Abstract
β-Lapachone, a plant product, has been shown to be a novel inhibitor of DNA topoisomerase. In this study, we performed experiments to examine the effects of β-lapachone on lipopolysaccharide (LPS)-induced inducible nitric oxide (NO) synthase (iNOS) in rat alveolar macrophages and aortic rings.
In alveolar macrophages, incubation with LPS (10 μg ml−1) for various time intervals resulted in a significant increase in nitrite production and iNOS protein synthesis, that was inhibited by co-incubation with β-lapachone (1–4.5 μM) without any cytotoxic effects. However, addition of β-lapachone after induction of NO synthase by LPS failed to affect the nitrite production.
Treatment with LPS (10 μg ml−1) for 6 h resulted in significant expression of mRNA for iNOS which was significantly inhibited in the presence of β-lapachone (3 μM) in alveolar macrophages.
In endothelium-intact rings of thoracic aorta, β-lapachone (1 and 3 μM) markedly inhibited the hypocontractility to phenylephrine in aortic rings treated with LPS (10 μg ml−1) for 4 h. When β-lapachone was added 3 h after LPS into the medium, the contractions evoked by phenylephrine were not significantly different in the presence or absence of β-lapachone.
Treatment with LPS (10 μg ml−1) for 4 h resulted in a significant increase in iNOS protein synthesis which was inhibited in the presence of β-lapachone (3 μM), but did not affect the constitutive (endothelial and neuronal) NOS forms in aortic rings.
These results indicate that β-lapachone is capable of inhibiting expression and function of iNOS in rat alveolar macrophages and aortic rings. It is considered that β-lapachone can be developed as a potential anti-inflammatory agent in the future.
Keywords: Lipopolysaccharide, nitric oxide, β-lapachone, inducible NO synthase
Introduction
β-Lapachone (3,4 dihydro-2,2-dimethyl-2H-naphtho-[1,2-b] pyran-5,6-dione), a novel DNA topoisomerase inhibitor (Li et al., 1993), is synthesized by simple sulphuric acid treatment of a natural plant product ‘lapachol', which is readily extracted from Tabecuia avellanedae growing mainly in Brazil, or is easily synthesized from lomatiol, isolated from seeds of lomatia growing in Australia (Goncalves et al., 1980; Schaffner-Sabba et al., 1984). β-Lapachone and its derivatives have been shown to exhibit a number of pharmacological actions including antibacterial, antifungal and antitrypanocidal activities (Goncalves et al., 1980; Guiraud et al., 1994).
Nitric oxide (NO), induced by bacterial lipopolysaccharide or cytokines, plays an important role in macrophage killing of cells (Hibbs et al., 1987; Stuerhr & Nathan, 1989). NO is recognized as an intercellular messenger in the cardiovascular, nervous, muscular and immune systems (Moncada et al., 1991; Murphy et al., 1993; Kobzik et al., 1994). It is through the activation of inducible NO synthase (iNOS) produced in large amounts of NO during endotoxaemia and has been suggested as a mediator of endotoxaemic shock (Lowenstein & Snyder 1992; Vallance & Moncada, 1993) and many other inflammatory conditions (Morris & Billiar, 1994). Drugs, that inhibit iNOS expression or enzyme activity resulting in decreasing NO generation, have beneficial therapeutic effects; examples of such agents include glucocorticoids (Moncada et al., 1991), anti-fungal imidazoles (Bogle et al., 1994) and some tyrosine kinase inhibitors (Joly et al., 1997). In this study, we performed experiments to examine the effects of β-lapachone on the regulation and effects of lipopolysaccharide (LPS)-iNOS in rat alveolar macrophages and aortic rings.
Methods
Rat alveolar macrophage cultures
Alveolar macrophages were obtained by tracheal lavage using modifications of the technique described by Brain & Frank (1968). Briefly, adult male Wistar rats (250–300 g) were anaesthetized with sodium pentobarbital (50 mg kg−1). The trachea was cannulated, and the lungs lavaged repetitively with 5-ml aliquots of sterile cold phosphate buffer solution (PBS). All aliquots were centrifuged for 10 min at 200×g at 4°C. The cells were resuspended in RPMI 1640 medium with 10% foetal bovine serum, penicillin (100 U ml−1) and streptomycin (100 μg ml−1), and cultured at a density of 1.2×106 cells per 60-mm dish at 37°C in 5% CO2 in moist air. Giemsa staining revealed that the alveolar cells were more than 95% macrophages. Cell viability was measured by exclusion of trypan blue. Macrophages grown and treated with either control vehicle (dimethyl sulphoxide, DMSO, less than 0.1% v/v) or LPS (10 μg ml−1) in the presence or absence of β-lapachone (1–4.5 μM) for various time intervals (3, 6, 12, 24, and 48 h).
Nitrite assay
Measurement of nitrite production as an assay of NO release was performed. Accumulation of nitrite in the medium was determined by colorimetric assay with Griess reagent (Green et al., 1982). Aliquots of conditioned media were mixed with an equal volume of Griess reagent (1% sulphanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride in 2% phosphoric acid). Nitrite concentrations were determined by comparison with OD550 of standard solutions of sodium nitrite prepared in cell culture medium.
RT–PCR for iNOS expression
The expression of iNOS was determined by reverse transcription-polymerase chain reaction (RT–PCR) analysis. Approximately 3×106 alveolar macrophages treated with LPS (10 μg ml−1) in the presence or absence of β-lapachone were homogenized with 1 ml of Trizol reagent (GIBCO-BRL), and total RNA was isolated according to the manufacturer's protocol. The first strand cDNA was synthesized by extension of (dT) primers with 200 U of SuperScript II reverse transcriptase (GIBCO) in a mixture containing 1 μg of total RNA digested by RNase-free DNase (2 U μg−1 of RNA) for 15 min at 37°C. The cDNA was then used as a template in a PCR using the Perkin Elmer DNA Thermal Cycler Model 480. PCR was performed in a final volume of 50 μl containing all four dNTPs, MgCl2 (1.5 mM), 2.0 U of AmpliTaq (GIBCO), and each primer (commercial mouse macrophage inducible NO synthase amplimer set, CLONTECH) at 0.4 μM. The amplification cycles were 94°C for 45 s, 65°C for 45 s, and 72°C for 2 min. The PCR products were separated by electrophoresis on a 1.8% agarose gel after 30–35 cycles and visualized by ethidium bromide staining. The mRNA of β-actin served as control for sample loading and integrity.
Rat thoracic aortic rings
Wistar rats (250–300 g) were stunned and killed by exsanguination. Rings, 4–5 mm wide, of thoracic aorta were suspended between two hooks connected to a transducer (Grass FT.03) for the measurement of isometric force. Endothelium-intact rings were used. The rings were suspended in 10 ml organ baths containing oxygenated (95% O2+5% CO2), warmed (37°C) Krebs solution containing (composition in mM) NaCl 118.3, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25.0 and glucose 11.1. Basal tension was set at 1.0 g. The rings were washed every 20 min for three times before a concentration-response curve to phenylephrine was obtained. The rings were incubated with either control vehicle (DMSO), LPS (10 μg ml−1), β-lapachone (1 and 3 μM) or LPS and β-lapachone under resting tension. After 4 h incubation concentration-response curves to phenylephrine (10−9–10−5 M) were constructed. In some experiments, β-lapachone (3 μM) was added 3 h after LPS into the medium. On the other hand, for NOSs immunoblotting studies, the rings after 4 h incubation with either control vehicle (DMSO), LPS (10 μg ml−1), β-lapachone (3 μM) or LPS and β-lapachone, were homogenized in buffer containing (composition in mM) Hepes 20, EDTA 0.5, dithiothreitol 2, phenylmethylsulphonyl fluoride (PMSF) 1, sucrose (0.25 M), 10 μg ml−1 leupeptin and 10 μg ml−1 aprotinin, pH 7.5, and centrifuged at 10,000 r.p.m. for 20 min at 4°C to remove debris. Proteins were determined according to Lowry et al. (1951) with bovine serum albumin as standard.
Western blot analysis
A 30–50 μg sample of cellular lysate protein was subjected to electrophoresis on 8% SDS–polyacrylamide gels for detecting inducible (macrophage and aorta samples) and constitutive (endothelial (eNOS) and neuronal (nNOS), aorta sample) NOS forms. The samples were then electroblotted onto nitrocellulose paper. After blocking, blots were incubated with anti-NOSs antibodies (Transduction Laboratories, U.S.A.) in PBS/Tween 20 for 1 h followed by two washes in PBS/Tween 20 and then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Cappel, U.S.A.) for 30 min. The levels of NOSs protein expression were determined using the enhanced chemiluminescence kit (Amersham, U.K.). The cellular lysates as positive controls were from LPS-treated RAW 264.7 cells (for iNOS), human endothelial cells (for eNOS) and rat pituitary cells (for nNOS). All these positive controls were provided by Transduction Laboratories (U.S.A.). Moreover, α-tubulin served as control for sample loading and integrity.
Cytotoxicity assay
Cytotoxicity was measured by the tetrazolium salt MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay using the modifications of the technique described by Mosmann (1983). Briefly, cells were cultured on 96-well plastic plates in a concentration of 105 cells per well and allowed to grow overnight in RPMI 1640 medium and 10% foetal bovine serum. At 24 h treatment with tested drugs, MTT solution (0.2 mg ml−1) was added to all wells of an assay, and plates were incubated at 37°C for 4 h. The cells were then treated with DMSO for 30 min. The plates were read on a Dynatech MR5000 multiwell scanning spectrophotometer using a test wavelength of 570 nm.
Materials
β-Lapachone was prepared according to the procedures described by Schaffner-Sabba et al. (1984). β-Lapachone was dissolved in DMSO as a stock solution at 10 mM concentration and stored in aliquots at −20°C. The following reagents were obtained from Sigma Chemical (U.S.A.): lipopolysaccharide (E. coli, 055:B5, lyophilized powder prepared by TCA extraction procedure), phenylephrine, sodium nitrite, NG-nitro-L-arginine methyl ester hydrochloride and MTT (tetrazolium salt).
Statistics
The values given are means±s.e.mean. The significance of difference from the respective controls for each experimental test condition was assessed by using one-way analysis of variance (ANOVA) followed by Dunnett's test for each paired experiment. P values<0.05 were regarded as indicating significant differences.
Results
Production of nitrite and iNOS expression
The rat alveolar macrophages were used to study the effects of LPS or combination with β-lapachone on the production of nitrite (a stable oxidation product of NO). The exposure of alveolar macrophages to LPS (10 μg ml−1) for several hours was associated with the accumulation of nitrite in the incubation medium. Treatment of alveolar macrophages with β-lapachone alone had no significant effects on basal levels of nitrite, while elicited a concentration-dependent inhibition of LPS-induced nitrite production (Figure 1a). This inhibitory effect of β-lapachone on LPS-induced nitrite production also appeared to be time dependent. A time course of nitrite accumulation in macrophages treated with LPS (10 μg ml−1) and inhibition by co-incubation with β-lapachone (3 μM) was shown in Figure 1b. However, when macrophages were pre-activated with LPS (10 μg ml−1) for 24 h following which the LPS was removed, and subsequently treated with fresh medium containing β-lapachone (4.5 μM) for a further 24 h, no inhibition of nitrite production was observed (Figure 1c).
Figure 1.
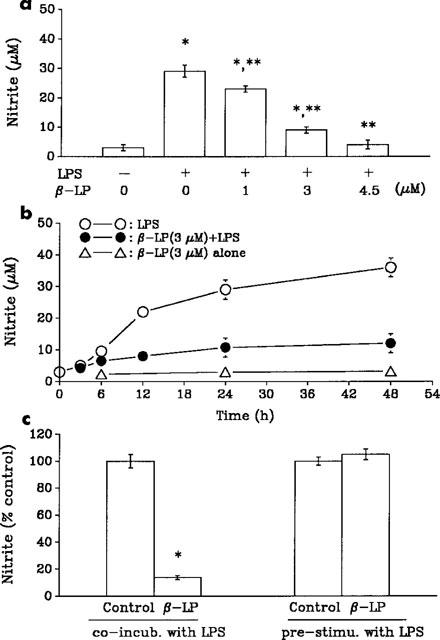
Inhibition of LPS-induced nitrite production. (a) Effects of increasing concentrations of β-lapachone (β-LP) on production of nitrite from alveolar macrophages exposed to LPS (10 μg ml−1) for 24 h. (b) β-LP (3 μM) inhibits the time-dependent increase of LPS-induced NO production in alveolar macrophages. (c) Nitrite production was measured 24 h after co-incubation (co-incub.) of macrophages with LPS and β-LP (4.5 μM) or pre-stimulated (pre-stimu.) of macrophages with LPS for 24 h after which LPS was removed and macrophages subsequently incubated in medium containing β-LP (4.5 μM) for a further 24 h. Data are presented as means±s.e.mean of 3–5 separate experiments performed in triplicate. *P<0.05 as compared with control. **P<0.05 as compared with LPS alone.
For further understanding the effects of β-lapachone on iNOS protein and mRNA expression, Western blot analysis and RT–PCR were used to determine the protein and iNOS mRNA in alveolar macrophages treated with LPS. Exposure of alveolar macrophages to LPS (10 μg ml−1) for 24 h resulted in an induction of iNOS protein. β-Lapachone elicited a concentration-related inhibition of LPS-induced iNOS (Figure 2a). Moreover, as shown in Figure 2b, treatment with LPS (10 μg ml−1) for 6 h resulted in significant expression of mRNA for inducible NO synthase which was significantly inhibited in the presence of β-lapachone (3 μM) in alveolar macrophages.
Figure 2.
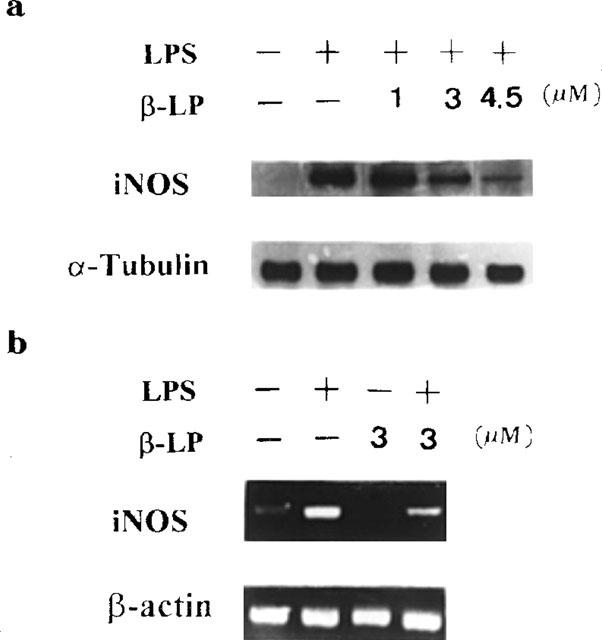
Inhibition of LPS-induced iNOS protein and iNOS mRNA expression. Alveolar macrophages were stimulated with LPS (10 μg ml−1) in the presence or absence of β-lapachone (β-LP, 1–4.5 μM). Cells were harvested at 24 h for Western blot analysis (a) and at 6 h for iNOS mRNA analysis (b). The internal controls of iNOS protein and mRNA were α-tubulin and β-actin respectively. Data are typical of three separate experiments.
On the other hand, treatment with LPS (10 μg ml−1), LPS+β-lapachone (3 and 4.5 μM) or β-lapachone alone (3 and 4.5 μM) for 24 h, did not induce the cytotoxicity in alveolar macrophages using the colorimetric MTT assay (Table 1).
Table 1.
Cytotoxicity assay for β-lapachone in control and LPS-treated rat alveolar macrophages

Studies on rat aortic rings
In intact endothelium aortic rings, phenylephrine caused a concentration-dependent increase in contraction (Figure 3a). Incubation with LPS (10 μg ml−1) for 4 h, shifted the concentration-contraction curve evoked by phenylephrine significantly to the right and decreased the maximal contractile response (Figure 3a). Co-incubation with β-lapachone (1 and 3 μM) significantly reversed the LPS-induced changes in the response to phenylephrine (Figure 3a). The calculated EC50 values and maximal contractile responses in these experiments were shown in Table 2. When β-lapachone (3 μM) was added 3 h after LPS into the medium, the contractions evoked by phenylephrine were not significantly different in the presence (pEC50, 6.35±0.05) or absence (pEC50, 6.42±0.06) of β-lapachone. Treatment of aortic rings with β-lapachone (3 μM) alone had no significant effects on contractions evoked by phenylephrine (data not shown).
Figure 3.

Inhibition of LPS-induced hypovasocontractility and aortic iNOS protein. Rat aortic rings with endothelium were stimulated with LPS (10 μg ml−1) in the presence or absence of β-lapachone (β-LP, 1 and 3 μM) for 4 h. Effects of β-LP on contractions evoked by phenylephrine (a) or inducible and constitutive (endothelial and neuronal) NOS forms (b) in LPS (10 μg ml−1)-treated rings were shown. In Western blot analysis, the cellular lysates as positive controls (C(+)) were from LPS-treated RAW 264.7 cells (iNOS), human endothelial cells (eNOS) and rat pituitary cells (nNOS) and α-tubulin served as control for sample loading and integrity. Data are presented as means±s.e.mean of 3–5 separate experiments in (a) and are typical of three separate experiments in (b).
Table 2.
Effects of β-lapachone on vascular reactivity evoked by phenylephrine in LPS-treated rat aorta rings

For further understanding the involvement of NOSs including inducible and constitutive forms in the aortic ring system, Western blot analysis was used to determine the NOSs proteins expression in the LPS-treated aortic rings in the presence or absence of β-lapachone. The results were shown in Figure 3b, treatment with LPS (10 μg ml−1) for 4 h resulted in a significant increase in iNOS protein synthesis which was inhibited in the presence of β-lapachone (3 μM), but did not affect the constitutive NOSs (eNOS and nNOS) in aortic rings.
Discussion
In this study, we performed experiments to examine the effects of β-lapachone on NO production and iNOS expression induced by LPS in rat alveolar macrophages. In addition, the effects of β-lapachone on LPS-induced aortic relaxation and iNOS protein expression were also studied. We found that low concentrations of β-lapachone were capable of inhibiting all of these effects induced by LPS. These results may have implication for the design of a novel anti-inflammatory agent working through the L-arginine-nitric oxide pathway.
The exposure of rat alveolar macrophage to LPS for several hours was associated with the accumulation of nitrite in the incubation medium. β-Lapachone was capable of inhibiting the production of NO in alveolar macrophages when co-incubated with LPS in a time and concentration-dependent manner, without any evidence for a cytotoxic effect, but was ineffective once inducible NO synthase is expressed by pre-activation with LPS. These results imply that β-lapachone may inhibit induction of NO synthase gene expression. To further investigate this possibility, the protein and mRNA for inducible NO synthase was examined. We found that the iNOS protein and gene expression in alveolar macrophages treated with LPS could be inhibited by β-lapachone. These results indicate that β-lapachone inhibits the induction rather than the activity of NO synthase. This profile of action is similar to that of glucocorticoids which probably act by inhibiting gene expression or the transcription of inducible NO synthase mRNA (Radomski et al., 1990). On the other hand, there is an iNOS induction leading to widespread vasodilatation and reduced responsiveness to vasoconstrictors in septic shock (Vallance & Moncada, 1993). Our results showed that the stimulation of isolated rat aortic rings with LPS for several hours reduced their responsiveness to phenylephrine, as results similar to previous studies (Bogle & Vallance, 1996; Joly et al., 1997), which demonstrated induction of iNOS in vascular rings by LPS. Co-incubation with β-lapachone prevented this vascular hypocontractility. However, β-lapachone failed to inhibit the hypocontractility to phenylephrine once NO synthase was induced by LPS in contrast NG-nitro-L-arginine methyl ester (100 μM), an inhibitor of NOS, did significantly inhibit this response (data not shown). Furthermore, treatment with LPS for 4 h resulted in a significant increase in iNOS protein synthesis which was inhibited in the presence of β-lapachone, but did not affect the constitutive NOSs (eNOS and nNOS) in aortic rings. Thus, β-lapachone likely prevents the induction of a functional active iNOS in the vessel wall as well as macrophages and thus might contribute to the anti-inflammatory effect of this drug.
β-Lapachone has been identified as a potent inhibitor of topoisomerase I (Li et al., 1993). Recent studies showed that β-lapachone is capable of inducing human promyelocytic leukaemia or human prostate cancer cells to undergo programmed cell death (Planchon et al., 1995; Li et al., 1995), but it (1–5 μM) did not induce apoptosis in normal human lymphocytes (Chau et al., 1998) or rat alveolar macrophages (data not shown). DNA topoisomerase has been reported to be involved in the noncytotoxic and cytotoxic cellular processes mediated by tumour necrosis factor (Baloch et al., 1990) or as a cofactor for activator-dependent transcription by RNA polymerase II (Kretzschmar et al., 1993). The inhibition of topoisomerase was also implicated as the mechanism of the immunosuppressive activity of streptonigrin on B-cell proliferation induced by LPS (Suzuki et al., 1996). Thus, the inhibition of topoisomerase may be involved in the action of β-lapachone on LPS-iNOS. However, further studies are required to determine the precise mechanism by which β-lapachone inhibits the induction of NO synthase activity in alveolar macrophages and aorta.
In conclusion, the results of this study demonstrate that the topoisomerase inhibitor β-lapachone prevents the induction of active NO synthase in rat alveolar macrophages and isolated vessels in response to LPS. This finding provides a potential drug for the development of novel anti-inflammatory agents.
Acknowledgments
This work was supported by research grants from National Science Council of the Republic of China (NSC 85 – 2331 – B – 002 – 292).
References
- BALOCH Z., COHEN S., COFFMAN F.D. Synergistic interactions between tumor necrosis factor and inhibitors of DNA topoisomerase I and II. J. Immunol. 1990;145:2908–2913. [PubMed] [Google Scholar]
- BOGLE R.G., SOO S.-C., WHITLEY G.ST.J., JOHNSTONE A.P., VALLANCE P. Effects of anti-fungal imidazoles on mRNA levels and nitric oxide synthesis in cultured murine macrophages. Br. J. Pharmacol. 1994;105:919–923. doi: 10.1111/j.1476-5381.1994.tb14881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGLE R.G., VALLANCE P. Functional effects of econazole on inducible nitric oxide synthase: production of a calmodulin-dependent enzyme. Br. J. Pharmacol. 1996;117:1053–1058. doi: 10.1111/j.1476-5381.1996.tb16696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAIN J.D., FRANK R. Recovery of free cells from rat lungs by repeated washings. J. Appl. Physiol. 1968;25:63–69. doi: 10.1152/jappl.1968.25.1.63. [DOI] [PubMed] [Google Scholar]
- CHAU Y.P., SHIAH S.G., DON M.J., KUO M.L. Involvement of hydrogen peroxide in topoisomerase inhibitor β-lapachone-induced apoptosis and differentiation in human leukaemia cells. Free Radical Biol. Med. 1998;24:660–670. doi: 10.1016/s0891-5849(97)00337-7. [DOI] [PubMed] [Google Scholar]
- GONCALVES A.M., VASCONELLOS M.E., DECOMPO R., CRUZ N.S., SOUZA W.R., LEON W. Evaluation of the toxicity of 3-ally-beta-lapachone against Trypanosoma cruzi bloodstream forms. Mol. Biochem. Parasitol. 1980;1:167–176. doi: 10.1016/0166-6851(80)90015-8. [DOI] [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI K., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S.R. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- GUIRAUD P., STEIMAN R., CAMPOS-TAKAKI G.M., SEIGLE-MURANDI F., SIMEN DE BUOCHBERG M. Comparison of antibacterial and antifungal activities of lapachol and beta-lapachone. Planta Med. 1994;60:373–374. doi: 10.1055/s-2006-959504. [DOI] [PubMed] [Google Scholar]
- HIBBS J.B.J., TAINTOR R.R., VAVRIN F. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- JOLY G.A., AYRES M., KILBOURN R.G. Potent inhibition of inducible nitric oxide synthase by geldanamycin, a tyrosine kinase inhibitor, in endothelial, smooth muscle cells, and in rat aorta. FEBS Lett. 1997;403:40–44. doi: 10.1016/s0014-5793(97)00004-5. [DOI] [PubMed] [Google Scholar]
- KOBZIK L., REID M.B., BREDT D.S., STAMLER J.S. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- KRETZSCHMAR M., MEISTERERNST M., ROEDER R.G. Identification of human DNA topoisomerase I as a cofactor for activator-dependent transcription by RNA polymerase II. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11508–11512. doi: 10.1073/pnas.90.24.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.J., AVERBOUKH L., PARDEE A.B. Beta-lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J. Biol. Chem. 1993;268:22463–22468. [PubMed] [Google Scholar]
- LI C.J., WANG C., PARDEE A.B. Induction of apoptosis by beta-lapachone in human prostate cancer cells. Cancer Res. 1995;55:3712–3715. [PubMed] [Google Scholar]
- LOWENSTEIN C.J., SNYDER S.H. Nitric oxide, a novel biologic messenger. Cell. 1992;70:705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MORRIS S.M., BILLIAR T.R. New insights into the regulation of inducible nitric oxide synthesis. Am. J. Physiol. 1994;266:E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- MURPHY S., SIMMONS M.L., AGULLO L., GARCIA A., FEINSTEIN D.L., GALEA E., REIS D.J., MINC-GOLOMB D., SCHWARTZ J.P. Synthesis of nitric oxide in CNS glial cells. Trend Neurosci. 1993;16:323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- PLANCHON S.M., WUERZBERGER S., FRYDMAN B., WITIAK D.T., HUTSON P., CHURCH D.R., WILDING G., BOOTHMAN D.A. Beta-lapachone-mediated apoptosis in human promyelocytic leukemis (HL-60) and human prostate cancer cells: A p53-independent response. Cancer Res. 1995;55:3706–3711. [PMC free article] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFFNER-SABBA K., SCHMIDT-RUPPIN K.H., WEHRIL W., SCHUERCH A.R., WASLEY J.W.F. Beta-lapachone: synthesis of derivatives and activities in tumor models. J. Med. Chem. 1984;27:990–994. doi: 10.1021/jm00374a010. [DOI] [PubMed] [Google Scholar]
- STUEHR D.J., NATHAN C.F. Nitric oxide: a macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI H., YAMASHITA M., LEE J.C., KATAOKA T., MAGAE J., NAGAI K. Immunosuppressive activity of streptonigrin in vitro and in vivo. Biosci. Biotech. Biochem. 1996;60:789–793. doi: 10.1271/bbb.60.789. [DOI] [PubMed] [Google Scholar]
- VALLANCE P., MONCADA S. The role of endogenous nitric oxide in septic shock. New Horizons. 1993;1:77–86. [PubMed] [Google Scholar]


