Abstract
Using fura-2 fluorometry, the effects of FK506, an immunosuppressant, on changes in cytosolic Ca2+ concentrations ([Ca2+]i) and tension were investigated in porcine coronary arterial strips. The effects of FK506 on the activity of voltage-operated Ca2+ channels were examined by applying a whole cell patch clamp to the isolated smooth muscle cells of porcine coronary artery.
FK506 inhibited the sustained increases in both [Ca2+]i and tension induced by 118 mM K+ depolarization and 100 nM U46619 in a concentration-dependent manner (1–30 μM). The extent of inhibition of the K+-induced contraction was greater than that of the U46619-induced contraction. The increases in [Ca2+]i and tension induced by histamine and endothelin-1 in the presence of extracellular Ca2+ were also inhibited by 10 μM FK506.
FK506 (10 μM) had no effect on Ca2+ release induced by caffeine or by histamine in the Ca2+-free solution.
FK506 (10 μM) had no effect on the [Ca2+]i-tension relationships of the contractions induced by cumulative increases of extracellular Ca2+ during K+ depolarization or stimulation with U46619.
In the patch clamp experiments, FK506 (30 μM) partially inhibited the inward current induced by depolarization pulse from −80 mV to 0 mV.
In conclusion, FK506 induces arterial relaxation by decreasing [Ca2+]i mainly due to the inhibition of the L-type Ca2+ channels, with no effect on the Ca2+ sensitivity of the contractile apparatus.
Keywords: FK506, porcine coronary artery, vasorelaxation, cytosolic Ca2+ concentration, voltage-operated Ca2+ channel
Introduction
FK506 (tacrolimus, (−)-(1R, 9S, 12S, 13R, 14S, 17R, 18E, 21S, 23S, 24R, 25S, 27R)-17-allyl-1, 14-dihydroxy-12-[(E)-2-[(1R,3R,4R)- 4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl]-23 , 25- dimethoxy- 13, 19, 21, 27 - tetramethyl - 11, 28 - dioxa - 4- azatricyclo [22.3.1.04.9] octacos-18-ene-2, 3, 10, 16-tetrone hydrate, C44H69NO12·H2O, M.W.: 822.05) is an immunosuppressant widely used in organ transplantations (Starzl et al., 1989). FK506 binds to its cytosolic receptor, FK506 binding protein (FKBP), and the resulting complex inhibits the type 2B Ca2+-calmodulin-dependent protein phosphatase, calcineurin, which is essential in T cell activation (Clipstone & Crabtree, 1992; Liu et al., 1991; 1992; O'Keefe et al., 1992). It is also suggested that calcineurin is involved in other signal transduction pathways regulated by Ca2+ (Guerini, 1997). For example, the Ca2+-dependent inactivation of L-type Ca2+ channels was partially inhibited by cyclosporin A, another widely used immunosuppressant (Schuhmann et al., 1997). Alteration of the Ca2+ sensitivity of the contractile apparatus as well as cytosolic Ca2+ concentration ([Ca2+]i) has recently been suggested as one of the regulatory mechanisms of smooth muscle contraction (Somlyo & Somlyo, 1994). It is suggested that protein phosphatases, especially type 1 phosphatase, play an important role in regulation of the Ca2+ sensitivity of the contractile apparatus of smooth muscle (Somlyo & Somlyo, 1994). However the role of type 2B phosphatase in the regulation of smooth muscle contraction remains to be elucidated.
Recently, it has been proposed that FKBPs form functional complexes with Ca2+ release channels and modulate their activity (Marks, 1997). These channels play crucial roles in many cellular functions including smooth muscle contraction, excitation-contraction coupling in striated muscle, T cell activation and fertilization (Himpens et al., 1995; Marks, 1992; 1997). FKBPs have been shown to be associated with ryanodine receptors (RyR) and to modulate channel activity, possibly by enhancing cooperation between its four subunits. Interaction with FKBPs stabilized the channel activity of RyR, namely it decreased open probability and increased mean open time of the channel after caffeine activation, and also increased the full conductance level (Brillantes et al., 1994; Jayaraman et al., 1992; Kaftan et al., 1996; Timerman et al., 1993). FK506 as well as the related compound, rapamycin, reversed the stabilizing effect of FKBPs, enhanced the caffeine-induced Ca2+ release and, in the case of skeletal muscle, enhanced contractility (Brillantes et al., 1994; Kaftan et al., 1996). Recently, it was also shown that FKBP12 was associated with IP3R and stabilized its activity in rat cerebellum (Cameron et al., 1995).
Hypertension is one of the common side effects of FK506, indicating that FK506 may directly modulate vascular smooth muscle contractility (Alessiani et al., 1993; Armitage et al., 1991; Fung et al., 1991). However, the effect of FK506 on the tension development of vascular smooth muscle has never been studied.
In the present study, in order to investigate the effect of FK506 on arterial contraction and to elucidate the role of the type 2B phosphatases in the regulation of smooth muscle contraction, we simultaneously measured [Ca2+]i and tension in porcine coronary arterial strips using the fura-2 front surface fluorometry method. Unexpectedly, FK506 was found to induce arterial relaxation by decreasing [Ca2+]i level mainly due to inhibition of Ca2+ influx in the porcine coronary artery. Therefore, we further evaluated the effects of FK506 on the Ca2+ channel activity with a patch clamp technique.
Methods
Preparation of medial strips of porcine coronary artery and fura-2 loading
Fresh pig hearts were obtained from a local slaughterhouse. The left circumflex branches of coronary arteries were immediately isolated and transported to the laboratory in ice cold physiological salt solution (PSS). The composition of normal PSS (mM) was; NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25, and D-glucose 11.5. A segment of the coronary artery 2–3 cm from the origin were excised. After removing the adventitia, the segment was opened longitudinally, and the endothelium was removed by gently rubbing the internal surface with a cotton swab. The medial preparation was cut into 1 mm width×5 mm long strips under a binocularscope. The lack of functional endothelium was confirmed by the observation that the addition of 1 μM bradykinin during contraction induced by 118 mM K+ depolarization did not induce relaxation.
Vascular strips without endothelium were loaded with the Ca2+ indicator dye, fura-2, by incubation in oxygenated Dulbecco's modified Eagle medium containing 25 μM fura-2 acetoxymethyl ester (fura-2/AM) and 5% w/v foetal bovine serum for 3–4 h at 37°C. The strips were then washed in normal PSS for more than 1 h to remove the dye remaining in the extracellular space and to equilibrate the strips before starting the specific measurements.
Simultaneous measurement of [Ca2+]i and tension of the porcine coronary arterial strips
The changes in [Ca2+]i and tension of the fura-2 loaded vascular strips were simultaneously measured at 37°C as previously described (Hirano et al., 1990). The fura-2 loaded strips were mounted vertically to a strain gauge connected at one end of the strip (model TB-612T, Nihon Koden, Tokyo, Japan) whilst the other end was connected to a clamp in a quartz organ bath. Changes in the fluorescence intensity of the fura-2-Ca2+ complex were monitored with a front surface fura-2 fluorometer equipped with optic fibres (model CAM-OF-2, Japan Spectroscopic, Tokyo, Japan). The quartz optic fibres were used to transmit alternating (400 Hz) 340 nm and 380 nm excitation light from a xenon lamp to the strips. The surface fluorescence of the strips was collected with glass optic fibres and passed through a 500 nm band-pass filter into a photomultiplier. The quartz and glass optic fibres were arranged in a concentric inner circle (3 mm diameter) and an outer circle (7 mm diameter) at one end of the optic fibres facing the strip. The fluorescence intensities (500 nm emission) at 340 nm and 380 nm excitation were monitored and their ratio (F340/F380) was recorded as an indicator of [Ca2+]i. During a fura-2 equilibration period, the strips were stimulated with 118 mM K+ deplolarization at 15 min intervals and the resting tension was increased in a stepwise manner. The resting tension was finally adjusted to approximately 300 mg (=2.97 mN) in normal PSS. This was the minimal resting tension yielding maximum tension development in response to depolarization with 118 mM K+.
Before each experimental protocol, the response to 118 mM K+ depolarization was recorded as control. Both changes in fluorescence ratio and tension were expressed as a percentage, assigning values in normal PSS (5.9 mM K+) and at 10 min after the stimulation with 118 mM K+ depolarization to be 0 and 100%, respectively.
Electrophysiological recordings
Small segments of coronary artery similar to those used for the tension study were incubated in Ca2+-free solution containing 0.05% w/v collagenase P and 0.15% w/v bovine serum albumin (fraction V, essentially fatty acid free) in a shaking water bath at 37°C for 35 min. Thereafter, the tissue was gently agitated with a blunt-tipped pipette to disperse the smooth muscle cells. The debris was filtered and the cells collected by centrifugation at 1000 r.p.m. for 2 min and suspended in fresh Ca2+-free solution containing 0.2% w/v bovine serum albumin and 0.1% w/v trypsin inhibitor (type IIs). The cell suspension was stored at 10°C and experiments were performed at room temperature (25–28°C) within 5 h after harvest. The composition of the Ca2+-free solution (in mM) was NaCl 140, KCl 5.4, MgCl2 1.2, glucose 12, N-[-2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid] (HEPES) 10 (pH=7.3–7.4).
Patch electrode was manipulated using a three dimensional micromanipulator (Manipulater E; Leitz, Wetzler, Germany). For recording the Ca2+ channel currents, high Ba2+ solution was superfused in the bath and the pipette was filled with high Cs+ solution with the following compositions (in mM), respectively; Ba2+ solution, BaCl2 90 and HEPES 5; Cs+ solution, CsCl 135, MgCl2 5, EGTA 5, Na2ATP 5, glucose 12 and HEPES 10; (pH 7.3–7.4). For recording the K+ channel currents, Ca2+-free solution (described above) was superfused in the bath and the pipette was filled with high K+ solution with the following ionic composition (in mM); high K+ solution, KCl 120, glucose 20, MgCl2 5, EGTA 5, HEPES 10; (pH=7.3–7.4). The membrane currents were recorded by a whole cell voltage clamp configuration (Hamill et al., 1981) through an amplifier (Axopatch 200, Axon Instruments, Burlingame, CA, U.S.A.). Patch electrodes (3–5 MΩ) were prepared with an electrode puller (P-97, Sutter Instrument Co., Novato, CA, U.S.A.), and heat polisher (MF-83, Narishige Scientific Instrument Laboratory, Tokyo, Japan). Data acquisition was compiled using pCLAMP software (Axon Instruments). In the present experiments, the membrane potential was kept at −80 mV and depolarizing pulse to 0 mV was repetitively applied to the cell (300 ms duration, 15 s interval). In preliminary experiments, we confirmed that ethanol (less than 0.1% v/v) did not affect Ca2+ channel currents.
Solutions and chemicals
The Ca2+-free PSS used in the fluorometry experiments contained 2 mM ethyleneglycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) instead of 1.25 mM CaCl2. High (118 mM) K+ PSS was prepared by replacing an equimolar substitution of KCl for NaCl. The solutions were gassed with a mixture of 5% CO2 and 95% O2 and the resulting pH was 7.4.
FK506 was kindly donated by Fujisawa Pharmaceutical Co., Ltd (Osaka, Japan). FK506 was dissolved in ethanol as a stock solution of 10 or 100 mM. The final concentration of ethanol was less than 0.1% v/v. This final concentration of ethanol, per se, had no effects on the [Ca2+]i and tension of the porcine coronary medial strips as previously described (Kuroiwa et al., 1993). Fura-2/AM was purchased from Dojindo Laboratories (Kumamoto, Japan). Bovine serum albumin, endothelin-1, nicardipine and trypsin inhibitor were purchased from Sigma (St. Louis, MO, U.S.A.). Bradykinin was purchased from the Peptide Institute, Inc. (Osaka, Japan). U46619 (9,11-dideoxy-9α,11α-methanoepoxy-prostaglandin F2α, C21, H34O4, M.W.: 350.5) was purchased from Funakoshi (Tokyo, Japan). Collagenase P was purchased from Boehringer-Manheim (Germany). All other chemicals were of the highest grade commercially available.
Data analysis
All data from the simultaneous measurements of [Ca2+]i and tension were collected by a computerized data acquisition system (MacLab; Analog Digital instruments, Castle Hill, Australia: Macintosh, Apple Computer, Cupertino, CA, U.S.A.). The data for the representative traces shown in the figures were printed directly from the computer using a laser printer (Laser-Writer II NTX-J, Apple Computer). The data are expressed as the means±s.e.means of the indicated numbers of experiments. One strip obtained from one animal was used for each experiment, therefore the number of experiments (n value) indicates the number of animals. Statistical analysis was performed using unpaired Student's t-tests and P values of less than 0.05 were considered to be significant.
Results
The effect of FK506 on the increases in [Ca2+]i and tension induced by high K+ depolarization
Figure 1 shows the effect of 10 μM FK506 on the increases in [Ca2+]i and tension induced by 118 mM K+ depolarization in porcine coronary arterial medial strips. As shown in Figure 1a, when the bathing solution was changed from normal (5.9 mM K+) PSS to 118 mM K+ PSS, [Ca2+]i rapidly increased to produce a sharp peak and then declined slightly to the plateau phase within 10 min. Tension also developed rapidly to the plateau phase. The levels of [Ca2+]i and tension at the plateau phase were both assigned as 100%. The bathing solution was then changed to normal PSS. At 30 min incubation in normal PSS, subsequent stimulation with 118 mM K+ depolarization in the absence of FK506 yielded responses of [Ca2+]i (96.4±2.3%, n=10) and tension (103.6±1.5%, n=10) similar to those obtained for the first response to 118 mM K+ depolarization (Figure 1a).
Figure 1.
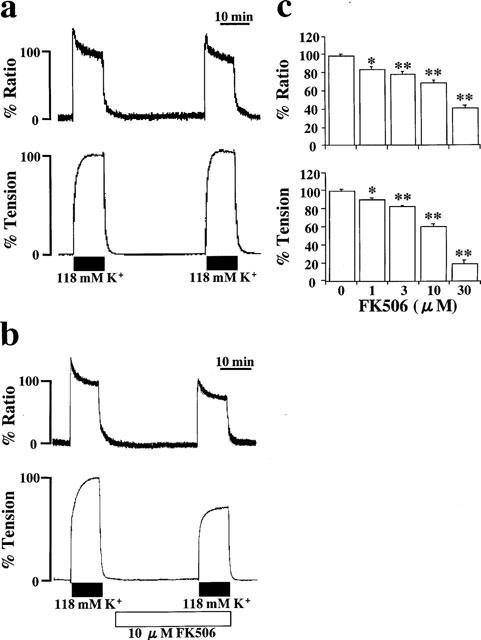
Effects of FK506 on the contraction induced by 118 mM K+-depolarization. (a and b) Representative recordings showing the changes in [Ca2+]i and tension induced by 118 mM K+ depolarization in the absence (a) and the presence (b) of 10 μM FK506. FK506 was applied 30 min prior to the stimulation of 118 mM K+-depolarization. (c) Concentration-dependent effects of FK506 on the increases in [Ca2+]i (upper panel) and tension (lower panel) induced by 118 mM K+-depolarization. Data are mean±s.e.mean. (n=4–10). *, **, significantly different from the values obtained in the absence of FK506 (*P<0.05: **P<0.01).
The application of 10 μM FK506 had no effect on [Ca2+]i or tension at the resting state (Figure 1b). The subsequent stimulation with 118 mM K+ depolarization at 30 min after application of FK506 caused increases in [Ca2+]i and tension, which were smaller than those observed in the absence of FK506. The levels of [Ca2+]i and tension at the plateau phase of contraction in the presence of 10 μM FK506 were 68.6±3.7% and 61.4±2.4% (n=7), respectively. Despite the removal of FK506 from the bathing solution, the inhibitory effect on the contraction remained for about 1 h and was thereafter completely reversed (data not shown). There was no difference in the inhibitory effect on high K+-depolarization-induced contraction between 60 min and 30 min pretreatment with FK506, while there was only an apparently smaller inhibition in the case of 15 min pretreatment (data not shown). Therefore, the effect of FK506 was evaluated using a 30 min pretreatment with FK506.
Figure 1c summarizes the concentration-dependent effect of FK506 on the increases in both [Ca2+]i and tension induced by 118 mM K+ depolarization. A significant inhibition of both [Ca2+]i and tension were observed at 1 μM and higher concentrations. The maximum inhibition was not observed at 30 μM FK506, which was the highest concentration available in less than 0.1% v/v ethanol vehicle. The profile of the concentration-dependent inhibition [Ca2+]i appeared to be similar to that observed on the tension response. Thus, the decreases in tension were well correlated with the decrease in [Ca2+]i in the FK506-induced relaxation.
The effects of FK506 on [Ca2+]i elevation and tension development induced by U46619
In order to compare the effect of FK506 on the voltage-operated Ca2+ channels with that on other Ca2+-influx pathways, U46619, a thromboxane A2 mimetic, was used to induce contraction in porcine coronary artery. Thromboxane A2 is a platelet-derived potent vasoconstrictor and could play an important role in cardiac allograft rejection (Khirabadi et al., 1985), in which blood coagulation and platelet aggregation are occasionally seen. In the porcine coronary artery, the application of 100 nM U46619 induced considerable rapid increases in both [Ca2+]i and tension, which reached the steady state level within 10–15 min. The steady state level was maintained for more than 1 h (Figure 2a). At 70 min of application, the level of tension (95.4±1.8%, n=5) induced by 100 nM U46619 was similar, while the level of [Ca2+]i (57.7±6.8%, n=5) was significantly lower than those obtained with 118 mM K+ depolarization. FK506 (30 μM) was applied at 10 min and during the steady state of U46619-induced contraction, inducing gradual decreases in [Ca2+]i and tension (Figure 2b). At 60 min, the inhibitory effect on [Ca2+]i and tension reached a maximal and steady level. The effect of FK506 was thus evaluated at 60 min after the application. Figure 2c summarizes the concentration-dependent inhibitory effects of FK506 on the contraction induced by U46619. FK506 (1 μM) had no effect on the increase in [Ca2+]i and tension induced by 100 nM U46619. At 10 μM, FK506 inhibited the tension developed from 95.4±1.8% (n=5) to 78.9±2.8% (n=7). FK506 thus inhibited the contraction induced by 118 mM K+ depolarization more potently than that induced by 100 nM U46619.
Figure 2.
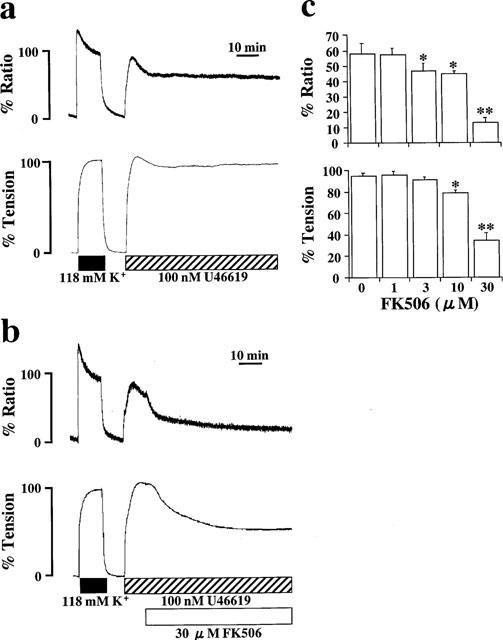
Effects of FK506 on the contraction induced by U46619. (a and b) Representative recordings of the changes in [Ca2+]i and tension induced by 100 nM U46619 in the absence (a) and the presence (b) of 30 μM FK506. FK506 was applied 10 min after the application of 100 nM U46619. (c) Concentration-dependent effects of FK506 on the steady-state [Ca2+]i elevation and tension development induced by 100 nM U46619. The data were obtained at 60 min after the application of FK506. Data are mean±s.e.mean. (n=4–7). *, **, significantly different from the values obtained in the absence of FK506 (*P<0.05; **P<0.01).
The effects of FK506 on [Ca2+]i and tension development induced by endothelin-1 or histamine in normal PSS
The effects of FK506 on contractions induced by endothelin-1 or histamine were examined (Figure 3). FK506 was applied 30 min prior to and during the agonist-induced contractions. Stimulation of strips with 10 nM endothelin-1 in the absence of FK506 induced rapid increases in [Ca2+]i and tension followed by a slight decline (Figure 3a). The maximum levels of [Ca2+]i increase and tension development induced by 10 nM endothelin-1 were 91.8±3.9% and 136.9±6.0% (n=7), respectively. At 60 min of application, the levels of [Ca2+]i and tension were 71.9±3.7% and 108.0±7.0% (n=7), respectively. In the presence of 10 μM FK506, although the initial increase in [Ca2+]i and tension was similar, [Ca2+]i and tension during the steady state were significantly smaller than those obtained in the absence of FK506. The levels of [Ca2+]i and tension at the peak were 88.3±12.1% and 122.7±3.8% (n=9), respectively. The levels of [Ca2+]i and tension at 60 min were 45.4±4.1% and 69.0±8.6% (n=9), respectively. On the other hand, 10 μM histamine induced rapid increases in both [Ca2+]i and tension followed by a gradual decline (Figure 3b). In the absence of FK506, the maximum levels of [Ca2+]i increase and tension development induced by 10 μM histamine were 103.4±5.7% and 122.2±6.8% (n=7), respectively. The levels of [Ca2+]i and tension at 60 min were 33.9±7.7% and 32.3±6.9% (n=7), respectively. Treatment with 10 μM FK506 significantly inhibited both the initial contraction (80.7±4.0% for [Ca2+]i; 110.0±5.4% for tension, n=11) and the following sustained contraction (25.0±5.8% for [Ca2+]i; 18.6±5.1% for tension at 60 min, n=11).
Figure 3.
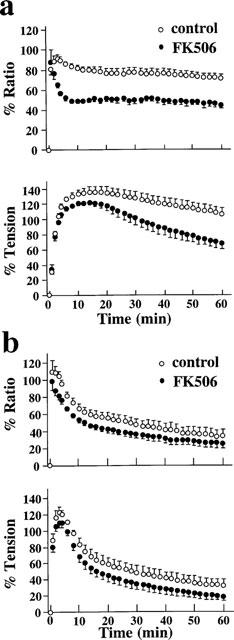
Effects of 10 μM FK506 on the increases in [Ca2+]i and tension induced by 10 nM endothelin-1 (a) and 10 μM histamine (b) in normal PSS. Time courses of changes in [Ca2+]i and tension in the presence and absence of FK506 were summarized. FK506 was applied 30 min prior to the applications of endothelin-1 or histamine. Data are mean±s.e.mean. (n=5–9).
The effects of FK506 on the Ca2+ release induced by caffeine and histamine in the Ca2+-free solution
The effect of FK506 on Ca2+ release from intracellular stores was examined by using caffeine and histamine as stimuli to induce two different mechanisms of Ca2+ release. Figure 4a shows representative recordings showing the effect of 20 mM caffeine on [Ca2+]i and tension of the porcine coronary arterial strips in the Ca2+-free PSS. Changing the bathing solution (normal PSS) to the Ca2+-free PSS containing 2 mM EGTA decreased basal [Ca2+]i level to −24.8±1.2% (n=5) in 10 min, whereas the resting tension was unchanged. The following stimulation with 20 mM caffeine induced transient increases in [Ca2+]i and tension (Figure 4a). The peak levels of [Ca2+]i and tension were 8.5±2.4% and 5.6±0.8% (n=5), respectively (Figure 4c). FK506 (10 μM) was applied 30 min before the application of caffeine (Figure 4b). The basal [Ca2+]i level decreased to −22.3±1.7% (n=6) in Ca2+-free PSS containing 10 μM FK506. The peak levels of [Ca2+]i and tension induced by 20 mM caffeine in the presence of 10 μM FK506 were 7.1±2.6% and 3.6±0.8% (n=6), respectively (Figure 4c). These values did not significantly differ from those obtained in the absence of FK506. The caffeine induced increases in [Ca2+]i and tension were concentration-dependent with the minimum concentration required to induce a maximum response being 20 mM (data not shown). The effects of FK506 on caffeine-induced contractions were also examined at the submaximal concentration, i.e., 10 mM caffeine. The peak levels of [Ca2+]i elevation and tension induced by 10 mM caffeine were −4.2±0.7% and 1.8±0.8% (n=4), respectively (Figure 4d). Treatment with 10 μM FK506 had no significant effects on these levels (−5.1±3.9% for [Ca2+]i; 1.7±0.5% for tension, n=5).
Figure 4.
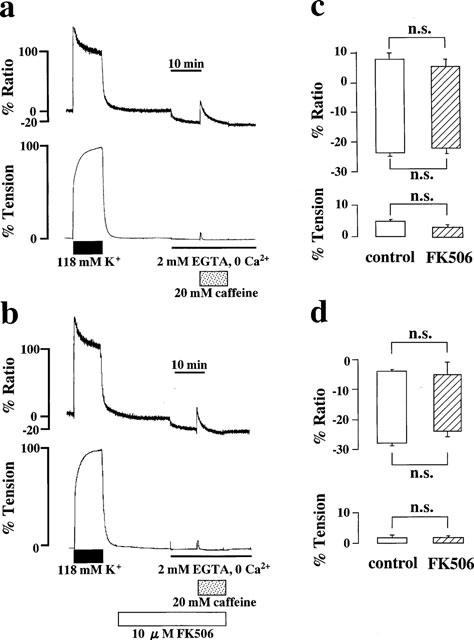
Effects of FK506 on the Ca2+ release induced by caffeine. (a and b) Representative recordings showing the elevation of [Ca2+]i and tension induced by 20 mM caffeine in Ca2+-free PSS containing 2 mM EGTA, in the absence (a) and presence (b) of 10 μM FK506. FK506 was applied 30 min prior to the application of caffeine. (c and d) Summary of the transient elevations in [Ca2+]i and tension induced by 20 mM (c) and 10 mM (d) caffeine in Ca2+-free PSS containing 2 mM EGTA in the absence and presence of 10 μM FK506. Bottom of the column indicates the level of [Ca2+]i and tension just before the application of caffeine. Top of the column indicates the peak level of [Ca2+]i and tension induced by caffeine. Data are mean±s.e.mean. (n=6). n.s., not significant difference.
The effects of FK506 on the Ca2+ release induced by 10 μM histamine were examined similarly (Figure 5). Stimulation with 10 μM histamine in the Ca2+-free PSS containing 2 mM EGTA induced transient increases in [Ca2+]i and tension, with the peak levels being 8.9±5.5% and 49.8±4.5% (n=9), respectively (Figure 5a and c). In the presence of 10 μM FK506, histamine-induced increases in [Ca2+]i(−1.3±5.1%, n=11) appeared smaller than that observed in the absence of FK506 but there was no significant difference between them. Tension development seen in the presence of FK506 (34.5±2.7%, n=11) was significantly smaller than that seen in the absence of FK506 (Figure 5b and c).
Figure 5.
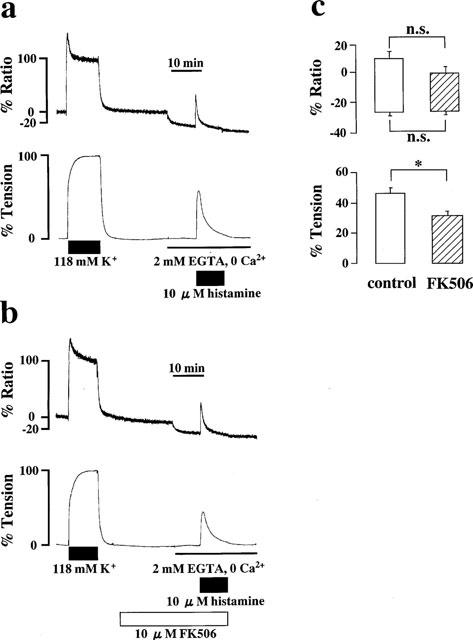
Effects of FK506 on the Ca2+ release induced by histamine. (a and b) Representative recordings of the [Ca2+]i elevation induced by 10 μM histamine in the Ca2+-free PSS in the absence (a) and the presence (b) of 10 μM FK506. (c) Summary of the transient elevations of [Ca2+]i and tension induced by 10 μM histamine in Ca2+-free PSS containing 2 mM EGTA, in the absence and the presence of 10 μM FK506. Bottom of the column indicates the level of [Ca2+]i and tension observed just before the application of 10 μM histamine. Top of the column indicates the peak level of [Ca2+]i and tension induced by 10 μM histamine. Data are mean±s.e.mean. (n=9–11). *P<0.05. n.s., not significant difference.
The effects of FK506 on the [Ca2+]i-tension relationships during the contraction induced by high K+ depolarization and U46619
To clarify the effect of FK506 on the Ca2+ sensitivity of the contractile apparatus of the coronary arterial smooth muscle, we examined the [Ca2+]i-tension relationship of the contractions induced by the cumulative applications of extracellular Ca2+ during stimulation with 118 mM K+ depolarization and 100 nM U46619. Figure 6 shows representative recordings of the changes in [Ca2+]i and tension observed in the absence of FK506. The strips were first exposed to Ca2+-free PSS containing 2 mM EGTA for 10 min and then to Ca2+-free PSS without EGTA for 5 min before stimulation with 118 mM K+ (Figure 5a) and U46619 (Figure 5b). When extracellular Ca2+ was applied cumulatively from 0–2.5 mM, graded elevations of [Ca2+]i and tension were observed. During exposure to 118 mM K+ depolarization, [Ca2+]i and tension increased to 110.3±6.9% and 111.9±3.3% (n=10), respectively at an extracellular Ca2+ concentration of 2.5 mM. During stimulation with 100 nM U46619, the levels of [Ca2+]i and tension reached 68.2±5.2% and 91.8±7.3% (n=6), respectively at 2.5 mM extracellular Ca2+. When the effects of FK506 on these contractions were examined, 10 μM FK506 was applied 30 min prior to the cumulative application of extracellular Ca2+. Treatment with 10 μM FK506 inhibited the increases in [Ca2+]i and tension following exposure to both 118 mM K+ depolarization and 100 nM U46619. [Ca2+]i and tension increased to 69.3±5.4% and 67.9±4.2% (n=8) at 2.5 mM extracellular Ca2+ during 118 mM K+ depolarization in the presence of FK506, respectively. During the stimulation with U46619 in the presence of FK506, [Ca2+]i and tension increased 52.1±4.6% and 78.1±10.1% (n=6) at 2.5 mM extracellular Ca2+, respectively.
Figure 6.
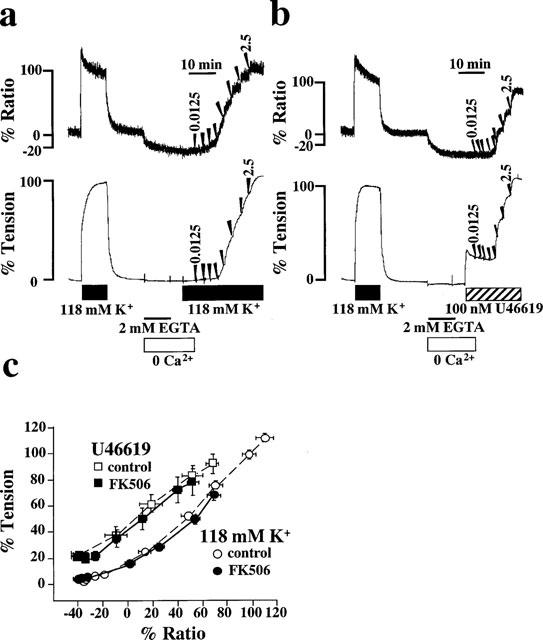
Effects of FK506 on the [Ca2+]i-tension relationships in porcine coronary arterial smooth muscle. (a and b) Representative recordings of the changes in [Ca2+]i and tension induced by the cumulative applications of extracellular Ca2+ from 0 to 2.5 mM during 118 K+ depolarization (a) and the stimulation with 100 nM U46619 (b). After 10 min incubation in the Ca2+-free PSS containing 2 mM EGTA, the strip was exposed to the Ca2+-free PSS without EGTA for 5 min and then the extracellular Ca2+ was cumulatively applied to obtain concentations of 0.0125, 0.025, 0.05, 0.125, 0.25, 0.5, 1.25 and 2.5 mM. When the effects of FK506 were examined, 10 μM FK506 was applied 30 min prior to the application of extracellular Ca2+. (c) The [Ca2+]i-tension relationships of the contractions induced by the cumulative application of extracellular Ca2+ during 118 mM K+ depolarization and the stimulation with 100 nM U46619 in the presence or absence of 10 μM FK506. Data are mean±s.e.mean. (n=6–11).
The effect of FK506 on the Ca2+ sensitivity was evaluated by examining the [Ca2+]i-tension relationships of these contractions (Figure 6c). In the absence of FK506, the [Ca2+]i-tension relation curve of contraction obtained during stimulation with U46619 was to the left of that obtained with 118 mM K+ depolarization. In both cases, the [Ca2+]i-tension relation curves obtained in the presence of 10 μM FK506 overlapped with those obtained in the absence of FK506. Thus, FK506 did not shift these [Ca2+]i-tension relation curves (Figure 6c).
The effect of FK506 on the Ca2+ channel current in the porcine coronary arterial smooth muscle cells
To investigate the mechanism of FK506-induced inhibition of [Ca2+]i elevation, we performed whole cell voltage clamp experiments of the dispersed porcine coronary arterial smooth muscle cells. When 90 mM Ba2+ solution was superfused in the bath and the pipette was filled with 135 mM Cs+solution, a depolarizing pulse to 0 mV from the holding potential of −80 mV evoked an inward current. The mean amplitude of the inward current was 236±79 pA (n=6). Nicardipine (1 μM) completely inhibited this inward current, indicating the involvement of a Ca2+ channel current (data not shown). Figure 7a shows the effects of FK506 on the inward current of the porcine coronary artery. Application of 30 μM FK506 immediately inhibited the peak amplitude of the inward current (Figure 7a inset) and the maximum inhibition of the current was obtained 5–10 min after application of FK506 (Figure 7b). Current recovery was not observed within 10 min following removal of FK506. The mean amplitude in the presence of 30 μM FK506 was 78.6±8.5% of the control (n=6; peak height). A lower concentration of FK506 (10 μM) did not show any significant inhibition (105.1±12.5% of the control, n=3) (Figure 7c). We also examined the effect of FK506 on the K+ channel current. An outward K+ current was evoked by a depolarizing pulse to 0 mV, and FK506 (30 μM) inhibited this current (Figure 7b inset). The outward K+ current was restored by removal of FK506 (Figure 7b). The mean amplitude in the presence of 30 μM FK506 was 59.7±6.1% of the control (n=6; peak height), and of 10 μM FK506 was 81.3±6.3% of the control (n=3; peak height) (Figure 7c).
Figure 7.
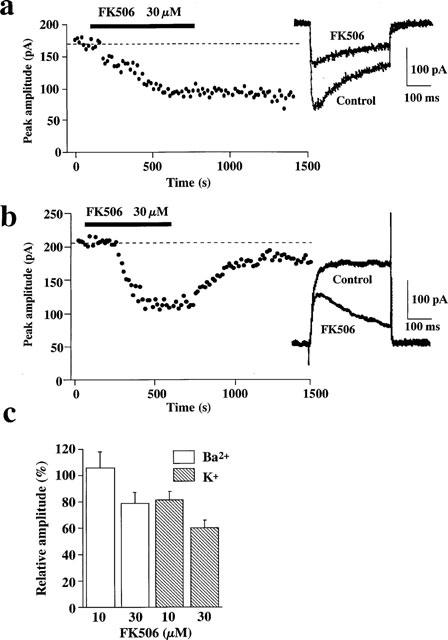
Effects of FK506 on the Ba2+ and K+ current evoked by a depolarizing pulse to 0 mV (the holding potential of −80 mV). (a and b) Time course of the Ba2+ (a) and K+ (b) currents inhibition induced by 30 μM FK506. Each point indicates the peak amplitude of the current. A depolarizing pulse to 0 mV was applied every 15 s. The broken line indicates mean amplitude of the Ba2+ and K+ currents before application of FK506 (control). FK506 (30 μM) was applied during the periods indicated by bar. Representative traces obtained in the presence and absence of 30 μM FK506 are shown in inset. Each trace is an average of ten traces before (control) and 7–10 min after application of FK506. (c) Inhibitory effects of FK506 on the Ba2+ and K+ currents. The amplitudes of both currents were expressed in a relative manner, assigning the values recorded prior to the application of FK506 (control) to be 100%. Data are mean±s.e.mean. (n=3–6).
Discussion
In the present study, we found that FK506, an immunosuppressant widely used in organ transplantation, relaxed porcine coronary artery. The relaxing effect of FK506 observed in this study is consistent with previous observations on the canine basilar artery (Nishizawa et al., 1993). In the present study, by using the front surface fluorometry of fura-2 and electrophysiological measurement, we found that; (1) FK506 decreased [Ca2+]i and caused relaxation during the contractions induced not only by high K+ depolarization but also by agonists such as U46619, histamine and endothelin-1. FK506 decreased [Ca2+]i during the sustained phase of contractions which are dependent on extracellular Ca2+, suggesting that the decrease in [Ca2+]i was due to inhibition of Ca2+ influx. (2) There were no effects on the relationship between [Ca2+]i and tension, indicating that the reduction of [Ca2+]i is the major mechanism of FK506-induced relaxation. Ca2+ sensitivity of the contractile apparatus was not affected. (3) The Ca2+ release induced by caffeine was not altered by FK506. (4) FK506 inhibited inward current induced by depolarizing pulse in the whole cell voltage clamp experiments, indicating that FK506 inhibits a voltage-operated L-type Ca2+ channel (VOC). It is thus suggested that the mechanism of relaxation induced by FK506 is analogous to that of Ca2+ channel blockers such as diltiazem and verapamil (Hirano et al., 1990).
High external K+ solution depolarizes membrane potential, activates VOC and induces sustained increases in [Ca2+]i and tension in vascular smooth muscle (Hirano et al., 1990). In the present study, FK506 inhibited the sustained phase of [Ca2+]i elevation induced by high K+ depolarization, indicating the inhibition of VOC by FK506. In the case of agonist-induced contractions, at least four different mechanisms should be considered for Ca2+ influx pathways. The first and second mechanisms are activation of VOC directly by agonist-induced membrane depolarization, and indirectly by agonist-activated intracellular second messengers or trimeric G proteins (Casteels & Suzuki, 1980; Miyoshi & Nakaya, 1991; Pacaud et al., 1991; Scornik & Toro, 1992). U46619 was shown to depolarize membrane potential of porcine coronary artery (Scornik & Toro, 1992). The third mechanism involves so-called receptor-operated Ca2+channels (Bolton, 1979) and the fourth mechanism involves the capacitative Ca2+ influx pathway (Parekh & Penner, 1997; Putney, 1990). FK506 had no significant effects on the activity of the capactitative Ca2+ influx induced by thapsigargin (data not shown). Since FK506 inhibited K+ depolarization-induced contractions more potently than U46619-induced contractions, FK506 was suggested to inhibit mainly VOC following contraction induced by depolarization and by agonists. The inhibitory effects of FK506 on VOC was clarified in the electrophysiological experiments. FK506 also inhibited K+ channel current (Figure 7b and c). Thus, the effects of FK506 on ion channels were not selective to VOC. However, we consider the inhibition of Ca2+ channel to be essential for the relaxation of porcine coronary arterial smooth muscle cells because inhibition of K+ channel current in the depolarized state is regarded to have no effect on the activity of VOC or on the membrane potential. On the other hand, inhibition of Ca2+ channels, which is a direct cause of decrease in [Ca2+]i induces relaxation of smooth muscle in the present study. However, the precise mechanism of inhibition of Ca2+ influx by FK506 remains to be elucidated. There is no similarity in chemical structure between FK506 and known Ca2+ channel blockers. The type 2B protein phosphatase was shown to be involved in the Ca2+-dependent inactivation of L-type Ca2+ channels in smooth muscle (Schuhmann et al., 1997). Since the concentration of FK506 required to inhibit VOC was much higher than that required to inhibit the phosphatase, it is possible that the FK506-induced inhibition of the Ca2+ influx is not mediated by inhibition of the phosphatase.
It has been recently shown that FKBP12 and FKBP12.6 are associated with the RyR and stabilized the activity of Ca2+ release channels in skeletal and cardiac muscle, respectively (Brillantes et al., 1994; Kaftan et al., 1996). In skeletal muscle, FK506 dissociated FKBP from RyR, enhanced Ca2+release and increased contractility (Brillantes et al., 1994). However, in the present study, FK506 had no effect on the caffeine-induced Ca2+ release. This finding suggests that, in smooth muscle cells, FKBP may play only a minor role, if any, in the regulation of the channel activity of RyR. It was reported that smooth muscle cells express RyR3, which is different from skeletal (RyR1) or cardiac (RyR2) isoforms (McPherson & Campbell, 1993). This difference in RyR isoform may be linked to the difference in sensitivity to inhibition by FK506. On the contrary, FK506 significantly inhibited the tension development induced by histamine in the Ca2+-free PSS, while the inhibition of [Ca2+]i elevation was not statistically significant. In the presence of extracellular Ca2+, the initial increases in [Ca2+]i and tension induced by histamine were inhibited by FK506. It has been shown that FKBP12 formed a functional complex with IP3R isolated from rat cerebellum and that FK506 dissociated this complex and increased Ca2+ flux through IP3R (Cameron et al., 1995). Therefore, it is possible that the inhibition of histamine-induced Ca2+ release is not due to the dissociation of an FKBP-IP3R complex. There is a possibility that FK506 inhibits either histamine binding to the receptor, receptor-G protein interaction or phospholipase C. These possibilities remain to be elucidated. However, since the transient contraction induced by endothelin-1 was not inhibited by FK506, it is unlikely that FK506 worked as a non-selective antagonist. Regarding the observation that the endothelin-induced Ca2+ release, as well as the caffeine-induced Ca2+ release is resistant to FK506, it is noteworthy that endothelin-1 induced Ca2+ release from the caffeine-sensitive store site in the cultured rat aortic smooth muscle cells (Kai et al., 1989).
Alteration of the Ca2+ sensitivity of the contractile apparatus is now considered to be one of the important regulatory mechanisms of smooth muscle contraction (Somlyo & Somlyo, 1994). However, the regulatory mechanisms of Ca2+ sensitivity remain to be elucidated. Protein phosphatases were shown to play an important role in regulation of Ca2+ sensitivity. Inhibitors of type 1 and type 2A protein phosphatases such as okadaic acid and calyculin-A have been shown to alter Ca2+ sensitivity of the contractile apparatus (Hirano et al., 1989). Myosin phosphatases were isolated from smooth muscle and categorized as type 1 phosphatase (Shimizu et al., 1994; Somlyo & Somlyo, 1994). Thus, type 1 phosphatase is considered to be the major phosphatase involved in regulation of the Ca2+ sensitivity. In the present study, FK506, an inhibitor of type 2B phosphatase, had no effect on the [Ca2+]i-tension relationship. This argues against a major contribution of type 2B phosphatase to the regulation of Ca2+sensitivity of the contractile apparatus of smooth muscle.
There was an apparent discrepancy in potency of FK506 between the tension study and patch clamp experiment. In the patch clamp experiment, gradual and progressive decline of Ca2+ channel activity (run down) made it difficult to examine the effects of FK506 after treatment for more than 30 min. In the tension study, however, the vasorelaxing effects of FK506 required at least 30 min treatment to reach steady state. Therefore, it was necessary to use a higher concentration of FK506 to observe the inhibition of Ca2+ channel activity with shorter treatment in the patch clamp experiment. The differences in temperature (37°C in the tension study vs room temperature in the patch clamp experiment) may have contributed to the difference in potency of FK506 because it has been reported that the binding properties of ligands to Ca2+ channel are affected by temperature (Maan & Hosey, 1987). Moreover, in addition to the inhibition of VOC which was confirmed in the patch clamp experiment, other mechanisms such as inhibition of agonist-induced Ca2+ influx or Ca2+release could have contributed to decrease in [Ca2+]i and force in the tension study. These additional effects of FK506 might have caused apparent higher potency in the vasorelaxation than in the inhibition of channel activity.
The plasma concentrations of FK506 in organ recipients are in the range 0.6–25 nM (Alessiani et al., 1993; Japanese FK506 study group, 1991). These values are consistent with the Ki value for inhibition of type 2B phosphatase activity (Liu et al., 1992). Thus, the concentration shown to induce smooth muscle relaxation was much higher than these values but are similar to those required to dissociate FKBP from RyR and to enhance Ca2+ release in cardiac muscle (Kaftan et al., 1996). However, the high lipophilicity of FK506 and its repetitive usage in patients might cause its accumulation in cells. It is noteworthy that the intracytoplasmic concentrations of FK506 in mouse splenic T cells and Jurkat cells were 10–900 times higher than extracellularly added concentrations (Dumont et al., 1994).
In conclusion, FK506 has been shown to cause relaxation of smooth muscle by decreasing [Ca2+]i mainly via inhibition of Ca2+ influx through VOC. FK506 had no effect on the Ca2+ sensitivity of contractile apparatus and the extent of relaxation was to be expected from the observed reduction in [Ca2+]i. Thus, the mechanism of relaxation induced by FK506 is analogous to that induced by Ca2+ channel blockers. It is unlikely that type 2B protein phosphatase mediates the inhibition of Ca2+ influx by FK506 nor that type 2B protein phosphatase plays an important role in the regulation of the Ca2+sensitivity of contractile apparatus in smooth muscle.
Acknowledgments
We thank Dr Timothy D. Keeley for reading the manuscript. This study was supported in part by Grant-in-aid for Scientific Research (A) (07407022) and (B) (10557072), for Scientific Research on Priority Areas (A) (10177222; 10177228), for Encouragement of Young Scientists (A) (10770308), and for Creative Basic Research Studies of Intracellular Signaling Network from the Ministry of Education, Science, Sports and Culture, Japan, and by The Vehicle Racing Commemorative Foundation, Kimura Memorial Heart Foundation Research Grant, Kaibara Morikazu Medical Science Promotion Foundation, Kanae Foundation for Life & Socio-Medical Science, and Japan Heart Foundation Research Grant.
Abbreviations
- [Ca2+]i
cytosolic Ca2+ concentration
- EGTA
ethyleneglycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid FKBP, FK506 binding protein
- fura-2/AM
fura-2 acetoxymethyl ester
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- IP3
inositol 1,4,5-trisphosphate
- IP3R
IP3 receptor
- PSS
physiological salt solution
- RyR
ryanodine receptor
- VOC
voltage-operated L-type Ca2+ channel
References
- ALESSIANI M., CILLO U., FUNG J.J., IRISH W., ABU-ELMAGD K., JAIN A., TAKAYA S., VAN THIEL D., STARZL T.E. Adverse effects of FK 506 overdosage after liver transplantation. Transplant. Proc. 1993;25:628–634. [PMC free article] [PubMed] [Google Scholar]
- ARMITAGE J.M., KORMOS R.L., FUNG J., STARZL T.E. The clinical trial of FK 506 as primary and rescue immunosuppression in adult cardiac transplantation. Transplant. Proc. 1991;23:3054–3057. [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- BRILLANTES A.-M.B., ONDRIAS K., SCOTT A., KOBLINSKY E., ONDRIASOVA E., MOSCHELLA M.C., JAYARAMAN T., LANDERS M., EHRLICH B.E., MARKS A.R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- CAMERON A.M., STEINER J.P., SABATINI D.M., KAPLIN A.I., WALENSKY L.D., SNYDER S.H. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., SUZUKI H. The effect of histamine on the smooth muscle cells of the ear artery of the rabbit. Pflügers Arch. 1980;387:17–25. doi: 10.1007/BF00580839. [DOI] [PubMed] [Google Scholar]
- CLIPSTONE N.A., CRABTREE G.R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- DUMONT F.J., KASTNER C., IACOVONE F., JR, FISCHER P.A. Quantitative and temporal analysis of the cellular interaction of FK-506 and rapamycin in T-lymphocytes. J. Pharmacol. Exp. Ther. 1994;268:32–41. [PubMed] [Google Scholar]
- FUNG J., ABU-ELMAGD K., JAIN A., GORDON R., TZAKIS A., TODO S., TAKAYA S., ALESSIANI M., DEMETRIS A., BRONSTER O., MARTIN M., MIELES L., SELBY R., REYES J., DOYLE H., STIEBER A., CASAVILLA A., STARZL T. A randomized trial of primary liver transplantation under immunosuppression with FK 506 vs cyclosporine. Transplant. Proc. 1991;23:2977–2983. [PMC free article] [PubMed] [Google Scholar]
- GUERINI D. Calcineurin: not just a simple protein phosphatase. Biochem. Biophys. Res. Commun. 1997;235:271–275. doi: 10.1006/bbrc.1997.6802. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HIMPENS B., MISSIAEN L., CASTEELS R. Ca2+ homeostasis in vascular smooth muscle. J. Vasc. Res. 1995;32:207–219. doi: 10.1159/000159095. [DOI] [PubMed] [Google Scholar]
- HIRANO K., KANAIDE H., ABE S., NAKAMURA M. Effects of diltiazem on calcium concentrations in the cytosol and on force of contractions in porcine coronary arterial strips. Br. J. Pharmacol. 1990;101:273–280. doi: 10.1111/j.1476-5381.1990.tb12700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRANO K., KANAIDE H., NAKAMURA M. Effects of okadaic acid on cytosolic calcium concentrations and on contractions of the porcine coronary artery. Br. J. Pharmacol. 1989;98:1261–1266. doi: 10.1111/j.1476-5381.1989.tb12672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAPANESE FK506 STUDY GROUP Japanese study of FK 506 on kidney transplantation: results of an early phase II study. Transplant. Proc. 1991;23:3071–3074. [PubMed] [Google Scholar]
- JAYARAMAN T., BRILLANTES A.-M., TIMERMAN A.P., FLEISCHER S., ERDJUMENT-BROMAGE H., TEMPST P., MARKS A.R. FK506 binding protein associated with the calcium release channel (ryanodine receptor) J. Biol. Chem. 1992;267:9474–9477. [PubMed] [Google Scholar]
- KAFTAN E., MARKS A.R., EHRLICH B.E. Effects of rapamycin on ryandodine receptor/Ca2+-release channels from cardiac muscle. Circ. Res. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- KAI H., KANAIDE H., NAKAMURA M. Endothelin-sensitive intracellular Ca2+ store overlaps with caffeine-sensitive one in rat aortic smooth muscle cells in primary culture. Biochem. Biophys. Res. Commun. 1989;158:235–243. doi: 10.1016/s0006-291x(89)80203-7. [DOI] [PubMed] [Google Scholar]
- KHIRABADI B.S., FOEGH M.L., RAMWELL P.W. Urine immunoreactive thromboxane B2 in rat cardiac allograft rejection. Transplantation. 1992;39:6–8. [PubMed] [Google Scholar]
- KUROIWA M., AOKI H., KOBAYASHI S., NISHIMURA J., KANAIDE H. Role of GTP-protein and endothelium in contraction induced by ethanol in pig coronary artery. J. Physiol. 1993;470:521–537. doi: 10.1113/jphysiol.1993.sp019873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J., ALBERS M.W., WANDLESS T.J., LUAN S., ALBERG D.G., BELSHAW P.J., COHEN P., MACKINTOSH C., KLEE C.B., SCHREIBER S.L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- LIU J., FARMER JR J.D., LANE W.S., FRIEDMAN J., WEISSMAN I., SCHREIBER S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- MAAN A.C., HOSEY M.M. Analysis of the properties of binding of calcium-channel activators and inhibitors to dihydropyridine receptors in chick heart membranes. Circ. Res. 1987;61:379–388. doi: 10.1161/01.res.61.3.379. [DOI] [PubMed] [Google Scholar]
- MARKS A.R. Calcium channels expressed in vascular smooth muscle. Circulation. 1992;86 suppl III:III-61–III-67. [PubMed] [Google Scholar]
- MARKS A.R.Intracellular calcium-release channels: regulators of cell life and death Am. J. Physiol. 1997272H597–H605.(Heart Circ. Physiol., 41) [DOI] [PubMed] [Google Scholar]
- MCPHERSON P.S., CAMPBELL K.P. The ryanodine receptor/Ca2+ release channel. J. Biol. Chem. 1993;268:13765–13768. [PubMed] [Google Scholar]
- MIYOSHI Y., NAKAYA Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem. Biophys. Res. Commun. 1991;181:700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- NISHIZAWA S., PETERSON J.W., SHIMOYAMA I., IWASAKI K., UEMURA K. Therapeutic effect of a new immunosuppressant, FK-506, on vasospasm after subarachnoid hemorrhage. Neurosurgery. 1993;32:986–992. doi: 10.1227/00006123-199306000-00018. [DOI] [PubMed] [Google Scholar]
- O'KEEFE S.J., TAMURA J., KINCAID R.L., TOCCI M.J., O'NEILL E.A. FK-506 and CsA-sensitive activtion of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- PACAUD P., LOIRAND G., BARON A., MIRONNEAU C., MIRONNEAU J. Ca2+ channel activation and membrane depolarization mediated by Cl− channels in response to noradrenaline in vascular myocytes. Br. J. Pharmacol. 1991;104:1000–1006. doi: 10.1111/j.1476-5381.1991.tb12540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.J., BIRD G.S. The signal for capactitative calcium entry. Cell. 1993;75:199–201. doi: 10.1016/0092-8674(93)80061-i. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., JR Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- SCHUHMANN K., ROMANIN C., BAUMGARTNER W., GROSCHNER K. Intracellular Ca2+ inhibits smooth muscle L-type Ca2+ channels by activation of protein phosphatase type 2B and by direct interaction with the channel. J. Gen. Physiol. 1997;110:503–513. doi: 10.1085/jgp.110.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCORNIK F.S., TORO L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am. J. Physiol. 1992;262:C708–C713. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- SHIMIZU H., ITO M., MIYAHARA M., ICHIKAWA K., OKUBO S., KONISHI T., NAKA M., TANAKA T., HIRANO K., HARTSHORNE D.J., NAKANO T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J. Biol. Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- STARZL T.E., TODO S., FUNG J., DEMETRIS A.J., VENKATARAMMAN R., JAIN A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIMERMAN A.P., OGUNBUMNI E., FREUND E., WIEDERRECHT G., MARKS A.R., FLEISCHER S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. J. Biol. Chem. 1993;268:22992–22999. [PubMed] [Google Scholar]


