Abstract
For the further elucidation of the central functions of nociceptin/orphanin FQ (noc/OFQ), the endogenous ligand of the G protein-coupled opioid receptor-like receptor ORL1, centrally acting specific antagonists will be most helpful. In this study it was found that the hexapeptide acetyl-RYYRIK-NH2 (Ac-RYYRIK-NH2), described in literature as partial agonist on ORL1 transfected in CHO cells, antagonizes the stimulation of [35S]-GTPγS binding to G proteins by noc/OFQ in membranes and sections of rat brain. The antagonism of the peptide was competitive, of high affinity (Schild constant 6.58 nM), and specific for noc/OFQ in that the stimulation of GTP binding by agonists for the μ-, δ-, and κ-opioid receptor was not inhibited. The hexapeptide also fully inhibited the chronotropic effect of noc/OFQ on neonatal rat cardiomyocytes. It is suggested that Ac-RYYRIK-NH2 may provide a promising starting point for in vivo tests for antagonism of the action of noc/OFQ and for the further development of highly active and specific antagonists.
Keywords: Nociceptin, orphanin FQ, [35S]-GTPγS, rat brain membranes, rat cardiomyocytes, acetyl-RYYRIK-NH2, antagonism
Introduction
The isolation of the endogenous ligand for the opioid receptor-like receptor ORL1, the heptadecapeptide FGGFTGARKSARKLANQ, named nociceptin (Meunier et al., 1995) or orphanin FQ (Reinscheid et al., 1995), marked the beginning of a new area in opioid and brain research (Rowe, 1996). Although nociceptin/orphanin FQ (noc/OFQ) shows some structural analogy to opioid peptides, particularly dynorphin A, and acts at the molecular and cellular levels in almost the same way as the classical μ-, δ-, and κ-opioids do, it produces pharmacological effects that oppose, or at least differ from, those of opioids. In the rat, noc/OFQ supraspinally suppresses opioid-mediated analgesia but seems to act spinally as an analgesic. In brain, it suppresses spatial learning, impairs motor performance, influences the release of pituitary hormones, and induces feeding. When administered intravenously, it exhibits smooth muscle relaxant, antinatriuretic and diuretic properties. Furthermore, stimulated immune function appears also to be regulated by noc/OFQ (reviewed by Meunier, 1997; Darland et al., 1998; Taylor & Dickenson, 1998). The multiple functions of noc/OFQ, which may be deduced from its pharmacological effects, should provide new insights into brain physiology and may lead to therapeutic applications, e.g., in the management of pain. However, our present knowledge about the pharmacological profile of the peptide is likely to be incomplete and additional functions may be expected.
For the further elucidation of the function of noc/OFQ, specific antagonists will be most helpful. From a combinatorial library of acetylated hexapeptide amides, Dooley et al. (1997) identified some hexapeptides that showed partial agonist activity in Chinese hamster ovary (CHO) cells transfected with ORL1 and in the inhibition of electrically induced contractions of mouse vas deferens. We thought that such short peptides might provide a promising starting point for the development of specific antagonists of noc/OFQ. Surprisingly, we found in this study that in a native preparation of rat brain membranes one of these peptides identified by Dooley et al. (1997), Ac-RYYRIK-NH2, is a specific antagonist for the activation of G proteins, as measured by stimulation of binding of a GTP analogue, by the activated noc/OFQ receptor. Furthermore, the peptide was also found to antagonize the chronotropic effect of noc/OFQ on spontaneously beating cultured neonatal rat heart cells.
Methods
Noc/OFQ and the hexapeptide Ac-RYYRIK-NH2 were synthesized in our institute. The selective agonists for the μ- and δ-opioid receptor, [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) and [D-Pen2, D-Pen5]-enkephalin (DPDPE), respectively, were obtained from Sigma (Deisenhofen, Germany), the κ-opioid receptor agonist U-504,88 was from RBI (Massachusetts, U.S.A.). Guanosine 5′-O-(γ-[35S]-thio)triphosphate ([35S]-GTPγS) was from NEN (Boston, MA, U.S.A.).
Noc/OFQ- and opioid-stimulated [35S]-GTPγS binding to cerebral cortex membranes and coronal brain sections obtained from male Wistar rats was determined as described earlier (Albrecht et al., 1998). Briefly, 20 μg of membrane protein was incubated with the compounds in 50 mM Tris (pH 7.4) containing: 100 mM NaCl, 1 mM MgCl2, 0.15 mM bacitracin, 100 μM GDP and 1 mg ml−1 BSA and about 70 pM [35S]-GTPγS at 30°C for 120 min with and without the hexapeptide Ac-RYYRIK-NH2. The binding of the GTP tracer to the membranes was determined after filtration through Whatman GF/B filters. Slide-mounted sections were incubated in buffer containing 2 mM GDP (Sim et al., 1995) with the compounds and [35S]-GTPγS for 120 min at 25°C. The slides were exposed to UR-imaging plates (FUJI Photo Film Co., Japan) for 16 h, together with 14C-microscales (Amersham) for quantification. The plates were scanned and analysed using the Bio-Imaging Analyzer System BAS-3000 (FUJI) linked to the micro computer imaging device system from Imaging Research Inc. (St. Catherines, Canada). Rat heart cells were obtained from ventricles of 1–2-day-old Wistar rats and cultured as described earlier (Wallukat et al., 1991). The chronotropic response was measured 5 min after the cumulative addition of noc/OFQ with and without Ac-RYYRIK-NH2.
Data are expressed as means±s.e.mean. EC50 values for the stimulation of [35S]-GTPγS binding by noc/OFQ in absence and presence of different concentrations of Ac-RYYRIK-NH2 were calculated from the concentration-response curves by nonlinear regression using the program GraphPad Prism 2.01 (GraphPad Software, Inc., San Diego, U.S.A.), and the constant for the inhibition by Ac-RYYRIK-NH2 was calculated by linear regression of the corresponding Schild plot.
Results
The EC50 value for the stimulation of binding of [35S]-GTPγS to membranes of rat cortex by noc/OFQ was found to be 11.36±1.83 nM (n=5), which compares well with the EC50 of 9.11 nM as found earlier (Albrecht et al., 1998). Increasing concentrations of Ac-RYYRIK-NH2 shifted the concentration-response curve for noc/OFQ to the right (Figure 1), and from the corresponding Schild plot the inhibition constant Ka of the hexapeptide was calculated to be 6.58±0.69 nM (n=5). The peptide did not antagonize the stimulation of GTPγS binding by agonists of the μ-, δ-, and κ-opioid receptor in membranes of rat cortex (Figure 2) as well as in sections of rat brain at the level of striatum (Figures 3 and 4, data for δ- and κ-agonists not shown). With membranes the high concentration of 10 μM Ac-RYYRIK-NH2, 1000 fold higher than its inhibitory constant, produced a small effect by its own, which added to the amount of bound [35S]-GTPγS stimulated by the opioid agonists (Figure 2). With much lower potency, as compared to the stimulation of GTP binding in brain membranes, noc/OFQ increased the beating rate of spontaneously beating cardiomyocytes in cultured neonatal rat heart cells, the maximum effect reaching 65% of that of the β-adrenergic agonist isoprenaline. Ac-RYYRIK-NH2 (1 and 10 μM) did not influence the beating rate but blocked the noc/OFQ-stimulated response (Figure 5).
Figure 1.
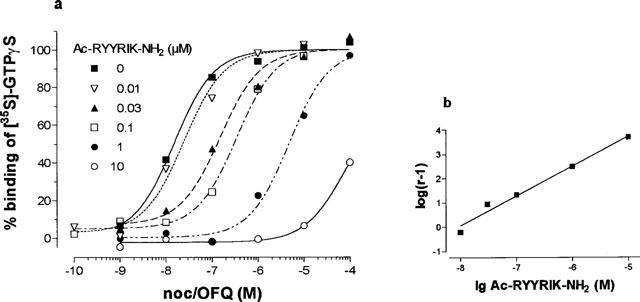
Effect of increasing concentrations of the hexapeptide Ac-RYYRIK-NH2 on the concentration-response curve for the noc/OFQ-stimulated binding of 70 pM [35S]-GTPγS to rat cortex membranes (a) and the corresponding Schild plot (b). Values in (a) are expressed as binding above basal and are normalized with the saturation value at 10 μM noc/OFQ taken as 100%. The data shown are representative of five separate experiments.
Figure 2.
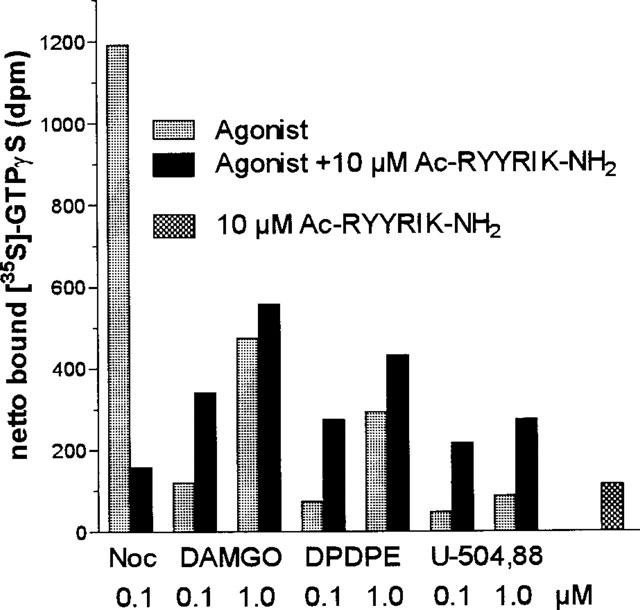
Effect of the hexapeptide Ac-RYYRIK-NH2 on the stimulation of binding of [35S]-GTPγS to rat cortex membranes by noc/OFQ and agonists for the μ-(DAMGO), δ-(DPDPE), and κ-opioid (U-504,88) receptor. Data are expressed as differences between binding in presence and absence of the compounds tested and are representative of three separate experiments.
Figure 3.
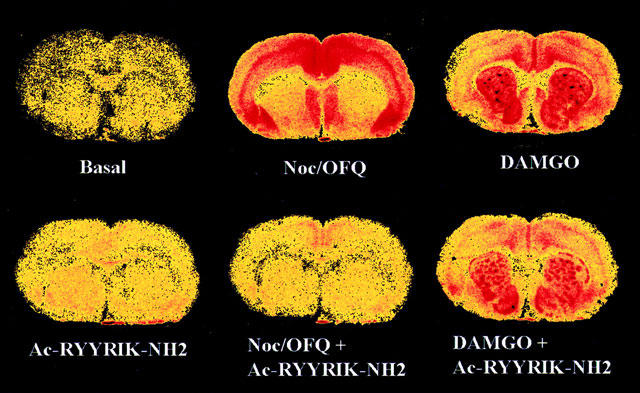
Distributions of basal, 1 μM noc/OFQ- and 5 μM DAMGO-stimulated [35S]-GTPγS binding in absence and presence of 10 μM (noc/OFQ) or 20 μM (DAMGO) hexapeptide Ac-RYYRIK-NH2 in representative sections of rat brain at the level of the caudate putamen. The sections were incubated in Tris buffer/2 mM GDP/100 mM NaCl with 80 pM tracer for 2 h at 25°C.
Figure 4.
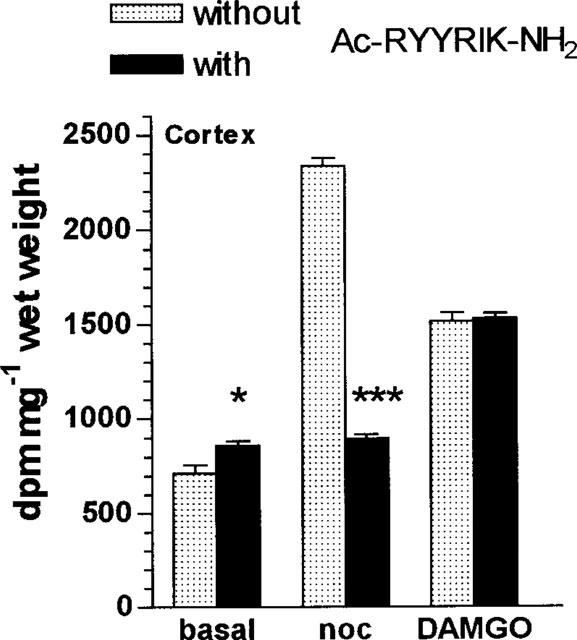
Influence of the hexapeptide Ac-RYYRIK-NH2 on basal, noc/OFQ-, and DAMGO-stimulated [35S]-GTPγS binding to cortex in adjacent coronal sections (six for each sample) obtained from rat brain. Conditions as in Figure 3. *P<0.05 and ***P<0.001 versus samples without Ac-RYYRIK-NH2.
Figure 5.
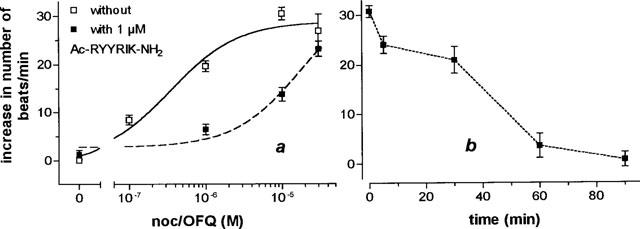
Concentration-response curve for the influence of noc/OFQ on the beating rate of spontaneously beating cardiomyocytes in cultured neonatal rat heart cells in absence and presence of the hexapeptide Ac-RYYRIK-NH2 (a) and kinetics of the inhibiting effect of 10 μM hexapeptide Ac-RYYRIK-NH2 on the beating rate induced by 10 μM noc/OFQ before (b). The basal beating rate was 150±4.5 beats min−1; n=20–38.
Discussion
For the further elucidation of the function of noc/OFQ, specific antagonists will be most helpful. Presently, no centrally acting specific antagonist is available. A truncated analogue of noc/OFQ, [Phe1 Ψ(CH2-NH)Gly2]nociceptin(1-13)-NH2, was found to act as specific competitive antagonist in two peripheral noc/OFQ-sensitive preparations, the electrically stimulated guinea-pig ileum and mouse vas deferens (Guerrini et al., 1998), but not at central sites where it acted as full agonist (Xu et al., 1998). The hexapeptide Ac-RYYRIK-NH2 was earlier identified as partial agonist in the stimulation of GTPγS binding to G proteins by the activated ORL1 receptor when transfected in CHO cells (Dooley et al., 1997). In our study the peptide was found to antagonize the stimulation of GTPγS binding by noc/OFQ, but not by agonists of the classical opioid receptors μ, δ, and κ, in vitro in rat brain preparations (Figures 1, 2, 3 and 4) with high affinity, as seen from the low Schild constant of 6.58 nM. Clearly, the hexapeptide is here a competitive receptor antagonist since it did not depress the maximal effect of noc/OFQ (Figure 1). Furthermore, in competition experiments using 3H-Tyr14-noc/OFQ as labelled ligand (Albrecht et al., 1998), we found that Ac-RYYRIK-NH2 had an affinity for the noc/OFQ receptor in rat brain membranes which was only 3 fold lower when compared with noc/OFQ (data not shown). However, we found the affinity of Ac-RYYRIK-NH2 to be much higher (KD 0.3 nM) than reported for mouse ORL1 transfected into CHO cells (KD 1.5 nM, Dooley et al., 1997). As stimulation of GTP binding to G proteins reflects the activation of G proteins by the receptor, and the coupling of receptor and G proteins can influence receptor affinity, it is suggested that the exact mechanism of this coupling differs between native membrane preparations and cells transfected with the receptor. This may lead to the observed differences in the receptor affinities and in the relations of the intrinsic efficacies of noc/OFQ and Ac-RYYRIK-NH2 between the two systems.
Because effects of peripherally administered noc/OFQ on cardiovascular parameters are becoming increasingly recognized (see review by Meunier, 1997), we tested its action on cultured neonatal rat heart cells and found that it dose-dependently evoked a positive chronotropic response by elevating the beating rate of the cardiomyocytes (Figure 5). The exact mechanism and significance of this activity remains to be elucidated. It seems, however, to be clear, that the chronotropic response is receptor-mediated, since the receptor antagonist Ac-RYYRIK-NH2 inhibited the response (Figure 5).
By specifically antagonizing the activation of G proteins by the noc/OFQ receptor in rat brain membranes, the hexapeptide Ac-RYYRIK-NH2 inhibits the primary events in triggering the biological effects of noc/OFQ. Therefore, this peptide should now provide a starting point for in vivo tests for antagonism of the action of noc/OFQ in the CNS and, considering its antagonistic action on cardiomyocytes, also in other tissues. Furthermore, its short sequence and the fact that it does not contain a free N- and C-terminal make it a convenient starting structure for the further development of highly active and specific antagonists.
Acknowledgments
The authors thank M. Georgi, H. Hans and M. Wegener for technical assistance and Dr M. Beyermann for the synthesis of noc/OFQ.
Abbreviations
- Ac-RYYRIK-NH2
acetyl-RYYRIK-NH2
- DAMGO
[D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin
- DPDPE
[D-Pen2, D-Pen5]-enkephalin
- GTPγS
guanosine 5′-O-(γ-thio)triphosphate
- noc/OFQ
nociceptin/orphanin FQ
- ORL1
opioid receptor-like receptor.
References
- ALBRECHT E., SAMOVILOVA N.N., OSWALD S., BAEGER I., BERGER H. Nociceptin (orphanin FQ): High-affinity and high-capacity binding site coupled to low-potency stimulation of guanylyl-5′-O-(gamma-thio)-triphosphate binding in rat brain membranes. J. Pharmacol. Exp. Ther. 1998;286:896–902. [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- DOOLEY C.T., SPAETH C.G., BERZETEIGURSKE I.P., CRAYMER K., ADAPA I.D., BRANDT S.R., HOUGHTEN R.A., TOLL L. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J. Pharmacol. Exp. Ther. 1997;283:735–741. [PubMed] [Google Scholar]
- GUERRINI R., CALO G., RIZZI A., BIGONI R., BIANCHI C., SALVADORI S., REGOLI D. A new selective antagonist of the nociceptin receptor. Br. J. Pharmacol. 1998;123:163–165. doi: 10.1038/sj.bjp.0701640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNIER J.C. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur. J. Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL(1) receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., CIVELLI O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- ROWE P.M. Excitement over orphanin FQ/nociceptin. Lancet. 1996;347:606. doi: 10.1016/s0140-6736(96)91296-9. [DOI] [PubMed] [Google Scholar]
- SIM L.J., SELLEY D.E., CHILDERS S.R. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[S-35]thio]triphos-phate binding. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR F., DICKENSON A. Nociceptin/orphanin FQ. A new opioid, a new analgesic. Neuroreport. 1998;9:R65–R70. [PubMed] [Google Scholar]
- WALLUKAT G., NEMECZ G., FARKAS T., KUEHN H., WOLLENBERGER A. Modulation of the beta-adrenergic response in cultured rat heart cells. I. Beta-adrenergic supersensitivity is induced by lactate via a phospholipase A2 and 15-lipoxygenase involving pathway. Mol. Cell. Biochem. 1991;102:35–47. doi: 10.1007/BF00232156. [DOI] [PubMed] [Google Scholar]
- XU I.S., WIESENFELDHALLIN Z., XU X.J. [Phe1 Psi(CH2-NH)Gly(2)]-nociceptin-(1-13)NH2, a proposed antagonist of the nociceptin receptor, is a potent and stable agonist in the rat spinal cord. Neurosci. Lett. 1998;249:127–130. doi: 10.1016/s0304-3940(98)00411-x. [DOI] [PubMed] [Google Scholar]


