agents and treatment with inhibitors are summarized in Figure 1. Briefly, at day 2 of culture, cells were challenged for 2 h with various agents (either α,βmeATP, AA, PGE2 or PGD2), washed and placed in drug-free medium until the end of the experiment (day 5 of culture). In selected experiments where effects of inhibitors were examined, either acetylsalicylic acid (ASA) or NS-398 were added to cultures 30 min before α,βmeATP and maintained in the medium throughout the challenge with the purine analogue (short protocol). To assess the role of inducible COX-2 in purine-induced reactive astrogliosis, cells challenged with α,βmeATP in the presence of NS-398 were washed and maintained in medium containing NS-398 at the same concentration up to cell fixing (long protocol). Neither NS-398 nor ASA had any effect on process length when tested alone.
Figure 1.
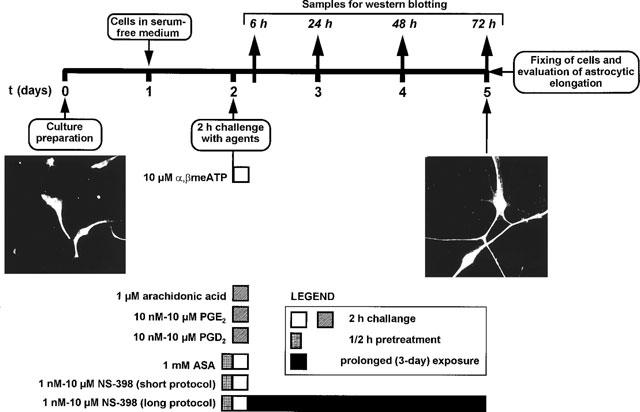
The experimental protocol used to assess a role for COXs in purine-mediated reactive astrogliosis. Rat striatal astrocytic cells were initially established in complete medium, placed in serum-free medium at day 1 to favour morphological differentiation resembling reactive astrogliosis, and challenged at day 2 with either purine analogues (e.g. α,βmeATP) or other agents (see text). After challenge, cells were grown for a further 3 days in drug-free medium, fixed, stained with an anti-GFAP antibody for determination of the elongation of astrocytic processes. Left panel: micrograph depicting control astrocytes with typical bipolar shape. Right panel: activated astrocytes showing increased length of astrocytic processes. In selected experiments, samples for evaluation of COX-2 by Western blot were taken at various periods after challenge with α,βmeATP, as indicated. To evaluate a role of constitutive COXs, ASA and NS-398 were added to the cultures 30 min before challenge with the purine analogue and maintained for the 2 h challenge period (short protocol). To evaluate the role of inducible COX-2, NS-398 was also maintained in the culture medium for the entire duration of the experiment (long protocol).
Immunofluorescence staining and analysis of GFAP-positive astrocytic processes
Astrocytes were identified by indirect immunofluorescence staining using rabbit anti-GFAP immunoglobulins (1 : 500, DAKO) followed by biotinylated donkey anti-rabbit secondary antibody and streptavidin-fluorescein (Bolego et al., 1997). The length of GFAP-positive astrocytic processes (mean μm cell−1±s.e.mean) was measured using a Zeiss fluorescence microscope equipped with a fluorescein filter and connected to an image video system (video camera, NIH Image 1.47 software on a Macintosh computer). The cellular processes of at least 75 cells sample−1 were analysed under blind conditions. Cells were chosen at random. The absolute control value was 141±5.6 μm cell−1 (mean±s.e.mean of 24 independent experiments). To enable a more immediate evaluation, data were expressed as per cent of a corresponding control set.
Western blot analysis
This was performed as previously described (Abbracchio et al., 1997). Briefly, 20 mg of total protein from each sample (see Figure 1) was loaded on 11% sodium-dodecylsulphate polyacrylamide gels and blotted onto nitrocellulose filters. Filters were incubated with goat polyclonal anti-rat anti-COX-2 antibody (1 : 3,000, Santa Cruz Biotechnology) followed by a secondary anti-goat antibody (conjugated to horseradish peroxidase, 1 : 4000) and visualized with ECL Western blotting kit (Amersham U.K.). Semiquantitative evaluation of autoradiograms was performed by densitometric analysis on a computerized image analyser (see above).
Results
The experimental protocol utilized in the present study is shown in Figure 1. Exposure of cultures to α,βmeATP under conditions that elicited the formation of reactive astrocytes (Figure 1) resulted, 24 h after agonist challenge, in increased expression of COX-2, as evaluated by Western blot analysis (Figure 2a). The amount of enzyme was still increased with respect to control cultures 48 h after challenge and returned to basal levels 72 h after challenge with the purine analogue. Under these experimental conditions a marked elongation of GFAP-positive astrocytic processes was detected (see Figure 1 and solid column in Figure 2b). Maintenance of cells in the continuous presence of the selective COX-2 inhibitor NS-398 (Futaki et al., 1994) after challenge with the purine analogue (see ‘long protocol' in Figure 1) resulted in a concentration-dependent attenuation of astrocytic elongation (Figure 2b). A trend to decreased reactive astrogliosis was already observed in the presence of NS-398 (0.001 μM); inhibition was statistically significant at 0.01 μM; complete abolition of α,βmeATP-induced reactive astrogliosis was observed at all other concentrations of inhibitor tested (Figure 2b), suggesting a specific role for the inducible enzyme in the formation of reactive astrocytes. Other non-selective inhibitors of COX-2 (e.g., dexamethasone, which also blocks phospholipase A2) also abolished purine-induced reactive astrogliosis (data not shown).
Figure 2.
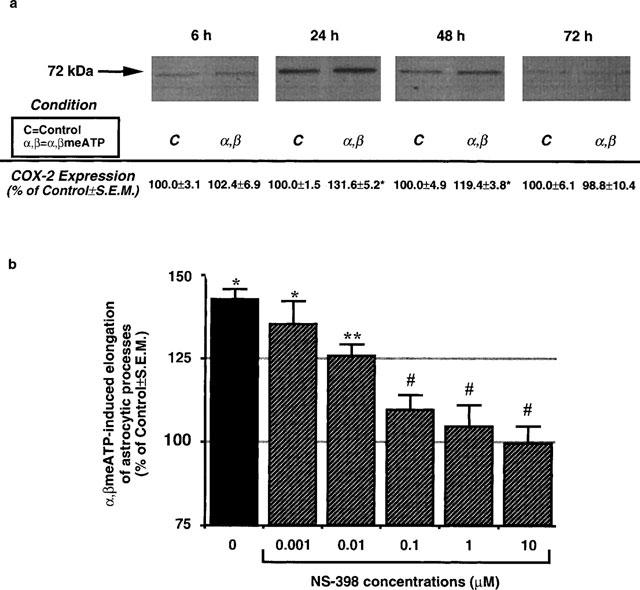
Involvement of inducible COX-2 in P2Y receptor-mediated reactive astrogliosis. (a) Cells were challenged with α,βmeATP (10 μM) for 2 h, placed in agonist-free medium, and the presence of COX-2 assessed at 6, 24, 48 and 72 h by immunoblot analysis with a specific ant-COX-2 antibody. Results (area×optical density of the 72 kDa MW COX-2 band) are expressed as mean per cent±s.e.mean of the corresponding control value from four independent experiments run in triplicate. COX-2 was significantly increased at 24 and 48 h (*P<0.05, 1-way ANOVA, Scheffé's F-test). (b) Under the experimental conditions, a marked astrocytic activation was found, as demonstrated by increased elongation of GFAP-positive astrocytic processes (expressed as percentage of control mean μm cell−1±s.e.mean; see solid column; *P<0.05, 1-way ANOVA, F-test). If, after challenge with the purine analogue, cells were maintained in the presence of NS-398 to selectively inhibit the inducible enzyme (long protocol, Figure 1), a concentration-dependent inhibition of α,βmeATP-induced astrocytic elongation was found (hatched columns; **P<0.05 vs both control and α,βmeATP alone, #P<0.05 vs α,βmeATP alone and not statistically different from control, 1-way ANOVA, F-test).
Western blot experiments revealed a low, but significant, amount of COX-2 also in control unstimulated astrocytes (Figure 2a), suggesting that COX-2 may be constitutively expressed in these cells. Therefore, we designed specific experiments aimed at testing a possible role for constitutive COXs in purine-induced astrogliosis. Cultures were exposed to NS-398 (0.001–10 μM) to selectively block constitutive COX-2, or to ASA (1 mM) (which irreversibly inhibits both COX-1 and COX-2; Kalgutkar et al., 1998) immediately before and throughout challenge with α,βmeATP (short protocol). ASA was used at a concentration high enough to irreversibly inactivate all the COXs enzymes present during challenge with the purine analogue, i.e., to ensure abolition of the contribution made by the constitutively expressed enzymes. After challenge, cells were washed, placed in drug-free medium, and grown for a further 3 days before morphological analysis. Under these conditions, no inhibition of purine-induced astrocytic elongation was detected (Table 1), hence ruling out a role for constitutive COXs and suggesting that any effect seen after treatment with the purine analogue must result from cloning of new enzyme. Data with NS-398 (Figure 2b) suggest that this is indeed COX-2.
Table 1.
Effect of NS-398 and acetylsalicylic acid (ASA) inhibition of COX-2 on elongation of astrocytic processes
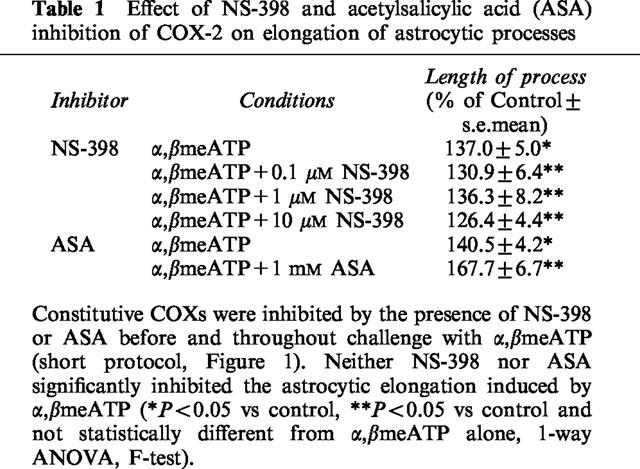
We have previously shown that, in a similar way to ATP analogues, exposure of cultures to AA elicits the formation of reactive astrocytes (Bolego et al., 1997). Since PGE2 and PGD2 have been implicated as functionally important COX products in brain (Blom et al., 1997; Minghetti et al., 1997), in the present study we have also examined these prostaglandins for their ability to trigger reactive astrogliosis. The exogenous addition of the inflammatory PGE2 (but not PGD2) mimicked the effects evoked by P2Y receptor activation, resulting in statistically significant and concentration-dependent elongation of astrocytic processes (Figure 3). This suggests that PGE2 is likely to represent the main AA metabolite involved in purine-induced reactive astrogliosis.
Figure 3.
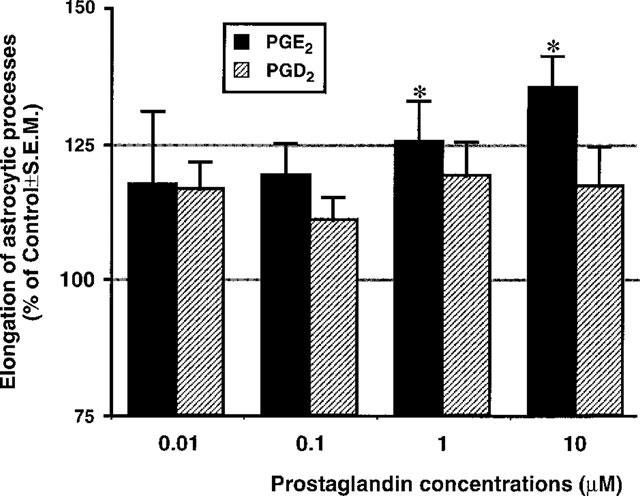
Exogenously applied PGE2, but not PGD2, can mimic P2Y receptor-mediated reactive astrogliosis. Cultures were challenged for 2 h with the concentrations of prostaglandins indicated, washed, grown for a further 3 days in drug-free medium, fixed, stained and assessed for elongation of the astrocytic processes. Results are the mean±s.e.mean of three independent experiments run in quadruplicate. *P<0.05, 1-way ANOVA, F-test.
Discussion
Our results show that ATP may trigger reactive astrogliosis via activation of a P2Y receptor linked to induction of COX-2. Transductional signalling to COX-2 induction involves an early activation of phospholipase A2 and AA release; this may hence act as an inducer of the COX-2 gene via the protein kinase-C/mitogen-activated kinase pathway (Neary et al., 1996). Regulation of COX-2 transcription by activator protein-1 (AP-1) has been reported (Wu, 1996); interestingly we and others have previously shown that exposure of astrocytes to ATP results in induction of the Jun and Fos transcriptional factors (Neary et al., 1996; Bolego et al., 1997) and formation of AP-1 complexes (Neary et al., 1996). Data also point to PGE2 as a main COX-2 metabolite in P2Y receptor-mediated reactive astrogliosis; this is consistent with previous results demonstrating increases of this inflammatory mediator in a human post-mortem astrocytic culture (Blom et al., 1997).
A relationship between extracellular P2 receptors and prostanoid production was proposed back in the 1970s (Burnstock et al., 1975). The ability of astroglial cells to release prostanoids upon activation of P2 receptors was demonstrated in the late 1980s (Pearce et al., 1989). Our results establish for the first time a specific correlation between activation of G protein-coupled P2Y receptors on astrocytes and induction of COX-2. Enhanced COX-2 activity in neurological diseases may contribute to neuronal damage via production of free radicals during AA conversion (Katsuki & Okuda, 1995). In addition, COX-2 products may potentiate brain damage by increasing oedema and by delivery of proinflammatory cells into the brain. Moreover, whereas neuron-derived prostanoids seem to be more involved in functions related to synaptic transmission and neural plasticity, reactive astrocytes and microglial cells are likely to be the major sources of prostaglandins in pathological conditions (Minghetti et al., 1997). A specific pathogenic role for astroglial COX-2 is also suggested by the demonstration that PGE2 largely potentiated release of pro-inflammatory cytokine interleukin-6 from both astroglioma cell lines and post-mortem human astrocytes (Blom et al., 1997). PGE2 has been also recently associated with release of reactive oxygen and other radicals in neurotoxicity induced by the prion protein (Brown et al., 1996). On this basis, selective inhibitors of COX-2 have been suggested to be beneficial for the therapy of central nervous system diseases characterized by neurodegenerative events (Ohtsuki et al., 1996; Blom et al., 1997), chronic inflammation and pain (Dolan, et al., 1998), while avoiding the gastrointestinal, renal and haematopoietic adverse effects typical of mixed COX-1/COX-2 inhibitors (Flower, 1996). Our results suggest that such a goal may be achieved by selectively blocking astroglial P2Y receptors mediating reactive astrogliosis. In previous work, we have shown that the non-selective P2 receptor antagonist suramin can concentration-dependently block the formation of reactive astrocytes by ATP analogues (Bolego et al., 1997). However, suramin also has a variety of additional activities on membrane channels, G proteins and enzymes. Selective antagonists of the astroglial P2Y receptor devoid of effects on other biological targets are hence highly desirable: these may represent a novel class of anti-inflammatory agents.
Acknowledgments
This work was partially supported by the European Union BIOMED2 Programme BMH4 CT96-0676. The authors are grateful to Professor G.C. Folco, Institute of Pharmacological Sciences, Milan, Italy, for invaluable suggestions on the experimental protocol, to Dr Alicia Hernandez (ibidem) for useful discussion, and to Roy Jordan for editorial assistance.
Abbreviations
- AA
arachidonic acid
- AP-1
activator protein-1
- ASA
acetylsalicylic acid
- COX
cyclo-oxygenase
- GFAP
glial fibrillary acidic protein
- α,βmeATP
α,β-methylene ATP
- β,γmeATP
β,γ-methylene ATP
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., RAINALDI G., GIAMMARIOLI A.M., CERUTI S., BRAMBILLA R., CATTABENI F., BARBIERI D., FRANCESCHI C., JACOBSON K.A., MALORNI W. The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-XL: studies in human astroglioma cells. Biochem. Biophys. Res. Commun. 1997;241:297–304. doi: 10.1006/bbrc.1997.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOM M.A.A., VAN TWILLERT M.G., DE VRIES S.C., ENGEL S.F., FINCH C.E., VEERHUIS R., EIKELENBOOM P. NSAIDS inhibit the IL-1β-induced IL-6 release from human post-mortem astrocytes: the involvement of prostaglandin E2. Brain Res. 1997;777:210–218. doi: 10.1016/s0006-8993(97)01204-3. [DOI] [PubMed] [Google Scholar]
- BOLEGO C., CERUTI S., BRAMBILLA R., PUGLISI L., CATTABENI F., BURNSTOCK G., ABBRACCHIO M.P. Characterization of the signalling pathways involved in ATP and basic fibroblast growth factor-induced astrogliosis. Br. J. Pharmacol. 1997;121:1692–1699. doi: 10.1038/sj.bjp.0701294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREITNER J.C.S., GAU B.A., WELSH K.A., PLASSMAN B.L., MCDONALD W.M., HELMS M.J., ANTHONY J.C. Inverse association of anti-inflammatory treatments and Alzheimer's disease: initial results of a control co-twin study. Neurology. 1994;44:227–232. doi: 10.1212/wnl.44.2.227. [DOI] [PubMed] [Google Scholar]
- BROWN D.R., SCHMIDT B., KRETZSCHMAR H.A. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature. 1996;380:345–347. doi: 10.1038/380345a0. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., COCKS T., PADDLE B., STASZEWSKA-BARCZAK J. Evidence that prostaglandin is responsible for the ‘rebound contraction' following stimulation of non-adrenergic, non-cholinergic (‘purinergic') inhibitory nerves. Eur. J. Pharmacol. 1975;31:360–362. doi: 10.1016/0014-2999(75)90060-6. [DOI] [PubMed] [Google Scholar]
- CENTEMERI C., BOLEGO C., ABBRACCHIO M.P., CATTABENI F., PUGLISI L., BURNSTOCK G., NICOSIA S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br. J. Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLAN S., O'SHAUGHNESSY P.J., NOLAN A.M. Up-regulation of COX-2 and prostaglandin EP3 receptor mRNA expression in spinal cord in a clinical model of chronic inflammation. Eur. J. Neurosci. 1998;10:77. [Google Scholar]
- FLOWER R.J. New directions in cyclooxygenase research and their implications for NSAID-gastropathy. Ital. J. Gastroenterol. 1996;28:23027. [PubMed] [Google Scholar]
- FUTAKI N., TAKAHASHI S., YOKOYAMA M., ARAI I., HIGUCHI S., OTOMO S. NS-398, a new anti-inflammatory agent selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- KALGUTKAR A.S., CREWS B.C., ROWLINSON S.W., GARNER C., SEIBERT K., MARNETT L.J. Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science. 1998;280:1268–1270. doi: 10.1126/science.280.5367.1268. [DOI] [PubMed] [Google Scholar]
- KATSUKI H., OKUDA S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. [DOI] [PubMed] [Google Scholar]
- MINGHETTI L., POLAZZI E., NICOLINI A., CREMINON C., LEVI G. Up-regulation of cyclooxygenase-2 expression in cultured microglia by prostaglandin E2, cyclic AMP and non-steroidal anti-inflammatory drugs. Eur. J. Neurosci. 1997;9:934–940. doi: 10.1111/j.1460-9568.1997.tb01444.x. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., RATHBONE M.P., CATTABENI F., ABBRACCHIO M.P., BURNSTOCK G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- OHTSUKI T., KITAGAWA K., YAMAGATA K., MANDAI K., MABUCHI T., MATSUSHITA K., YANAGIHARA T., MATSUMOTO M. Induction of cyclooxygenase-2 mRNA in gerbil hippocampal neurons after transient forebrain ischaemia. Brain Res. 1996;736:353–356. doi: 10.1016/0006-8993(96)00948-1. [DOI] [PubMed] [Google Scholar]
- PEARCE B., MURPHY S., JEREMY J., MORROW C., DANDONA P. ATP-evoked Ca2+ mobilization and prostanoid release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J. Neurochem. 1989;52:971–977. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- RIDET J.L., MALHOTRA S.K., PRIVAT A., GAGE F.H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- TOCCO G., FREIRE-MOAR J., SCHREIBER S.S., SAKHI S.H., AISEN P.S., PASINETTI G.M. Maturational regulation and regional induction of cyclooxygenase-2 in rat brain: implications for Alzheimer's disease. Exp. Neurol. 1997;144:339–349. doi: 10.1006/exnr.1997.6429. [DOI] [PubMed] [Google Scholar]
- WU K.H. Cyclooxygenase 2 induction: molecular mechanisms and pathophysiological roles. J. Lab. Clin. Med. 1996;128:242–245. doi: 10.1016/s0022-2143(96)90023-2. [DOI] [PubMed] [Google Scholar]


