Abstract
Antioxidants attenuate noncholinergic airway constriction. To further investigate the relationship between tachykinin-mediated airway constriction and oxygen radicals, we explored citric acid-induced bronchial constriction in 48 young Hartley strain guinea-pigs, divided into six groups: control; citric acid; hexa(sulphobutyl)fullerenes+citric acid; hexa(sulphobutyl)fullerenes+phosphoramidon+citric acid; dimethylthiourea (DMTU)+citric acid; and DMTU+phosphoramidon+citric acid. Hexa(sulphobutyl)fullerenes and DMTU are scavengers of oxygen radicals while phosphoramidon is an inhibitor of the major degradation enzyme for tachykinins.
Animals were anaesthetized, paralyzed, and artificially ventilated. Each animal was given 50 breaths of 4 ml saline or citric acid aerosol. We measured dynamic respiratory compliance (Crs), forced expiratory volume in 0.1 (FEV0.1), and maximal expiratory flow at 30% total lung capacity (V[dot above]max30) to evaluate the degree of airway constriction.
Citric acid, but not saline, aerosol inhalation caused marked decreases in Crs, FEV0.1 and V[dot above]max30, indicating marked airway constriction. This constriction was significantly attenuated by either hexa(sulphobutyl)fullerenes or by DMTU. In addition, phosphoramidon significantly reversed the attenuating action of hexa(sulphobutyl)fullerenes, but not that of DMTU.
Citric acid aerosol inhalation caused increases in both lucigenin- and t-butyl hydroperoxide-initiated chemiluminescence counts, indicating citric acid-induced increase in oxygen radicals and decrease in antioxidants in bronchoalveolar lavage fluid. These alterations were significantly suppressed by either hexa(sulphobutyl)fullerenes or DMTU.
An elastase inhibitor eglin-c also significantly attenuated citric acid-induced airway constriction, indicating the contributing role of elastase in this type of constriction.
We conclude that both oxygen radicals and elastase play an important role in tachykinin-mediated, citric acid-induced airway constriction.
Keywords: Airway constriction, bronchial reactivity, oxygen radicals, tachykinins
Introduction
Satoh et al. (1993) found that citric acid-induced airway constriction is mediated via tachykinin neurokinin-2 (NK-2) but not NK-1 receptors. We demonstrated previously that oxygen radicals are involved in the activation of afferent C-fibres which release tachykinins. The involvement of oxygen radicals has been found in several types of noncholinergic airway constriction such as that caused by capsaicin (Lai, 1990), hyperventilation (Fang & Lai, 1993) and exsanguination (Zhang & Lai, 1994). However, it is not clear whether oxygen radicals are involved in citric acid-induced airway constriction. This study was thus conducted to test the role of oxygen radicals by direct measurement of their activities and using antioxidants to antagonize the radicals (Halliwell, 1992).
Two types of antioxidants were employed in this study. We demonstrated previously that water-soluble derivatives, such as fullerenol-1 synthesized by Chiang et al. (1995), ameliorate airway constriction induced by exsanguination (Lai & Chiang, 1997) and by xanthine-xanthine oxidase (Lai et al., 1997). The former, but not the latter, airway constriction was mediated by release of tachykinins from C-fibres. Fullerenol-1 was later modified to become hexa(sulphobutyl)fullerenes (Chi et al., 1998), which is more potent and has fewer side effects compared to fullerenol-1. Similarly, we found that dimethylthiourea (DMTU) inhibits tachykinin-mediated noncholinergic airway constriction induced by capsaicin (Lai, 1990) and hyperventilation (Fang & Lai, 1993). To further investigate the relationship between tachykinin-mediated airway constriction and oxygen radicals, novel water-soluble hexa(sulphobutyl)fullerenes and DMTU were used in this study.
In a previous study using an elastase inhibitor eglin-c (Lai & Lin, 1998), we found that endogenous elastase plays an important role in hyperpnea-induced airway constriction. It is not clear whether elastase plays a role in citric acid-induced airway constriction. It is possible that endogenous elastase could cause airway constriction directly (Suzuki et al., 1996) or indirectly via its enhancement of the release of bronchoconstrictors and/or oxygen radicals. To test this hypothesis, eglin-c was used to suppress endogenous elastase and to see if this suppression attenuates citric acid-induced airway constriction.
Methods
Animal preparations
Forty-eight young Hartley strain guinea-pigs weighing 217±9 g were divided into six groups of eight animals each: control; citric acid; hexa(sulphobutyl)fullerenes+citric acid; hexa(sulphobutyl)fullerenes+phosphoramidon+citric acid; DMTU+citric acid; DMTU+phosphoramidon+citric acid. Following anaesthesia with sodium pentobarbitone (30–40 mg kg−1), each animal's trachea, carotid artery and jugular vein were cannulated. After being paralyzed with gallamine triethiodide (4 mg kg−1), the animal was artificially ventilated. To ensure that the animal was anaesthetized during paralysis, we administered gallamine according to the following plans. (l) Gallamine was only given when its active period (40 min) was within the effective duration of pentobarbitone (1–2 h). If it was necessary to administer gallamine beyond this effective period of the anaesthetic, supplemental doses of pentobarbitone were given before any more gallamine treatment. (2) If an additional dose of gallamine was needed after a single dose, we first examined the level of anaesthesia and made sure that the expected anaesthesia could be maintained longer than the effective duration of gallamine. The second injection of gallamine was then given to the animal. Each animal in the control group received 50 breaths of 4 ml saline aerosol while those in all citric acid groups were given citric acid aerosol (50 breaths of 4 ml aerosol generated from 0.6M citric acid). Both aerosols were generated from a nebulizer (Ultra-Neb99, DeVilbiss Co., Somerset, PA, U.S.A.). According to our previous method of administering fullerenol-1 (Lai & Chiang, 1997), each animal in all hexa(sulphobutyl)fullerenes groups was injected peritoneally with hexa(sulphobutyl)fullerenes (10 mg kg−1) for 2 days prior to the functional study. In addition, 2 mg kg−1 of hexa(sulphobutyl)fullerenes were also injected intravenously 30 min prior to citric acid aerosol inhalation. Phosphoramidon, the inhibitor of neutral endopeptidase (the degradation enzyme for tachykinins), was intravenously injected (1 mg kg−1) 5 min prior to the citric acid aerosol inhalation. DMTU was given to the animals by intraperitoneal injection for 3 days before the study. The three consecutive daily doses of DMTU were 750, 250 and 250 mg kg−1 (Lai, 1990).
Evaluation of bronchial function
Each anaesthetized-paralyzed and ventilated animal was placed supine inside a whole-body plethysmograph. The flow rate was monitored with a Validyne DP45 differential pressure transducer as the pressure dropped across three layers of 325-mesh wire screen in the wall of the plethysmograph. Lung volume change was obtained via integration of flow. Airway opening pressure was measured with a Statham PM 131 pressure transducer. Maximal expiratory flow-volume (MEFV) manoeuvres were performed using the Buxco Pulmonary Maneuvers system (Sharon, CT, U.S.A.). The system consists of a pressure panel and a fast solenoid manifold, both of which are operated by a BioSystem for Manoeuvres software program in a 586 computer. Tracings of pressure, flow, volume, and the MEFV curve appeared on the computer screen and these tracings were stored and printed. For the MEFV manoeuvre, the lungs were inflated to total lung capacity (TLC, lung volume at airway opening pressure=30 cmH2O) four times. At peak volume during the fourth inflation, the solenoid valve for inflation was shut off and immediately another solenoid valve for deflation was automatically turned on. The deflation valve was connected to a 20-L container, which maintained a subatmospheric pressure of −40 cmH2O. The negative pressure of 40 cmH2O produced maximal expiratory flow (Lai, 1988). During the baseline period, we first performed MEFV manoeuvres two to three times to obtain the baseline TLC. Subsequently, MEFV manoeuvres were carried out 20 min after inhalation of saline or citric acid aerosol and maximal expiratory flow at 30% vital capacity (V[dot above]max30) was obtained. At the same time, the program picked the forced expiratory volume in 0.1 s (FEV0.1) from the volume-time tracing. Airway opening pressure (Pao) and tidal volume (VT) were measured during artificial ventilation, and both parameters were recorded on a polygraph. Dynamic respiratory compliance (Crs) was calculated as the ratio of the VT-to-Pao difference between the end of expiration and the end of inspiration. Before and after each MEFV manoeuvre, functional residual capacity (FRC) (the lung volume at Pao=0) was determined using a modified neon dilution method (Lai, 1988). Starting from FRC, the lungs were inflated with a standard neon (0.5%) gas mixture to 50% vital capacity. Gas in the lungs, in the dead space of the instrument, and in the syringe was mixed thoroughly by repeating the injection and withdrawal of the gas mixture 10–20 times. The equilibrated gas mixture was withdrawn and analysed with a Varian gas chromatograph (Model 3300). The total volume (including FRC and instrumental dead space) was calculated. The FRC was obtained by subtracting the instrumental dead space from the total volume.
The general experimental procedure consisted of obtaining the values of Crs, FEV0.1 and V[dot above]max30 both before and 20 min after inhalation of saline or citric acid aerosol.
Collection of bronchoalveolar lavage (BAL) fluid
An additional 24 young guinea-pigs were divided into four groups of six animals each: saline control; citric acid; hexa(sulphobutyl)fullerenes+citric acid; and DMTU+citric acid. Preparations for these four groups of animals were the same as those described above. BAL fluid was collected about 3 min following saline or citric acid aerosol inhalation. To obtain BAL fluid, 3 ml of saline was instilled via the trachea 2 min after the inhalation of saline or citric acid aerosol. Saline in the lungs was withdrawn about 40 s following the instillation. Furthermore, to detect temporal changes in the production of oxygen radicals, an additional 12 animals were divided into two groups: saline control and citric acid. These animals were treated as described above except that BAL fluid was sampled 10 min after inhalation of either saline or citric acid aerosol. The obtained BAL fluid was immediately wrapped with aluminium foil and kept in the ice box until testing for chemiluminescence, which was usually done within 2 h.
Measurements of lucigenin-initiated and lucigenin-amplified t-butyl hydroperoxide (TBHP)-initiated chemiluminescence
Determinations of both lucigenin-initiated and lucigenin-amplified TBHP-initiated BAL fluid chemiluminescence were performed with the method of Sun et al. (1998) with some modifications. 0.1 ml physiological buffer solution (PBS, pH 7.4) was added to 0.2 ml BAL fluid in a stainless cell (5 cm in diameter). The chemiluminescence was then measured in an absolutely dark chamber of the Chemiluminescence Analyzing System (Tohoku Electronic Industrial Co., Sendai, Japan). This system contains a photon detector (Model CLD-110), chemiluminescence counter (Model CLC-10), water circulator (Model CH-20), and 32-bit IBM personal computer system. A cooler circulator was connected to the model CLD-110 photon detector to keep the temperature at 5°C. The Model CLD-110, according to the manufacturer's specifications, is so sensitive it is able to detect as low as 10−15 W of radiant energy. Photon emission from the BAL fluid was counted at 10-s intervals at 37°C and atmospheric conditions. At the 100-s time point, 1.0 ml of lucigenin (0.01 mM) in PSB was injected into the cell. The chemiluminescence in the BAL sample was continuously measured for a 600-s time period. Subsequently, at the 700-s time point, 0.1 ml of TBHP (Sigma Co., St. Louis, MO, U.S.A.) in PBS (pH=7.4) was injected into the cell. The chemiluminescence in the sample was continuously measured for a total of 200-s. The total amount of chemiluminescence was calculated by integrating the area under the curve and subtracting it from the background level, which was equivalent to the dark average. The assay was performed in duplicate for each sample and was expressed as chemiluminescence counts per 10 s.
Elastase inhibitor and citric acid-induced airway constriction
An additional 18 guinea-pigs were evenly divided into two groups: saline control and eglin-c. Each animal was intra-tracheally instilled with 0.25 ml of either saline or eglin-c (37.5 mg kg−1) 10 min prior to citric acid aerosol inhalation. The dose of eglin-c was according to our previous studies (Lai & Diamond, 1990; Lai & Zhou, 1997). The functional testing of airways was performed before as well as 10 and 20 min after the inhalation of citric acid aerosol, in the same fashion as that described above.
Drugs and chemicals
Hexa(sulphobutyl)fullerenes was synthesized by Chi et al. (1998). Eglin-c was kindly provided by Dr Hans Peter Schnebli, Ciba-Geigy Research Department, Basel, Switzerland. Citric acid, dimethylthiourea, gallamine triethiodide, lucigenin, phosphoramidon, sodium pentobarbitone, and t-butyl hydroperoxide were obtained from the Sigma Chemical Company (St. Louis, MO, U.S.A.).
Statistical analysis
All values are reported as means±s.e.mean. Analysis of variance was used to establish the statistical significance of differences among groups. If significant differences among groups were obtained using the analysis of variance, Duncan's multiple range test was used to differentiate differences between groups. Differences were considered significant if P<0.05.
Results
Body weight and baseline respiratory parameters in the guinea-pigs used for examining effects of antioxidants are listed in Table 1. Body weights, but not respiratory parameters, of animals treated with DMTU were lower than those of the animals in other groups. Differences in body weight between groups were caused by pretreatment with various drugs. To account for individual differences, we compared the results using per cent baseline values for each animal.
Table 1.
Body weight and baseline respiratory parameters in guinea-pigs
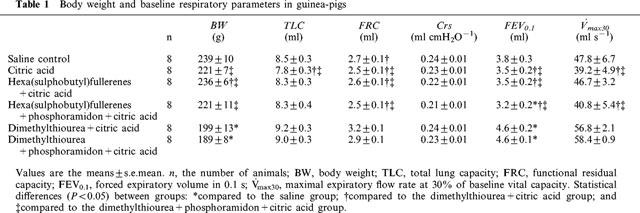
Effect of citric acid on bronchial function
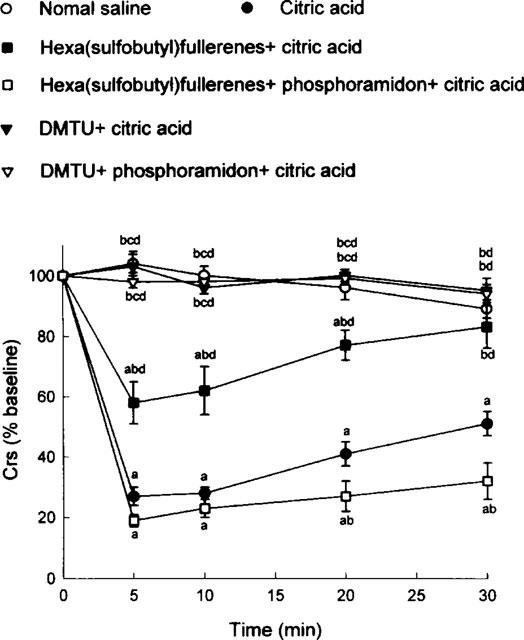
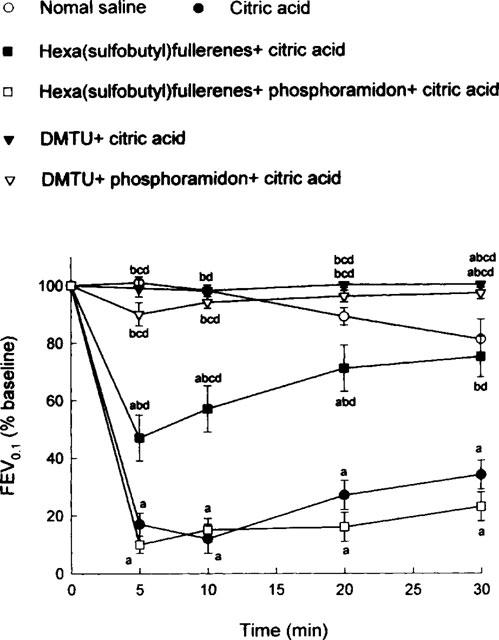
Saline aerosol inhalation did not induce any significant change (expressed as per cent baseline values) in Crs (Figure 1). On the other hand, citric acid aerosol inhalation caused a marked decrease in Crs (expressed as per cent baseline value), indicating severe airway constriction in the citric acid group (Figure 1). This constriction was significantly attenuated either by hexa(sulphobutyl)fullerenes or by DMTU; this attenuating effect was significantly larger with DMTU than with hexa(sulphobutyl)fullerenes. In addition, phosphoramidon significantly reversed the attenuating action of hexa(sulphobutyl)fullerenes but not that of DMTU (Figure 1). Using FEV0.1 (Figure 2) and V[dot above]max30 (Figure 3) as indicators, the effects of hexa(sulphobutyl)fullerenes and DMTU, as well as supplemental influences of phosphoramidon, appeared to be in the same manner as those of Crs values shown above.
Figure 1.
Citric acid-induced alterations in dynamic respiratory compliance (Crs), expressed as per cent baseline values, in six groups of guinea-pigs. DMTU=dimethylthiourea. Statistical differences (P<0.05) between groups: acompared to the saline control group; bcompared to the citric acid group; ccompared to the hexa(sulphobutyl)fullerenes+citric acid group; and dcompared to the hexa(sulphobutyl)fullerenes+phosphoramidon+citric acid group.
Figure 2.
Citric acid-induced alterations in forced expiratory volume in 0.1 s (FEV0.1), expressed as per cent baseline values, in six groups of guinea-pigs. DMTU=dimethylthiourea. Statistical differences (P<0.05) between groups: acompared to the saline control group; bcompared to the citric acid group; ccompared to the hexa(sulphobutyl)fullerenes+citric acid group; and dcompared to the hexa(sulphobutyl)fullerenes+phosphoramidon+citric acid group.
Figure 3.
Citric acid-induced alterations in maximal expiratory flow at 30% vital capacity (V[dot above]max30), expressed as per cent baseline values, in six groups of guinea-pigs. DMTU=dimethylthiourea. Statistical differences (P<0.05) between groups: acompared to the saline control group; bcompared to the citric acid group; ccompared to the hexa(sulphobutyl)fullerenes+citric acid group; and dcompared to the hexa(sulphobutyl)fullerenes+phosphoramidon+citric acid group.
Effect of citric acid on BAL samples
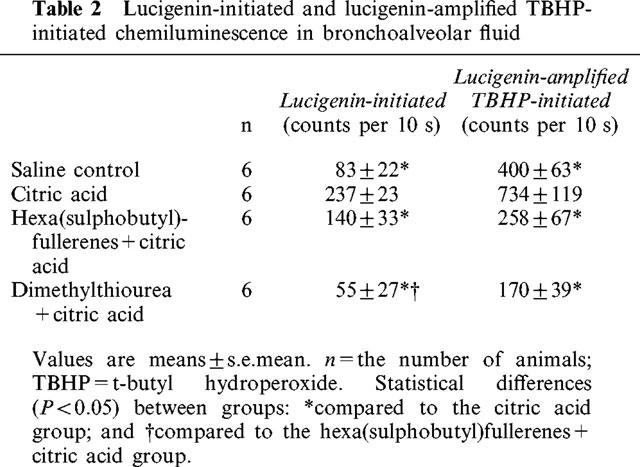
In the BAL samples obtained about 3 min after inhalation of saline or citric acid aerosol, the addition of lucigenin caused a small rise while adding TBHP caused an even larger increase in the chemiluminescence signal (Figure 4). Both increases were larger in the BAL fluid obtained from the animals who inhaled citric acid than that from the animals who inhaled saline aerosol (Figure 4). Group data of BAL samples obtained 3 min after aerosol inhalation are shown in Table 2. Inhalation of citric acid aerosol induced significant increases in both lucigenin- and TBHP-initiated chemiluminescence counts. These increases were, however, significantly attenuated by either hexa(sulphobutyl)fullerenes or DMTU.
Figure 4.
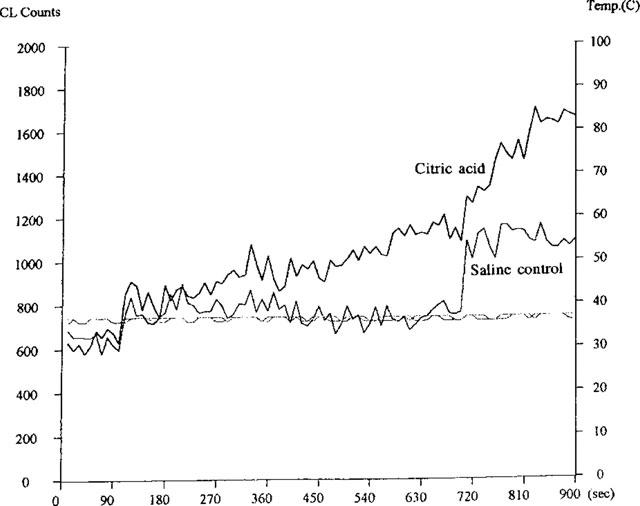
Examples of lucigenin-initiated (100–700-s) and lucigenin-amplified, t-butyl hydroperoxide (TBHP)-initiated (700–900-s) chemiluminescence signals of bronchoalveolar (BAL) lavage fluid obtained from a saline control and citric acid-treated animals. Lucigenin was added at 100-s and TBHP added at 700-s time points. BAL samples were maintained constantly around 37°C as shown in the two temperature lines parallel to the x-axis baseline.
Table 2.
Lucigenin-initiated and lucigenin-amplified TBHP-initiated chemiluminescence in bronchoalveolar fluid
In the BAL samples obtained 10 min after inhalation of citric acid aerosol, the addition of lucigenin and TBHP caused smaller increases in the chemiluminescence signals compared to those of the 3 min samples. Chemiluminescence counts (per 10 s) initiated by lucigenin were: saline control, 78±12; and citric acid, 156±41. At the same time, TBHP-initiated chemiluminescence counts per 10 s were: saline control, 287±50; and citric acid, 328±107. No significant differences were found in the above two values between the citric acid and saline control groups.
Effect of eglin-c on citric acid-induced airway constriction
Body weight and baseline respiratory parameters in guinea-pigs used for examining the effects of the elastase inhibitor eglin-c are listed in Table 3. No significant differences in either body weight or respiratory parameters between groups were found. Similar to Figure 1, citric acid caused marked decreases in Crs (Figure 5), FEV0.1 (Figure 6) and V[dot above]max30 (Figure 7), indicating severe airway constriction in saline control animals. This airway constriction was significantly attenuated by pretreatment with eglin-c at 20 and/or 30 min time points (Figure 5–7).
Table 3.
Body weight and baseline respiratory parameters in guinea-pigs
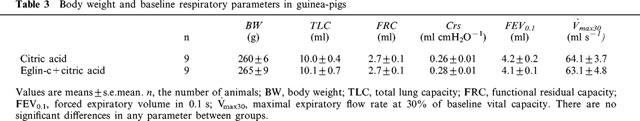
Figure 5.
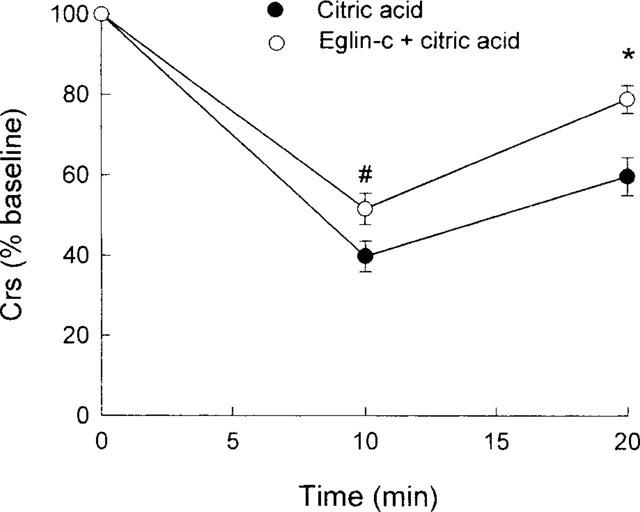
Citric acid-induced alterations in dynamic respiratory compliance (Crs), expressed as per cent baseline values, in two groups of guinea-pigs. Statistical differences between groups: #P<0.05; *P<0.01.
Figure 6.
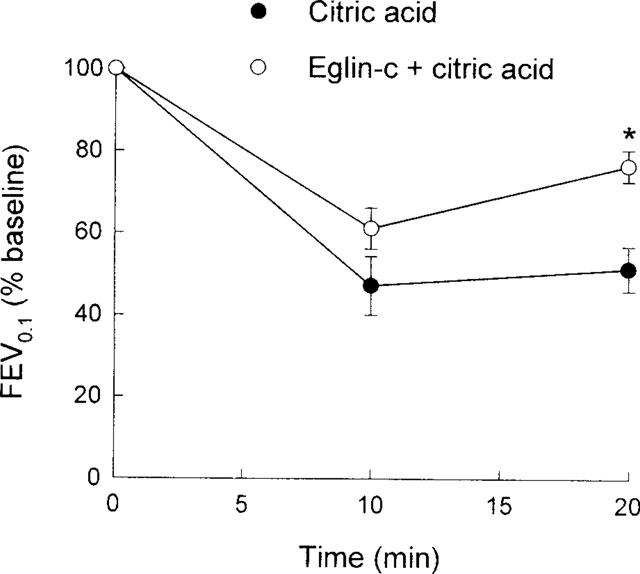
Citric acid-induced alterations in forced expiratory volume in 0.1 s (FEV0.1), expressed as per cent baseline values, in two groups of guinea-pigs. Statistical differences between groups: *P<0.01.
Figure 7.
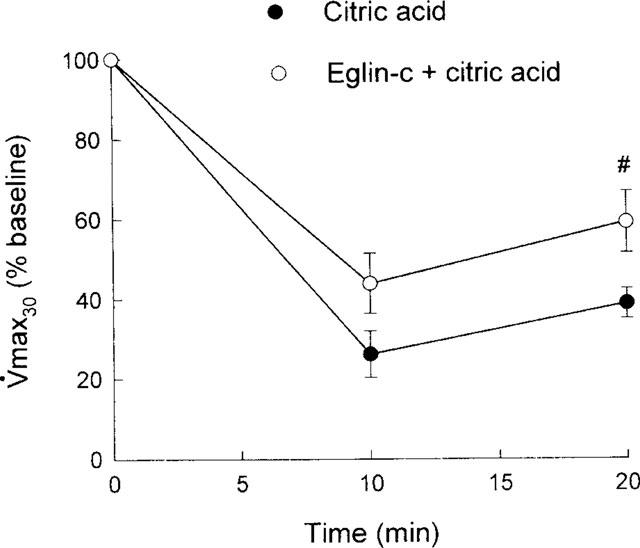
Citric acid-induced alterations in maximal expiratory flow at 30% vital capacity (V[dot above]max30), expressed as per cent baseline values, in two groups of guinea-pigs. Statistical differences between groups: #P<0.05.
Discussion
We demonstrated that citric acid-induced bronchoconstriction was significantly attenuated by hexa(sulphobutyl)fullerenes and DMTU, the attenuating effect of DMTU being more potent than that of the hexa(sulphobutyl)fullerenes. In addition, the inhibitor for neutral endopeptidase (the major degradation enzyme of tachykinins) significantly reversed the attenuating effect of hexa(sulphobutyl)fullerenes but not that of DMTU. Furthermore, citric acid aerosol inhalation caused increases in lucigenin- and TBHP-induced chemiluminescence. We showed also that eglin-c suppressed citric acid-induced airway constriction. Several features of these results will be discussed below.
Tachykinins in citric acid-induced airway constriction
It is known that acid aspiration induces airway constriction (Goldman et al., 1992). Inhalation of citric acid aerosol causes coughing (Girard et al., 1995), airway hyperresponsiveness (Girard et al., 1996), bronchoconstriction (Satoh et al., 1993), and plasma extravasation in the lungs (Satoh et al., 1993). These effects of citric acid-induced alterations have been attenuated or prevented by capsaicin pretreatment to deplete tachykinins (Girard et al., 1996; Satoh et al., 1993), the selective capsaicin antagonist capsazepine (Satoh et al., 1993), and the NK-2 receptor antagonist SR 48968 (Girard et al., 1996; Satoh et al., 1993). Capsazepine interferes with proton-sensitive ion channels (Bevan & Yeats, 1991) via occupation of the proposed capsaicin receptor site (Szallasi & Blumberg, 1990). Thus, the above citric acid-induced changes are related closely with the activation of afferent C-fibres in the lungs via capsaicin receptors. The sequence of this action may be depicted as follows. Citric acid stimulates afferent C-fibres which, in turn, release tachykinins. Released tachykinins act on effectors via three types of neurokinin (NK) receptors: NK-1, NK-2, and NK-3 (Khawaja & Rogers, 1996). NK-2 receptor can be blocked by SR 48968, which prevents citric acid-induced airway constriction (Girard et al., 1996; Satoh et al., 1993). However, the NK-1 receptor antagonist CP 96345 did not prevent this type of airway constriction, indicating the specific NK-2 receptor in mediating the constriction. In addition to airway constriction, released tachykinins cause also coughing and characteristics of neurogenic inflammation, including increases in airway responsiveness (Girard et al., 1996), plasma extravasation (Lei et al., 1996; Martling & Lundberg, 1988), and airway secretion (Ramnarine et al., 1994).
Phosphoramidon is an inhibitor for neutral endopeptidase which is the major degradation enzyme for tachykinins (Borson, 1991). Theoretically, administration of phosphoramidon should prevent the degradation of tachykinins and thus augment citric acid-induced airway constriction. In the presence of hexa(sulphobutyl)fullerenes, our results were according to this expectation. Again, this fact supports the idea that citric acid-induced airway constriction is mediated via tachykinins. Following DMTU treatment, however, phosphoramidon did not augment citric acid-induced airway constriction. The failure of phosphoramidon to augment the airway constriction might be related to an adrenergic β2-agonist property of DMTU (Lin & Lai, 1998).
Oxygen radicals in tachykinin-mediated, citric acid-induced airway constriction
Goldman et al. (1992) found that acid aspiration caused increases in both reactive oxygen species and lung permeability. In agreement with their results, we observed that citric acid inhalation induced an increase in chemiluminescence counts and airway constriction. Lucigenin-initiated chemiluminescence is an effective monitor of mitochondrial superoxide generation (Rembish & Trush, 1994). On the other hand, TBHP-initiated chemiluminescence is an effective monitor of lipid peroxide generation (Boveris et al., 1980; Nakano et al., 1975; Sugioka & Nakano, 1976) and has been used to detect decreased levels of endogenous antioxidants in liver and cardiac tissues (Cadenas et al., 1981; Prasad et al., 1992). From the present study, it is suggested that the decreased antioxidant activity following citric acid inhalation is partially caused by the superoxide pathway, while remote pathophysiological events are mediated by defective scavenging defences. The generation of free radicals in the presence of defective scavenging defences might be the cause of the stimulation of afferent C-fibres, resulting in noncholinergic airway constriction.
It is not clear why there was a time-lag between the increase in oxygen radical production and airway constriction. We detected increases in oxygen radicals within 3 min, but not at 10 min, and airway constriction was detected between 3 min to 20 min after the citric acid inhalation. It is apparent that oxygen radicals are needed for the initiation of the release of tachykinins and subsequent airway constriction. This is believed because the action of oxygen radicals was significantly prevented by either DMTU or hexa(sulphobutyl)fullerenes (Figure 123). Compared to hexa(sulphobutyl)fullerenes, DMTU caused a larger inhibition of citric acid-induced increases in oxygen radicals and airway constriction. This effect of DMTU might be related to its smaller molecular size and its ability to penetrate the cell (Fox, 1984). In addition, DMTU might have the properties of an adrenergic β2-agonist (Lin & Lai, 1998), which suppresses both the production of oxygen radicals and airway constriction.
Elastase in citric acid-induced bronchoconstriction
We found that eglin-c inhibits citric acid-induced airway constriction, indicating the important role of endogenous elastase in this type of bronchoconstriction. It is possible that endogenous elastase may cause airway constriction in two ways. In addition to a direct constricting effect of elastase (Suzuki et al., 1996), serine elastase can enhance the release of bronchoconstrictors. For example, serine proteinases augment the release of histamine in sheep (Molinari et al., 1996), rats (Emadi-Khiav & Pearce, 1994) and humans (Hultsch et al., 1988). It is interesting to mention that this type of released histamine causes bronchoconstriction in vivo in sheep (Molinari et al., 1996). Beside its direct action, histamine can also trigger the release of tachykinins (Saria et al., 1988). The source for endogenous elastase is speculative. It is possible that citric acid aerosol induces mucosal injury as well as degranulations of mast cells and leukocytes, which release elastase. It is not clear, however, whether there are simultaneous releases of elastase and oxygen radicals. Compared to the effect of antioxidants [hexa(sulphobutyl)fullerenes and DMTU] (Figure 123), however, eglin-c produced a smaller magnitude of suppression of citric acid-induced airway constriction (Figure 567). This may imply that oxygen radicals may be the main contributing factor to elicit tachykinin release following inhalation of citric acid aerosol.
In summary, we conclude that both oxygen radicals and elastase play an important role in tachykinin-mediated, citric acid-induced airway constriction.
Acknowledgments
We deeply appreciate Dr Hans Peter Schnebli, Ciba-Geigy Research Department, Basel, Switzerland for providing eglin-c for this study. This investigation was supported by the National Science Council (NSC 87-2314-B002-349 and NSC 87-2314-B002-121M41) and the Department of Health (DOH88-HR-833) and help from NHRI.
Abbreviations
- BAL
bronchoalveolar lavage
- Crs
dynamic respiratory compliance
- DMTU
dimethylthiourea
- FEV0.1
forced expiratory volume in 0.1 s
- FRC
functional residual capacity
- MEFV
maximal expiratory flow-volume
- NK
neurokinin
- PBS
physiological buffer solution
- TBHP
t-butyl hydroperoxide
- TLC
total lung capacity
- V[dot above]max30
maximal expiratory flow at 30% total lung capacity
References
- BEVAN S., YEATS J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurons. J. Physiol. 1991;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSON D.B. Roles of neutral endopeptidase in airways. Am. J. Physiol. 1991;260:L212–L225. doi: 10.1152/ajplung.1991.260.4.L212. [DOI] [PubMed] [Google Scholar]
- BOVERIS A., CADENAS E., REITER R., FILIPKOWSKI M., NAKASE Y., CHANCE B. Organ chemiluminescence: noninvasive assay for oxidative radical reaction. Proc. Natl. Acad. Sci. U.S.A. 1980;77:347–351. doi: 10.1073/pnas.77.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADENAS E., WEFERS H., SIES H. Low-level chemiluminescence of isolated hepatocytes. Eur. J. Biochem. 1981;119:531–536. doi: 10.1111/j.1432-1033.1981.tb05640.x. [DOI] [PubMed] [Google Scholar]
- CHI Y., BHONSLE J.B., CANTEENWALA T., HUANG J.-P., SHIEA J., CHEN B.-J., CHIANG L.Y. Novel water-soluble hexa(sulphobutyl)fullerenes as potent free radical scavengers. Chem. Lett. 1998. pp. 465–466.
- CHIANG L.Y., LU F.-J., LIN J.-T. Free radical scavenging activity of water-soluble fullerenols. J. Chem. Soc. Chem. Commun. 1995. pp. 1283–1284.
- EMADI-KHIAV B., PEARCE F.L. Involvement of a serine protease in mast cell activation. Agents Actions. 1994;41:C37–C38. doi: 10.1007/BF02007756. [DOI] [PubMed] [Google Scholar]
- FANG Z.X., LAI Y.-L. Oxygen radicals in bronchoconstriction of guinea-pigs elicited by isocapnic hyperpnea. J. Appl. Physiol. 1993;74:627–633. doi: 10.1152/jappl.1993.74.2.627. [DOI] [PubMed] [Google Scholar]
- FOX R.B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J. Clin. Invest. 1984;74:1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD V., NALINE E., VILAIN P., EMOND-ALT X., ADVENIER C. Effect of the two tachykinin antagonists, SR 48968 and SR 140333, on cough induced by citric acid in the unanesthetized guinea-pig. Eur. Respir. J. 1995;8:1110–1114. doi: 10.1183/09031936.95.08071110. [DOI] [PubMed] [Google Scholar]
- GIRARD V., YAVO J., EMOND-ALT X., ADVENIER C. The tachykinin NK2 receptor antagonist SR 48968 inhibits citric acid-induced airway hyperresponsiveness in guinea-pigs. Am. J. Respir. Crit. Care Med. 1996;153:1496–1502. doi: 10.1164/ajrccm.153.5.8630592. [DOI] [PubMed] [Google Scholar]
- GOLDMAN G., WELBOURN R., KOBZIK L., VALERI C.R., SHERPO D., HECHTMAN H.B. Reactive oxygen species and elastase mediate lung permeability after acid aspiration. J. Appl. Physiol. 1992;73:571–575. doi: 10.1152/jappl.1992.73.2.571. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- HULTSCH T., ENNIS M., HEIDTMANN H.H. The role of chymase in ionophore-induced histamine release from human pulmonary mast cells. Adv. Exper. Med. Biol. 1988;240:133–136. doi: 10.1007/978-1-4613-1057-0_16. [DOI] [PubMed] [Google Scholar]
- KHAWAJA A.M., ROGERS D.F. Tachykinins: receptor to effector. Int. J. Biochem. Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- LAI Y.-L. Maximal expiratory flow in the guinea-pig. Lung. 1988;166:303–313. doi: 10.1007/BF02714061. [DOI] [PubMed] [Google Scholar]
- LAI Y.-L. Oxygen radicals in capsaicin-induced bronchoconstriction. J. Appl. Physiol. 1990;68:568–573. doi: 10.1152/jappl.1990.68.2.568. [DOI] [PubMed] [Google Scholar]
- LAI Y.-L., LIN Y.J.Elastase in hyperpnea-induced guinea-pig airway constriction Eur. J. Pharmacol. 1998(In press) [DOI] [PubMed]
- LAI Y.-L., CHIANG L.Y. Water-soluble fullerene derivatives attenuate exsanguination-induced bronchoconstriction of guinea-pigs. J. Autonom. Pharmacol. 1997;17:229–235. doi: 10.1046/j.1365-2680.1997.00461.x. [DOI] [PubMed] [Google Scholar]
- LAI Y.-L., CHIOU W.-Y., CHIANG L.Y. Fullerene derivatives attenuate bronchoconstriction induced by xanthine-xanthine oxidase. Fullerene Sci. Technol. 1997;5:1057–1065. [Google Scholar]
- LAI Y.-L., DIAMOND L. Inhibition of porcine pancreatic elastase-induced pulmonary emphysema by eglin-c. Exp. Lung Res. 1990;16:547–557. doi: 10.3109/01902149009068826. [DOI] [PubMed] [Google Scholar]
- LAI Y.-L., ZHOU K.-R. Eglin-c prevents monocrotaline-induced ventilatory dysfunction. J. Appl. Physiol. 1997;82:324–328. doi: 10.1152/jappl.1997.82.1.324. [DOI] [PubMed] [Google Scholar]
- LEI Y.-H., BARNES P.J., ROGERS D.F. Involvement of hydroxyl radicals in neurogenic airway plasma exudation and bronchoconstriction in guinea-pigs in vivo. Br. J. Pharmacol. 1996;117:449–454. doi: 10.1111/j.1476-5381.1996.tb15211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN C.-W., LAI Y.-L. Tachykinins in propranolol-augmented, hyperpnea-induced bronchoconstriction in Taida guinea-pigs: effects of dimethylthiourea. J. Autonom. Pharmacol. 1998;18:139–147. doi: 10.1046/j.1365-2680.1998.1830139.x. [DOI] [PubMed] [Google Scholar]
- MARTLING C.-R., LUNDBERG J.M. Capsaicin sensitive afferents contribute to acute airway edema following tracheal instillation of hydrochloric acid or gastric juice in the rat. Anesthesiology. 1988;68:350–356. doi: 10.1097/00000542-198803000-00005. [DOI] [PubMed] [Google Scholar]
- MOLINARI J.F., SCURI M., MOORE W.R., CLARK J., TANAKA R., ABRAHAM W.M. Inhaled trypase causes bronchoconstriction in sheep via histamine release. Am. J. Respir. Crit. Care Med. 1996;154:649–653. doi: 10.1164/ajrccm.154.3.8810600. [DOI] [PubMed] [Google Scholar]
- NAKANO M., NOGUCHI T., SUGIOKA K., FUKUYAMA H., SATO M. Spectroscopic evidence for the generation of singlet oxygen in the reduced nicotinamide adenine dinucleotide phosphate-dependent microsomal lipid peroxidation system. J. Biol. Chem. 1975;250:2404–2406. [PubMed] [Google Scholar]
- PRASAD K., LEE P., MANTHA S., KALRA J., PRASAD M., GUPTA J.B. Detection of ischemia-reperfusion cardiac injury by cardiac muscle chemiluminescence. Molec. Cell Biochem. 1992;115:49–58. doi: 10.1007/BF00229095. [DOI] [PubMed] [Google Scholar]
- RAMNARINE S.I., HIRAYAMA Y., BARNES P.J., ROGERS D.F. ‘Sensory-efferent' neural control of mucus secretion: characterization using tachykinin receptor antagonists in ferret trachea in vitro. Br. J. Pharmacol. 1994;113:1183–1190. doi: 10.1111/j.1476-5381.1994.tb17122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMBISH S.J., TRUSH M.A. Further evidence that lucigenin-derived chemiluminescence monitors mitochondrial superoxide generation in rat alveolar macrophages. Free Radical Biol. Med. 1994;17:117–126. doi: 10.1016/0891-5849(94)90109-0. [DOI] [PubMed] [Google Scholar]
- SARIA A., MARTLING C.-R., YAN X., THEODORSSON-NORHEIM E., GAMSE R., LUNDBERG J.M. Release of multiple tachykinins from capsaicin-sensitive sensory nerves in the lung by bradykinin, histamine, dimethylphenyl piperazinium, and vagal nerve stimulation. Am. Rev. Respir. Dis. 1988;l37:l330–1335. doi: 10.1164/ajrccm/137.6.1330. [DOI] [PubMed] [Google Scholar]
- SATOH H., LOU Y.P., LUNDBERG J.M. Inhibitory effects of capsazepine and SR 48968 on citric acid-induced bronchoconstriction in guinea-pigs. Eur. J. Pharmacol. 1993;236:367–372. doi: 10.1016/0014-2999(93)90473-u. [DOI] [PubMed] [Google Scholar]
- SUGIOKA K., NAKANO M. A possible mechanism of the generation of singlet molecular oxygen in NADPH-dependent microsomal lipid peroxidation. Biochem. Biophys. Acta. 1976;423:203–216. doi: 10.1016/0005-2728(76)90179-1. [DOI] [PubMed] [Google Scholar]
- SUN J.-S., TSUANG Y.-H., CHEN I.-J., HUANG W.-C., HANG Y.-H., LU F.-J. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns. 1998;24:225–232. doi: 10.1016/s0305-4179(97)00115-0. [DOI] [PubMed] [Google Scholar]
- SUZUKI T., WANG W., LIN J.T., SHIRATO K., MITSUHASHI H., INOUE H. Aerosolized human neutrophil elastase induces airway constriction and hyperresponsiveness with protection by intravenous pretreatment with half-length secretory leukoprotease inhibitor. Am. J. Respir. Crit. Care Med. 1996;153:1405–1411. doi: 10.1164/ajrccm.153.4.8616573. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res. 1990;524:106–111. doi: 10.1016/0006-8993(90)90498-z. [DOI] [PubMed] [Google Scholar]
- ZHANG H.-Q., LAI Y.-L. Intratracheal antioxidants atten-uate exsanguination-induced bronchoconstriction in guinea-pigs. J. Appl. Physiol. 1994;76:553–559. doi: 10.1152/jappl.1994.76.2.553. [DOI] [PubMed] [Google Scholar]


