Abstract
We have tested our prediction that AM630 is a CB2 cannabinoid receptor ligand and also investigated whether L759633 and L759656, are CB2 receptor agonists.
Binding assays with membranes from CHO cells stably transfected with human CB1 or CB2 receptors using [3H]-CP55940, confirmed the CB2-selectivity of L759633 and L759656 (CB2/CB1 affinity ratios=163 and 414 respectively) and showed AM630 to have a Ki at CB2 receptors of 31.2 nM and a CB2/CB1 affinity ratio of 165.
In CB2-transfected cells, L759633 and L759656 were potent inhibitors of forskolin-stimulated cyclic AMP production, with EC50 values of 8.1 and 3.1 nM respectively and CB1/CB2 EC50 ratios of >1000 and >3000 respectively.
AM630 inhibited [35S]-GTPγS binding to CB2 receptor membranes (EC50=76.6 nM), enhanced forskolin-stimulated cyclic AMP production in CB2-transfected cells (5.2 fold by 1 μM), and antagonized the inhibition of forskolin-stimulated cyclic AMP production in this cell line induced by CP55940.
In CB1-transfected cells, forskolin-stimulated cyclic AMP production was significantly inhibited by AM630 (22.6% at 1 μM and 45.9% at 10 μM) and by L759633 at 10 μM (48%) but not 1 μM. L759656 (10 μM) was not inhibitory. AM630 also produced a slight decrease in the mean inhibitory effect of CP55940 on cyclic AMP production which was not statistically significant.
We conclude that AM630 is a CB2-selective ligand that behaves as an inverse agonist at CB2 receptors and as a weak partial agonist at CB1 receptors. L759633 and L759656 are both potent CB2-selective agonists.
Keywords: Cannabinoid CB1 and CB2 receptors, AM630, L759633, L759656, CB2-selective ligands, CB2-selective agonist, CB2-selective inverse agonist, [35S]-GTPγS, cyclic AMP
Introduction
Many cannabinoid effects are now known to be mediated by CB1 receptors that are present in the central nervous system as well as in certain neuronal and nonneuronal peripheral tissues or by CB2 receptors that are found mainly in cells of the immune system (Pertwee, 1997). Both receptor types are coupled to their effector systems through Gi/o proteins (Pertwee, 1997). These discoveries have led to the development of selective ligands for CB1 and CB2 receptors. Particularly important have been SR141716A, which behaves as a selective CB1 receptor inverse agonist (Bouaboula et al., 1997; MacLennan et al., 1998) and SR144528, which behaves as a selective CB2 receptor inverse agonist (Rinaldi-Carmona et al., 1998).
A less well characterized cannabinoid receptor ligand is 6-iodopravadoline (AM630) (Figure 1). This has been shown to be a selective, competitive antagonist of certain cannabinoid receptor agonists in the mouse isolated vas deferens (Pertwee et al., 1995) but to possess the properties of a weak cannabinoid CB1 receptor agonist in the myenteric plexus-longitudinal muscle preparation of guinea-pig small intestine (Pertwee et al., 1996). There are also reports that AM630 attenuates cannabinoid-induced stimulation of [35S]-GTPγS binding to mouse and guinea-pig brain membrane preparations (Hosohata et al., 1997a,1997b) and that it behaves as an inverse agonist in Chinese hamster ovary (CHO) cells stably transfected with CB1 receptors (Landsman et al., 1998).
Figure 1.
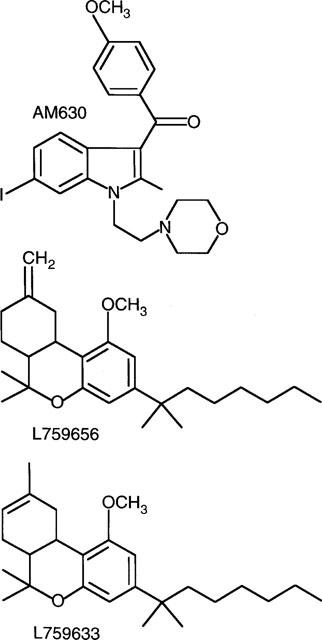
Structures of AM630, L759656 and L759633.
Although there is now good evidence that AM630 is a CB1 receptor ligand, the question remains as to whether it also interacts with CB2 receptor, and if so whether it is a CB2 receptor agonist, antagonist or inverse agonist. Our prediction was that AM630 would be a CB2 receptor ligand as it is a structural analogue of WIN55212-2, a cannabinoid receptor agonist that is already known to have some degree of selectivity for the CB2 receptor (Felder et al., 1995; Showalter et al., 1996). In this investigation we first compared the ability of AM630 to bind to CB1 and CB2 receptors and then attempted to establish whether it shows agonist, antagonist or inverse agonist activity at each of these receptor types. The pharmacological properties of two other putative cannabinoid receptor ligands were also investigated in the present study. These are L759633 and L759656 (Figure 1), both of which have been reported to have a markedly higher affinity for human CB2 than human CB1 receptors (Gareau et al., 1996). However, no other information about these compounds has yet been published. Since there is a need for CB2-selective agonists for use as pharmacological tools, we carried out experiments to establish whether either of these compounds show significant efficacy at CB1 and/or CB2 receptors.
Binding assays were performed with membrane preparations of CB1- or CB2-transfected CHO cells. The radioactive probe used in most of our binding experiments was [3H]-CP55940, which binds equally well to CB1 and CB2 receptors (Showalter et al., 1996). The functional assays used exploited the well-established ability of cannabinoid receptor agonists to inhibit forskolin-stimulated cyclic AMP production and of cannabinoid receptor agonists and inverse agonists to modulate [35S]-GTPγS binding (Pertwee, 1997; MacLennan et al., 1998). These assays were carried out with CHO cells stably transfected with CB1 or CB2 receptors.
Methods
CHO cells
These were stably transfected with cDNA encoding human CB1 or CB2 receptors. The CB1-transfected cells used in binding assays with [3H]-CP55940 or [3H]-WIN55212-2 were supplied by Euroscreen, Brussels (Bmax=23.8 pmol mg−1 protein) and were a gift from Organon. The CB2-transfected cells used in binding assays with [3H]-CP55940, [3H]-WIN55212-2 or [35S]-GTPγS (Bmax=72.5 pmol mg−1 protein) and the CB1- and CB2-transfected cells used in cyclic AMP assays (Bmax=3.5 pmol mg−1 protein and 50.7 pmol mg−1 protein respectively) were provided by Drs G. Disney and A. Green, GlaxoWellcome R&D, Medicines Research Centre, Stevenage. Untransfected CHO cells were a gift from Dr Min Zhao, University of Aberdeen. The clones used in cyclic AMP assays were the same as those used in the sPAP reporter assay described by Green et al. (1998). Cells were maintained at 37°C and 5% CO2 in DMEM (f-12 HAM) with 2 mM Glutamine, Geneticin (600 μg ml−1) and Hygromycin (300 μg ml−1). Because receptor overexpression may lead to the activation of effector mechanisms to which receptors in natural membranes are not normally coupled (see Kenakin, 1995), the cyclic AMP assays were performed with cells expressing fewer CB1 or CB2 receptors than the cells used in the binding assays.
Membrane preparation
CHO cells were suspended in 50 mM Tris buffer (pH 7.4) and 0.32 M sucrose and homogenized with an Ultra-Turrex homogenizer. The homogenate was diluted with 50 mM Tris buffer (pH 7.4) and centrifuged at 50,000×g for 1 h to isolate the membranes.
Binding experiments
A filtration procedure was used to measure [3H]-CP55940 and [3H]-WIN55212-2 binding. This is a modification of the method described by Compton et al. (1993). Binding assays were performed with [3H]-CP55940 or [3H]-WIN55212-2, 1 mM MgCl2, 1 mM EDTA, 2 mg ml−1 bovine serum albumin (BSA) and 50 mM Tris buffer, total assay volume 500 μl. Binding was initiated by the addition of cell membranes (20–30 μg protein). Assays were carried out at 30°C for 90 min before termination by addition of ice-cold wash buffer (50 mM Tris buffer, 1 mg ml−1 BSA) and vacuum filtration using a 12-well sampling manifold (Brandel Cell Harvester) and Whatman GF/B glass-fibre filters that had been soaked in wash buffer at 4°C for 24 h. Each reaction tube was washed three times with a 4 ml aliquot of buffer. The filters were oven-dried for 60 min and then placed in 5 ml of scintillation fluid (Ultima Gold XR, Packard). Radioactivity was quantified by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence and absence of 1 μM unlabelled cannabinoid. Protein assays were performed using a Bio-Rad Dc kit. Unlabelled and radiolabelled cannabinoids were each added in a volume of 50 μl following dilution in assay buffer (50 mM Tris buffer containing 10 mg ml−1 BSA). The concentration of [3H]-CP55940 or [3H]-WIN55212-2 used in displacement assays was 0.5 nM. The concentrations of cannabinoids that produced a 50% displacement of radioligand from specific binding sites (IC50 values) were calculated using GraphPad Prism (GraphPad Software, San Diego, U.S.A.). Competitive binding curves were fitted with minimum values for displacement of radioligand from specific binding sites constrained to zero. Dissociation constant (Ki values) were calculated using the equation of Cheng & Prusoff (1973) and dissociation constant values of [3H]-CP55940 and [3H]-WIN55212-2 shown in the footnote to Table 1.
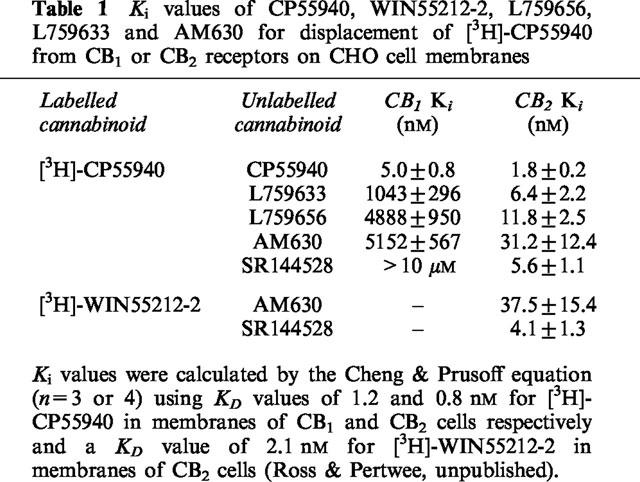
Table 1.
Ki values of CP55940, WIN55212-2, L759656, L759633 and AM630 for displacement of [3H]-CP55940 from CB1 or CB2 receptors on CHO cell membranes
Cyclic AMP assay
Cells (2×106 cells ml−1) were preincubated for 30 min at 37°C with cannabinoid and 3-isobutyl-1-methylxanthine (IBMX; 50 μM) in phosphate buffered saline containing 1 mg ml−1 BSA (assay buffer) followed by a further 30 min incubation with 2 μM forskolin in a total volume of 500 μl. The reaction was terminated by addition 0.1 M HCl and centrifuged to remove cell debris. The pH was brought to 8–9 using 1 M NaOH and cyclic AMP content was then measured using a radioimmunoassay kit (Biotrak, Amersham). Forskolin and IBMX were dissolved in DMSO.
[35S]-GTPγS assay
CB2 transfected cells were removed from flasks by scraping, resuspended in homogenization buffer (0.32 M sucrose and 50 mM Tris), and homogenized using an ultra-Turrex homogenizer. The homogenate was diluted with Tris buffer (50 mM, pH 7.4) and centrifuged at 50,000 ×g for 45 min. Cell membranes (20 μg) were incubated in assay buffer containing 2 mg ml−1 fatty acid free BSA, 20 μM GDP and 0.1 nM [35S]-GTPγS. The assay buffer contained 50 mM Tris, 10 mM MgCl2, 100 mM NaCl and 0.2 mM EDTA at pH 7.4. Incubations were carried out at 30°C for 90 min in a total volume of 500 μl. The reaction was terminated by the addition of 4 ml of ice-cold wash buffer (50 mM Tris and 1 mg ml−1 BSA, pH 7.4) followed by rapid filtration under vacuum through Whatman GF/B glass-fibre filters (pre-soaked in wash buffer) using a 12-tube Brandel cell harvester. The tubes were washed three times with 4 ml of wash buffer. Filters were oven dried, placed in 5 ml of scintillation fluid and bound radioactivity was determined by liquid scintillation counting. Basal binding of [35S]-GTPγS was determined in the presence of 20 μM GDP and absence of cannabinoid. Non-specific binding was determined in the presence of 10 μM GTPγS.
Analysis of data
Values have been expressed as means and variability as s.e.mean or as 95% confidence limits. Mean values have been compared using the Kruskall-Wallis test followed by Dunn's multiple comparison test. A P value <0.05 was considered to be significant. Effects of test compounds on forskolin-stimulated cyclic AMP production have been expressed in percentage terms. This was calculated from the equation [100×(f′−b)]/(f−b) where f ′, f and b are values of cyclic AMP production (pmol ml−1), f ′ in the presence of forskolin and the test compound, f in the presence of forskolin only and b in the absence of both forskolin and the test compound. Drug-induced inhibition of specific [35S]-GTPγS binding was expressed as the percentage decrease below the basal level of [35S]-GTPγS binding using the equation [100×(d′−d)]/d where d′ and d are d.p.m. in the presence and absence of the drug respectively. Values for EC50, IC50 and maximal effects (Emax) and the 95% confidence limits of these values have been calculated by non-linear regression analysis using GraphPad Prism (GraphPad Software, San Diego, U.S.A.).
The ability of AM630 to antagonize CP55940-induced inhibition of forskolin-stimulated cyclic AMP production in CB2 transfected cells is expressed in terms of the concentration ratio. This has been defined as the concentration of CP55940 that produces a particular degree of inhibition in the presence of AM630 at a concentration, B, divided by the concentration of CP55940 that produces an identical degree of inhibition in the absence of AM630. Since AM630 behaved as an inverse agonist at CB2 receptors (see Results), it was considered inappropriate to insert concentration ratio values into the Schild equation in order to obtain a KB value of AM630 at these receptors. Concentration ratio values and their 95% confidence limits have been determined by symmetrical (2+2) dose parallel line assays (Colquhoun, 1971), using responses to pairs of agonist concentrations located on the steepest part of each log concentration-response curve. This method was also used to establish whether log concentration-response curves of CP55940 constructed in the presence and absence of AM630 deviated significantly from parallelism.
Drugs
CP55940 {(−)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol} was supplied by Pfizer, WIN55212-2 {(R)-(+)-[2,3-dihydro-5-methyl-3-[(4-morpholino)methyl]pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl](1-naphthyl)methanone} by Research Biochemicals International, SR141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride] and SR144528 {N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide} by Sanofi Recherche and L759633 [(6aR,10aR)-3-(1,1-dimethyl-heptyl)-1-methoxy-6,6,9-trimethyl-6a,7,10,10a -tetrahydro -6H-benzo[c]chromene] and L759656 [(6aR,10aR)-3-(1,1-dimethyl-heptyl)-1-methoxy-6,6-dimethyl-9-methylene - 6a,7,8,9,10,10a-hexahydro-6H-benzo[c]chromene] by Merck Frosst. AM630 (6-iodopravadoline) was synthesized in the laboratory of Dr A. Makriyannis. [3H]-CP55940 (126 Ci mmol−1) and [3H]-WIN55212-2 (45 Ci mmol−1) were supplied by NEN Life Science Products. Cannabinoids were stored as 1 mg ml−1 stock solutions in ethanol and diluted in assay buffer. At the highest concentrations used, ethanol by itself had no detectable effect on specific binding of [3H]-CP55940, [3H]-WIN55212-2 or [35S]-GTPγS or on forskolin-stimulated cyclic AMP production (data not shown).
Results
Cannabinoid binding experiments
The radioligand binding data shown in Table 1 confirm that CP55940 is a high-affinity non-selective ligand for cannabinoid receptors. The data also confirm L759656 and L759633 to be markedly CB2-selective, with much lower Ki values in CB2 transfected cell membranes than in membranes of CB1 transfected cells. AM630 is also CB2-selective with a CB1/CB2 Ki ratio of 165 in transfected CHO cell membranes. Further experiments showed AM630 to be no less effective in displacing [3H]-CP55940 than [3H]-WIN55212-2 from CB2 receptors on CHO cell membranes (Table 1). SR144528 was also equally effective in displacing [3H]-CP55940 and [3H]-WIN55212-2 from CB2 receptors (Table 1).
Effects of CP55940, L759656, L759633 and AM630 on cyclic AMP production
Cyclic AMP concentrations in the absence and presence of 2 μM forskolin were 5.1±1.8 and 46.3±13.1 pmol ml−1 respectively in CB1-transfected cells (n=6) and 5.6±2.6 and 52.8±11.7 pmol ml−1 respectively in CB2-transfected cells (n=6). CP55940 was highly potent in inhibiting forskolin-stimulated cyclic AMP production in both CB1- and CB2-transfected cells (Table 2 and Figure 2). The CB1/CB2 EC50 ratio of 0.9 reflects the non-selective nature of this agonist. In contrast, L759633 and L759656 showed greater potency against forskolin-stimulated cyclic AMP production in CB2-transfected cells than in CB1-transfected cells (Table 2 and Figure 2). Indeed, forskolin-stimulated cyclic AMP production in CB1-transfected cells was not significantly inhibited by L759656 and was only inhibited by L759633 at the highest concentration used (10 μM) (Table 2). The maximal inhibitory effects of CP55940, L759633 and L759656 on forskolin-stimulated cyclic AMP production in CB2-transfected cells were not significantly different (Figure 2).
Table 2.
EC50 values for inhibition of forskolin-stimulated cyclic AMP production in CHO cells stably transfected with CB1 or CB2 receptors
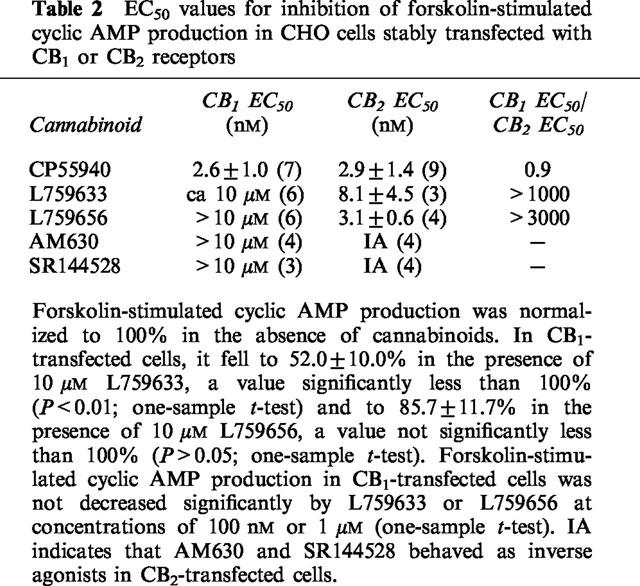
Figure 2.
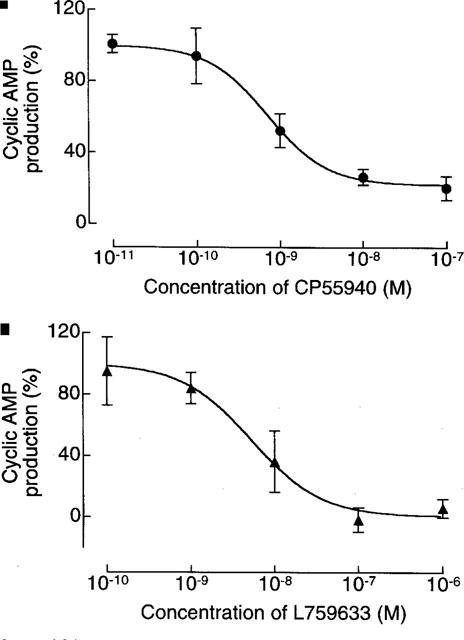
Inhibition by (a) CP55940 (n=9), (b) L759633 (n=3) and (c) L759656 (n=4) of forskolin-stimulated cyclic AMP production in CB2-transfected CHO cells. Each symbol represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean. Forskolin-stimulated cyclic AMP production in the absence of any cannabinoid has been normalized to 100%. Mean Emax values for CP55940, L759633 and L759656, with 95% confidence limits shown in brackets, were 21.9% (5.1 to 38.7), 0.5% (−25.3 to 26.3%) and 5.9% (−10.6 to 22.4%) respectively (GraphPad Prism).
AM630 produced a significant inhibitory effect on cyclic AMP production in CB1-transfected cells, albeit only at concentrations in the micromolar range (Figure 3). More specifically, AM630 concentrations of 1 and 10 μM caused forskolin-stimulated cyclic AMP production in this cell line to fall to 77.5±4.2% (n=4) and 54.1±12.4% (n=8) of the control value respectively (one-sample t-test). In CB2-transfected cells, AM630 enhanced the ability of forskolin to stimulate cyclic AMP production at 0.1, 1 and 10 μM, the highest of these concentrations provoking an increase in cyclic AMP production of 5.5 fold (Figure 3). In the absence of forskolin, AM630 had no effect on cyclic AMP production by CB1- or CB2-transfected cells (n=3; data not shown). Forskolin-stimulated cyclic AMP production in untransfected CHO cells was affected neither by AM630 nor by L759633 or L759656 (data not shown).
Figure 3.
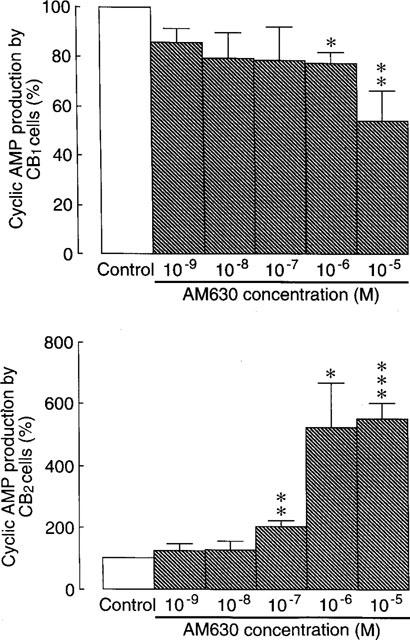
The effect of AM630 on forskolin-stimulated cyclic AMP production in CB1 and CB2-transfected CHO cells (±s.e.mean; n=3 to 8). Each column represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean. Forskolin-stimulated cyclic AMP production in the absence of AM630 has been normalized to 100%. Asterisks indicate values significantly less than 100% (*P<0.05; **P<0.01; ***P<0.001; one-sample t-test). The mean EC50 value of AM630 for enhancement of forskolin-stimulated cyclic AMP production in the CB2-transfected cells with its 95% confidence limits shown in brackets is 230.4 nM (48.4 and 1096 nM) (GraphPad Prism).
Effect of AM630 on GTPγS binding and on CP55940-induced inhibition of cyclic AMP production
In CB2-transfected cells, the ability of CP55940 to inhibit cyclic AMP production at concentrations of 1 μM or less was abolished by 1 μM AM630 which produced a non-parallel upward shift in the log concentration-response curve of the agonist (Figure 4). At the lower concentration of 100 nM, AM630 produced a significant dextral shift in the log concentration-response curve of CP55940 for inhibition of cyclic AMP production by CB2-transfected cells that did not deviate significantly from parallelism (Figure 5). The mean value of this shift, with its 95% confidence limits shown in brackets, was 21.3 (3.5 and 649.4). Also shown in Figure 5 is the ability of the established CB2 receptor antagonist, SR144528, to oppose CP55940-induced inhibition of cyclic AMP production by the same cell line. AM630 also shared the ability of SR144528 to produce a concentration-related inhibition of GTPγS binding to membranes from CB2-transfected cells (Figure 6). It was 7.4 times less potent than SR144528. However, the maximum degree of inhibition produced by the two compounds was essentially the same.
Figure 4.
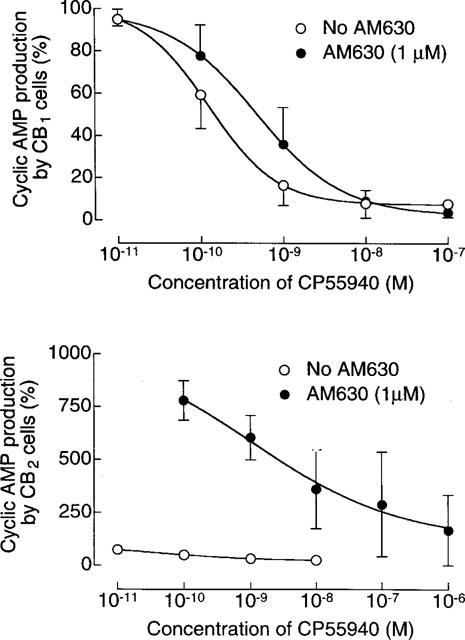
The effect of 1 μM AM630 on CP55940-induced inhibition of forskolin-stimulated cyclic AMP production in CB1 and CB2-transfected CHO cells. Each symbol represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean (n=3). Forskolin-stimulated cyclic AMP production in the absence of AM630 and CP55940 has been normalized to 100%. Mean EC50 values of CP55940 in the CB1-transfected cells with their 95% confidence limits shown in brackets are 0.5 nM (0.2 and 1.2 nM) in the presence of AM630 and 0.2 nM (0.1 and 0.4 nM) in its absence (GraphPad Prism).
Figure 5.
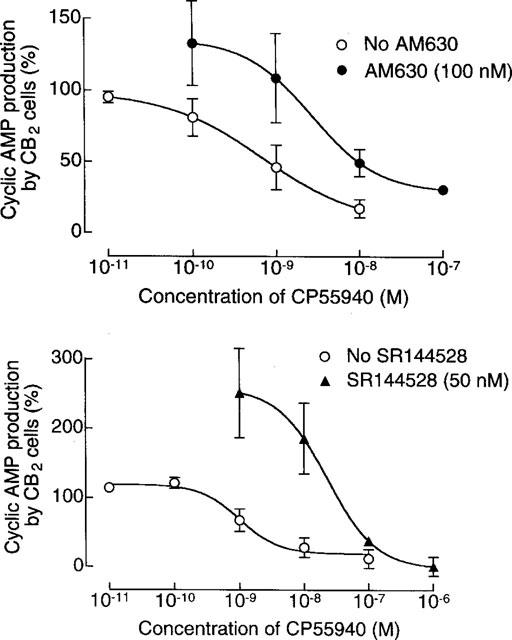
The effect of 100 nM AM630 or 50 nM SR144528 on CP55940-induced inhibition of forskolin-stimulated cyclic AMP production in CB2-transfected CHO cells. Each symbol represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean (n=3 or 4). Forskolin-stimulated cyclic AMP production in the absence of AM630, SR144528 and CP55940 has been normalized to 100%. The mean dextral shift in the log concentration-response curve of CP55940 produced by AM630 is 21.3 and its 95% confidence limits are 3.0 and 649.4 (symmetrical (2+2) dose parallel line assay). It did not deviate significantly from parallelism.
Figure 6.
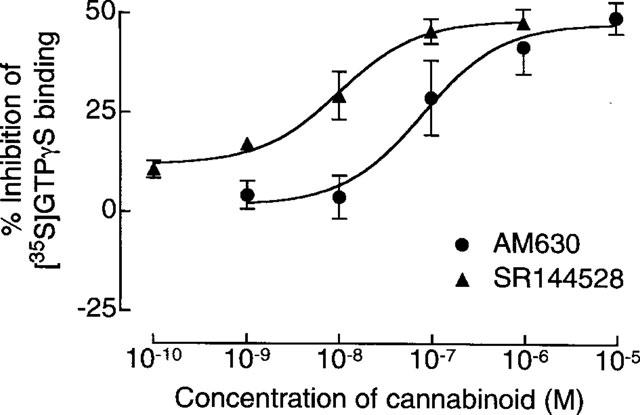
Effects of AM630 and SR144528 on specific binding of [35S]-GTPγS to CB2-transfected CHO cell membranes. Each symbol represents mean percentage decrease in binding±s.e.mean (n=3 to 5). The mean EC50 values of AM630 and SR144528 for inhibition of [35S]-GTPγS binding with their 95% confidence limits shown in brackets are 76.6 nM (16.5 and 356.2 nM) and 10.4 nM (3.6 and 29.7 nM) respectively. Corresponding values for the maximum degree of inhibition produced are 47.2±5.8% and 48.5±2.9% respectively (mean Emax values±s.e.mean; non-linear regression analysis; GraphPad Prism).
A further set of experiments with CB2-transfected cells (Figure 7), showed that inhibition of forskolin-stimulated cyclic AMP production by 10 nM CP55940 (85.5±3.4%; n=5) was concentration-dependently reversed by AM630. A 50% reversal was achieved by AM630 at a concentration (EC50) of 128.6±40.6 nM (n=5). This is the mean concentration of AM630 that increased forskolin-stimulated cyclic AMP production by CB2-transfected cells to a level midway between that observed in the presence of 10 nM CP55940 and that observed in the absence of this agonist. A similar method has been used by Rinaldi-Carmona et al. (1998) to calculate the concentration of SR144528 that produces a 50% reversal of CP55940-induced inhibition of forskolin-stimulated cyclic AMP production by CB2-transfected cells.
Figure 7.
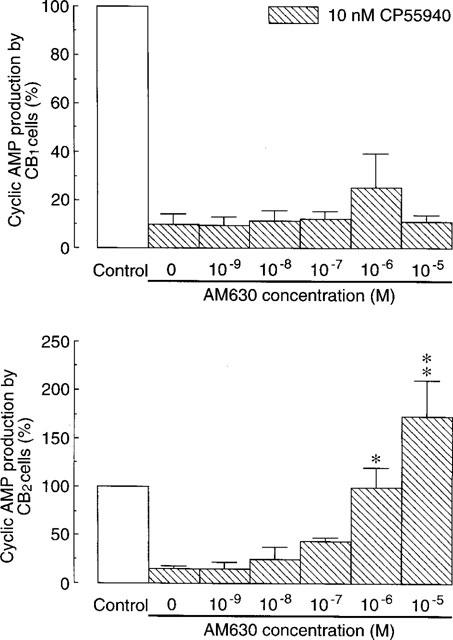
Effects of AM630 on inhibition of forskolin-stimulated cyclic AMP production by 10 nM CP55940 in CB1- and CB2-transfected CHO cells (±s.e.mean; n=4 to 6). Each column represents mean percentage change in forskolin-stimulated cyclic AMP production. Forskolin-stimulated cyclic AMP production in the absence of AM630 and CP55940 has been normalized to 100%. Asterisks indicate significant differences from forskolin-stimulated cyclic AMP production in the presence of CP55940 and absence of AM630 (*P<0.05; **P<0.01; Kruskall-Wallis test followed by Dunn's multiple comparison test).
In CB1-transfected cells, 1 μM AM630 appeared to produce a 3 fold decrease in the potency of CP55940 for inhibition of forskolin-stimulated cyclic AMP production (Figure 4). However, this effect of AM630 was not statistically significant. Similarly, the inhibitory effect of 10 nM CP55940 on forskolin-stimulated cyclic AMP production by this cell line (89.4±5.6%; n=4) appeared to be slightly reversed by 1 μM AM630 although not by higher or lower concentrations of this compound (Figure 7). Again, this effect was not statistically significant. In contrast, it was possible to detect reversal of this inhibitory effect in CB1-transfected cells by SR141716A. This compound produced a 50% reversal of the inhibitory effect of 10 nM CP55940 on forskolin-stimulated cyclic AMP production at 11.2±3.1 nM (n=5).
Discussion
The results obtained showed that L759633 and L759656 bind with higher affinity to CB2 than CB1 receptors. Although this confirms previously reported binding data for these compounds (Gareau et al., 1996), the CB2/CB1 affinity ratios of L759633 and L759656 were both higher in the previous investigation (793 and >1000 respectively) than reported here (163 and 414 respectively). Consistent with their binding properties, both L759633 and L759656 were markedly more potent as inhibitors of forskolin-stimulated cyclic AMP production in CB2- than CB1-transfected cells. Indeed, the CB1/CB2 EC50 ratios of both compounds in this functional bioassay (>1000 and >3000 respectively) were somewhat greater than their CB2/CB1 affinity ratios. Our results also confirm previous reports that CP55940 is a cannabinoid receptor agonist that acts with more or less equal potency at CB1 and CB2 receptors (Felder et al., 1995). The ability of L759633 and L759656 to behave as CB2-receptor agonists has not been reported previously. Clearly, both compounds will be useful pharmacological tools for the study of cannabinoid receptor pharmacology.
AM630 bound to both CB1 and CB2 receptors with a CB2/CB1 affinity ratio of 165, indicating it to have a similar selectivity for CB2 binding sites as L759656. Its affinity for CB2 receptors was 2.6 times less than that of L759656. The Ki value obtained for AM630 in these experiments with CB2-transfected cell membranes (31.2 nM) is similar to a previously reported Ki value of AM630 (11.2 nM) for displacement of [3H]-CP55940 from a membrane preparation of mouse spleen (Pertwee, 1998), a tissue known to express CB2 receptors (Munro et al., 1993). Both these Ki values are similar to KB values of AM630 reported by Pertwee et al. (1995) for antagonism of the inhibitory effects of CP55940 (17.3 nM) and WIN55212-2 (36.5 nM) on electrically-evoked contractions of the mouse isolated vas deferens, possibly reflecting the presence in this tissue of CB2-like cannabinoid receptors that can mediate twitch inhibition (Griffin et al., 1997).
Unlike L759633 and L759656, AM630 did not inhibit forskolin-stimulated cyclic AMP production by CB2-transfected cells. Instead, it readily reversed the ability of CP55940 to produce this inhibitory effect with an EC50 (128.6 nM) approximately one order of magnitude higher than the corresponding EC50 value (10 nM) of the established CB2-selective antagonist, SR144528 (Rinaldi-Carmona et al., 1998). In addition, AM630 alone produced a concentration-dependent enhancement of forskolin-stimulated cyclic AMP production by CB2-transfected cells. Possibly, AM630 is antagonizing a CB2 receptor agonist that is being spontaneously released by this cell line. Another possibility is that the CB2-transfected cells contain constitutively active CB2 receptors in equilibrium with inactive CB2 receptors and that AM630 is an inverse agonist that binds preferentially to the receptors in the inactive state thereby decreasing the proportion of constitutively active receptors. A similar mechanism has been proposed to explain the ability of the CB2 receptor antagonist, SR144528, to enhance forskolin-stimulated cyclic AMP production by CB2-transfected cells (Rinaldi-Carmona et al., 1998). In line with both suggested mechanisms is our finding that AM630 shares the ability of SR144528 to inhibit GTPγS binding to CB2 receptors. Thus CB2 receptors are G protein coupled (Pertwee, 1997) and it is to be expected that the binding of GTPγS to G proteins will be stimulated by agonists for G protein-coupled receptors and inhibited by inverse agonists for such receptors (Bouaboula et al., 1997; Breivogel et al., 1997; MacLennan et al., 1998). A third possibility is that AM630-induced enhancement of cyclic AMP production in CB2-transfected cells is mediated by Gs proteins. However, AM630 had no effect on cyclic AMP production by CB2-transfected cells in the absence of forskolin. Moreover, although there is evidence that, under certain conditions, CB1 receptors may be coupled to adenylate cyclase through Gs proteins, there is also evidence that CB2 receptors are not (Glass & Felder, 1997).
Although AM630 antagonizes CP55940 in CB2-transfected cells, it shares the ability of CP55940 to inhibit forskolin-stimulated cyclic AMP production by CB1-transfected cells, albeit with an EC50 above 1 μM. This finding is consistent with a previous observation that AM630 behaves as a CB1 receptor agonist in the myenteric plexus-longitudinal muscle preparation of guinea-pig small intestine with an EC50 value of 1.9 μM (Pertwee et al., 1996), a potency value that is reasonably close to the Ki of AM630 for displacement of [3H]-CP55940 from CB1-transfected cell membranes (Table 1). AM630 does not seem to behave as a CB1 receptor agonist in all CB1 receptor-containing preparations. Landsman et al. (1998) have reported that AM630 inhibits [35S]-GTPγS binding to CB1-transfected cell membranes with an EC50 of 0.9 μM. This effect is opposite in direction to that of CB1 receptor agonists which stimulate [35S]-GTPγS binding (Breivogel et al., 1997; MacLennan et al., 1998). Hosohata et al. (1997a,1997b) have found AM630 to antagonize cannabinoid-induced stimulation of [35S]-GTPγS binding to mouse and guinea-pig brain membrane preparations with KB values of 3.1 and 9.3 μM respectively. Thus it would seem that, depending on the CB1 receptor-containing preparation used, AM630 can behave as a CB1 receptor agonist, antagonist or inverse agonist. Further experiments are required to establish why this should be. It is noteworthy, however, that in the present investigation, a concentration of AM630 that inhibited forskolin-stimulated cyclic AMP production by CB1-transfected cells when administered alone (1 μM), appeared to produce a dextral shift in the log concentration-response curve of CP55940 for inhibition of forskolin-stimulated cyclic AMP production by CB1-transfected cells. This dextral shift was not statistically significant. However, this observation does raise the possibility that high concentrations of AM630 may produce a significant degree of antagonism in this cell line in which case AM630 would possess the mixed agonist-antagonist properties of a CB1 partial agonist.
In conclusion, our results confirm L759633 and L759656 to have significantly higher affinity for CB2 than CB1 receptors (see Introduction). In addition, they show that both these compounds are potent, high-efficacy CB2 receptor agonists, that L759656 lacks significant activity as a CB1 receptor agonist and that although L759633 can activate CB1 receptors, it does so only at micromolar concentrations that are above those at which it is capable of interacting with CB2 receptors. The pharmacological properties of AM630 are more complex. Thus, we found AM630 to behave as an inverse agonist at CB2 receptors but as a partial agonist at CB1 receptors. Further experiments are required to establish the structural features of AM630 responsible for each of these actions.
Acknowledgments
This work was supported by grants 039538 and 047980 from the Wellcome Trust (to R.G.P. and R.A.R.) and by grants DA3801 (to A.M.) and DA9158 (to A.M. and R.G.P.) from the National Institute on Drug Abuse. We thank Organon for CB1-transfected cells, GlaxoWellcome for CB1- and CB2-transfected cells, Merck Frosst for L759633 and L759656, Pfizer for CP55940 and Sanofi Recherche for SR141716A and SR144158.
Abbreviations
- AM630
6-iodopravadoline
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- CP55940
(−)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol
- IBMX
3-isobutyl-1-methylxanthine
- L759633
(6aR,10aR)-3-(1,1-dimethyl-heptyl)-1-methoxy-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromene
- L759656
(6aR,10aR)-3-(1,1-dimethyl-heptyl)-1-methoxy-6,6-dimethyl-9-methylene-6a,7,8,9,10,10a-hexahydro-6H-benzo[c]chromene
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- WIN55212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-[(4-morpholino)methyl]pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl](1-naphthyl)methanone
References
- BOUABOULA M., PERRACHON S., MILLIGAN L., CANAT X., RINALDI-CARMONA M., PORTIER M., BARTH F., CALANDRA B., PECCEU F., LUPKER J., MAFFRAND J.-P., LE FUR G., CASSELLAS P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., SIM L.J., CHILDERS S.R. Regional differences in cannabinoid receptor G-protein coupling in rat brain. J. Pharmacol. Exp. Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- CHENG Y.-C., PRUSOFF W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN D. Lectures on Biostatistics. Oxford University Press., Oxford; 1971. [Google Scholar]
- COMPTON D.R., RICE K.C., DE COSTA B.R., RAZDAN R.K., MELVIN L.S., JOHNSON M.R., MARTIN B.R. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J. Pharmacol. Exp. Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- GAREAU Y., DUFRESNE C., GALLANT M., ROCHETTE C., SAWYER N., SLIPETZ D.M., TREMBLAY N., WEECH P.K., METTERS K.M., LABELLE M. Structure activity relationships of tetrahydrocannabinol analogues on human cannabinoid receptors. Bioorg. Med. Chem. Letts. 1996;6:189–194. [Google Scholar]
- GLASS M., FELDER C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neuroscience. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN A., O'SHAUGHNESSY C., DISNEY G., WHITTINGTON A., REES S., LEE M., MARSHALL F. Reporter assays for human cannabinoid CB1 and CB2 receptors for the identification of novel agonists 1998Burlington, Vermont, International Cannabinoid Research Society; Symposium on the Cannabinoids102 [Google Scholar]
- GRIFFIN G., FERNANDO S.R., ROSS R.A., MCKAY N.G., ASHFORD M.L.J., SHIRE D., HUFFMAN J.W., YU S., LAINTON J.A.H., PERTWEE R.G. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur. J. Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- HOSOHATA K., QUOCK R.M., HOSOHATA Y., BURKEY T.H., MAKRIYANNIS A., CONSROE P., ROESKE W.R., YAMAMURA H.I. AM630 is a competitive cannabinoid receptor antagonist in the guinea pig brain. Life Sci. 1997a;61:PL115–118. doi: 10.1016/s0024-3205(97)00596-1. [DOI] [PubMed] [Google Scholar]
- HOSOHATA Y., QUOCK R.M., HOSOHATA K., MAKRIYANNIS A., CONSROE P., ROESKE W.R., YAMAMURA H.I. AM630 antagonism of cannabinoid-stimulated [35S]GTPγS binding in the mouse brain. Eur. J. Pharmacol. 1997b;321:R1–R3. doi: 10.1016/s0014-2999(97)00047-2. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Agonist-receptor efficacy I: mechanisms of efficacy and receptor promiscuity. Trends Pharmacol. Sci. 1995;16:188–192. doi: 10.1016/s0165-6147(00)89020-3. [DOI] [PubMed] [Google Scholar]
- LANDSMAN R.S., MAKRIYANNIS A., DENG H., CONSROE P., ROESKE W.R., YAMAMURA H.I. AM630 is an inverse agonist at the human cannabinoid CB1 receptor. Life Sci. 1998;62:PL109–PL113. doi: 10.1016/s0024-3205(97)01187-9. [DOI] [PubMed] [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., KWAN J., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoid receptors and their ligands in brain and other tissues Marihuana and Medicine 1998Totowa: Humana Press; ed. Nahas, G.G., Sutin, K.M. & Agurell, S.(in press) [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., NASH J.E., COUTTS A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R., GRIFFIN G., FERNANDO S., LI X., HILL A., MAKRIYANNIS A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995;56:1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.-M., CASSELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.-C., LE FUR G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- SHOWALTER V.M., COMPTON D.R., MARTIN B.R., ABOOD M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]


