Abstract
5-HT1A receptor agonists have proven to be effective antidepressant medications, however they suffer from a significant therapeutic lag before depressive symptoms abate. Flibanserin is a 5-HT1A receptor agonist and 5-HT2A receptor antagonist developed to possibly induce a more rapid onset of antidepressant action through its preferential postsynaptic 5-HT1A receptor agonism.
Flibanserin antagonized the effect of microiontophoretically-applied DOI in the medial prefrontal cortex (mPFC) following 2 days of administration, indicating antagonism of postsynaptic 5-HT2A receptors. This reduction in the effect of locally-applied DOI was no longer present following 7-day flibanserin administration.
Two-day flibanserin administration only marginally reduced the firing activity of dorsal raphe (DRN) 5-HT neurons. Following 7 days of administration, 5-HT neuronal firing activity had returned to normal and the somatodendritic 5-HT1A autoreceptors were desensitized.
The responsiveness of postsynaptic 5-HT1A receptors located on CA3 hippocampus pyramidal neurons and mPFC neurons, examined using microiontophoretically-applied 5-HT and gepirone, was unchanged following a 7-day flibanserin treatment.
As demonstrated by the ability of the 5-HT1A receptor antagonist WAY 100635 to selectively increase the firing of hippocampal neurons in 2- and 7-day treated rats, flibanserin enhanced the tonic activation of postsynaptic 5-HT1A receptors in this brain region.
The results suggest that flibanserin could be a therapeutically useful compound putatively endowed with a more rapid onset of antidepressant action.
Keywords: Flibanserin, 5-HT1A receptors, 5-HT2A receptors, BMY 7378, WAY 100635, LSD, dorsal raphe nucleus, medial prefrontal cortex, hippocampus, electrophysiology
Introduction
Serotonin (5-HT)1A receptor agonists have been shown to be effective antidepressant drugs despite some difficulty of titrating the dose to obtain this therapeutic action without inducing cumbersome side effects (see Robinson et al., 1990; Wilcox et al., 1996; Stahl et al., 1998). Nevertheless, these compounds suffer from the same therapeutic lag as other antidepressant medications used to date, i.e. 2–3 weeks of administration are required before antidepressant action is observed. It has been hypothesized that this delay in the therapeutic response results from the initial action of 5-HT1A agonists at the somatodendritic 5-HT1A autoreceptors located on the serotonergic neurons (Blier & de Montigny, 1987). Following their acute or short-term administration, 5-HT neuronal firing is diminished, thereby resulting in a decrease in endogenous 5-HT release in projection areas (Fornal et al., 1994; Blier & de Montigny, 1987; Dong et al., 1997; Bosker et al., 1996; Kreiss & Lucki, 1997). However, following long-term administration, the somatodendritic 5-HT1A autoreceptors are desensitized, thereby allowing normal firing activity and subsequent release of 5-HT despite the ongoing presence of 5-HT1A agonists (Blier & de Montigny, 1987; Dong et al., 1997; Kreiss & Lucki, 1997). Normalized levels of synaptic 5-HT plus the presence of 5-HT1A receptor agonists then leads to an enhanced activation of the postsynaptic 5-HT1A receptors located in limbic structures (Haddjeri et al., 1998). It has been hypothesized that this enhanced tonic activation underlies the antidepressant response in multiple classes of antidepressant treatments (Blier & de Montigny, 1994).
Recent advances in drug development have concentrated on shortening the therapeutic lag. Two major approaches have been delineated. First, attempts have been made to block, using the preferential presynaptic 5-HT1A receptor antagonist pindolol (Romero et al., 1996), the initial inhibition of 5-HT neuronal firing due to the activation of the somatodendritic 5-HT1A autoreceptors. In five of six double blind studies, conjunctive therapy of selective 5-HT reuptake inhibitors (SSRI) and pindolol has been shown to have a more rapid onset of antidepressant action (Pérez et al., 1997; Tome et al., 1997; Berman et al., 1997; Thomas et al., 1997; Zanardi et al., 1997; 1998). In addition, an open label study has found a similar reduction in the therapeutic lag of the 5-HT1A agonist buspirone by the concurrent administration of pindolol (Blier et al., 1997). The latter observation, if confirmed in a double-blind study, would indicate that an enhanced tonic activation of postsynaptic 5-HT1A receptors is truly a main determinant of the antidepressant response.
The second approach in drug development has been to bypass autoreceptor activation altogether by developing compounds with preferential activity at the postsynaptic 5-HT1A receptors (de Montigny & Blier, 1991). Flibanserin (BIMT 17) has been described as a 5-HT1A agonist with selective activity at postsynaptic 5-HT1A receptors as well as a 5-HT2A receptor antagonist (Ki of 7.3 and 6.9, respectively) but with no significant affinity for other receptors (Borsini et al., 1995a; 1995b). This combination of properties could lead to a rapid increase in 5-HT1A receptor-mediated inhibition as well as a decrease in 5-HT2 receptor-mediated excitation of postsynaptic neurons. Studies demonstrating an enhanced inhibition of the postsynaptic membrane with the coadministration of a 5-HT1A agonist and a 5-HT2 antagonist support this contention (Ashby et al., 1994). Acute experiments with flibanserin have been somewhat conflicting regarding the preferential activity of flibanserin at the postsynaptic membrane. Borsini et al. (1995a) demonstrated that 8-OH-DPAT increased the firing of cortical neurons at low doses and decreased the firing at high doses, a biphasic effect suggestive of pre- and postsynaptic components to the action of this compound, whereas flibanserin dose-dependently only inhibited cortical firing. The results of this indirect evaluation were interpreted as a preferential activity of flibanserin in some postsynaptic areas. However, this study was possibly complicated by the affinity of the comparison drug, 8-OH-DPAT, for the 5-HT7 receptor. In contrast, we found that, given intravenously, flibanserin was most potent on 5-HT neurons (Rueter et al., 1998b). In this same study, however, flibanserin was more potent at postsynaptic 5-HT1A receptors when applied locally and it acted as a partial agonist in the hippocampus but as a full agonist in the cortex. The present study was designed to further delineate the properties of flibanserin. In order to better mimic the conditions under which human patients would receive the compound, flibanserin was given systemically in a sustained fashion. The effects of 2- and 7-day treatments of flibanserin on 5-HT1A and/or 5-HT2A receptors in the dorsal raphe nucleus (DRN), medial prefrontal cortex (mPFC), and CA3 region of the hippocampus of the rat were investigated using in vivo single unit recording and microiontophoresis. In some experiments, the 5-HT1A receptor agonist gepirone was used as an active reference drug.
Methods
Animal preparation and drug administration
Under halothane anaesthesia, pairs of male Sprague-Dawley rats were implanted with osmotic minipumps (Alza, Palo Alto, CA, U.S.A) that delivered either flibanserin, gepirone or their vehicle. This mode of administration more closely approaches the blood levels of psychotropic drugs achieved in patients given the much faster metabolism of such agents in rodents that in humans. Flibanserin was dissolved in deionized water with several drops of acetic acid and gepirone in water only. The concentrations of flibanserin (2.5, 5, or 10 mg kg−1 day−1) and gepirone (15 mg kg−1 day−1) were determined based on the mean body weight of the animals during the 2- or 7-day treatment. All experiments were performed with the minipumps in place. After implantation, rats were housed in standard conditions with free access to food and water. Principles established by the Canadian Committee on Animal Care were followed at all times.
For electrophysiological experiments, rats were anaesthetized with chloral hydrate (400 mg kg−1, i.v.) and placed in a stereotaxic frame with the nose bar set 3 mm below the ear bars. In order to maintain a full anaesthetic state in which there was no reaction to a tail or paw pinch, chloral hydrate supplements of 100 mg kg−1 were given as needed. Burr holes were drilled over the DRN (anterior 0.9, lateral 0, in reference to interaural 0), the mPFC (anterior 3.0, lateral 0.8, in reference to bregma) or the hippocampus (anterior 4.0, lateral 4.0, in reference to lambda), and electrodes were lowered to the target areas (DRN DV: 5.0–6.5; mPFC DV: 1.0–3.5; hippocampus CA3 region DV: 3.5–4.2, in reference to dura; Paxinos & Watson, 1982). In order to limit the number of animals used, more than one site was examined in each rat.
For extracellular recordings in the DRN, single barrel glass electrodes were pulled in the conventional manner in order to achieve a tip with a 2–7 MΩ impedance and were filled with 2 M NaCl solution. DRN 5-HT neurons were identified based on their established characteristics (Aghajanian, 1978). For sampling the mean firing rate, neurons were recorded during five successive penetrations, 100–200 μm apart, formed in a star pattern.
Extracellular recordings in the mPFC and hippocampus were performed with five-barrel glass electrodes pulled to a tip with an impedance of 0.8–1.2 MΩ. The central barrel was filled with a 2 M NaCl solution and served as the recording barrel. Depending upon the experiment, two of the side barrels were filled with the following solutions: 5-HT creatinine sulphate (5 mM in 200 mM NaCl, pH 3.5–4.0), (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI; 50 mM in 200 mM NaCl, pH 4.0), BMY 7378 (50 mM in 200 mM NaCl, pH 3.0; RBI), or gepirone (25 mM in 200 mM NaCl, pH 5.0). The final two side barrels were filled with 2 M NaCl to serve as an automatic current balance and quisqualic acid (1.5 mM in 400 mM NaCl, pH 8.0) to activate or maintain the neuronal firing.
For all multibarrel electrodes, currents of −10 nA were used to retain the microiontophoretic solutions between experimental ejections. Medial PFC and hippocampus CA3 pyramidal neurons were identified based on their respective characteristics (Ashby et al., 1990; Kandel & Spencer, 1961). Because hippocampus CA3 pyramidal neurons and the majority of mPFC neurons do not discharge spontaneously in the anaesthetized rat, a leak or small to moderate ejection current of quisqualate (0 to −50 nA in the mPFC; +1 to −4 nA in the hippocampus) was used to activate the neurons within their physiological range without affecting their responsiveness to 5-HT and 5-HT receptor agonists (Ranck, 1975; Ashby et al., 1990). Microiontophoretic ejections were made for 50 s periods, with the exception of 5-HT during its concurrent application with BMY 7378. The antagonism of the effect of DOI applied by iontophoresis in the mPFC was carried out after the 2-day treatment period, presumably before adaptative changes would occur with more prolonged administration, to ensure that flibanserin was present in sufficient concentrations in the brain.
Intravenous drug administrations were made through a catheter inserted into a lateral tail vein. For consecutive injections, a minimum of 2 min elapsed before the subsequent injection. Lysergic acid diethylamide (LSD), dissolved in 0.9% saline, was used to assess the functioning of the presynaptic 5-HT1A autoreceptors because this intravenous probe has always provided results consistent with alterations of 5-HT1A receptor responsiveness, unlike the more selective 5-HT1A/7 agonist 8-OH-DPAT (Blier et al., 1987; Blier & de Montigny, 1987; Shen et al., 1993). The tonic inhibition of the postsynaptic hippocampal neurons was assessed using two methods. First, the ability of the microiontophoretic application of the 5-HT1A receptor antagonist, BMY 7378, (Chaput & de Montigny, 1988) to disinhibit hippocampal neurons was investigated in 2-day treated rats. Second, the 5-HT1A receptor antagonists BMY 7378 and WAY 100635 (Fletcher et al., 1996) were dissolved in 0.9% saline and used to assess the degree of 5-HT1A receptor-mediated inhibition of the postsynaptic neurons induced by the sustained administration of flibanserin for 2 and 7 days. The degree to which the antagonists could disinhibit the firing of hippocampal neurons has been determined to be a measure of the tonic activation of postsynaptic 5-HT1A receptors (Haddjeri et al., 1998). Two minutes prior to the intravenous administration of WAY 100635 or BMY 7378, the firing activity of the quisqualate-activated CA3 pyramidal neurons was decreased to about 5 Hz in order to more readily allow the detection of enhancements in firing following administration of the antagonists in control and treated rats.
The degree of tonic activation of postsynaptic 5-HT1A receptors in the mPFC was not determined in a manner analogous to that used in the hippocampus for the following reasons. First, both of the selective 5-HT1A antagonists available, BMY 7378 and WAY 100635, have been shown to inhibit mPFC neuronal firing when given alone (Rueter et al., 1998b). Second, the aim of the present study was to compare the effects of sustained flibanserin administration with other antidepressant drugs, and, to date, the tonic activation of postsynaptic receptors has only been assessed in the hippocampus.
Drugs
Flibanserin (BIMT 17; 1-[2-[4-(3-trifluoromethyl phenyl) piperazin-1-yl] ethyl] benzimidazol-[1H]-2-one; Boehringer Ingelheim, Ontario, Canada), chloral hydrate, gepirone (Bristol Myers Squibb, Wallingford, CT, U.S.A), LSD (lysergic acid diethylamide), 5-HT creatinine sulphate (Sigma, Mississauga, ON, Canada), DOI ((±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride; Research Biochemical International (RBI), Natick, MA, U.S.A), quisqualic acid, WAY 100635 (N - {2 - [4(2-methoxyphenyl) -1-piperazinyl]ethyl}-n-(2 - pyridinyl) cyclohexanecarboxamide trimydroxychloride; Wyeth Ayerst, Princeton, NJ, U.S.A), BMY 7378 (8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl] - 8-azaspirol [4,5] -decane-7,9-dione dihydrochloride Bristol-Myers Squibb, Wallingford, CT, U.S.A).
Data analysis
For 2-day administration of flibanserin, the means±s.e.mean of DRN 5-HT neuronal firing rates were determined for each dose of flibanserin and their paired controls and were analysed with a one-way analysis of variance (ANOVA) with post hoc Newman-Keuls. The firing rates following 7-day administration were analysed with a Student's t-test. The effect of LSD on DRN 5-HT neuronal firing activity was assessed, converted to percentage of baseline, and analysed with a Student's t-test. For the inhibition of the recorded neuron by microiontophoretic application of 5-HT, DOI and gepirone, the resultant inhibition was analysed online by computer. The effects of different ejection values upon the number of spikes suppressed in treated and control rats were analysed with two-way repeated measures ANOVA with post hoc Newman-Keuls. The degree of disinhibition of the recorded cell by the i.v. administration of BMY 7378 or WAY 100635 was measured and converted into percentage of baseline in order to allow comparison between cells and analysed using two-way repeated measures ANOVAs with post hoc Newman Keuls (2-day administration experiments) or a Student's t-test (7-day administration experiments).
Results
Effect of 2-day flibanserin treatment on DRN 5-HT neuronal firing activity
A 2-day administration of flibanserin (2.5, 5 and 10 mg kg−1 day−1) significantly decreased the firing rate of DRN 5-HT neurons by 20–27% (F3,186=3.1, P=0.03). There was no significant difference in the mean firing rate of 5-HT neurons between any of the doses of flibanserin (Table 1). Since the inhibitory effect of flibanserin appeared to be already maximal at 5 mg kg−1 day−1, this dose was chosen for further experiments after 2 and 7 days of administration.
Table 1.
Mean firing rates of DRN 5-HT neurons following 2-day systemic administration of flibanserin and control vehicle
Effects of microiontophoretically-applied 5-HT and DOI on mPFC neurons in 2-day flibanserin-treated and control rats
The microiontophoretic application of 5-HT current-dependently inhibited the firing of mPFC neurons in both flibanserin-treated (5 mg kg−1 day−1) and control rats (F2,35=24.9, P<0.0001). There was, however, no difference in the number of spikes suppressed by 5-HT between flibanserin-treated rats and their paired controls (F1,19=0.1, P=0.8; Figure 1).
Figure 1.

Effects of microiontophoretically-applied 5-HT on mPFC neurons in rats treated for 2 days with (b) 5 mg kg−1 day−1 flibanserin or (a) vehicle (delivered using an osmotic minipump implanted subcutaneously) as depicted in integrated firing rate histograms. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Quisqualate was used to activate the neurons. (c) Mean±s.e.mean of the number of spikes suppressed by microiontophoretically-applied 5-HT. Five to six rats were used in each treatment group. The number of neurons recorded is indicated in the boxes at the bottom of each column. *P<0.05 when compared to respective 5 and 15 nA ejection values.
The microiontophoretic application of DOI current-dependently inhibited the firing of mPFC neurons in both treated and control rats (F2,35=44.6, P<0.0001). The ability of DOI to inhibit mPFC neurons was significantly decreased in 2-day flibanserin treated rats (F1,25=5.4, P=0.03; Figure 2). These results support the notion that 5-HT exerts its inhibitory effect on neuronal firing rate in the mPFC via more than the 5-HT2A receptor subtype.
Figure 2.

Effects of microiontophoretically-applied DOI on mPFC neurons in rats treated for 2 days with (b) 5 mg kg−1 day−1 flibanserin or (a) vehicle (delivered using an osmotic minipump implanted subcutaneously) as depicted in integrated firing rate histograms. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Quisqualate was used to activate the neurons. (c) Mean±s.e.mean of the number of spikes suppressed by microiontophoretically-applied DOI. Five to six rats were used in each treatment group. The number of neurons recorded is indicated in the boxes at the bottom of each column. *P<0.05 when compared to respective 5 nA ejection value. **P<0.05 when compared to respective smaller ejection values. +P<0.05 for overall effect of treatment.
Effect of microiontophoretically-applied BMY7378 on hippocampus CA3 pyramidal neurons in rats treated for 2 days with flibanserin or gepirone
In a first series of experiments, the ability of the local application of the 5-HT1A receptor antagonist BMY 7378 to antagonize the actions of compounds at the postsynaptic 5-HT1A receptors in the hippocampus, the effect of microiontophoretically-applied BMY 7378 was determined during concurrent local application of 5-HT. The inhibition of hippocampal neurons induced by 5-HT (Figure 3a,b), an effect previously demonstrated to be entirely attributable to 5-HT1A receptor activation in this paradigm (Chaput & de Montigny, 1988; Blier et al., 1993a,1993b), was reversed by BMY 7378. In contrast, local application of BMY 7378 was not able to disinhibit neuronal firing when serotonergic compounds were given systemically in a sustained fashion (flibanserin: 2.5,5, and 10 mg kg−1 day−1; gepirone: 15 mg kg−1 day−1, Figure 3).
Figure 3.

Examples of integrated firing rate histograms of hippocampus CA3 pyramidal neurons in rats treated for 2 days with (a) vehicle control, (b) 5 mg kg−1 day−1 flibanserin, and (c) 15 mg kg−1 day−1 gepirone (delivered using an osmotic minipump implanted subcutaneously) during the microiontophoretic application of the 5-HT1A receptor antagonist BMY 7378. The experiments were carried out three times. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Note that BMY 7378 antagonizes the effects of locally-applied 5-HT (a and b) but not that of systemically-administered flibanserin or gepirone (b and c).
Effect of intravenous injection of WAY 100635 and BMY 7378 on the firing activity of hippocampus CA3 pyramidal neurons following 2 days of flibanserin or gepirone administration
In order to determine the extent to which the systemic 2-day administration of flibanserin (5 mg kg−1 day−1), and gepirone (15 mg kg−1 day−1) was enhancing the tonic activation of postsynaptic 5-HT1A receptors in the hippocampus, the ability of BMY 7378 to disinhibit the firing of these neurons was tested. Briefly, if the presence of the 5-HT1A receptor agonists is tonically inhibiting the hippocampal neurons, the administration of a 5-HT1A receptor antagonist should displace the agonists from the postsynaptic receptors thereby removing the inhibition. With the inhibition removed, the firing rate of the hippocampal neuron should increase (Haddjeri et al., 1998). For these experiments, BMY 7378 was initially chosen based on its consistent ability to antagonize the inhibitory effect of flibanserin on postsynaptic neuronal firing (Rueter et al., 1998Rueter et al., 1998). BMY 7378, in cumulative doses, inhibited the firing rate of hippocampal neurons in the control rats, indicative of it being a partial agonist in the hippocampus (Figures 4 and 5). Following the 2-day administration of flibanserin (2.5,5, and 10 mg kg−1 day−1) and gepirone (15 mg kg−1 day−1), BMY 7378 induced either a smaller inhibition, no change, or an increase in hippocampal firing rate. The relative increases in firing activity were dependent upon the increasing doses of the agonists (F4,17=4.0, P=0.02; Figures 4 and 5).
Figure 4.

Examples of integrated firing rate histograms of hippocampus CA3 pyramidal neurons in rats treated for 2 days with (a) vehicle control, (b) 5 mg kg−1 day−1 flibanserin, (c) 15 mg kg−1 day−1 gepirone (delivered using an osmotic minipump implanted subcutaneously) during the i.v. administration of cumulative doses of BMY 7378. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Arrows indicate the time of i.v. injections with the cumulative doses of BMY 7378 denoted above the arrow. Note the presence of BMY 7378 significantly antagonizes the effect of microiontophoretically-applied 5-HT.
Figure 5.

Effects of the cumulative i.v. administration of BMY 7378 on the percentage of baseline firing rate of hippocampus CA3 pyramidal neurons following 2-day administration of flibanserin, gepirone, and vehicle (delivered using an osmotic minipump implanted subcutaneously; mean±s.e.mean). Four to five rats were used in each treatment group. Due to the complexity of the figure, indicators of significant differences between the doses of BMY 7378 and between the treatments are not included here.
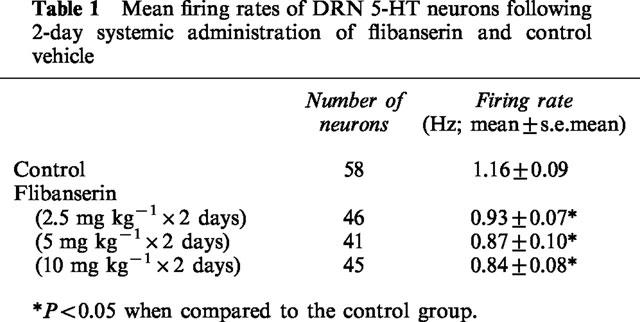
Due to the inhibition of hippocampal neurons caused by the partial agonistic properties of BMY 7378, further experiments were conducted in control and 2-day flibanserin treated rats (5 mg kg−1 day−1), utilizing a WAY 100635 pretreatment intended to minimize the inhibition induced by BMY 7378. However, it was found that WAY 100635 itself significantly increased the firing rate of hippocampal neurons in flibanserin-treated but not control rats (Fdose of 5-HT1A antagonist, 6,40=4.3, P=0.002; Ftreatment 1,7=6.7, P=0.04; Figure 6). The subsequent administration of cumulative doses of BMY 7378 did not further increase the firing activity.
Figure 6.
Effects of the i.v. administration of the 5-HT1A receptor antagonist WAY 100635 and cumulative doses of BMY 7378 on the basal firing rate of hippocampus CA3 pyramidal neurons in rats treated for 2 days with (b) 5 mg kg−1 day−1 flibanserin or (a) vehicle (delivered using an osmotic minipump implanted subcutaneously) as depicted in integrated firing rate histograms. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Arrows indicate the time of i.v. injections with the dose of WAY 100635 and the cumulative doses of BMY 7378 denoted above the arrow. (c) Mean±s.e.mean values for the percentage of baseline firing rate of hippocampal neurons in response to the i.v. administration of WAY 100635 and subsequent cumulative doses of BMY 7378. The open circles depict the results obtained in control rats and the filled circles those observed in flibanserin-treated rats. Five rats were used in each treatment group. *P<0.05 when compared to the preinjection value and to control values.
Effects of 7-day flibanserin treatment on DRN 5-HT neuronal firing activity and the functioning of the somatodendritic 5-HT1A autoreceptor
There was no difference in the firing rates of DRN 5-HT neurons between rats treated for 7 days with 5 mg kg−1 day−1 flibanserin or vehicle controls (0.97±0.07 versus 1.01±0.07 Hz, respectively; t103=0.4, P=0.7).
The ability of the 5-HT agonist LSD to inhibit the firing activity of DRN 5-HT neurons was significantly reduced in rats treated for seven days with flibanserin (t6=4.9, P=0.003; Figure 7). Whereas 10 μg kg−1 of LSD was sufficient to completely suppress the firing of 5-HT neurons in most control rats, at least 20 μg kg−1 was required to achieve the same effect in flibanserin-treated rats (% inhibition of neuronal firing activity induced by 10 μg kg−1 LSD: control 96±2%, n=4, flibanserin-treated 31±13%, n=4; t6=4.9, P=0.003).
Figure 7.

Effects of the i.v. administration of LSD on the firing rates of DRN 5-HT neurons in control (a) and 7 day flibanserin-treated rats (5 mg kg−1 day−1; b) (delivered using an osmotic minipump implanted subcutaneously) as depicted in integrated firing rate histograms. Arrows indicate the time of injection with the cumulative dose of LSD (μg kg−1) denoted above the arrows. Note the inhibitory effect of LSD was reversed by the i.v. administration of the selective 5-HT1A receptor antagonist WAY 100635.
Effect of microiontophoretically-applied gepirone and DOI on mPFC neurons in 7-day flibanserin-treated and control rats
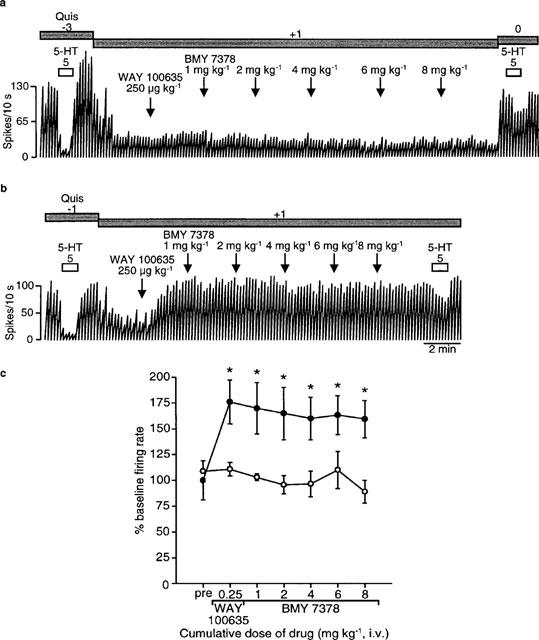
Microiontophoretically-applied gepirone current dependently inhibited the firing rate of mPFC neurons, measured as spikes suppressed, in both control and flibanserin treated rats (5 mg kg−1 day−1, F2,34=50.5, P<0.0001; Figure 8a, b and c). There was no difference in the ability of gepirone to inhibit firing between control and 7-day treated rats (F1,20=0.6, P=0.4; Figure 8c).
Figure 8.
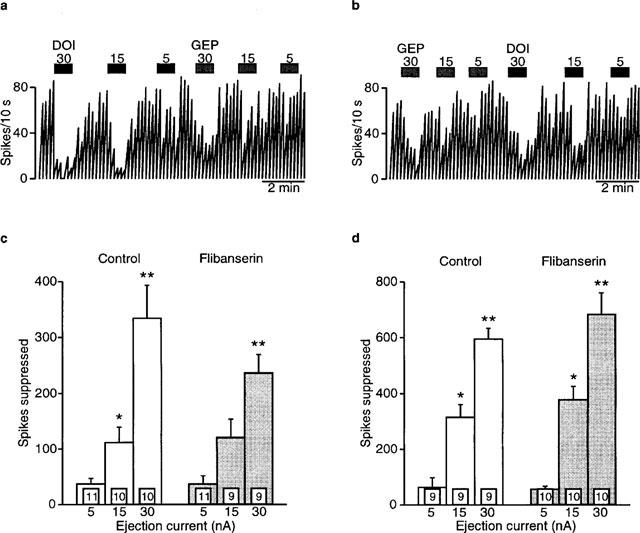
Effects of microiontophoretically-applied gepirone and DOI on mPFC neurons in rats treated for 7 days with (b) 5 mg kg−1 day−1 flibanserin or (a) vehicle (delivered using an osmotic minipump implanted subcutaneously) as depicted in integrated firing rate histograms. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Quisqualate was used to activate the neurons. (c) Mean±s.e.mean of the number of spikes suppressed by microiontophoretically-applied gepirone. (d) Mean±s.e.mean of the number of spikes suppressed by microiontophoretically-applied DOI. Four to five rats were used for each treatment group. The number of neurons recorded is indicated in the boxes at the bottom of each column. *P<0.05 when compared to respective 5 nA ejection value. **P<0.05 when compared to respective smaller ejection values.
Similarly, microiontophoretically-applied DOl current dependently inhibited the firing rate of mPFC neurons in both control and flibanserin treated rats (spikes suppressed; F2,33=72.8, P<0.0001: Figure 8a, b and d). However, unlike the results seen following 2-day flibanserin administration, there was no difference between control and treated animals (F1,18=1.0, P=0.3; Figure 8d).
Effect of microiontophoretically-applied 5-HT and gepirone on hippocampus CA3 pyramidal neurons in 7-day flibanserin-treated and control rats
Microiontophoretically-applied 5-HT current-dependently inhibited the firing activity of hippocampal neurons in both control and flibanserin-treated rats (5 mg kg−1 day−1, spikes suppressed; F2,40=32.4, P<0.0001; Figure 9a, b and c). There was no difference in the ability of 5-HT to suppress neuronal firing between the control and treated rats (F1,21=0.4, P=0.5: Figure 9c). In addition, microiontophoretically-applied gepirone current-dependently inhibited the firing rate of hippocampal neurons (spikes suppressed; F2,36=27.6, P<0.0001; Figure 9a, b and d), with there being no difference between the control and treated rats (F1,20=1.2, P=0.3; Figure 9d).
Figure 9.
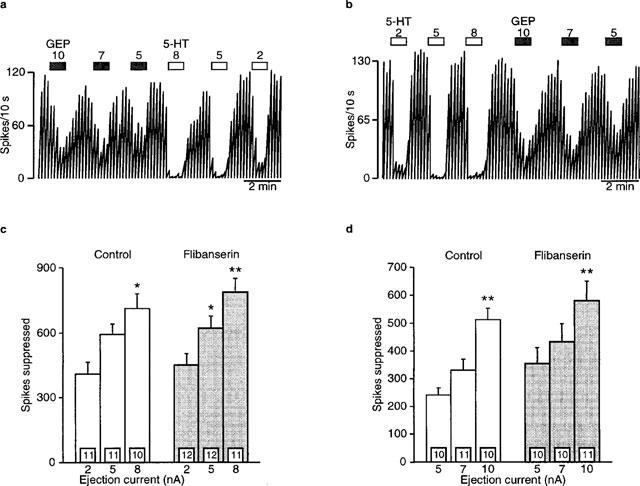
Effects of microiontophoretically-applied 5-HT and gepirone on hippocampus CA3 pyramidal neurons in rats treated for 7 days with (b) 5 mg kg−1 day−1 flibanserin or (a) vehicle (delivered using an osmotic minipump implanted subcutaneously) as depicted in integrated firing rate histograms. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Quisqualate was used to activate the neurons. (c) Mean±s.e.mean of the number of spikes suppressed by microiontophoretically-applied 5-HT. (d) Mean±s.e.mean of the number of spikes suppressed by microiontophoretically-applied gepirone. Six to seven rats were used for each treatment group. The number of neurons recorded is indicated in the boxes at the bottom of each column. *P<0.05 when compared to respective 5 nA ejection value. **P<0.05 when compared to respective smaller ejection values.
Effect of the intravenous injection of WAY 100635 on the firing activity of hippocampus CA3 pyramidal neurons following 7 days of flibanserin administration
WAY 100635 (250 μg kg−1, i.v.) significantly antagonized the inhibition induced by the microiontophoretic application of 5-HT (F1,6=81.1, P<0.001), and there was no difference in the antagonism induced by WAY 100635 between the flibanserin-treated (5 mg kg−1 day−1) animals and the controls (F1,6=1.0, P=0.4; Figure 10c). Furthermore, WAY 100635 did not alter the firing rate of hippocampal neurons in control rats (Figure 10a and d). In contrast, there was a significant increase of 34% in the firing rate of hippocampal neurons in the 7-day flibanserin-treated rats following the i.v. administration of WAY 100635 (t8=3.1, P=0.02: Figure 10b and d).
Figure 10.

Effects of the i.v. administration of the selective 5-HT1A receptor antagonist WAY 100635 on the basal firing rate of hippocampus CA3 pyramidal neurons in rats treated for 7 days with (b) 5 mg kg−1 day−1 flibanserin or (a) vehicle (delivered using an osmotic minipump implanted subcutaneously) as depicted in integrated firing rate histograms. The rectangles above the traces indicate the time of microiontophoretic ejections with the ejection values in nA denoted above the rectangles. Arrows indicate the time of i.v. injections with the dose of WAY 100635 denoted above the arrow. (c) Mean±s.e.mean values for the percentage decrease in the ability of microiontophoretically-applied 5-HT to inhibit the firing of hippocampal neurons after the administration of WAY 100635 (250 μg kg−1, i.v.). (d) Mean±s.e.mean values for the percentage of baseline firing rate of hippocampal neurons following the i.v. administration of 250 μg kg−1 WAY 100635. The number of neurons recorded is indicated in the boxes at the bottom of each column. *P<0.05.
Discussion
Sustained administration of the 5-HT1A receptor agonist/5-HT2A receptor antagonist flibanserin induced several changes in the 5-HT system of the rat. First, DRN 5-HT neuronal firing activity was reduced by only 25% following 2-day administration of flibanserin, and this inhibition of firing activity was not dose-dependent. Following 7 days of sustained flibanserin administration, 5-HT neuronal firing had recovered to normal levels probably due to the desensitization of the somatodendritic 5-HT1A autoreceptors. Second, as expected, a 2-day flibanserin treatment significantly attenuated the effect of microiontophoretically-applied DOI in the mPFC. However, this ability of flibanserin to antagonize DOI was no longer present following 7-day flibanserin administration indicating a fairly rapid change in the functioning of the postsynaptic 5-HT2A receptors in the mPFC. In contrast, there was no effect of flibanserin on the ability of microiontophoretically-applied 5-HT or the 5-HT1A receptor agonist gepirone to inhibit either mPFC or hippocampus CA3 pyramidal neurons after 2 or 7 days of flibanserin administration. Finally, sustained flibanserin administration, for either 2 or 7 days, significantly enhanced the tonic activation of the postsynaptic 5-HT1A receptors in the hippocampus.
The decrease in DRN 5-HT neuronal firing activity seen following 2 days of flibanserin administration and the recovery to normal following a longer period of treatment with the desensitization of the somatodendritic 5-HT1A autoreceptors was a finding similar to those reported for other 5-HT1A receptor agonists (Blier & de Montigny, 1987; Dong et al., 1997; Godbout et al., 1991). However, there were notable differences between flibanserin and these other 5-HT1A receptor agonists. First, the degree of inhibition of 5-HT neuronal firing activity seen following two days of administration of flibanserin (about 25%; 2.5–10 mg kg−1 day−1) was markedly smaller than that seen with either 15 mg kg−1 day−1 of gepirone (80%; Blier & de Montigny, 1987) and ipsapirone (75%; Dong et al., 1997), or 10 mg kg−1 day−1 of tandospirone (70%: Godbout et al., 1991). Second, the ability of flibanserin to inhibit DRN 5-HT neuronal firing activity was not dose-dependent, i.e. increasing the dose of flibanserin 2–4 fold did not significantly alter the degree of inhibition (Table 1), unlike the other 5-HT1A agonists mentioned above and BAY X 3702 (6%, 42%, 77% with 0.5, 1.0 and 1.25 mg kg−1 day−1 for 2 days; Dong et al., 1998). Finally, unlike a 7-day sustained administration with gepirone and ipsapirone, the firing rate of 5-HT neurons had recovered fully following 7 days of flibanserin administration. Nevertheless, the desensitization of the somatodendritic 5-HT1A autoreceptors seen with sustained flibanserin administration did develop as with other 5-HT1A agonists (Figure 7; Blier & de Montigny, 1987; Dong et al., 1997; Godbout et al., 1991). Recent studies investigating the time course of changes in the 5-HT1A autoreceptors in the DRN following sustained administration of the SSRI fluoxetine and paroxetine suggest that there is a gradual desensitization of the autoreceptors during periods of enhanced stimulation of 5-HT1A receptors (Le Poul et al., 1995; 1997). It is thus possible to hypothesize that the full recovery of 5-HT neuronal firing activity following 7 days of flibanserin administration was due to the fact that there was a limited degree of firing inhibition and, therefore, a desensitization of the somatodendritic 5-HT1A autoreceptors was sufficient to readily reverse the inhibitory effects of the compound.
Flibanserin significantly antagonized the inhibitory effect of DOI at the 5-HT2A receptors in the mPFC following 2 days of administration (Figure 2). This finding is consistent with the 5-HT2A receptor antagonistic properties of flibanserin. Since flibanserin has a greater affinity for 5-HT1A receptors than for 5-HT2A receptors, this result indicates that the dose of 5 mg kg−1 day−1 was most likely sufficient to act on both of these receptors (Figure 2; Borsini et al., 1995b). It is interesting to note that, while 5-HT2A receptors in the cortex have generally been described as excitatory, the microiontophoretic application of the 5-HT2 receptor agonist DOI inhibits the firing of mPFC neurons (Ashby et al., 1990; unpublished results from our laboratory). The ability of a 5-HT2A receptor antagonist to block this inhibition further suggests that at least a subgroup of 5-HT2A receptors in the mPFC may be inhibitory. The antagonism of cortical 5-HT2A receptors was not present following 7-day flibanserin administration (Figure 8). This suggests an increase in the sensitivity of the 5-HT2A receptors in cortex. Previous studies investigating the chronic administration of 5-HT2 receptor antagonists have consistently reported a decrease in the density of 5-HT2A receptors, but the findings regarding the sensitivity of these receptors following long-term treatment have been contradictory (Blackshear & Sanders-Bush, 1982; Akiyoshi et al., 1994; Stoltz et al., 1983; Darmani et al., 1992). Some studies have found a desensitization of the 5-HT2A receptors while others have shown a supersensitivity of behaviours mediated by these receptors during the withdrawal period. Indeed, it has been suggested that receptor responsiveness is a more accurate picture of the functioning of these receptors than receptor binding parameters (Smith et al., 1990). While none of these previous studies have reported an increased functioning of these receptors during the treatment with a 5-HT2 receptor antagonist, the present study suggests that a supersensitivity of 5-HT2A receptors may occur during 5-HT2A receptor antagonist administration. In contrast, it may be that the purported increased sensitivity of the 5-HT2A cortical receptors following the 7-day administration with flibanserin may not have been due to the 5-HT2A receptor antagonistic properties of flibanserin but rather to its 5-HT1A receptor agonistic properties. A 7-day administration with gepirone has indeed been shown to increase 5-HT2 receptor-mediated behaviours despite a decreased number of cortical 5-HT2 binding sites (Yocca et al., 1991).
There is one further intriguing aspect to the changes in cortical 5-HT2 receptors following long-term administration of 5-HT2 receptor antagonists. Kidd et al. (1990) have shown that chronic administration of the 5-HT2 receptor antagonist ritanserin, a treatment regimen that decreases the number of 5-HT2 receptor binding sites in cortex, decreased the inhibitory effect of the 5-HT1A agonist receptor 8-OH-DPAT on 5-HT release. Moreover, repeated administration of the 5-HT2 receptor agonist, DOI, which also downregulates 5-HT2 receptors in the cortex, attenuated the 5-HT1A receptor-mediated inhibitory action of 8-OH-DPAT on DRN 5-HT neuronal firing (Kidd et al., 1991). 8-OH-DPAT is known to inhibit 5-HT neuronal firing via a feedback loop, most likely extending from the cortex (Blier & de Montigny, 1987; Ceci et al., 1994). Thus, the sustained interaction of flibanserin on cortical 5-HT2A receptors may be contributing to the lack of a dose-dependent effect of flibanserin on the firing activity of DRN 5-HT neurons following 2-day administration and/or the normalization of DRN 5-HT neuronal firing activity seen following 7 days of flibanserin administration.
Several experiments in the present study were designed to examine the degree of tonic inhibition of hippocampus CA3 pyramidal neurons following 2-day flibanserin administration. BMY 7378 was chosen because, in acute administration studies, it was found to more consistently antagonize flibanserin than did the more selective 5-HT1A receptor antagonist WAY 100635 (Rueter et al., 1998). First, the ability of the local application of the 5-HT1A receptor antagonist BMY 7378 to reverse the ongoing inhibition of hippocampal neurons induced by flibanserin was investigated. While the local application of BMY 7378 could reverse the inhibition caused by the local application of 5-HT, it could not reverse the inhibition caused by the systemically-administered flibanserin (Figure 3). Given that systemically-administered flibanserin could be acting on 5-HT1A receptors on the hippocampal neuron located outside the small region affected by the microiontophoretic ejection of BMY 7378 (i.e. on the dendrites of pyramidal neurons, Blier et al., 1993a,1993b), this result was not necessarily surprising. The second set of experiments were designed to reverse the inhibition induced by systemically-administered flibanserin using the systemic injection of BMY 7378. However, results indicated that BMY 7378 significantly inhibited neuronal firing in controls. In the presence of flibanserin, the inhibitory effect of BMY 7378 was attenuated in a dose-dependent fashion. At the highest dose of flibanserin used, BMY 7378 actually increased hippocampus neuronal firing above baseline levels (Figures 4 and 5). Thus, these experiments demonstrated a tonic inhibition of CA3 pyramidal neurons by flibanserin mediated by the postsynaptic 5-HT1A receptors. Nevertheless, the inhibition of firing activity induced by the apparent partial agonistic properties of BMY 7378 was troubling. Therefore, a third set of experiments was performed, utilizing a pretreatment of WAY 100635 intended to block this putative agonistic action of BMY 7378, thereby leaving only the disinhibition of the hippocampal neuron. As expected, in control rats, pretreatment with WAY 100635 prevented the inhibitory action of BMY 7378. However, WAY 100635, by itself, significantly disinhibited the firing activity of the hippocampal neurons, further supporting an enhanced tonic activation of the postsynaptic 5-HT1A receptors following a 2-day flibanserin administration (Figure 6). An enhancement of the tonic activation of the postsynaptic 5-HT1A receptors has been demonstrated following long-term treatment with several types of antidepressant treatments (Haddjeri et al., 1998). Such an increased tonic activation after 2 days of treatment, as was the case with flibanserin, so far has been reported with the dual 5-HT/norepinephrine reuptake blockade, as well as with a monoamine oxidase inhibitor and pindolol, two strategies putatively endowed with a more rapid onset of action and/or a greater efficacy (Rueter et al., 1998a; Haddjeri et al., 1998).
Similar to the 2-day treatment, the disinhibition of hippocampus neuronal firing induced by the i.v. administration of WAY 100635 indicated that the 7-day flibanserin treatment increased the tonic activation of the postsynaptic 5-HT1A receptors in the CA3 region of the hippocampus (Figure 10). There was, however, no difference in the ability of WAY 100635 to block the effects of locally-applied 5-HT between flibanserin-treated and control animals (Figure 10c), suggesting the selective disinhibition of postsynaptic neurons was a reflection of the systemic levels of flibanserin combined with the presumably normal extracellular levels of 5-HT. It may be considered somewhat surprising that the disinhibition induced by the i.v. administration of WAY 100635 was not significantly larger following 7-day flibanserin administration when compared to the effects seen at 2 days of administration given that the DRN 5-HT neuronal firing had returned to normal levels. However, it is yet unclear whether there is a linear relationship between the degree of disinhibition and the clinical efficacy of an antidepressant treatment (Haddjeri et al., 1998).
In summary, flibanserin induced a desensitization of the somatodendritic 5-HT1A autoreceptors which allowed a recovery of normal 5-HT neuronal firing activity in a shorter time period than other 5-HT1A agonists. In addition, there was an enhanced tonic activation of the postsynaptic 5-HT1A receptors in the hippocampus at both 2 and 7 days of flibanserin administration, an effect obtained in a significantly shorter period of drug administration than that seen with other antidepressant compounds. However, one has to consider that the degree of activation of multiple subtypes of postsynaptic 5-HT receptors other than the 5-HT1A subtype by endogenous 5-HT was still dependent on the recovery of the firing rate of 5-HT neurons during sustained flibanserin administration. Nevertheless, these results suggest that flibanserin could be an effective antidepressant drug that may have a more rapid onset of action than classical antidepressant drugs.
Acknowledgments
This work was supported in part by the Medical Research Council of Canada (grant MT-11014), the Fonds de la Recherche en Santé de Québec and Boehringer Ingelheim. L.R. is the recipient of a Fellowship from the Royal Victoria Hospital (Montréal, Canada). P.B. is the recipient of a Medical Research Council of Canada Scientist Award.
Abbreviations
- DOI
(±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride
- DRN
dorsal raphe nucleus
- LSD
lysergic acid diethylamide
- mPFC
medial prefrontal cortex
- SSRI
selective serotonin reuptake inhibitors
- 5-HT
serotonin
References
- AGHAJANIAN G.K.Feedback regulation of central monoaminergic neurons: Evidence from single-cell recording studies Essays in Neurochemistry and Neuropharmacology 1978New York: Wiley; 1–32.ed. Youdim, M.B.H., Lovenberg, W., Sharman, D.F., & Lagnando, J.R. pp [PubMed] [Google Scholar]
- AKIYOSHI J., TSUCHIYAMA K., MIZOBE Y., NAKAMURA M., KURANAGA H., NAGAYAMA H. Effects of chronic mianserin administration on serotonin metabolism and receptors in the 5-hydroxytryptophan depression model. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 1994;18:165–179. doi: 10.1016/0278-5846(94)90033-7. [DOI] [PubMed] [Google Scholar]
- ASHBY C.R., JR, EDWARDS E., WANG R.Y. Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- ASHBY C.R., JR, JIANG L.H., KASSER R.J., WANG R.Y. Electrophysiological characterization of 5-hydroxytryptamine2 receptors in the rat medial prefrontal cortex. J. Pharmacol. Exp. Ther. 1990;252:171–178. [PubMed] [Google Scholar]
- BERMAN R.M., DARNELL A.M., MILLER H.L., ANAND A., CHARNEY D.S. Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: a double-blind, placebo-controlled trial. Am. J. Psychiat. 1997;154:37–43. doi: 10.1176/ajp.154.1.37. [DOI] [PubMed] [Google Scholar]
- BLACKSHEAR M.A., SANDERS-BUSH E. Serotonin receptor sensitivity after acute and chronic treatment with mianserin. J. Pharmacol. Exp. Ther. 1982;221:303–308. [PubMed] [Google Scholar]
- BLIER P., BERGERON R., DE MONTIGNY C. Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacology. 1997;16:333–338. doi: 10.1016/S0893-133X(96)00242-4. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: Electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C., TARDIF D. Short-term lithium treatment enhances responsiveness of postsynaptic 5-HT1A receptors without altering 5-HT autoreceptor sensitivity: An electrophysiological study in the rat brain. Synapse. 1987;1:225–232. doi: 10.1002/syn.890010302. [DOI] [PubMed] [Google Scholar]
- BLIER P., LISTA A., DE MONTIGNY C. Differential properties of pre- and postsynaptic 5-hydroxytryptamine1A receptors in the dorsal raphe and hippocampus: I – Effect of spiperone. J. Pharmacol. Exp. Ther. 1993a;265:7–15. [PubMed] [Google Scholar]
- BLIER P., LISTA A., DE MONTIGNY C. Differential properties of pre- and postsynaptic 5-hydroxytryptamine1A receptors in the dorsal raphe and hippocampus: II – Effect of pertussis and cholera toxins. J. Pharmacol. Exp. Ther. 1993b;265:16–23. [PubMed] [Google Scholar]
- BORDET R., THOMAS P., DUPUIS B. Effect of pindolol on onset of action of Paroxetine in the treatment of major depression: intermediate analysis of a double-blind, placebo-controlled trial. Am. J. Psychiat. 1998;155:1346–1351. doi: 10.1176/ajp.155.10.1346. [DOI] [PubMed] [Google Scholar]
- BORSINI F., CECI A., BIETTI G., DONETTI A. BIMT 17, a 5-HT1A receptor agonist/5-HT2A receptor antagonist, directly activates postsynaptic 5-HT inhibitory responses in the rat cerebral cortex. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995a;352:283–290. doi: 10.1007/BF00168558. [DOI] [PubMed] [Google Scholar]
- BORSINI F., GIRALDO E., MONFERINI E., ANTONINI G., PARENTI M., BIETTI G., DONETTI A. BIMT 17, a 5-HT2A receptor antagonist and 5-HT1A receptor full agonist in rat cerebral cortex. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995b;352:276–282. doi: 10.1007/BF00168557. [DOI] [PubMed] [Google Scholar]
- BOSKER F.J., DE WINTER T.Y.C.E., KLOMPMAKERS A.A., WESTENBERG H.G.M. Flesinoxan dose-dependently reduces extracellular 5-hydroxytryptamine (5-HT) in rat median raphe and dorsal hippocampus through activation of 5-HT1A receptors. J. Neurochem. 1996;66:2546–2555. doi: 10.1046/j.1471-4159.1996.66062546.x. [DOI] [PubMed] [Google Scholar]
- CECI A., BASCHIROTTO A., BORSINI F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- CHAPUT Y., DE MONTIGNY C. Effects of the 5-hydroxytryptamine1 receptor antagonist BMY 7378, on 5-hydroxytryptamine neurotransmission: Electrophysiological studies in the rat central nervous system. J. Pharmacol. Exp. Ther. 1988;246:359–370. [PubMed] [Google Scholar]
- DARMANI N.A., MARTIN B.R., GLENNON R.A. Behavioural evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J. Pharmacol. Exp. Ther. 1992;262:692–698. [PubMed] [Google Scholar]
- DE MONTIGNY C., BLIER P.Development of selective agonists of postsynaptic 5-HT1A receptors: a future direction in the pharmacotherapy of affective disorders Current Practices and Future Developments in the Pharmacotherapy of Mental Disorders 1991New York: Elsevier Science Publishers; 99–103.ed. Meltzer, H.Y, & Nerozzi, D. pp [Google Scholar]
- DONG J., DE MONTIGNY C., BLIER P. Effect of acute and repeated versus sustained administration of the 5-HT1A agonist ipsapirone: electrophysiological studies in the rat hippocampus and dorsal raphe. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:303–311. doi: 10.1007/pl00005055. [DOI] [PubMed] [Google Scholar]
- DONG J., DE MONTIGNY C., BLIER P.Full agonistic properties of Bay X 3702 on presynaptic and postsynaptic 5-HT1A receptors electrophysiological studies in the rat hippocampus and dorsal raphe J. Pharmacol. Exp. Ther. 1998(in press) [PubMed]
- FLETCHER A., FORSTER E.A., BILL D.J., BROWN G., CLIFFE I.A., HARTLEY J.E., JONES D.E., MCLENACHAN A., STANHOPE K.J., CRITCHLEY D.J.P., CHILDS K.J., MIDDEFELL V.C., LANFUMEY L., CORRADETTI R., LAPORTE A.-M., GOZLAN H., HAMON M., DOURISH C.T. Electrophysiological, biochemical, neurohormonal, and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- FORNAL C.A., LITTO W.J., METZLER C.W., MARROSU F., TADA K., JACOBS B.L. Single-unit responses of serotonergic dorsal raphe neurons to 5-HT1A agonist and antagonist drug administration in behaving cats. J. Pharmacol. Exp. Ther. 1994;270:1345–1358. [PubMed] [Google Scholar]
- GODBOUT R., CHAPUT Y., BLIER P., DE MONTIGNY C. Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine- I. Effects of acute and long-term administration of tandospirone on serotonin neurotransmission. Neuropharmacology. 1991;30:679–690. doi: 10.1016/0028-3908(91)90175-b. [DOI] [PubMed] [Google Scholar]
- HADDJERI N., BLIER P., DE MONTIGNY C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDEL E.R., SPENCER W.A. Electrophysiology of hippocampal neurons. II. Afterpotentials and repetitive firing. J. Neurophysiol. 1961;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- KIDD E.J., GARRATT J.C., MARSDEN C.A. Effects of repeated treatment with 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) on the autoregulatory control of dorsal raphe 5-HT neuronal firing and cortical 5-HT release. Eur. J. Pharmacol. 1991;200:131–139. doi: 10.1016/0014-2999(91)90675-g. [DOI] [PubMed] [Google Scholar]
- KIDD E.J., LEYSEN J.E., MARSDEN C.A. Chronic 5-HT2 receptor antagonist treatment alters 5-HT1A autoregulatory control of 5-HT release in rat brain in vivo. J. Neurosci. Meth. 1990;34:91–98. doi: 10.1016/0165-0270(90)90046-i. [DOI] [PubMed] [Google Scholar]
- KREISS D.S., LUCKI I. Chronic administration of the 5-HT1A receptor agonist 8-OH-DPAT differentially desensitizes 5-HT1A autoreceptors of the dorsal and median raphe nuclei. Synapse. 1997;25:107–116. doi: 10.1002/(SICI)1098-2396(199702)25:2<107::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- LE POUL E., LAARIS N., DOUCET E., LAPORTE A.M., HAMON M., LANFUMEY L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn-Schmeideberg's Arch. Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- LE POUL E., LAARIS N., HAMON M., LANFUMEY L. Fluoxetine-induced desensitization of somatodendritic 5-HT1A autoreceptors in independent of gluococorticoid(s) Synapse. 1997;27:303–312. doi: 10.1002/(SICI)1098-2396(199712)27:4<303::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- PÉREZ V., GILABERTE I., FARIES D., ALVAREZ E., ARTIGAS F. Randomised, double-blind, placebo-controlled trial of pindolol in combination with fluoxetine antidepressant treatment. Lancet. 1997;349:1594–1597. doi: 10.1016/S0140-6736(96)08007-5. [DOI] [PubMed] [Google Scholar]
- RANCK J.B.Behavioural correlates and firing repertories of neurons in the dorsal hippocampal formation of unrestrained rats The Hippocampus 1975New York: Plenum; 207–244.ed. Isaacson, I. & Robert, L. pp [Google Scholar]
- ROBINSON D.S., RICKELS K., FEIGHNER J., FABRE L.F., JR, GAMMANS R.E., SHROTRIYA R.C., ALMS D.R., ANDARY J.J., MESSINA M.E. Clinical effects of the 5-HT1A partial agonists in depression: A composite analysis of buspirone in the treatment of depression. J. Clin. Psychopharmacol. 1990;10:67S–76S. doi: 10.1097/00004714-199006001-00013. [DOI] [PubMed] [Google Scholar]
- ROMERO L., BEL N., ARTIGAS F., DE MONTIGNY C., BLIER P. Effect of pindolol at pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology. 1996;15:349–360. doi: 10.1016/0893-133X(95)00240-E. [DOI] [PubMed] [Google Scholar]
- RUETER L.E., DE MONTIGNY C., BLIER P. Electrophysiological characterization of the effect of long-term duloxetine administration on the rat serotonergic and noradrenergic systems. J. Pharmacol. Exp. Ther. 1998a;285:404–412. [PubMed] [Google Scholar]
- RUETER L.E., DE MONTIGNY C., BLIER P. In vivo electrophysiological assessment of the agonistic properties of flibanserin at pre- and postsynaptic 5-HT1A receptors in the rat brain. Synapse. 1998b;29:392–405. doi: 10.1002/(SICI)1098-2396(199808)29:4<392::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J., METCALF M.A., JOSE P.A., HAMBLIN M.W., SIBLEY D.W. Molecular cloning and expression of a 5-hydroxytryramine 7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- SMITH R.L., BARRETT R.J., SANDERS-BUSH E. Adaptation of brain 5-HT2 receptors after mianserin treatment: receptor sensitivity, not receptor binding, more accurately correlates with behaviour. J. Pharmacol. Exp. Ther. 1990;254:484–488. [PubMed] [Google Scholar]
- STAHL S.M., KAISER L., ROESCHEN J., KEPPEL HESSELINK J.M., ORAZEM J. Effectiveness of ipsapirone, a 5-HT-1A partial agonist, in major depressive disorder: support for the role of 5-HT-1A receptors in the mechanism of action of serotonergic antidepressants. Int. J. Neurpsychopharmacol. 1998;1:11–18. doi: 10.1017/S1461145798001059. [DOI] [PubMed] [Google Scholar]
- STOLTZ J.F., MARSDEN C.A., MIDDLEMISS D.N. Effect of chronic antidepressant treatment and subsequent withdrawal on [3H]-5-hydroxytryptamine and [3H]-spiperone binding in rat frontal cortex and serotonin receptor mediated behaviour. Psychopharmacology. 1983;80:150–155. doi: 10.1007/BF00427959. [DOI] [PubMed] [Google Scholar]
- TOME M.B., ISAAC M.T., HARTE R., HOLLAND C. Paroxetine and pindolol: a randomized trial of serotonergic autoreceptor blockade in the reduction of antidepressant latency. Int. Clin. Psychopharmacol. 1997;12:81–89. [PubMed] [Google Scholar]
- WILCOX C.S., FERGUSON J.M., DALE J.L., HEISER J.F. A double-blind trial of low- and high-dose ranges of gepirone-ER compared with placebo in the treatment of depressed outpatients. Psychopharmacol. Bull. 1996;32:335–342. [PubMed] [Google Scholar]
- YOCCA F.D., EISON A.S., HYSLOP D.K., RYAN E., TAYLOR D.P., GIANUTSOS G. Unique modulation of central 5-HT2 receptor binding sites and 5-HT2 receptor-mediated behaviour by continuous gepirone treatment. Life Sci. 1991;49:1777–1785. doi: 10.1016/0024-3205(91)90478-t. [DOI] [PubMed] [Google Scholar]
- ZANARDI R., FRANCINI L., GASPERINI M., PEREZ J., SMERALDI E. Selective serotonin reuptake inhibitors alone and in association with pindolol in the treatment of delusional depression. Eur. Neuropsycholpharmacol. 1998;8 Suppl. 2:S98. [Google Scholar]
- ZANARDI R., ARTIGAS F., FRANCHINI L., SFORZINI L., GASPERINI M., SMERALDI E., PEREZ J. How long pindolol should be associated to paroxetine to improve the antidepressant response. J. Clin. Psychopharmacol. 1997;17:446–450. doi: 10.1097/00004714-199712000-00002. [DOI] [PubMed] [Google Scholar]


