Abstract
Atypical neuroleptics produce fewer extrapyramidal side-effects (EPS) than typical neuroleptics. The pharmacological profile of atypical neuroleptics is that they have equivalent or higher antagonist affinity for 5-HT2 than for dopamine D2 receptors. Our aim was to identify which 5-HT2 receptor contributed to the atypical profile. Catalepsy was defined as rats remaining immobile over a horizontal metal bar for at least 30 s, 90 min after dosing. Radioligand binding assays were carried out with homogenates of human recombinant 5-HT2A, 5-HT2B and 5-HT2C receptors expressed in Human Embryo Kidney (HEK293) cells. Haloperidol (1.13 mg kg−1 i.p.) induced catalepsy in all experiments. The selective 5-HT2C/2B receptor antagonist, SB-228357 (0.32–10 mg kg−1 p.o.) significantly reversed haloperidol-induced catalepsy whereas the 5-HT2A and 5-HT2B receptor antagonists, MDL-100907 (0.003–0.1 mg kg−1 p.o.) and SB-215505 (0.1–3.2 mg kg−1 p.o.) respectively did not reverse haloperidol-induced catalepsy. The data suggest a role for 5-HT2C receptors in the anticataleptic action of SB-228357.
Keywords: 5HT2C and Dopamine D2 receptor antagonists, catalepsy
Introduction
Clozapine and the new generation of ‘atypical' neuroleptics such as olanzapine are less liable than typical neuroleptics to cause extrapyramidal side-effects (EPS) when used to treat schizophrenia (Meltzer, 1996). As well as being dopamine D2 receptor antagonists, the atypical neuroleptics are high affinity antagonists at 5HT2 receptors. This property may be responsible for their atypical profile (Meltzer et al., 1989). Therefore, we have investigated whether 5-HT2A/2B/2C receptor subtypes modulate haloperidol-induced catalepsy.
Methods
Male Sprague Dawley rats (200–250 g; Charles River) were housed in groups of six under a 12 h light dark cycle (lights on 0700 h) with free access to food and water. To test for catalepsy, rats were positioned so that their hindquarters were on the bench and their forelimbs rested on a 1 cm diameter horizontal bar, 10 cm above the bench. The length of time the rats maintained this position was recorded by stopwatch to a maximum of 120 s. This procedure occurred 30, 60 and 90 min after drug administration. Rats were judged to be cataleptic, and assigned a score of ‘1' if they maintained this position for 30 s or more; otherwise, they were assigned a score of ‘0'. As the raw data were derived from a non-linear quantal scoring scheme, a logistic regression analysis in SAS-RA© (SAS Institute Inc.) was used to analyse the data at the 90 min time point. MDL-100907 (R-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl) ethyl]-4-piperidinemethanol) (0.003–0.1 mg kg−1), SB-215505 (6-chloro-5-methyl-1-(5-quinolylcarbamoyl) indoline) (0.1 – 3.2 mg kg−1) and SB-228357 (1-5[-fluoro-3-(3-pyridyl)phenylcarbamoyl] -5- methoxy-6-trifluoromethylindoline) (0.32–10 mg kg−1) were ground in one drop of BRIJ-35 and diluted in 1% methylcellulose and administered at 2 ml kg−1 p.o. Haloperidol was dissolved with an equal weight of tartaric acid and injected at 1 ml kg−1 i.p. immediately after the 5HT2 receptor antagonists. In all experiments, each treatment group consisted of six rats and the assessor was blind to the treatments.
Radioligand binding assays were carried out with homogenates of human recombinant 5-HT2A, 5-HT2B and 5-HT2C receptors expressed in Human Embryo Kidney (HEK293) cells (Kennett et al., 1997). Washed membranes were incubated with ten concentrations (1×10−11 M–1×10−5 M) of test compounds and 0.5 nM [3H]-ketanserin (5-HT2A), 8 nM [3H]-5-HT (5-HT2B) or 0.6 nM [3H]-mesulergine (5-HT2C) for 30–45 min at 37°C in a pH 7.4 50 mM tris buffer. Ki (dissociation equilibrium constant, mol l−1 of the drug) values were calculated from the IC50 (Cheng & Prusoff, 1973) using the KD: 5-HT2A 0.7 nM, 5-HT2B 11.0 nM and 5-HT2C 0.58 nM. pKi values were calculated as the negative logarithm of the molar Ki.
Results
Haloperidol produced a significant cataleptic response in the range 0.38–3.76 mg kg−1 (Figure 1, top panel). Maximal effect (6/6 cataleptic rats) occurred at a dose of 1.13 mg kg−1 (P<0.01) and this dose was used in subsequent experiments for challenge with the 5-HT2 receptor antagonists. SB-228357 significantly attenuated haloperidol-induced catalepsy at 0.32–10 mg kg−1. At 10 mg kg−1 catalepsy was only seen in one of the six rats (P<0.01; Figure 1 bottom panel). Neither MDL-100907 nor SB-215505 reduced haloperidol-induced catalepsy (P>0.05 in each case; Figure 2).
Figure 1.
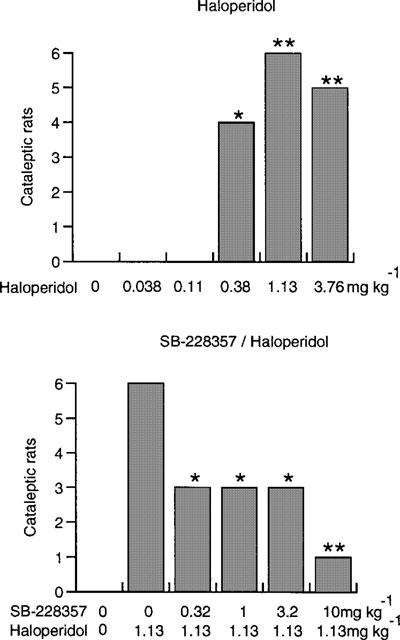
Each bar shows the number of cataleptic rats, 90 min after administration of haloperidol (top) and after pretreatment with SB-228357 (bottom). Significant haloperidol-induced catalepsy (top), or significant antagonism of the haloperidol effect (bottom) denoted by *=P<0.05; **=P<0.01.
Figure 2.
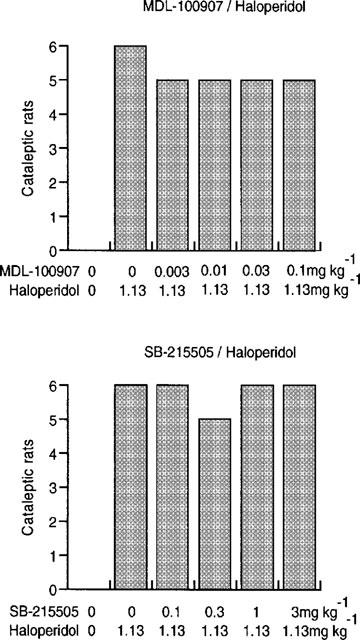
Each bar shows the number of cataleptic rats 90 min after administration of haloperidol and pretreatment with MDL-100907 and SB-215505.
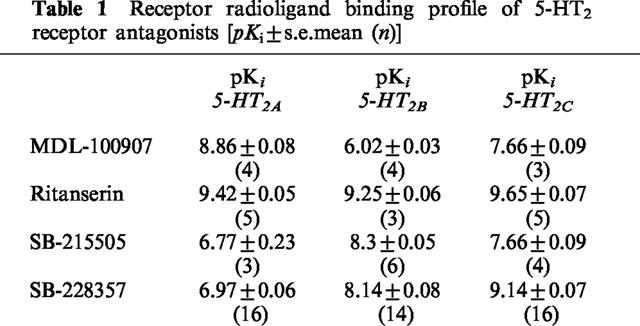
In the radioligand binding studies (Table 1), SB-228357 had a high affinity for the 5-HT2C receptor with 100 and 10 fold selectivity over the 5-HT2A and 5-HT2B receptors respectively. MDL-100907 was at least 700 and 16 fold selective for the 5-HT2A receptor over the 5-HT2B and 5-HT2C receptor respectively. SB-215505 was 30 fold selective for the 5-HT2B over the 5-HT2A receptor, and only marginally selective over the 5-HT2C receptor. Ritanserin had a high affinity for all three receptors (Table 1).
Table 1.
Receptor radioligand binding profile of 5-HT2 receptor antagonists [pKi±s.e.mean (n)]
Discussion
Serotoninergic mechanisms are known to influence neuroleptic-induced catalepsy (Balsara et al., 1979), and 5-HT2 receptor antagonism has been suggested to confer a favourable side-effect profile to neuroleptics (Meltzer et al., 1989). There is clinical evidence to support this as the mixed 5-HT2A/2C receptor antagonist, mianserin has been shown to reduce neuroleptic-induced akathisia (Poyurovsky & Weizman, 1997).
Other workers have attempted to discover if 5-HT2 receptor antagonists modulate catalepsy. Kalkman et al. (1998) used SB-200646 and failed to show attenuation of loxepine-induced catalepsy. However, SB-200646 has only moderate affinity (pKi=6.9) and selectivity for the 5-HT2C receptor (Kennett et al., 1994). Bligh-Glover et al. (1995) showed that ritanserin attenuated the catalepsy induced by low doses (0.25–0.375 mg kg−1) of haloperidol but not high doses (0.75 mg kg−1). However while ritanserin had a high affinity for 5-HT2 receptors (Table 1) it is a non-selective compound. The recent discovery of high affinity and more selective 5-HT2 receptor antagonists has enabled further investigation of the role of these receptors in catalepsy.
Further evidence has recently been provided that 5-HT2C receptor antagonism may produce a favourable outcome in states of motor disturbance. Thus, oro-facial dyskinesias elicited by stimulation of subthalamic 5-HT2C receptors are blocked by the 5-HT2C receptor antagonist, SDZ SER 082 (Eberle-Wang et al., 1996), and injection of the 5-HT2C receptor antagonist, SB-206553 (5-methyl-1-(3-pyridylcarbamoyl)-2,3-dihydropyrrolo[2,3-f]indole), into the substantia nigra zona reticulata produces an antiparkinsonian effect in the rat (Fox et al., 1998). Furthermore, 60% of striatal 5-HT-mediated phosphoinositide hydrolysis is accounted for by 5-HT2C receptors despite there being 3 fold fewer striatal 5-HT2C than 5-HT2A receptors (Wolf & Schutz, 1997).
MDL-100907, has been reported to have 100 fold selectivity for the 5HT2A over the 5-HT2C receptor (5-HT2A Ki=0.85 nM; 5-HT2C Ki=87 nM, Kehne et al., 1996), although our data (Table 1) show this compound to have less than 100 fold selectivity. MDL-100907 is in clinical development for schizophrenia, following an ‘atypical' profile in preclinical tests (Kehne et al., 1996). In these experiments, antagonism of apomorphine-induced stereotypy was used as a method of predicting EPS liability. In our study, MDL-100907 failed to attenuate haloperidol-induced catalepsy at doses that were active in the rat 5-HTP head twitch model (Kehne et al., 1996). Kalkman et al. (1998) have shown that MDL-100151 (R-(±)-α-(2,3- dimethoxyphenyl)-1- [2 -(4-fluorophenyl)ethyl]-4-piperidinemethanol), the racemate of MDL-100907, is cataleptogenic at a dose of 0.3 mg kg−1. This could explain why MDL-100907 failed to attenuate catalepsy. In contrast, SB-228357 had a pronounced anticataleptic profile. This compound had a high affinity for the 5-HT2C receptor and is 100 and 10 fold selective over the 5-HT2A and 5-HT2B receptor respectively (Table 1). This suggests that 5-HT2C receptor antagonism, or possibly mixed 5-HT2C/2B receptor antagonism is the optimum profile for antagonising haloperidol-induced catalepsy. However, there was no evidence that SB-215505 blocked the cataleptic response. This compound has a high affinity for the 5-HT2B receptor and is moderately selective over both 5-HT2A and 5-HT2C receptors (Table 1).
By deduction, our data suggest that 5-HT2C receptor antagonism, and not 5-HT2A or 5-HT2B receptor antagonism, is likely to be the mechanism by which ‘atypical' antipsychotic drugs lack EPS.
Abbreviations
- D2
dopamine 2
- 5-HT2
serotonin 2
- EPS
extrapyramidal side-effects
- HEK
human embryo kidney
- IC50
inhibitor constant
- KD
dissociation equilibrium constant
- Ki
inhibitor constant
- pKi
negative logarithm of inhibitor constant
References
- BALSARA J.J., JADHAV J.H., CHANDOKAR A.G. Effect of drugs influencing central serotonergic mechanisms on haloperidol-induced catalepsy. Psychopharmacol. 1979;62:67–69. doi: 10.1007/BF00426037. [DOI] [PubMed] [Google Scholar]
- BLIGH-GLOVER W., JASKIW G.E., VRTUNSKI B., UBOGY D., MELTZER H.Y. 5-HT2 receptor antagonists can attenuate submaximal haloperidol-induced catalepsy in rats. Schiz. Res. 1995;15:153–154. [Google Scholar]
- CHENG Y.C., PRUSSOF W.H. Relationship between inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;92:881–894. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- EBERLE-WANG K., LUCKI I., CHESSELET M.-F. A role for the subthalamic nucleus in 5-HT2C-induced oral dyskinesia. Neuroscience. 1996;72:117–128. doi: 10.1016/0306-4522(95)00548-x. [DOI] [PubMed] [Google Scholar]
- FOX S.H., MOSER B., BROTCHIE J.M. Behavioural effects of 5-HT2C receptor antagonism in the substantia nigra zona reticulata of the 6-hydroxydopamine-lesioned rat model of Parkinson's Disease. Exp. Neurol. 1998;151:35–49. doi: 10.1006/exnr.1998.6792. [DOI] [PubMed] [Google Scholar]
- KALKMAN H.O., NEUMANN V., NOZULAK J., TRICKLEBANK M.D. Cataleptogenic effect of subtype selective 5-HT receptor antagonists in the rat. Eur. J. Pharmacol. 1998;343:201–207. doi: 10.1016/s0014-2999(97)01554-9. [DOI] [PubMed] [Google Scholar]
- KEHNE J.H., BARON B.M., CARR A.A., CHANEY S.F., ELANDS J., FELDMAN D.J., FRANK R.A., VAN GIERSBERGEN P.L.M., MCCLOSKEY T.C., JOHNSON M.P., MCCARTY D.R., POIROT M., SENYAH Y., SIEGEL B.W., WIDMAIER C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J. Pharmacol. Exp. Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., BRIGHT F., TRAIL B., RILEY G., HOLLAND V., AVENELL K.Y., STEAN T., UPTON N., BROMIDGE S., FORBES I.T., BROWN A.M., MIDDLEMISS D.N., BLACKBURN T.P. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., GLEN A., GREWAL S., FORBES I., GADRE A., BLACKBURN T.P. In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist. Br. J. Pharmacol. 1994;111:797–802. doi: 10.1111/j.1476-5381.1994.tb14808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELTZER H.Y. Pre-clinical pharmacology of atypical antipsychotic drugs: a selective review. Br. J. Psychiat. 1996;168 Suppl. 29:23–31. [PubMed] [Google Scholar]
- MELTZER H.Y., MATSUBARA S., LEE M.A. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- POYUROVSKY M., WEIZMAN A. Serotonergic agents in the treatment of acute neuroleptic-induced akathisia: open-label study of buspirone and mianserin. Int. Clin. Psychopharmacol. 1997;12:263–268. doi: 10.1097/00004850-199709000-00003. [DOI] [PubMed] [Google Scholar]
- WOLF W.A., SCHUTZ L.J. The serotonin 5-HT2C receptor is a prominent serotonin receptor in basal ganglia: evidence from functional studies on serotonin-mediated phosphinositide hydrolysis. J. Neurochem. 1997;69:1449–1458. doi: 10.1046/j.1471-4159.1997.69041449.x. [DOI] [PubMed] [Google Scholar]


