Abstract
Previous studies show that linking acetylated glucosamine to S-nitroso-N-acetyl-D,L-penicillamine (SNAP) stabilizes the molecule and causes it to elicit unusually prolonged vasodilator effects in endothelium-denuded, isolated rat femoral arteries. Here we studied the propanoyl (SNPP; 3 carbon side-chain), valeryl (SNVP; 5C) and heptanoyl (SNHP; 7C) N-substituted analogues of SNAP (2C), to further investigate other molecular characteristics that might influence chemical stability and duration of vascular action of S-nitrosothiols.
Spectrophotometric analysis revealed that SNVP was the most stable analogue in solution. Decomposition of all four compounds was accelerated by Cu(II) and cysteine, and neocuproine, a specific Cu(I) chelator, slowed decomposition of SNHP. Generation of NO from the compounds was confirmed by electrochemical detection at 37°C.
Bolus injections of SNAP (10 μl; 10−8–10−3 M) into the perfusate of precontracted, isolated rat femoral arteries taken from adult male Wistar rats (400–500 g), caused concentration-dependent, transient vasodilatations irrespective of endothelial integrity. Equivalent vasodilatations induced by SNVP and SNHP were transient in endothelium-intact vessels but failed to recover to pre-injection pressures at moderate and high concentrations (10−6–10−3 M) in those denuded of endothelium. This sustained effect (>1 h) was most prevalent with SNHP and was largely reversed by the NO scavenger, haemoglobin.
We suggest that increased lipophilicity of SNAP analogues with longer sidechains facilitates their retention by endothelium-denuded vessels; subsequent slow decomposition within the tissue generates sufficient NO to cause prolonged vasodilatation. This is a potentially useful characteristic for targeting NO delivery to areas of endothelial damage.
Keywords: Nitric oxide, S-nitrosothiols, vasodilatation, SNAP analogues
Introduction
Nitric oxide (NO) synthesized in the endothelium of blood vessels (Palmer et al., 1987; 1988; Palmer & Moncada, 1989) is recognized to be an important factor in control of local blood flow and of blood pressure in animals (Aisaka et al., 1989; Rees et al., 1989; Gardiner et al., 1990; Chu et al., 1991) and man (Vallance et al., 1989; Haynes et al., 1993). In addition, NO is known to inhibit platelet adhesion and aggregation (Radomski et al., 1987a,1987b; 1990), smooth muscle mitogenesis (Garg & Hassid, 1989) and monocyte adhesion (Lefer, 1997). Endothelial dysfunction resulting in reduced NO synthesis is thought to play an important role in atherogenesis (Chappell et al., 1987; Harrison et al., 1987; Forstermann et al., 1988; Guerra et al., 1989), and physical damage to the endothelium during percutaneous transluminal coronary angioplasty (PTCA) is a major contributory factor in the high incidence of thrombus formation and restenosis following this procedure (Langford et al., 1994). Current NO donor drugs, including the organic nitrates (such as glyceryl trinitrate; GTN) and sodium nitroprusside (SNP) do not improve outcome in patients with unstable angina or myocardial infarction or following PTCA.
S-Nitrosothiols (general formula R-S-N=O) undergo thermal decomposition in solution to disulphides, generating NO in the process (Williams, 1985). They present a potential alternative to existing NO donors, particularly as they do not appear to engender vascular tolerance (Harowitz et al., 1983; Bauer & Fung, 1991), an undesirable feature of prolonged administration of organic nitrates. Other potential advantages over current NO donors are their relative platelet (De Belder et al., 1994) and arterial selectivity (MacAllister et al., 1995) which might make them particularly attractive in the treatment of thrombotic and arterial disease. However, the therapeutic potential of the investigated S-nitrosothiols, such as S-nitroso-N-acetyl-D,L-penicillamine (SNAP; Figure 1a) and S-nitrosoglutathione (GSNO), is limited by the unpredictable nature of their decomposition, due in part to the catalytic effect of trace Cu(I) ions (Dicks et al., 1996; Gordge et al., 1996; Al-Sa'doni et al., 1997). Accelerated decomposition in the presence of Cu(II) (De Man et al., 1996) is now thought to be mediated by prior reduction to Cu(I) by thiols and might contribute to copper-mediated inhibition of atherogenesis (Ferns et al., 1997). In vivo, decomposition may also be accelerated by direct transfer of NO+ to reduced tissue thiols (transnitrosation; Askew et al., 1995) and by enzyme-dependent mechanisms (Askew et al., 1995; Gordge et al., 1996). Therapeutic effects of existing S-nitrosothiols might be improved by increasing their stability in vitro and by introducing a means of targeting delivery to damaged vessels deprived of endogenous NO.
Figure 1.
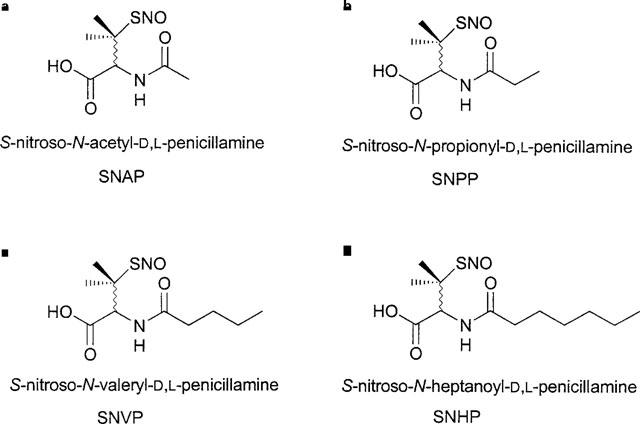
Structural formulae and full generic names for (a) SNAP, (b) SNPP, (c) SNVP and (d) SNHP.
Recently, we reported that a novel S-nitrosated glyco-amino acid, consisting of SNAP coupled to acetylated glucosamine (RIG200), was substantially more stable than the parent compound in vitro. Furthermore, we showed that it caused prolonged (>4 h), NO-mediated vasodilatation in endothelium-denuded rat isolated femoral arteries, whilst responses to SNAP itself were transient (Megson et al., 1997). Both RIG200 and SNAP caused transient vasodilatation in endothelium-intact vessels. We speculated that removal of the endothelium facilitates retention of RIG200 and suggested that lipophilicity of the compound by the acetylated glucosamine might be responsible for the effect. Here, we further test our hypothesis using novel N-substituted analogues of SNAP synthesized with different carbon side-chain lengths (C3–7; Figure 1b,c and d). We envisaged that increasing alkyl side-chain length would increase lipophilicity and in vitro stability. We also anticipated that SNAP analogues with longer side-chains would be more likely to cause sustained vasodilatation in endothelium-denuded vessels, similar to RIG200. Selectivity for endothelium-denuded vessels could lead to targeting of NO donors to vessels with endothelial injury caused by atherosclerosis or surgical procedures such as PTCA.
Methods
Decomposition of SNAP analogues in vitro
2.5 mM solutions of SNAP and RIG200 in oxygenated (95% O2, 5% CO2) Krebs buffer (composition in mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 5.5, were incubated in the dark at 24°C. Care was taken to use the same Krebs solution to dilute SNAP and its analogues in order to ensure identical copper ion content. The decrease in absorbance at a wavelength (λ) of 341 nm was measured using a Phillips PU 8720 ultraviolet/visible scanning spectrophotometer (path length=1 cm).
Experiments at 24°C were repeated in the presence of either an intermediate concentration of CuSO4 (1 μM; Askew et al., 1995), or the specific Cu(I) chelator, neocuproine (NCu; 1 μM; (Dicks et al., 1996; Al Sa'doni et al., 1997) in order to establish the effect of Cu(I) on the decomposition rate. Decomposition was also compared in the presence of the reduced thiol, cysteine (Cys; 1 mM). Rate constants were derived according to the following equation:
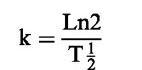 |
where T½=the observed half-life of S-nitrosothiols in solution.
NO release from S-nitrosothiols (10−5 M) in Krebs buffer solution at 37°C was confirmed using an isolated NO electrode (World Precision Instruments, Aston, Hertfordshire, U.K.). The electrode was calibrated with NO generated in situ from NaNO2 (10−7–10−6 M) acidified in ascorbic acid (1 mM). The role of Cu(I) and reduced thiols in decomposition was assessed using CuSO4 (1 μM), NCu (10 μM) and Cys (10 μM).
Ionization constants and lipophilicity parameters in n-octanol/water of N-substituted analogues of SNAP
The pH-metric method (Avdeef, 1993) was used to measure the ionization constant (pKa) and the logarithm of the partition coefficient in n-octanol/water of the neutral form of SNAP and SNVP (log PN). The method is based on the principle that there is a shift in the aqueous acid-base titration curve of a protogenic substance when a second phase (namely n-octanol) is added (Figure 2). The technique requires two successive titrations; first, the solute in water is titrated against standard acid or base to deduce the ionization constant (pKa). The titration is then repeated in the presence of n-octanol and a new ionization constant p0Ka is determined. The shift in the ionization constant is in response to the partitioning of some of the substance into the organic phase. This shift in pKa for a generic acid (HA) is used in the calculation of PN as shown in the following equation:
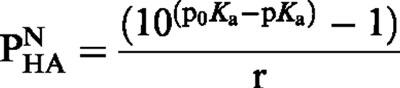 |
where r is the ratio of n-octanol to water.
Figure 2.
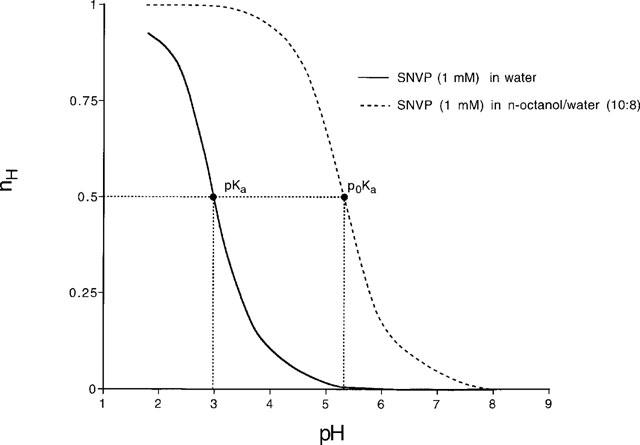
Difference (Bjerrum) plots for SNVP (1 mM) in water and in n-octanol/water (10 : 8; both n=4) where nH is the proportion of SNVP that is not ionised. These pKa values were used to derive the partition coefficient (log PN) for SNVP. Similar experiments were carried out for SNAP and results are presented in Table 2.
All potentiometric titrations were performed using GLpKa apparatus (Sirius Analytical Instruments Ltd, Forrest Row, East Sussex, U.K.).
For the determination of the pKa of SNAP and SNVP, 15 ml aqueous solutions (n=4) were initially acidified to pH 1.8 with HCl. The solutions were then titrated under nitrogen at 25.0±0.1°C with standardized KOH to pH 8. pKa values were obtained by difference (Bjerrum) plots (Figure 2) and the values obtained were refined by a weighted nonlinear least-squares procedure.
For the experimental determination of log PN, titrations of SNAP and SNVP (1 mM), containing volumes of n-octanol (1 ml organic solvent/15 ml water to 10 ml organic solvent/8 ml water) were performed in the pH range 1.8–8.0 (n=4). The same conditions and calculation procedures used for the determination of pKa values were adopted. The pH-metric approach does not permit measurement of log P lower than −0.5, thus the log P of the anionic species (log PA) could not be experimentally determined.
The experimental log PN value for SNAP (0.96) was used to calculate the corresponding values for the other SNAP analogues by a Rekker type approach (Avdeef, 1993; Mannhold et al., 1995; Caron et al., 1997).
Biological activity
Preparation
Experiments were performed on isolated segments of femoral artery from adult male Wistar rats (400–500 g; n=36) using the perfusion technique described previously (Flitney et al., 1992; Megson et al., 1997). Briefly, animals were sacrificed by cervical dislocation and both femoral arteries were cannulated immediately distal to the epigastric arterial branch. Arterial segments (7–8 mm long) were dissected free and transferred to perspex organ bath chambers (1 ml volume) at 37°C where they were perfused (0.6 ml min−1; Gilson minipuls 3, Anachem, Luton, U.K.) and superfused (1 ml min−1; Watson Marlow 302S; Watson Marlow, Falmouth, U.K.) with fresh oxygenated Krebs buffer solution. Twin vessels were precontracted with phenylephrine (PE) and perfusion pressure was monitored by a differential pressure transducer (T; Sensym SCX 15ANC, Farnell Electronic Components, Leeds, U.K.) located upstream.
The apparatus permits exclusive drug delivery to the luminal surface of the vessel by bolus injection (10 μl) through a resealable rubber septum into the perfusate immediately upstream of the vessel (transit time to artery ∼3 s, through lumen ∼300 ms). Injections of vehicle (Krebs buffer) had no effect on perfusion pressure. Vasodilator responses in control vessels could be compared to those perfused with supra-maximal concentrations of the recognized NO scavenger, ferrohaemoglobin (Martin et al., 1985). Where possible, two vessels from each animal were used in parallel; one being denuded of endothelium, the other with endothelium intact.
Experimental protocols
All experiments were carried out in a darkened laboratory in order to protect photolabile drugs and to prevent photorelaxation of vessels (Megson et al., 1995). Drugs were dissolved and diluted in PE-containing Krebs solution and kept on ice prior to use.
Endothelial function of precontracted arteries was assessed using ferrohaemoglobin (Hb; 10 μM); Hb scavenges endogenous NO and the resultant vasoconstriction is a measure of basal NO activity. This technique was preferred to cholinergic, endothelium-dependent relaxation because, in this perfusion system, the endothelium is already highly stimulated to produce NO by flow, and responses to cholinergic stimuli are, as a result, typically small (Flitney et al., 1992; Megson et al., 1997). In addition, a significant proportion of ACh-induced vasodilatation is believed to be due to release of endothelium-derived hyperpolarizing factor (EDHF; Cohen & Vanhoutte, 1995). In experiments where the endothelium was removed, air was passed through the lumen until such time as the vessel was unresponsive to Hb (5–10 min). Denudation invariably caused an increase in pressure due to loss of endothelium-derived NO. Pressure was restored to its original level by appropriate reduction in PE concentration (∼0.5×original concentration). Selected vessels were taken for immunohistochemical staining to confirm endothelial denudation (5 μm paraffin sections, fixed in formalin (10%; 24 h), treated with biotinylated constitutive NO synthase antibodies coupled to avidin (avidin-biotin complex) and visualized (n=8 endothelium-denuded; n=8 endothelium-intact).
Vasodilator responses to bolus injections of SNAP and its analogues
Bolus injections of increasing concentrations of SNAP, SNPP, SNVP or SNHP (10 μl; 10−8–10−3 M) were made sequentially into the perfusate of precontracted, endothelium-intact or -denuded vessels. Responses were deemed to have recovered once pressure was maintained for more than 5 min. Time intervals between injections of S-nitrosothiol were matched between intact and denuded vessels for each individual experiment. Responses to 10−3 M concentrations were allowed to recover for a period of 1 h, after which vessels were perfused with Hb (10 μM). In order to assess the role of extracellular NO in vasodilatation induced by SNAP and its N-substituted analogues in endothelium-denuded vessels, sequential bolus injections of S-nitrosothiols were also made into perfusate containing Hb (10 μM).
Drugs and reagents
All chemicals except the S-nitrosothiols were obtained from Sigma Ltd. (Poole, Dorset, U.K.). Met-Hb was reduced to the ferro-form using sodium dithionite as described previously (Martin et al., 1985).
SNAP was prepared using an established method (Field et al., 1978). Ultraviolet/visible spectral analysis confirmed an absorption peak at λ=341 nm, characteristic for S-nitrosothiols. The extinction coefficient (ε) at this wavelength was 1168 M−1 cm−1. SNAP is soluble in Krebs buffer at concentrations up to 10 mM. The N-substituted analogues of SNAP were synthesized by the acylation of D,L-penicillamine followed by S-nitrosation (details will be published elsewhere). All were soluble to concentrations up to 2.5 mM after ultrasonication.
Analysis of results
Signals from the pressure transducers were processed by a MacLab/4e analogue-digital converter and displayed through ‘Chart' software (AD Instruments, Sussex, U.K.) on a Macintosh Performa 630 microcomputer. Vasodilator response amplitude was expressed as a percentage of PE-induced pressure existing prior to the first in a series of drug application (percentage pressure change; negative values represent relaxation, positive represent constriction). Data are given for percentage pressure change both at the peak of responses and following response recovery as defined earlier. Mean values are given±s.e.mean.
P values stated in the text were obtained using two-factor, repeated dose ANOVAs except where otherwise specified. P<0.05 was accepted as statistically significant.
Results
Decomposition of SNAP analogues in vitro
Rate constants derived from spectrophotometric studies of decomposition indicated that SNAP was the most stable of the analogues in Krebs buffer alone at 24°C (Table 1). The rate of SNAP and SNPP decomposition was noticeably more variable than SNVP and SNHP. SNVP proved the most stable in Krebs buffer; decomposition was <1% in 1 h, preventing calculation of a rate constant.
Table 1.
Rate constants for N-substituted analogues of SNAP in Krebs buffer alone (control) and in the presence of NCu (10 μM), CuSO4 (1 μM) and Cys (1 mM)
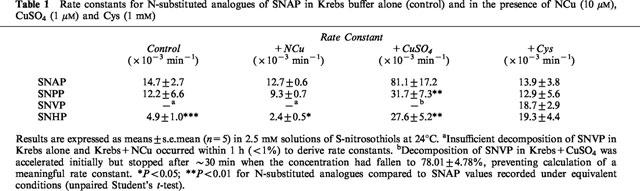
Decomposition of all four analogues was accelerated by Cu2+ (1 μM; Table 1). SNAP decomposition in the presence of Cu2+ was significantly faster than that of the other S-nitrosothiols (P<0.05; unpaired student's t-test). Interestingly, SNVP decomposition under these conditions stopped after ∼30 min, when the concentration reached 78.01±4.78 of its initial value, preventing determination of a meaningful rate constant. Only the rate of decomposition of SNHP was significantly inhibited by NCu (10 μM; Table 1; P<0.05), although the variabilty seen with SNAP and SNPP in Krebs alone was not evident in the presence of NCu.
Cys (1 mM) accelerated decomposition of all four analogues and the rate of decomposition in the presence of Cys was not significantly different between the analogues (Table 1).
Generation of NO from the decomposition of SNAP and its analogues (10 μM) was confirmed at 37°C using an isolated NO electrode. The results largely support the spectrophotometric data in that SNAP generates NO significantly more rapidly than the other analogues and that NO generation is accelerated by addition of Cu2+ or Cys (results for SNVP are illustrated in Figure 3; similar experiments with other analogues are summarized in Table 2). Addition of Cu2+ and Cys together greatly accelerated decomposition; NO generation reached a peak value of ∼1.5–2.5 μM after 2 min (Figure 3c). Addition of an excess of NCu (10 μM) in experiments involving Cu2+ reversed the Cu-mediated accelerated NO release (Figure 3b) but did not affect NO generated spontaneously or in the presence of Cys. Addition of Hb (10 μM) at any point during experiments caused a rapid (<1 min) fall in NO concentration to levels at or near zero (Figure 3a and c).
Figure 3.
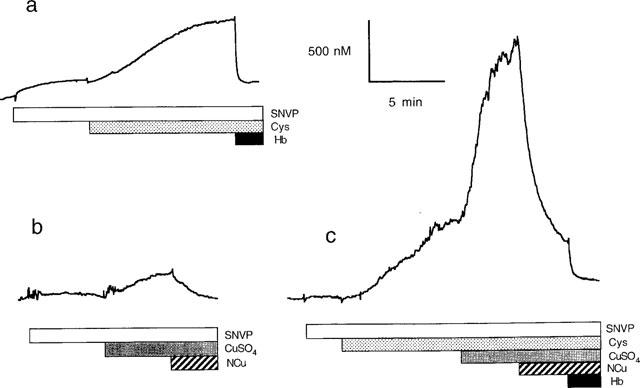
Representative trace for NO concentration detected in solutions of SNVP (10 μM) in saline at 37°C, using an isolated NO electrode. Cys (10 μM; a, c), CuSO4 (1 μM; b, c), NCu (10 μM; b, c) and Hb (10 μM; a, c) were added to the solution as indicated by the horizontal bars.
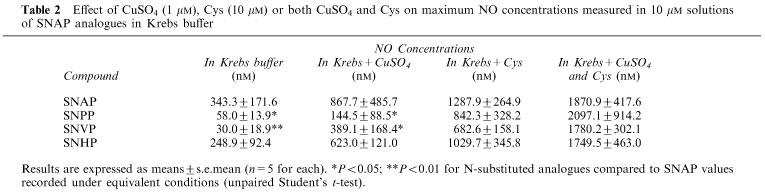
Table 2.
Effect of CuSO4 (1 μM), Cys (10 μM) or both CuSO4 and Cys on maximum NO concentrations measured in 10 μM solutions of SNAP analogues in Krebs buffer
Biological activity
Vessel parameters
Vessels were pre-contracted to pressures of 101.2±3.7 mmHg with PE (7.89±0.52 μM; n=68). Pressure in endothelium intact vessels increased by 78.1±9.0% (n=32; P<0.001; unpaired student's t-test) on perfusion with Hb (10 μM). Pressure in denuded vessels failed to increase significantly above pre-contraction levels on perfusion with Hb (+1.2±3.8%; n=36).
Vasodilator responses in endothelium-intact vessels
Bolus microinjections of all four S-nitrosothiols caused dose-dependent vasodilatation in endothelium-intact arteries (Figures 4 and 5a). PD2 values were calculated as 5.83±0.17 (SNAP; n=8), 5.74±0.39 (SNPP; n=8), 5.09±0.31 (SNVP; n=8) and 5.66±0.23 (SNHP; n=8). There was no significant difference between responses (P>0.05, ANOVA) or PD2 values (P>0.05, Student's t-test) for these compounds. Vasodilator responses to S-nitrosothiols recovered to or above pre-injection pressures at all but the highest injected concentration (Figures 4a and 6a). Responses to 10−3 M bolus injections of SNPP and SNHP failed to recover fully after washout. Perfusion with Hb 1 h after washout of 10−3 M bolus injections of SNAP analogues caused perfusion pressure to rise significantly above pre-injection pressure (Figure 4a; +71.7±14.5% after SNAP, n=8; +57.3±7.1% for SNPP, n=8; +51.9±7.5% for SNVP, n=8 and +49.0±16.9% for SNHP; n=8).
Figure 4.
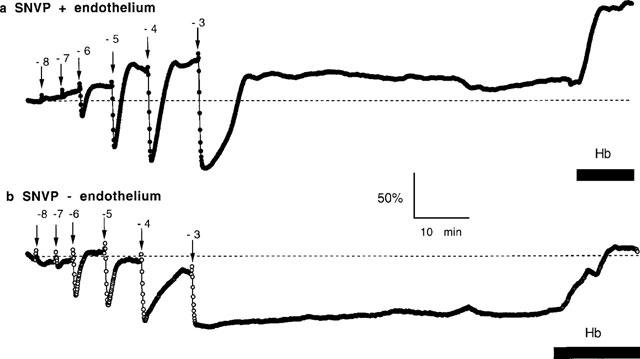
Representative pressure recordings of vasodilatations elicited by sequential bolus injections (10 μl) of SNVP (log M concentrations as indicated) in (a) endothelium-intact and (b) endothelium-denuded rat femoral arteries. Perfusion with 10 μM Hb is indicated by the horizontal bar.
Figure 5.
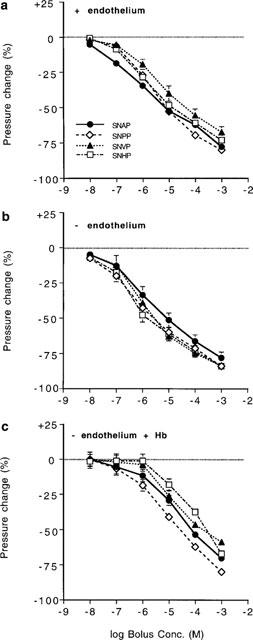
Concentration-response curves for peak amplitude of responses to bolus injections (10 μl) of N-substituted S-nitrosothiols into the perfusate of (a) endothelium-intact vessels, (b) endothelium-denuded vessels and (c) endothelium-denuded vessels perfused with 10 μM Hb (n=8 for all compounds under each condition). There was no statistical significant difference between responses to any of the SNAP analogues in either (a) or (b) (P>0.05; repeated dose, 2 factor ANOVAs). Responses to SNVP and SNHP, but not the other analogues, were significantly enhanced in (b) compared to (a) and responses to all four analogues were significantly inhibited by Hb (c; P<0.05).
Figure 6.
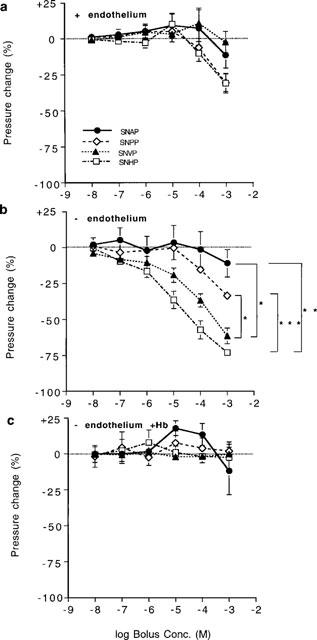
Concentration-recovery curves (means±s.e.mean) for bolus injections (10 μl) of N-substituted S-nitrosothiols into the perfusate of (a) endothelium-intact vessels, (b) endothelium denuded vessels and (c) endothelium-denuded vessels perfused with 10 μM Hb (n=8 for all compounds under each condition). Significant differences in (b) are as shown (*P<0.05, **P<0.01, ***P<0.001). Responses to the four analogues were not significantly different from each other in (a) and (c; P>0.05; repeated dose, 2 factor ANOVAs).
Vasodilator responses in endothelium-denuded vessels
Peak amplitude of responses to SNVP and SNHP compounds was significantly enhanced in endothelium-denuded vessels compared to intact vessels (P<0.01 and P<0.05 respectively, n=8 for both compounds) but not for SNAP and SNPP (P>0.05). The extent to which SNAP and SNPP responses recovered following bolus injections was not different in endothelium denuded vessels compared with intact vessels (Figure 6b; P>0.05 for both compounds). However, in denuded vessels, responses to SNVP and SNHP were sustained at concentrations >10−6 M (Figure 6b; P<0.01 for both compounds). Sustained vasodilatation was clearly related to the length of the N-substituted side-chain, and was most pronounced with SNHP. Sustained responses were largely reversed by Hb to 99.0±7.1% of pre-injection pressure following SNAP; 82.6±7.2% following SNPP; 94.2±7.9% following SNVP and 81.6±7.0% following SNHP (n=8 for all four compounds).
Inhibition of responses in endothelium-denuded vessels by Hb
Vasodilator responses in endothelium-denuded vessels during perfusion with Hb (10 μM) were significantly attenuated (Figure 5c; P<0.05, n=7 for all four compounds) and recovered to, or above, pre-injection levels (Figure 6c) with all four analogues; sustained vasodilatation was not evident in Hb-perfused vessels.
Lipophilicity and sustained vasodilatation
Excellent agreement was achieved between experimental and calculated values for SNVP (2.53 and 2.47±0.022 respectively), confirming the validity of the Rekker-type approach for determining partition coefficients and demonstrates the lack of intramolecular effects in this series of S-nitrosothiols. Ionization constants do not vary significantly from shorter to longer molecules, thus all the derivatives have the same degree of ionization and are 99.9% ionized at physiological pH. The log P of the anionic species (log PA) is generally assumed to be three orders of magnitude lower than log PN (Avdeef, 1996). In addition, according to their chemical structures, it is reasonable to assume that the anionic forms are not affected by any intramolecular effects, thus their logarithm of partion coefficient (log PA) are linearly correlated with log PN (Fruttero et al., 1998) which can be used as the general lipophilicity descriptors for SNAP and its derivatives.
A logarithmic plot of calculated values for PN against response recovery following a bolus injection (10−3 M) for each of the S-nitrosothiols (Figure 7), shows a linear correlation (r2=0.998), suggesting that lipophilicity (Testa et al., 1996) might be an important factor in determining the degree of sustained vasodilatation exhibited by this group of compounds.
Figure 7.

Logarithmic plot of partition coefficient (PN) of N-substituted analogues of SNAP against response recovery to bolus injections of compounds (10 μl; 10−3 M). Recovery is expressed as the pressure attained at t=60 min after bolus washout as a percentage of pre-injection pressure (n=8). The relationship is linear, with a correlation coefficient (r2) of 0.998.
Discussion
Our results show that the N-substituted chain length of SNAP analogues is an important determinant of the rate of decomposition of the compounds in solution, their lipophilicity, and their ability to cause sustained vasodilatation in endothelium-denuded isolated rat femoral arteries.
Increasing the length of the alkyl sidechain of SNAP clearly affected in vitro stability (Table 1), with SNVP proving the least prone to decomposition. The stability of S-nitrosothiols is affected by steric factors as it involves the dimerization of two thiyl radicals (RS•, Bainbrigge et al., 1997) and it is apparent that this process is most effectively retarded by a 5C side chain. Decomposition of SNAP, SNPP, and SNHP were greatly accelerated in the presence of Cu2+, as would be predicted from previous observations (De Man et al., 1996; Dicks et al., 1996; Gordge et al., 1996; Al-Sa'doni et al., 1997). However, SNVP decomposition, though accelerated initially, stopped when the concentration reached ∼80% of its original value. The reason for this apparent resistance of SNVP to Cu-mediated decomposition is unknown but it may involve chelation of Cu ions by the resulting disulphide, in a process similar to that reported previously for GSNO (Swift, 1989). NCu significantly inhibited decomposition of SNHP and greatly reduced the variability in decompostion seen with SNAP and SNPP in Krebs buffer alone (Table 1). This result suggests that sufficient trace Cu(I) in Krebs buffer exists to accelerate SNHP decomposition, and that changing Cu(I) levels from day-to-day are responsible for inconsistencies in SNAP and SNPP decomposition. Interestingly, the exquisite sensitivity of SNAP to trace Cu(I) is not apparently shared by SNVP, again implying that the valeryl sidechain imparts resistance to Cu(I) catalysis.
The rate of decomposition of SNAP analogues was accelerated by Cys and, interestingly, decomposition of all four compounds in the presence of Cys occurred at similar rates (Table 1). This observation might help to explain why S-nitrosothiols with widely varying thermal stabilities in vitro have similar biological properties, and suggests that transnitrosation to Cys might be a key mechanism for decomposition in vivo.
SNVP, of all the compounds, generated NO most slowly in Krebs buffer alone (Table 2), as predicted by spectrophotometric studies. Accelerated NO generation in the presence of either Cu2+ or Cys (Figure 3a and b) reflected Cu+-mediated catalysis and transnitrosation respectively. We propose that the markedly accelerated NO generation seen in the presence of both Cys and Cu2+ (Figure 3 and Table 2) is due to Cys-mediated reduction of Cu2+ to Cu+, facilitating Cu+-mediated catalysis of S-nitrosothiol decomposition. This, added to transnitrosation from SNAP analogues to Cys, forming unstable S-nitrosocysteine, would explain the rapid release of NO under these conditions. The results indicate that in the presence of Cys, different SNAP analogues generate similar amounts of NO, perhaps explaining comparable biological effects.
Vasodilator responses to bolus injections of SNAP analogues
The lack of discrimination of arteries to bolus injections of SNAP and its analogues (Figure 5a), despite large variations in thermal stability (Table 1), lends weight to the argument that S-nitrosothiol decomposition in vascular tissue is accelerated by a process common to all of these compounds. In general, responses to all four compounds were transient in intact vessels (Figures 4 and 6), with only responses to the highest dose on occasion failing to recover fully to pre-injection pressure (Figure 6a). The rebound contraction sometimes seen in endothelium intact vessels following bolus injections (Figure 4a) were similar to those seen with RIG200 (Megson et al., 1997); an effect that might be due to desensitization of vessels to endothelium-derived NO following delivery of exogenous NO. Hb-mediated vasoconstriction at the end of experiments reflected scavenging of endothelium-derived NO and confirmed the endothelial integrity of the vessels (Figure 4a).
Peak amplitudes of vasodilator responses to SNVP and SNHP were significantly increased in endothelium-denuded vessels (Figure 5b), perhaps reflecting hypersensitivity of vessels to exogenous NO once deprived of endothelium-derived NO (Moncada et al., 1991). However, these two analogues were also the ones that caused sustained vasodilatation in endothelium-denuded vessels (Figure 6b), suggesting that the apparent increase in peak amplitude of responses might be due to the falling perfusion pressure caused by the sustained effects of preceding injections, rather than to hypersensitivity (Figures 4b and 6b). The prolonged responses to moderate and high concentrations (10−6–10−3 M) of SNVP and SNHP (Figure 6b), which lasted for >1 h after washout, were largely reversed by Hb and were therefore predominently NO-mediated (Figure 4b). Some vessels (n=5/16) still had reduced tone (<17%) even during Hb perfusion, perhaps suggesting an alternative mechanism for sustained vasodilatation, involving either NO from a source inaccessible to Hb, or an NO-independent mechanism. However, this possibility is not supported by evidence from our other experiments in which vasodilatations in response to S-nitrosothiols in endothelium-denuded vessels during Hb perfusion, invariably recovered to pre-injection pressure (Figure 6c). The peak amplitude of responses in endothelium-denuded vessels to all four compounds was significantly inhibited by perfused Hb (Figure 5c), implying that the transient element of responses is, at least in part, due to NO released at a site accessible to intraluminal Hb.
The precise mechanism by which some S-nitrosothiols cause sustained vasodilatation clearly requires further investigation but our studies suggest that the effect is correlated to lipid solubility (Figure 7). We hypothesize that sustained vasodilatation is due to retention of lipophilic S-nitrosothiols in lipid-rich compartments of sub-endothelial layers of denuded arteries. Retained compounds decompose slowly to release NO which causes dilatation of vessels for >1 h after bolus washout. Penetration of compounds is sufficiently shallow to facilitate scavenging of NO by intraluminal Hb, which does not penetrate the basement membrane of arteries (Hongo et al., 1988).
These properties require confirmation in human vessels and ultimately in vivo, but S-nitrosothiols that cause prolonged vasodilatation specifically in vessels with endothelial injury could be beneficial following PTCA, particularly if they prove to have anti-mitogenic properties and inhibitory effects on platelet aggregation. S-nitrosothiols could also be useful in the treatment of atherosclerosis where compounds might not only be selectively retained in areas of endothelial damage but, in light of their lipophilicity, also in regions of lipid deposition.
Acknowledgments
We acknowledge support from the BHF (Junior Research Fellowship to ILM; FS/95061), from the Rollo Trust (St Andrews) and from MURST (Torino). Professor D.J. Webb is supported by a Research Leave Fellowship from the Wellcome Trust (WT0526330).
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- Cys
cysteine
- EDHF
endothelium-derived hyperpolarizing factor
- Hb
ferrohaemoglobin
- GTN
glyceryl trinitrate
- T½
half-life
- NCu
neocuproine
- NO
nitric oxide
- SNP
sodium nitroprusside
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
- SNHP
S-nitroso-N-heptanoyl-D,L-penicillamine
- SNPP
S-nitroso-N-propanoyl-D,L-penicillamine
- SNVP
S-nitroso-N-valeryl-D,L-penicillamine
- P
partition coefficient
- PTCA
percutaneous transluminal coronary angioplasty
- PE
phenylephrine
References
- AISAKA K., GROSS S.S., GRIFFITH O.W., LEVI R. NG-Methyl arginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo. Biochem. Biophys. Res. Commun. 1989;160:881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- AL-SA'DONI H.H., MEGSON I.L., BISLAND S.K., BUTLER A.R., FLITNEY F.W. Neocuproine, a selective Cu(I) chelator, and the relaxation of rat vascular smooth muscle by S-nitrosothiols. Br. J. Pharmacol. 1997;121:1047–1050. doi: 10.1038/sj.bjp.0701218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASKEW S.C., BUTLER A.R., FLITNEY F.W., KEMP G.D., MEGSON I.L. Chemical mechanism underlying the vasodilator and platelet anti-aggregating properties of S-nitroso-N-acetyl-D,L-penicillamine and S-nitrosoglutathione. Bioorg. Med. Chem. 1995;3:1–9. doi: 10.1016/0968-0896(94)00139-t. [DOI] [PubMed] [Google Scholar]
- AVDEEF A.Assessment of distribution-pH profiles Lipohilicity in Drug Action and Toxicology 1996Weinheim, VCH Publishers; ed. Pliska, V., Testa, B. & van de Waterbeemd, H. [Google Scholar]
- AVDEEF A. pH-metric Log P: refinement of partition coefficients and ionization constants of multiprotic substances. J. Pharm. Sci. 1993;82:183–190. doi: 10.1002/jps.2600820214. [DOI] [PubMed] [Google Scholar]
- BAINBRIGGE N., BUTLER A.R., GORBITZ C.H. The thermal stability of S-nitrosothiols: experimental studies and ab initio calculations on model compounds. J. Chem. Soc. Perkin Trans., 2. 1997;2:351–353. [Google Scholar]
- BAUER J.A., FUNG H.L. Differential hemodynamic effects and tolerance properties of nitroglycerin and an S-nitrosothiol in experimental heart failure. J. Pharmacol. Exp. Ther. 1991;256:249–254. [PubMed] [Google Scholar]
- CARON G., GAILLARD P., CARRUPT P.A., TESTA B. Lipophilicity behaviour of model and medicinal compounds containing a sulfide, sulfoxide or sulfone moiety. Helvetica. Chimica. Acta. 1997;80:449–462. [Google Scholar]
- CHAPPELL S.P., LEWIS M.J., HENDERSON A.H. Effect of lipid feeding on endothelium-dependent relaxation in rabbit aorta preparations. Cardiovasc. Res. 1987;21:34–38. doi: 10.1093/cvr/21.1.34. [DOI] [PubMed] [Google Scholar]
- CHU A., CHAMBERS D.E., LIN C.-C., KEUHL W.D., PALMER R.M.J., MONCADA S., COBB F. Effects of inhibition of nitric oxide formation on basal vasomotion and endothelium-dependent responses of the coronary arteries in awake dogs. J. Clin. Invest. 1991;87:1964–1968. doi: 10.1172/JCI115223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN R.A., VANHOUTTE P.M. Endothelium-dependent hyperpolarisation: beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- DE BELDER A.J., MACALLISTER R., RADOMSKI M.W., MONCADA S. Effects of S-nitroso-glutathione in the human forearm circulation: evidence for selective inhibition of platelet activation. Cardiovasc. Res. 1994;28:691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]
- DE MAN G., DE WINTER B.Y., BOECKXSTAENS G.E., HERMAN A.G., PELCKMANS P.A. Effect of Cu2+ on relaxations to the nitrergic neurotransmitter, NO and S-nitrosothiols in the rat fundus. Br. J. Pharmacol. 1996;119:990–996. doi: 10.1111/j.1476-5381.1996.tb15769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKS A.P., SWIFT H.R., WILLIAMS D.L.H., BUTLER A.R., AL-SA'DONI H.H., COX B.G. Identification of Cu+ as the effective reagent in nitric oxide formation from S-nitrosothiols (RSNO) J. Chem. Soc. Perkin. Trans. 1996;2:481–487. [Google Scholar]
- FERNS G.A.A., LAMB D.J., TAYLOR A. The possible role of copper ions in atherogenesis: the Blue Janus. Atherosclerosis. 1997;133:139–252. doi: 10.1016/s0021-9150(97)00130-5. [DOI] [PubMed] [Google Scholar]
- FIELD L., DILTS R.V., RAVICHANCHRAN R., LENHERT P.G., CARNAHAN G.E. An unusually stable thionitrite from N-acetyl-D,L-penicillamine: X-ray structure and molecular structure of 2-(acetylamino)-2-carboxy-1, 1-dimethylethylthionitrite. J. Chem. Soc. Chem. Commun. 1978. pp. 249–250.
- FLITNEY F.W., MEGSON I.L., FLITNEY D.E., BUTLER A.R. Iron-sulphur cluster nitrosyls, a novel class of nitric oxide generator: mechanism of vasodilator action on rat isolated tail artery. Br. J. Pharmacol. 1992;107:842–848. doi: 10.1111/j.1476-5381.1992.tb14534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORSTERMANN U., MUGGE A., ALHEID U., HAVERICH A., FROLICH J.C. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ. Res. 1988;62:185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- FRUTTERO R., CARON G., FORNATTO E., BOSCHI D., ERMONDI G., GASCO A., CARRUPT P.A., TESTA B. Mechanisms of liposomes/water partitioning of (p-methylbenzyl)alkylamines. Pharm. Res. 1998;15:1407–1413. doi: 10.1023/a:1011953622052. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br. J. Pharmacol. 1990;101:625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARG U.C., HASSID A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDGE M.P., MEYER D.J., HOTHERSHALL J., NEILD G.H., PAYNE N.N., NORONHA-DUTRA A. Role of a copper (I)-dependent enzyme in the anti-platelet action of S-nitrosoglutathione. Br. J. Pharmacol. 1996;114:1083–1089. doi: 10.1111/j.1476-5381.1996.tb15704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERRA R.J., BROTHERTON A.F.A., GOODWIN P.J., CLARK C.R., ARMSTRONG M.L., HARRISON D.G. Mechanism of abnormal endothelium-dependent relaxation in atherosclerosis: implications for altered autocrine and paracrine functions of EDRF. Blood Vessels. 1989;26:300–314. doi: 10.1159/000158779. [DOI] [PubMed] [Google Scholar]
- HAROWITZ J.D., ANTMAN E.M., LORELL B.H., BARRY W.H., SMITH T.W. Potentiation of the cardiovascular effects of nitroglycerin by N-acetylcysteine. Circulation. 1983;68:1247–1253. doi: 10.1161/01.cir.68.6.1247. [DOI] [PubMed] [Google Scholar]
- HARRISON D.G., ARMSTRONG M.L., FRIEMAN P.C., HEISTAD D.D. Restoration of endothelium-dependent relaxation by dietary treatment in atherosclerosis. J. Clin. Invest. 1987;80:1808–1811. doi: 10.1172/JCI113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYNES W.G., NOON J.P., WALKER B.R., WEBB D.J. L-NMMA increases blood pressure in man. Lancet. 1993;342:931–932. doi: 10.1016/0140-6736(93)91981-q. [DOI] [PubMed] [Google Scholar]
- HONGO K., OGAWA H., KASSELL N., NAKAGOMI T., SASAKI T., TSUKAHARA T., LEHMAN M. Comparison of intraluminal and extraluminal inhibitory effects of haemoglobin on endothelium-dependent relaxation of rabbit basilar artery. Stroke. 1988;19:1550–1555. doi: 10.1161/01.str.19.12.1550. [DOI] [PubMed] [Google Scholar]
- LANGFORD E.J., BROWN A.S., WAINWRIGHT R.J., DE BELDER A.J., THOMAS M.R., SMITH R.E.A., RADOMSKI M.W., MARTIN J.F., MONCADA S. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994;344:1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- LEFER A.M. Nitric oxide: nature's naturally occurring leukocyte inhibitor. Circulation. 1997;95:553–554. doi: 10.1161/01.cir.95.3.553. [DOI] [PubMed] [Google Scholar]
- MACALLISTER R.J., CALVER A.L., RIEZEBOS J., COLLIER J., VALLANCE P. Relative potency of nitrovasodilators on human blood vessels: an insight into the targetting of nitric oxide delivery. J. Pharmacol. Exp. Ther. 1995;273:1529–1537. [PubMed] [Google Scholar]
- MANNHOLD R., REKKER R.F., SONNTAG C., TERLAAK A.M., DROSS K. Comparative evaluation of the predictive power of calculation procedures for molecular lipophilicity. J. of Pharm. Sci. 1995;84:1410–1419. doi: 10.1002/jps.2600841206. [DOI] [PubMed] [Google Scholar]
- MARTIN W., VILLANI G.M., JOTHIANANDAN D., FURCHGOTT R.F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin, and by methylene blue in the rabbit aorta. J. Pharmacol. Exp. Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- MEGSON I.L., FLITNEY F.W., BATES J., WEBSTER R.N. “Repriming” of vascular smooth muscle photorelaxation is dependent on endothelium-derived nitric oxide. Endothelium. 1995;3:39–46. [Google Scholar]
- MEGSON I.L., GREIG I.R., GRAY G.A., WEBB D.J., BUTLER A.R. Prolonged effect of a novel S-nitrosated glyco-amino acid in endothelium-denuded rat femoral arteries: potential as a slow release nitric oxide donor drug. Br. J. Pharmacol. 1997;122:1617–1624. doi: 10.1038/sj.bjp.0701557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., REES D.D., SCHULZ R., PALMER R.M.J. Development and mechanism of a specific supersensitivity to nitrovasodilators following inhibition of nitric oxide synthase in vivo. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M.J., ASHTON D., MONCADA S. Vascular endothelial cells synthesise nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of EDRF. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., MONCADA S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;158:348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987a;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987b;148:1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. An L-arginine: nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., MONCADA S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIFT H. 1989. PhD Thesis. Department of Chemistry, University of Durham, Durham, U.K.
- TESTA B., CARRUPT P.A., GAILLARD P., TSAI R.S.Intramolecular interactions encoded in lipophilicity: their nature and significance Lipophilicity in Drug Action and Toxicology 1996Weinheim: VCH Publishers; 49–71.ed. Pliska, V., Testa, B. & van de Waterbeemd, H. pp [Google Scholar]
- VALLANCE P., COLLIER J., MONCADA S. Effects of endothelium-derived nitric oxide on peripheral arterial tone in man. Lancet. 1989;334:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- WILLIAMS D.L.H. S-nitrosation and the reactions of S-nitroso compounds. Chem. Soc. Rev. 1985;14:171–196. [Google Scholar]


