Abstract
The effect of prolonged administration of a carboxypeptidase Y-like kininase inhibitor, ebelactone B (EB) (2-ethyl-3, 11-dihydroxy-4, 6, 8, 10, 12-pentamethyl-9-oxo-6-tetradecenoic 1, 3-lactone), on the development of deoxycorticosterone acetate (DOCA)-salt hypertension was tested.
The systolic blood pressure (SBP) of non-treated 6-week-old Sprague-Dawley strain rats was gradually increased by DOCA-salt treatment from 137±2 mmHg (n=11) to 195±7 mmHg at 10 weeks of age.
With daily oral administration of lisinopril (5 mg kg−1, twice a day), which is an inhibitor of angiotensin converting enzyme, a major kininase in plasma, the development of hypertension was not suppressed.
By contrast, administration of EB (5 mg kg−1, twice a day), completely inhibited the development of hypertension (SBP: 146±1 mmHg, n=5, 10 weeks old). The reduced SBP at 10 weeks of age was equal to the SBP before any treatment (142±1 mmHg, n=5).
Direct determination of mean blood pressure (MBP) in conscious, unrestrained rats confirmed that MBP elevation was completely inhibited by EB.
Continuous subcutaneous infusion (5 mg kg−1 day−1) of HOE140, a bradykinin B2 receptor antagonist, restored the elevation of SBP, which was suppressed by EB.
The weights of left ventricle of DOCA-salt treated rats 10-weeks-old (0.36±0.02 g 100 g body weight−1, n=11) was significantly reduced by EB (0.27±0.01, n=5), as were the sodium levels in serum, cerebrospinal fluid and erythrocyte.
These findings suggested that EB is effective in preventing salt-related hypertension presumably by eliminating sodium retention.
Keywords: Ebelactone B, deoxycorticosterone acetate-salt hypertension, bradykinin, kininase
Introduction
A wide variety of antihypertensive agents can reduce established genetic or secondary hypertension, and several antihypertensive agents capable of improving the patient's quality of life are now available. However, fewer drugs have been developed for the prevention of hypertension. Some researchers have tried to predict high blood pressure in childhood so that action can be taken to prevent hypertension in the adults (Zinner et al., 1971; Klein et al., 1977; Holland & Beresford, 1977; Higgins et al., 1980; Levine et al., 1980; Lauer et al., 1991). Even in a genetically hypertensive model, namely, the spontaneously hypertensive rats, it was suggested that the initial phase is critical in the development of hypertension (Unger & Rettig, 1990). In the present experiment, we administered a urinary kininase inhibitor with the aim of preventing the development of hypertension in models in which short-duration administration was effective in reducing high blood pressure (Majima et al., 1995).
We previously reported that the renal kallikrein kinin system showed an antihypertensive action, suppressing the development of hypertension when sodium retention in the body was induced (Majima & Katori, 1994). This was due to kinin generated through the action of kallikrein secreted from the connecting tubules of the kidney (Scicli & Carretero, 1986), and may be potentiated when kinin degradation in the kidney is inhibited. In rat urine, we found a novel urinary kininase, a carboxypeptidase Y (CPY)-kininase, some of whose characteristics resembled those of a carboxypeptidase from yeast (Kuribayashi et al., 1993). This serine protease was a major kininase in rat urine in terms of kinin-degrading activity (Kuribayashi et al., 1993), and is also secreted in human urine (Saito et al., 1995). In addition, a microbial product, ebelactone B (EB), was found. It was isolated from Actinomycetes, and is a potent inhibitor of carboxypeptidase Y-like kininase (Majima et al., 1994a). EB showed kinin-dependent diuretic and natriuretic actions in anaesthetized rats (Majima et al., 1994a), and transiently but significantly reduced the high blood pressure in a deoxycorticosterone acetate (DOCA)-salt model on short-term administration (Majima et al., 1995).
In the present experiment, we tested the preventive effect on the development of hypertension by a prolonged administration of EB from the first day of DOCA-salt treatment.
Methods
Animals
Male Sprague-Dawley strain (SD) rats (specific pathogen-free, 6-weeks-old, SLC, Hamamatsu, Japan) were used. All animals were housed at a constant humidity (60±5%) and temperature (25±1°C), and kept on a 12-h light/12-h dark cycle throughout the experiments. All rats were given normal rat chow containing 0.3% sodium, NMF (Oriental Yeast Corp., Tokyo, Japan). The number of animals (n) used for each experiment is stated in the corresponding section. This study was performed in accordance with the guidelines for animal experiments of Kitasato University School of Medicine.
Induction of hypertension and administration of kininase inhibitors
At 6 weeks of age, the drinking water was replaced with 1% NaCl solution after resection of the left kidney, and weekly subcutaneous administration of deoxycorticosterone acetate solution (5 mg kg−1 week−1, 5 mg ml−1 in physiological saline containing 50 mg ml−1 of gum arabic) was started for 4 weeks as reported previously (Majima et al., 1991).
One day after the start of DOCA-salt treatment, EB (5 mg kg−1, suspended in 1% CMC at a concentration of 15 mg ml−1; a gift from the Institute of Microbial Chemistry, Tokyo, Japan), lisinopril (5 mg kg−1, suspended in 1% CMC at a concentration of 15 mg ml−1; a gift from Shionogi Pharmaceutical Corp., Osaka, Japan) or BP102 (sinorphan, 30 mg kg−1, dissolved in 1% CMC at a concentration of 90 mg/ml, a gift from Shionogi Pharmaceutical Corp., Osaka, Japan) was administered twice a day for 4 weeks by oral administration. BP102 was developed as a prodrug of the neutral endopeptidase (NEP) inhibitor thiorphan, which was the first synthetic inhibitor of NEP (Roques et al., 1980). Control animals received only vehicle solution, and two further control groups were prepared. One group is unilateral nephrectomised rats without 1% NaCl solution and subcutaneous injection of DOCA. Another group is unilateral nephrectomised rats with DOCA-treatment without giving 1% NaCl solution.
Doses used in the present experiment were selected as follows. The previous report (Majima et al., 1995), we administered EB at doses of 5 and 15 mg kg−1 (twice a day). The hypotensive effects were not increased with higher doses. Thus, we selected the dose of 5 mg kg−1. In case of BP102, diuretic effects were not different between the doses of 30 and 100 mg kg−1 (twice a day), suggesting that 30 mg kg−1 was a maximal dose. In the preliminary experiments, lisinopril (5 mg kg−1, twice a day) completely blocked the development of hypertension in young spontaneously hypertensive rats. Thus, this dose was selected in these experiments.
Measurement of blood pressure
The systolic blood pressure (SBP) of unanaesthetized rats was determined twice a week with a tail-cuff plethysmograph (Ueda model UR1000, Ueda Seisakusho, Tokyo Japan) as reported previously (Majima et al., 1991; 1993a; 1994b) twice a week for 4 weeks from the start of experiment. Mean arterial blood pressure (MBP) was determined for 1 h in conscious and unrestrained rats, as reported previously (Majima et al., 1993a; 1994b) 1 day after determination of the SBP. The rats were anaesthetized with light ether anaesthesia soon after the SBP determination by tail-cuff method, and a polyethylene cannula (PE-10, Clay Adams, Parsippany, NJ, U.S.A.) was inserted into the abdominal aorta through the femoral artery under light ether anaesthesia and the cannula was connected to a PE-50 cannula (Clay Adams, Parsippany, NJ, U.S.A.) and exteriorized in the interscapular region.
On the next day, a blood pressure transducer (TP-200T, Nihon Kohden, Tokyo, Japan) was attached to the other end of the intra-arterial cannula, and the mean arterial blood pressure was monitored on a polygraph (WS-641-G, Nihon Kohden, Tokyo, Japan) (Majima et al., 1995). Starting 30 min after the connection of the transducer, recordings were made for over 1 h in the rats, which were kept in separate cages.
Weights of left ventricle of heart and of kidney
Immediately after blood collection, rats were exsanguinated and the hearts and kidneys were excised under ether anaesthesia, and were fixed with a 10% formaldehyde solution. After removal of the atrium and right ventricle from the fixed hearts, the left ventricles were weighed (Majima et al., 1993a). The hearts (non-treated) from rats without nephrectomy, which received no salt water were also weighed. The kidneys were also weighed after removal of the capsula fibrosa.
Blood collection
One hour after MBP determination, a half ml of blood was collected from the carotid artery at 10 weeks of age through a cannula into glass tubes without anticoagulant under light ether anaesthesia. Collected blood was left at room temperature for 2 h, and then centrifuged at 1500×g for 15 min at 25°C in order to obtain serum. Blood (1 ml) was also collected directly into tubes containing ice-chilled iso-osmotic lithium chloride solution, for determination of the sodium concentration of the erythrocytes. During the blood collection, there was no volume replacement.
Collection of urine and measurement of urinary levels of sodium
Twenty-four hour urine samples from individual rats were collected using metabolic cages 24 h after determination of SBP at 7 and 9 weeks of age. The volume of urine and drinking water were recorded at the end of the 24 h period. Urinary sodium levels were determined electrometrically using electrodes selective for sodium ions, respectively (Majima et al., 1993a). Sodium balance was approximately calculated as the amounts of sodium excreted in urine over 24 h subtracted from the sodium intake.
Collection of urine and measurement of urinary active kallikrein
Twenty-four hour urine samples from individual rats were collected using metabolic cages after determination of SBP at 7 and 9 weeks of age. The volume of urine and drinking water were recorded at the end of the 24 h period.
The active kallikrein in the 24 h urine was measured using a peptidyl fluorogenic substrate selective for glandular kallikrein, Pro-Phe-Arg-methyl-coumarinylamide (Peptide Institute, Minoh, Osaka, Japan), as reported previously (Majima et al., 1993a). One arbitrary unit was defined as the amount of urinary kallikrein that released 1×10−10 mol 7-amino-4-methylcoumarin from 1 μl of urine in 10 min at 37°C.
Measurement of urinary kinin secretion
Free kinin was measured in the urine collected via catheters (PE-10, Clay Adams, Parsippany, NJ, U.S.A.) inserted into ureters of rats under sodium pentobarbitone anaesthesia (60 mg kg−1, s.c.). During urine collection, physiological saline was infused (6 ml kg−1 h−1) to the femoral vein. The kinin levels were determined with a bradykinin enzyme immunoassay kit (Markit-M, Dainippon Pharmaceutical Corp., Osaka, Japan) after extraction with ethanol (Majima et al., 1991; 1993a; 1994b). Extracted kinin fraction was purified with a Sep-Pak C18 column (Waters Associates, Milford, MA, U.S.A.) (Kauker et al., 1984). Kinin was separated by high-performance liquid chromatography by the method reported previously (Shima et al., 1992; Majima et al., 1993a). Four weeks after the start of DOCA-salt treatment (10-weeks-old), EB (5 mg kg−1, suspended in 1% CMC at a concentration of 15 mg ml−1) was orally administered. Urinary kinin excretion during the first 30 min was determined. The excretion of urinary kinin in control rats which did not receive EB, was also measured.
Measurement of sodium levels in serum and in erythrocytes
The levels of sodium in the sera were determined by ion-selective electrodes, as reported previously (Majima et al., 1991). The sodium concentration in the erythrocytes (RBC[Na]i), which was a marker for sodium retention, was determined using atomic absorption spectrophotometry (McCormic et al., 1989), as reported previously (Majima et al., 1993a; 1994b; 1995). The sodium concentrations in the erythrocytes were expressed as mmol l−1 RBC.
Measurement of sodium levels in cerebrospinal fluid
Immediately before the blood collection, cerebrospinal fluid from rats (Waynfortii, 1988), was obtained by aspiration from the cisterna magna with a 26-gauge needle under light ether anaesthesia. All rats were kept on the sloped stand which was prepared to aspirate the cerebrospinal fluid easily (Waynfortii, 1988). The levels of sodium in the cerebrospinal fluid were determined with an atomic absorption spectrophotometer, as a marker for the sodium retention (Majima et al., 1994b).
Continuous administration of a bradykinin antagonist
A bradykinin antagonist, HOE140 (5 mg kg−1 day−1, dissolved in physiological saline, infusion rate; 12 μl day−1, Peptide Institute) was administered from the first day of DOCA-salt treatment by continuous subcutaneous infusion using a micro-osmotic pump (Alzet model 2002, Alza Corp, Palo Alto, CA, U.S.A.) implanted under the skin of back. Control animals received physiological saline (12 μl day−1) using the same type pump. From the first day of DOCA-salt treatment, EB (5 mg kg−1, twice a day, suspended in 1% CMC at a concentration of 15 mg ml−1) was administered twice a day for 2 weeks by oral administration. SBP was determined by tail cuff method and urinary sodium levels were determined using the method described above (Majima et al., 1993a).
Statistical analysis
Values were expressed as means±s.e.mean. Factorial ANOVA and repeated measures ANOVA with the post-hoc test were used to evaluate the significance of differences. For comparison between two groups, Student's t-test was used. A P value less than 0.05 was considered to be significant.
Results
Effects of kininase inhibitors on systemic blood pressure of DOCA-salt treated rats
The SBP of non-treated SD strain rats (6-weeks-old) was 137±2 mmHg, and DOCA-salt treatment gradually increased the SBP to 155±3 and 195±7 mmHg, at 8 and 10 weeks of age, respectively (Figure 1). The daily oral administration of the angiotensin converting enzyme (ACE) inhibitor lisinopril from the first day of DOCA-salt treatment did not suppress the development of hypertension (156±5 and 210±10 mmHg, at 8 and 10 weeks of age, respectively). The SBP of rats treated with BP102 (30 mg kg−1, p.o.), which is an inhibitor of neutral endopeptidase, remained at low levels (146±1 and 160±4 mmHg, at 8 and 10 weeks of age, respectively). At 10 weeks of age, SBP of BP102-treated rats was slightly increased, and the difference of SBP between at 6 weeks of age and at 10 weeks of age was statistically significant (ANOVA was used to evaluate the significance of differences). By contrast, administration of EB (5 mg kg−1, p.o.), an inhibitor for rat urinary kininase, completely inhibited the development of hypertension (SBP: 138±1 and 146±1 mmHg, at 8 and 10 weeks of age, respectively).
Figure 1.
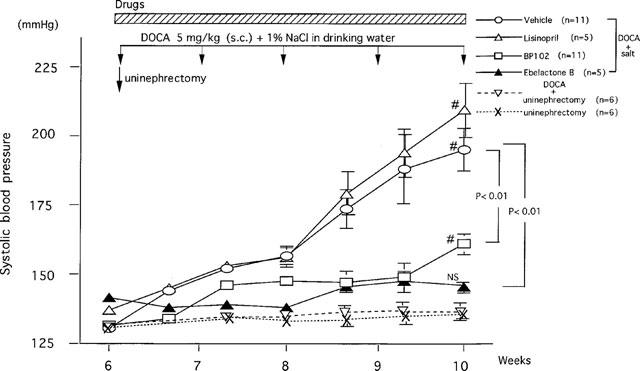
Effects of ebelactone B, BP102 and lisinopril on the development of deoxycorticosterone acetate-salt hypertension. Values are means±s.e.mean. After unilateral nephrectomy at 6 weeks of age, deoxycorticosterone acetate (5 mg kg−1, s.c.) was administered once a week, and ebelactone B (5 mg kg−1), BP102 (30 mg kg−1), or lisinopril (5 mg kg−1) was administered orally twice a day from immediately after the surgery for 4 weeks. Values in rats receiving these three compounds are shown in comparison with those in rats receiving vehicle. The results of unilateral nephrectomized group with DOCA and unilateral nephrectomized group without DOCA-salt also showed. # comparison of values at 6 and 10 weeks of age. ANOVA was used to evaluate the significance of differences.
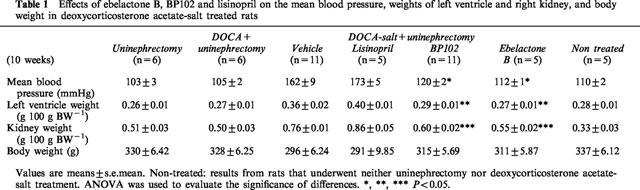
The results from the direct determination of blood pressure in conscious, unrestrained rats 10-weeks-old (Table 1) were confirmed by those from the tail cuff determination. MBP was reduced by EB (112±1 mmHg) to that in the non-treated rats (110±2 mmHg). There was no significant difference between the control groups (non-treated rats, uninephrectomized rats, and uninephrectomized rats with DOCA treatment) and EB-treated rats.
Table 1.
Effects of ebelactone B, BP102 and lisinopril on the mean blood pressure, weights of left ventricle and right kidney, and body weight in deoxycorticosterone acetate-salt treated rats
Effects of kininase inhibitors on weights of left ventricles of heart and kidneys
The left ventricle weight of the vehicle-treated rats (0.36±0.02 g 100 g body weight−1), 4 weeks after the start of DOCA-salt treatment (10-weeks-old) was significantly higher than the left ventricle weight of the EB-treated rats (0.27±0.01 g 100 g body weight−1). The left ventricle weight of the EB-treated rats was the same as the left ventricle weight of non-treated rats (0.28±0.01 g 100 g body weight−1) (Table 1). The left kidney weight of vehicle-treated rats (0.76±0.01 g 100 g body weight−1) (10-weeks-old) was significantly higher than the left kidney weight of the EB-treated rats (0.55±0.01 g 100 g body weight−1).
Effects of kininase inhibitors on water intake, urine volume, and balance of water and sodium
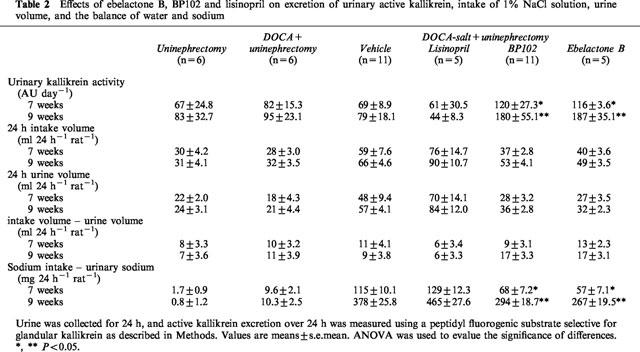
The urine volume at both 7 and 9 weeks of age were not changed significantly with these kininase inhibitors (Table 2). The same was true in water intake in these animals (Table 2). The tentatively calculated values of sodium balance are shown in Table 2. The amounts of sodium excreted in urine over 24 h were subtracted from the sodium intake, which were derived from the drinking water and food in each rat. As shown in Table 2, sodium retention seen in vehicle- and lisinopril-treated rats was significantly suppressed by BP102 and EB at both 7 and 9 weeks of age.
Table 2.
Effects of ebelactone B, BP102 and lisinopril on excretion of urinary active kallikrein, intake of 1% NaCl solution, urine volume, and the balance of water and sodium
Effects of kininase inhibitors on urinary kallikrein excretions
Urinary active kallikrein secretions in BP102-treated rats were 174 and 226% of those in vehicle rats at 7 and 9 weeks of age, respectively, and in EB-treated rats, were 168 and 236% of those in vehicle rats at 7 and 9 weeks of age, respectively. By contrast, lisinopril (89 and 55% of vehicle rats at 7 and 9 weeks of age, respectively) did not increase the excretion of urinary active kallikrein at either age (Table 2).
Effects of a urinary kininase inhibitor, EB on urinary kinin excretion
The amounts of urinary kinin in DOCA-salt treated rats (10-weeks-old) without EB were 113±29 pg 30 min−1 (n=4). With EB (5 mg kg−1, p.o.), urinary kinin excretion was increased to 477±154 pg 30 min−1 during the first 30 min.
Effects of kininase inhibitors on sodium concentration in serum, cerebrospinal fluid and erythrocytes
The serum sodium levels in rats treated with EB (138±1 mmol l−1) and BP102 (136±1 mmol l−1) were significantly reduced, compared with the levels of vehicle-treated rat (142±1 mmol l−1); whereas lisinopril treatment (144± 1 mmol l−1) had no effect on the serum sodium levels (Table 3).
Table 3.
Effects of ebelactone B, BP102 and lisinopril on the sodium levels in the serum, cerebrospinal fluid and erythrocytes
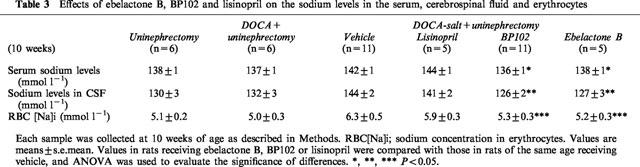
The sodium levels in cerebrospinal fluid in rats treated with EB (127±3 mmol l−1) and BP102 (126±2 mmol l−1) were also less than those in vehicle-treated rats (144±2 mmol l−1). Lisinopril (141±2 mmol l−1) did not reduce the levels (Table 3).
The sodium levels in the erythrocytes of rats treated with EB (5.2±0.3 mmol l−1) and BP102 (5.3±0.3 mmol l−1) were also reduced, compared with vehicle-treated rats (6.3±0.5 mmol l−1). However, lisinopril (5.9±0.3 mmol l−1) showed no significant effect (Table 3).
Effects of HOE140 on systemic blood pressure in EB-treated rats
Continuous subcutaneous infusion of HOE140 to DOCA-salt treated rats, which received oral administration of EB, caused more rapid increase in SBP (167±3 mmHg, n=5, at 8 weeks of age), in comparison with SBP in vehicle-treated rats (145±3 mmHg, n=5, at 8 weeks of age) (Figure 2).
Figure 2.
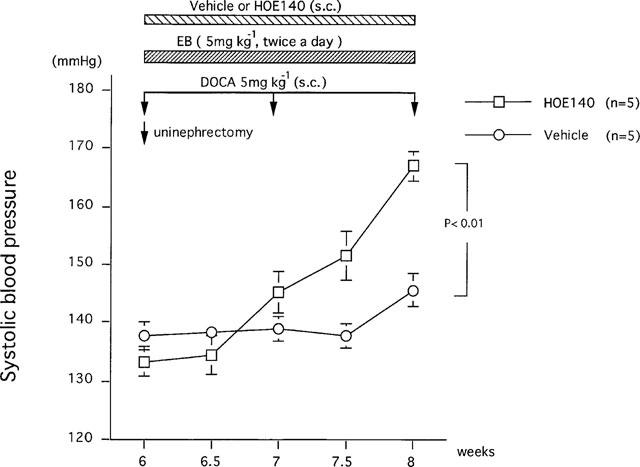
Effects of HOE140 on the development of deoxycorticosterone acetate-salt hypertension in rats under ebelactone B treatment. Values are means±s.e.mean obtained. After uninephrectomy at 6 weeks of age, deoxycorticosterone acetate (5 mg kg−1, s.c.) was administered once a week. Ebelactone B (5 mg kg−1) was administered orally twice a day from the first day of deoxycorticosterone acetate-salt treatment. HOE140 was continuously infused subcutaneously using a micro-osmotic pump (5 mg kg−1 day−1, dissolved in physiological saline). ANOVA was used to evaluate the significance of differences.
Discussion
We previously reported that Brown Norway Katholiek (BN-Ka) rats, which secrete no kinin in the urine because of a deficiency of kininogens, developed DOCA-salt hypertension more rapidly than in normal rats of the same strain (Brown Norway Kitasato rats, BN-Ki rats) (Majima et al., 1991). We found that a major kininase in urine was CPY-like exopeptidase, and was inhibited by a microbial product, ebelactone B, isolated from Actinomycetes (Majima et al., 1994a). EB did not inhibit kininases in plasma. The short-term administration of EB (4–7 days) to DOCA-salt treated rats resulted in transient but significant reductions in SBP and MBP during the developmental stages of DOCA-salt hypertension in normal BN-Ki and SD strain rats, which can generate kinin in the urine, however, kininogen-deficient BN-Ka rats showed no reduction in blood pressure (Majima et al., 1995). These results suggested that EB reduced blood pressure through an action on the kallikrein-kinin system. EB abolished the retention of sodium in the body, with concomitant increases in urinary sodium excretion even in short-term experiments (Majima et al., 1995).
In the present experiment, to test the effects of prolonged administration of EB on the initiation of hypertension in a DOCA-salt hypertensive model, we administered EB from the first day of DOCA-salt treatment throughout the 4-week experimental period (Figure 1). EB completely inhibited the development of hypertension throughout the experimental period and increased urinary kinin excretions. The preventive effect on hypertension development was kinin-dependent, judging from the effect of continuous and simultaneous infusion of HOE140 (Figure 2). By contrast, the ACE inhibitor lisinopril did not reduce the blood pressure. These effects of kininase inhibitors were confirmed from the mean blood pressure determined through the indwelling cannula (Table 1). Furthermore, the weights of left ventricle treated with EB were the same as those of rats without DOCA-salt treatment, although significant increases in the weights of left ventricle were observed in DOCA-salt treated rats receiving only vehicle solutions (Table 1). The increase in left ventricular weight may be a consequence of the increased after-load caused by the elevated arterial blood pressure. The kidney weight in vehicle and lisinopril-treated rats were larger than those in EB- and BP102-treated rats. This may be due to the dilatation of renal tubules which was usually observed in the kidney of DOCA-salt treated rats, which secreted a greater volume of urine.
The lack of any antihypertensive effect of ACE inhibitors in DOCA-salt hypertension in rats, which had been reported already by others (Pham et al., 1993), was also confirmed in the present experiment, even though the administration was started at the pre-hypertensive stage (Figure 1). Although ACE (kininase II) is a predominant kininase in rat plasma (Majima et al., 1993b), and though administration of the ACE inhibitor, captopril causes a significant increase in blood kinin levels (Majima et al., 1994a; Nakagawa & Nasjletti, 1988), lisinopril induced no hypotensive response, suggesting that the increased blood kinin levels were not related to the hypotensive response. Plasma renin activity was suppressed markedly in this DOCA-salt model (Majima et al., 1991). These may be reasons for the lack of the antihypertensive effect of ACE inhibitors in DOCA-salt hypertension.
EB decreased the sodium levels in serum together with those in the cerebrospinal fluids and erythrocytes (Table 3), suggesting that EB prevented sodium retention in the body. The increase in sodium concentration of erythrocyte was reported to be a good marker of sodium retention (McCormic et al., 1989), and the increase in sodium levels in cerebrospinal fluid after intracisternal infusion of high sodium solution caused a continuous increase in systemic blood pressure with the concomitant increase in the sympathetic nerve discharge. The 24 h urine volume in rats receiving vehicle solutions or lisinopril tended to be larger than that in rats treated with EB, possibly because of pressure diuresis, or the increased intake of sodium from drinking water (Table 2). The sodium levels in the cerebrospinal fluid and the erythrocytes may be reduced as a result of the lack of sodium retention. In another hypertensive model, spontaneously hypertensive rats, we preliminary tested the effect of EB. Two weeks administration of EB (15 mg kg−1 day−1) to spontaneously hypertensive rats (4-weeks-old) reduced SBP from 165±3 (vehicle) to 146±3 mmHg (EB) (n=5, P<0.05). These suggested that EB may be effective in a model other than DOCA-salt hypertension.
NEP has been reported to be another major kininase in rat urine (Kuribayashi et al., 1993). BP102, a prodrug of the NEP inhibitor thiorphan, significantly inhibited the development of hypertension (Figure 1). However, its potency was weaker than that of the CPY-like kininase inhibitor EB in spite of using the maximal dosage of BP102 and EB. Since the optimal pH of NEP was around 8, and that of CPY-like kininase around 6, the pH of the urine of rats fed normal chow (around 6) was more suitable for CPY-like kininase to show high protease activity. The actual contribution of CPY-like kininase to the degradation of endogenous kinin may be greater than that of NEP. Thus, EB may exert more complete suppression of the development of DOCA-salt hypertension than BP102. Although the difference of kinetic parameters such as bioavailability was not tested in the present study, the diuretic action of EB was also greater than that of BP102 (Majima et al., 1994a; Nakajima et al., 1998).
We have previously reported that renal kallikrein secretion from DOCA-salt treated rats was increased compared with that from rats receiving no DOCA-salt treatment, and that the kallikrein secretion from DOCA-salt treated animals peaked 3 weeks after the start of DOCA-salt treatment and thereafter declined (Katori et al., 1992). It was reported that DOCA-salt treatment caused renal injury in parallel with the development of hypertension (Dworkin et al., 1984; Raij et al., 1989). The reduction in kallikrein secretion in the late phase of this hypertensive model may be a reflection of the damage to the renal tubules from which the kallikrein was secreted. In the present experiments, there was no significant reduction in the secretion of urinary kallikrein during ACE inhibitor treatments, although some researchers have reported the reduction in the urinary kallikrein secretion after ACE inhibitors (Zacharieva et al., 1996). The reduction in high blood pressure by EB and BP102 may prevent renal injury, because as shown in the present study, urinary secretion of renal kallikrein remained at a higher level in rats receiving these kininase inhibitors (Table 2).
In conclusion, the present results indicated that EB, a urinary CPY-like kininase inhibitor, is a promising agent in terms of the novel concept of preventing the development of hypertension by abolishing sodium retention through the inhibition of kinin degradation.
Abbreviations
- ACE
angiotensin converting enzyme
- CPY
carboxypeptidase Y
- DOCA
deoxycorticosterone acetate
- EB
ebelactone B
- MBP
mean blood pressure
- NEP
neutral endopeptidase
- SBP
systolic blood pressure
- SD
Sprague-Dawley
References
- DWORKIN L.D., HOSTETTER T.H., RENNKE H.G., BRENNER B.M. Hemodynamic basis for glomerular injury in rats with deoxycorticosterone-salt hypertension. J. Clin. Pharmacol. 1984;73:1448–1461. doi: 10.1172/JCI111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGGINS M.W., KELLER J.B., METZNER H.L., MOORE F.E., OSTRANDER L.D. Studies of blood pressure in Tecumseh, Michigan. II. Antecedents in childhood of high blood pressure in young adults. Hypertension. 1980;2 suppl I:I-117–I-123. [PubMed] [Google Scholar]
- HOLLAND W.W., BERESFORD S.A.A.Factors influencing blood pressure in children Epidemiology and Control of Hypertension 1977New York: Grune & Stratton, Inc; 375–386.ed. Paul O. pp [Google Scholar]
- KATORI M., MAJIMA M., MOHSIN SSJ., HANAZUKA M., MIZOGAMI S., OH-ISHI S. Essential role of kallikrein-kinin system in suppression of blood pressure rise during the developmental stage of hypertension induced by deoxycorticosterone acetate-salt in rats. Agent Action. 1992;38 suppl III:235–242. [PubMed] [Google Scholar]
- KAUKER M.L., CROFTON J.T., SHARE L., NASJLETTI A. Role of vasopressin in regulation of renal kinin excretion in Long- Evans and diabetes insipidus rats. J Clin. Invest. 1984;73:824–831. doi: 10.1172/JCI111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN B.E., HENNEKENS C.H., JESSE M.J., GOURLEY J.E., BLUMENTHAL S.Longitudinal studies of blood pressure in offspring of hypertensive mothers Epidemiology and Control of Hypertension 1977New York, Grune & Stratton, Inc; 387–395.Paul O. (ed). pp [Google Scholar]
- KURIBAYASHI Y., MAJIMA M., KATORI M., KATO H. Major kininases in rat urine are neutral endopeptidase and carboxypeptidase Y-like exopeptidase. Biomed Res. 1993;14:191–201. [Google Scholar]
- LAUER R.M., BURNS T.L., CLARKE W.R., MAHONEY L.T. Childhood predictors of future blood pressure. Hypertension. 1991;18 3(suppl I):I-74–I-81. doi: 10.1161/01.hyp.18.3_suppl.i74. [DOI] [PubMed] [Google Scholar]
- LEVINE R.S., HENNEKENS C.H., DUNCAN R.C., ROBERTSON E.G., GOURLEY J.E., CASSADY J.C., GELBAND H. Blood pressure in infant twins: Birth to 6 months of age. Hypertension. 1980;2 suppl:29–33. doi: 10.1161/01.hyp.2.4_pt_2.i29. [DOI] [PubMed] [Google Scholar]
- MAJIMA M., IKEDA Y., KURIBAYASHI Y., MIZOGAMI S., KATORI M., AOYAGI T. Ebelactone B, an inhibitor of urinary carboxypeptidase Y-like kininase, prevents the development of deoxycorticosterone acetate-salt hypertension in rats. Eur. J. Pharmacol. 1995;284:1–11. doi: 10.1016/0014-2999(95)00320-k. [DOI] [PubMed] [Google Scholar]
- MAJIMA M., KATORI M. Sodium accumulation induces hypertension in kininogen-deficient Brown Norway Katholiek rats. Jpn. Heart J. 1994;35:494–495. [Google Scholar]
- MAJIMA M., KATORI M., HANAZUKA M., MIZOGAMI S., NAKANO T., NAKAO Y., MIKAMI R., URYU H., OKAMURA R., MOHSIN SSJ., OHISHI S. Suppression of rat deoxycorticosterone-salt hypertension by the kallikrein-kinin system. Hypertension. 1991;17:806–813. doi: 10.1161/01.hyp.17.6.806. [DOI] [PubMed] [Google Scholar]
- MAJIMA M., KURIBAYASHI Y., IKEDA K., ADACHI H., KATO M., KATORI M., AOYAGI T. Diuretic and natriuretic effect of ebelactone B in anesthetized rats by inhibition of a urinary carboxypeptidase Y-like kininase. Jap. J. Pharmacol. 1994a;65:79–82. doi: 10.1254/jjp.65.79. [DOI] [PubMed] [Google Scholar]
- MAJIMA M., MIZOGAMI S., KURIBAYASHI Y., KATORI M., OH-ISHI S. Hypertension induced by a nonpressor dose of angiotensin II in kininogen-deficient rats. Hypertension. 1994b;24:111–120. doi: 10.1161/01.hyp.24.1.111. [DOI] [PubMed] [Google Scholar]
- MAJIMA M., SHIMA C., SAITO M., KURIBAYASHI Y., KATORI M., AOYAGI T. Poststatin, a novel inhibitor of bradykinin-degrading enzymes in rat urine. Eur. J. Pharmacol. 1993b;232:181–190. doi: 10.1016/0014-2999(93)90772-a. [DOI] [PubMed] [Google Scholar]
- MAJIMA M., YOSHIDA O., MIHARA H., MUTO T., MIZOGAMI S., KURIBAYASHI Y., KATORI M., OH-ISHI S. High sensitivity to salt in kininogen-deficient Brown Norway Katholiek rats. Hypertension. 1993a;22:705–714. doi: 10.1161/01.hyp.22.5.705. [DOI] [PubMed] [Google Scholar]
- MCCORMIC C.P., HENNESSY J.F., RAUCH A.L., BUCKALEW V.M. Erythrocyte sodium concentration and Rb uptake in weaning Dahl rats. Am. J. Hypertens. 1989;2:604–609. doi: 10.1093/ajh/2.8.604. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA M., NASJLETTI A. Plasma kinin concentration in deoxycorticosterone-salt hypertension. Hypertension. 1988;11:411–415. doi: 10.1161/01.hyp.11.5.411. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA S., MAJIMA M., ITO H., HAYASHI I., YAJIMA Y., KATORI M. Effects of a neutral endopeptidase inhibitor, BP102, on the development of deoxycorticosterone acetate-salt hypertension in kininogen-deficient Brown Norway Katholiek rats. Int. J. Tiss. Reac. 1998;XX:45–56. [PubMed] [Google Scholar]
- PHAM I., GOZALEZ W., AMRANI A., OURNIE-ZALUSKI M., PHILIPPE M., LABOULANDINE I., ROQUES B., MICHEL J. Effects of converting enzyme inhibitor and neutral endopeptidase inhibitor on blood pressure and renal function in experimental hypertension. J. Pharmacol. Exp. Ther. 1993;265:1339. [PubMed] [Google Scholar]
- RAIJ L., DALMASSO A.P., STANLEY N.A., FISH A.J. Renal injury in DOCA-salt hypertensive C5-sufficient and C5-deficient mice. Kidney Int. 1989;36:582–592. doi: 10.1038/ki.1989.234. [DOI] [PubMed] [Google Scholar]
- ROQUES B.P., FOURNIE-ZALUSKI M.C., SOROCA E., LECOMTE J.M., MALFROY B., LLORENS C., SCHWARTZ J.C. The enkephalinase inhibitor thiorphan shows antinociceptive activity in mice. Nature. 1980;288:286. doi: 10.1038/288286a0. [DOI] [PubMed] [Google Scholar]
- SAITO M., MAJIMA M., KATORI M., SANJOU Y., SUYAMA I., SHIOKAWA H., KOSHIBA K., AOYAGI T. Degradation of bradykinin in human urine byarboxypeptidase Y-like exopeptidase and neutral endopeptidase and their inhibition by ebelactone B and phosphoramidon. Int. J. Tiss. Reac. 1995;XVII:181–190. [PubMed] [Google Scholar]
- SCICLI A.G., CARRETERO O.A. Renal kallikrein-kinin system. Kidney Int. 1986;29:120–130. doi: 10.1038/ki.1986.14. [DOI] [PubMed] [Google Scholar]
- SHIMA C., MAJIMA M., KATORI M. A stable metabolite, Arg-Pro-Pro-Gly-Phe, of bradykinin in the degradation pathway in human plasma. Jpn. J. Pharmacol. 1992;60:111–119. doi: 10.1254/jjp.60.111. [DOI] [PubMed] [Google Scholar]
- UNGER T., RETTIG R. Development of genetic hypertension: Is there a critical phase. Hypertension. 1990;16:615–616. doi: 10.1161/01.hyp.16.6.615. [DOI] [PubMed] [Google Scholar]
- WAYNFORTII H.B. Experimental and Surgical Technique in the Rat. London, UK, Academic Press; 1988. pp. 59–61. [Google Scholar]
- ZACHARIEVA S., TORBOVA S., ORBETZOVA M., BORISSOVA A.M., ANDONOVA K., SHEITANOVA S. Effects of perindopril treatment on plasma and urine of kallikrein activity and the stable metabolite of prostaglandin E2 in patients with essential hypertension. Meth. Findings Exper. Clin. Pharm. 1996;18:205–209. [PubMed] [Google Scholar]
- ZINNER S.H., LEVEY P.S., KASS E.H. Familial aggregation of blood pressure in childhood. N. Engl. J. Med. 1971;284:401–404. doi: 10.1056/NEJM197102252840801. [DOI] [PubMed] [Google Scholar]


