Abstract
Differences in the acetylcholine (ACh)-induced endothelium-dependent relaxation and hyperpolarization of the mesenteric arteries of Wistar Kyoto rats (WKY) and stroke-prone spontaneously hypertensive rats (SHRSP) were studied.
Relaxation was impaired in preparations from SHRSP and tendency to reverse the relaxation was observed at high concentrations of ACh in these preparations.
Relaxation was partly blocked by NG-nitro-L-arginine (L-NOARG, 100 μM) and, in the presence of L-NOARG, tendency to reverse the relaxation was observed in response to higher concentrations of ACh, even in preparations from WKY. The relaxation remaining in the presence of L-NOARG was also smaller in preparations from SHRSP.
The tendency to reverse the relaxation observed at higher concentrations of ACh in preparations from SHRSP or WKY in the presence of L-NOARG were abolished by indomethacin (10 μM).
Elevating the K+ concentration of the incubation medium decreased relaxation in the presence of both indomethacin and L-NOARG.
Relaxation in the presence of L-NOARG and indomethacin was reduced by the application of both apamin (5 μM) and charybdotoxin (0.1 μM). This suggests that the relaxation induced by ACh is brought about by both endothelium-derived relaxing factor (EDRF, nitric oxide (NO)) and hyperpolarizing factor (EDHF), which activates Ca2+-sensitive K+ channels.
Electrophysiological measurement revealed that ACh induced endothelium-dependent hyperpolarization of the smooth muscle of both preparations in the presence of L-NOARG and indomethacin; the hyperpolarization being smaller in the preparation from SHRSP than that from WKY.
These results suggest that the release of both NO and EDHF is reduced in preparations from SHRSP. In addition, indomethacin-sensitive endothelium-derived contracting factor (EDCF) is released from both preparations; the release being increased in preparations from SHRSP.
Keywords: Mesenteric artery, stroke-prone spontaneously hypertensive rats, nitric oxide, contracting factor, hyperpolarizing factor
Introduction
Vascular contraction is controlled by endothelium-derived factors such as relaxing (EDRF), contracting (EDCF), and hyperpolarizing factors (EDHF) (Pearson & Vanhoutte, 1993). Changes in these factors can be causes of changes in blood pressure. For example, it has been reported that endothelium-dependent relaxation is impaired in the blood vessels of hypertensive rats (Winquist, 1988; Lüscher & Vanhoutte, 1986). Reduced amounts of EDRF, EDHF, or increased amounts of EDCF can impair the relaxation.
In the mesenteric artery, which is thought to be a resistance artery, impaired relaxation has also been observed in the preparation from hypertensive rats (Watt & Thurston, 1989; Jameson et al., 1993; Li & Bukoski, 1993; Li et al., 1994; Takase et al., 1994; Diedrich et al., 1990). This is thought to be brought about by the increased release of EDCF, which is a product of arachidonic acid cascade synthesized via the cyclo-oxygenase pathway, since relaxation can be restored by agents such as indomethacin (Watt & Thurston, 1989; Jameson et al., 1993; Li & Bukoski, 1993; Li et al., 1994) or meclofenamate (Takase et al., 1994; Diedrich et al., 1990), which are known to block that pathway (Mizuno et al., 1982).
Decreased release of EDRF can also be a cause of impaired relaxation as described above, and as suggested in the aorta of stroke-prone spontaneously hypertensive rats (SHRSP) (Sunano et al., 1992). The EDRF in the aorta is known to be nitric oxide (NO), and synthesis of NO can be blocked by agents such as NG-nitro-L-arginine (L-NOARG) (Moore et al., 1990) and NG-methyl-L-arginine (L-NMMA) (Palmer et al., 1988). In the mesenteric artery, however, acetylcholine can still induce relaxation in the presence of these agents, indicating the involvement of a factor(s) other than NO. One of the factors that can induce the relaxation of the mesenteric artery is EDHF (Nagao et al., 1992; Parsons et al., 1994; Hwa et al., 1994; Waldron & Garland, 1994).
In the present experiments, differences in the effects of L-NOARG and indomethacin on endothelium-dependent relaxation between mesenteric arteries from Wistar Kyoto rats (WKY) and SHRSP were studied. In addition, the involvement of EDHF in relaxation and its changes in the preparation from SHRSP were also studied. SHRSP were used, as endothelium-dependent relaxation is more prominently impaired than in conventional spontaneously hypertensive rats (SHR) (Sunano et al., 1989), and WKY were used as the control normotensive rats, since SHRSP were originally established from WKY (Okamoto et al., 1974).
Methods
Sixteen-week-old SHRSP and age matched WKY were used in these experiments. They were obtained from Dr Okamoto (Okamoto et al., 1974) and bred successively in our animal facility. They were fed a normal chow (Funabashi SP) and tap water was given freely. Room temperature was kept at 22°C, with 60% humidity, and a 12 h light-and-dark cycle.
The blood pressure of the rats was measured by means of the tail cuff method. Prior to measurement, the rats were warmed in a cage kept at 40°C for 10 min. This procedure was required to obtain constant and stable blood pressure values.
The rats were killed by bleeding from vena cava after anaesthetizing with CO2. The mesenterium including the superior mesenteric artery and its branches were excised and immediately immersed in a modified Tyrode's solution. The composition of the modified Tyrode's solution was as follows (mM): NaCl, 137; KCl, 5.4; CaCl2, 2.0; MgCl2, 1.0; NaHCO3, 11.9; NaH2PO4, 0.4; glucose, 5.6; which was equilibrated with a gas mixture of 95% O2 and 5% CO2 at 37°C. The pH of the solution under these conditions was 7.3. K+-Tyrode's solution was made by replacing all NaCl in the modified Tyrode's solution with KCl, and the high-K+ Tyrode's solution containing the desired concentration of K+ was made by mixing the Tyrode's and K+-Tyrode's solutions.
Ring preparations of 1.5 mm in width were made from the second branches of the superior mesenteric artery, and they were kept in the modified Tyrode's solution. In six preparations respectively from WKY and SHRSP, the endothelium was damaged by perfusing the vessels with the modified Tyrode's solution containing 0.3% 3-[(3-Cholamidopropyl) dimethylammonio]-1-propane-sulphonate (CHAPS) for 2.5 min. The rings were mounted in an organ bath (MOB-1, Technical Supply, Osaka) under a stretch tension of 1 mN, and tension changes were measured isometrically with a force-displacement transducer (Shinkoh, Nagano, Japan). The temperature of the apparatus was kept at 37°C by circulating water of the same temperature.
The preparations were equilibrated in the modified Tyrode's solution for at least 60 min, and then they were subjected twice to high K+-induced contractions by changing the solution from the modified to the high-K+ solution containing 80 mM K+ for 5 min with an interval of 15 min in between. Endothelium-dependent relaxation was induced by applying acetylcholine (ACh) cumulatively to preparations pre-contracted with 5 μM noradrenaline (NA). We ascertained that this concentration of NA induced submaximal contraction of the preparations and that maximum contraction was achieved at a concentration of 10 μM. Synthesis of NO and the products of the cyclo-oxygenase pathway of the arachidonic acid cascade were blocked with L-NOARG (100 μM) and indomethacin (10 μM), respectively. The involvement of EDHF in relaxation was investigated by increasing the K+ concentration in the modified Tyrode's solution or by applying tetraethylammonium (TEA), glibenclamide, apamin or charybdotoxin. At the end of the experiments, the preparations were completely relaxed by applying verapamil (10 μM) and papaverine (100 μM), and all tensions were measured in respect to this relaxed level.
Changes in membrane potential were measured by means of microelectrode technique. In this method, glass microelectrodes which had tip resistance of 40–80 MΩ when filled with 3 M KCl were used. Mesenteric artery was mounted on organ bath of 1 ml volume with insect pins. The preparation was continuously superfused with Tyrode's solution at flow rate of 4 ml min−1. Microelectrode was inserted into smooth muscle from outer surface of the preparation.
The drugs used in this experiment were: 3-[(3-Cholamidopropyl) dimethylammonio]-1-propane-sulphate (CHAPS, Sigma, St Louis, MO, U.S.A.), noradrenaline bitartrate (NA, Sigma, St Louis, MO, U.S.A.), sodium nitroprusside (SNP, Sigma, St Louis, MO, U.S.A.), acetylcholine hydrochloride (ACh, Wako, Osaka, Japan), NG-nitro-L-arginine (Sigma, St Louis MO, U.S.A.), indomethacin (Sigma, St Louis MO, U.S.A.), SQ 29,548 (Cayman Chemical, MI, U.S.A.), tetraethylammonium (TEA, Wako, Osaka, Japan), glibenclamide (Sigma, St Louis, MO, U.S.A.), apamin (Sigma, St Louis, MO, U.S.A.), charybdotoxin (Peptid Inst., Osaka, Japan), verapamil (Wako, Osaka, Japan) and papaverine (Wako, Osaka, Japan).
The values obtained were expressed as the means±s.e.mean. These values were analysed by the Student's t-test and Newman-Keul's multiple comparison test, where P values of less than 0.05 were considered to be significant.
Results
Body weight and systolic blood pressure of the rats
Body weights of SHRSP and WKY at 16 weeks of age were 309±4.9 g (n=30) and 395±4.6 g (n=30), respectively, the former being significantly smaller (P<0.01). The systolic blood pressures of SHRSP and WKY were 245±3.5 mmHg (n=30) and 135±1.1 mmHg (n=30), respectively. The systolic blood pressure of the former was significantly higher than those of the latter (P<0.01).
Relaxation of mesenteric arteries induced by sodium nitroprussid (SNP)
SNP induced relaxation of NA (5 μM)-precontracted endothelium-removed preparations both from WKY and SHRSP. The concentration-response curve for the relaxation was almost identical in both preparations; the maximal relaxation observed at 1 mM SNP was 97±1.5 and 98±1.2% of precontraction in preparations from WKY and SHRSP, respectively (data was not shown).
Relaxation induced by ACh
The amplitudes of the precontraction induced by 5 μM NA in the preparations from WKY and SHRSP were 87.2±6.6% (n=10) and 90.6±4.8% (n=10) of 80 mM K+-induced contraction, respectively; the difference between these values were not significant. The application of ACh to preparations precontracted with 5 μM NA, caused a concentration-dependent relaxing response (Figure 1). In preparations from WKY, the concentration-dependent relaxing response was observed in response to ACh concentrations of up to 1 mM. The maximal relaxation (91.8±1.0% of the pre-contracted value (n=12)) was observed at a concentration of 0.1 μM, and then gradually increased up to 98.3±0.6% (n=12) at 1 mM. In preparations from SHRSP, only low to moderate concentrations of ACh induced relaxation, and tendency to reverse the relaxation appeared as the concentrations of the drug were increased further. Thus, maximal relaxation (65.8±7.7% of the pre-contracted value (n=8)) was observed at an ACh concentration of 0.1 μM. The relaxation by ACh of concentrations higher than 3 nM were significantly smaller in the preparation from SHRSP than that from WKY (P<0.05).
Figure 1.
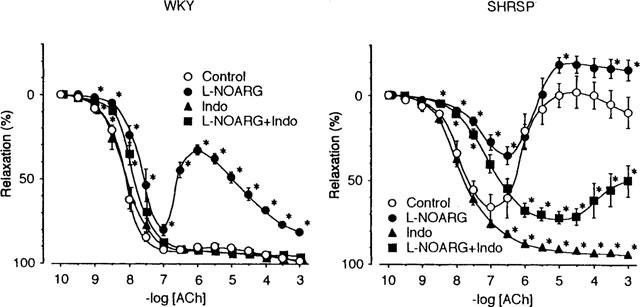
The concentration-response curves for acetylcholine (ACh)-induced relaxation and effects of L-NOARG and indomethacin in mesenteric arteries from WKY and SHRSP. WKY and SHRSP indicate the preparations from WKY and SHRSP, respectively. The preparations were pre-contracted in the presence of 5 μM of noradrenaline (NA), and then ACh was added cumulatively. The relaxation induced by ACh was expressed as a percentage of the NA-induced pre-contraction. Data are means±s.e.mean from 8–12 preparations. Control, L-NOARG, Indo and L-NOARG+Indo indicate the ACh concentration-response curves in the absence of, in the presence of L-NOARG (100 μM), indomethacin (10 μM) and both, respectively. Asterisks indicate significant differences from respective Control values (*P<0.05).
In CHAPS-treated preparations from both WKY and SHRSP, relaxation induced by ACh was markedly reduced, and only 20.2±5.4% (n=6) relaxation was observed even in preparations from WKY.
Effects of L-NOARG and methylene blue on the action of ACh
L-NOARG (100 μM) showed prominent effect on the relaxation of preparations from WKY, especially by ACh at concentrations higher than 0.1 μM (Figure 1, WKY). Relaxation was attenuated, and the preparations tended to contract at higher concentrations of ACh, similarly to that observed in preparations from SHRSP in the absence of L-NOARG. Relaxation at lower concentrations of ACh remained, although its amplitude was reduced. In preparations from SHRSP, L-NOARG attenuated ACh-induced relaxation and the tendency to contract in response to higher concentrations of ACh was augmented (Figure 1, SHRSP). However, the relaxation induced by lower concentrations of ACh still remained, although its magnitude was reduced.
Methylene blue (10 μM) had similar effects to L-NOARG in preparations from both WKY and SHRSP (data was not shown).
Effects of indomethacin and SQ 29,548 on the action of ACh
The ACh-induced relaxation of mesenteric arteries was augmented by indomethacin at a concentration of 10 μM. Augmentation was prominent in preparations from SHRSP, and the tendency to reverse the relaxation at higher concentrations of ACh disappeared (Figure 1, SHRSP). Augmentation of the relaxation induced by indomethacin was not observed in preparations from WKY (Figure 1, WKY). Thus, differences in the responses of preparations from WKY and SHRSP were minimized in the presence of indomethacin.
The effects of indomethacin on preparations from SHRSP were similar to the effects of the drug on preparations from WKY in the presence of L-NOARG (Figure 1). Reduced relaxation of the preparation from WKY in the presence of L-NOARG was recovered, and the tendency to reverse the relaxation observed at higher concentrations of ACh was abolished by the addition of indomethacin (10 μM). In preparations from SHRSP, reduced relaxation in the presence of L-NOARG was also restored, and the tendency to contract was abolished by the addition of indomethacin (Figure 1, SHRSP). Thus, only the relaxation was observed in response to ACh in the presence of both L-NOARG and indomethacin. When the relaxation induced by ACh 1 mM in the presence of L-NOARG and indomethacin were compared between preparations from WKY and SHRSP, they were significantly smaller in preparations from the latter (96.2±1.1% (n=12) vs 50.0±9.3% (n=12), P<0.01).
Similarly, SQ 29,548 (100 μM) augmented the relaxation induced by ACh and minimized the tendency to reverse the relaxation in preparations from SHRSP, and attenuated them in both preparations in the presence of L-NOARG, although the effect was less prominent than that of indomethacin (Figure 2).
Figure 2.
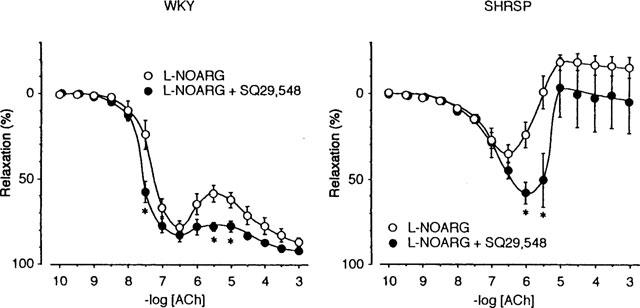
Effect of SQ 29,548 on the relaxation induced by acetylcholine (ACh) in the presence of L-NOARG. ACh was applied in the presence of 100 μM L-NOARG (○) or 100 μM L-NOARG and 100 μM SQ 29,548 (•). Data are means±s.e.mean from 12 preparations. The others are the same as those in Figure 1. Note that SQ 29,548 augmented the relaxation and minimized the tendency to reverse the relaxation observed at high concentrations of ACh.
Influence of increasing K+ concentration and TEA
This experiment was performed in the presence of L-NOARG and indomethacin. As shown in Figure 3, relaxation induced by ACh was attenuated by increasing K+ concentration in the incubation medium in both preparations. Similar but weaker effects were observed with 10 mM TEA. In preparations from SHRSP, the effect of this concentration of TEA was similar to that of increasing K+ concentration to 10 mM. In the preparation from WKY, the amplitude of the maximum relaxation was not altered, although the concentration of ACh required for relaxation increased (data was not shown). Experiments with higher concentrations of TEA could not be performed because of the contractile effects of the drug.
Figure 3.
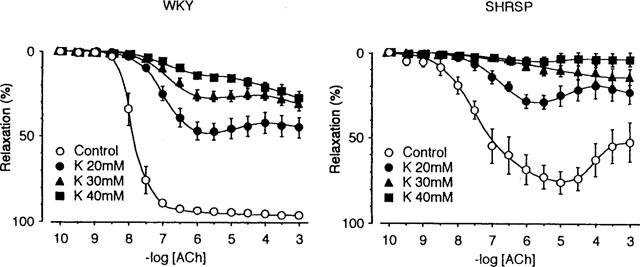
The effect of increasing the K+ concentration on the response to acetylcholine (ACh). These experiments were performed in the presence of 100 μM L-NOARG and 10 μM indomethacin so that the involvement of nitric oxide and the cyclo-oxygenase pathway of the arachidonic acid cascade could be excluded. The K+ concentration in the modified Tyrode's solution was increased to 20 mM (•), 30 mM (▴) and to 40 mM (▪). The noradrenaline concentration was 5 μM in the control, 3 μM in the K+ 20 mM, 2 μM in the K+ 30 mM, and 1 μM in the K+ 40 mM Tyrode's solution was used to adjust the contraction amplitude. Data are means±s.e.mean from 12 preparations. Relaxations of ACh of the concentrations higher than 10 nM were significantly smaller in the presence of elevated K+ than control (5.4 mM K+). The others are the same as those in Figure 1.
Effects of glibenclamide, apamin and charybdotoxin
Glibenclamide (10 μM) did not significantly affect relaxation induced by ACh in the presence of L-NOARG and indomethacin (data was not shown).
Apamin, up to 5 μM, had no effect on ACh-induced relaxation in the presence of L-NOARG and indomethacin in preparations from WKY (Figure 4, WKY), while it attenuated relaxation and enhanced the tendency to reverse the relaxation at high concentrations of ACh in preparations from SHRSP (Figure 4, SHRSP). Charybdotoxin (ChTX 0.1 μM) shifted ACh concentration-response curve in the presence of L-NOARG and indomethacin to the right but did not alter the maximum relaxation in the preparation from WKY (Figure 4, WKY). The effect of ChTX on the preparation from SHRSP was basically similar to that of apamin and it was stronger than that of apamin (Figure 4, SHRSP). The simultaneous application of apamin and ChTX at the concentrations described above, however, attenuated relaxation markedly in preparations from WKY, and almost blocked relaxation in preparations from SHRSP (Figure 4).
Figure 4.
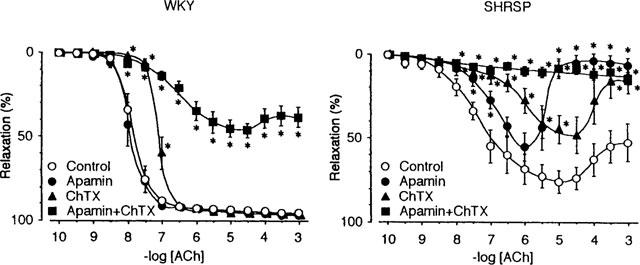
Concentration-response curves for acetylcholine (ACh) in the presence of L-NOARG and indomethacin, and the effects of apamin and charybdotoxin. Control indicates the relaxation curve in the presence of L-NOARG (100 μM) and indomethacin (10 μM). Apamin, ChTX and Apamin+ChTX indicate curves in the presence of apamin (5 μM), charybdotoxin (0.1 μM) and both, respectively in addition to L-NOARG and indomethacin. Data are means±s.e.mean of 12 preparations. Asterisks indicate significant difference between control value and values in the presence of respective drug or drugs (*P<0.05). The others are the same as in Figure 1.
ACh-induced hyperpolarization of smooth muscle
The resting membrane potentials of the smooth muscle of the mesenteric arteries of WKY and SHRSP were −63±1.3 mV (n=72) and −59±0.8 mV (n=84), respectively. The membrane potential of the latter was significantly smaller (P<0.01). ACh 1 μM hyperpolarized the membrane by 13.9±1.5 mV (n=11) and 8.0±1.1 mV (n=9) in the arteries of WKY and SHRSP, respectively. The amplitude of hyperpolarization was smaller in preparations from SHRSP (P<0.01).
Changes of membrane potential were observed also in the presence of NA (5 μM), L-NOARG (100 μM) and indomethacin (10 μM); the condition was the same that the relaxation by hyperpolarization was studied. Under this condition, the membrane potential was −61±1.0 mV (n=23) and −42±0.6 mV (n=43), respectively in preparations from WKY and SHRSP. The difference in the membrane potential was also significant (P<0.01). Application of 1 μM ACh under the same condition caused hyperpolarization of the smooth muscle membrane in both preparations (Figure 5). The hyperpolarization induced by the application of ACh (1 μM) to preparations from WKY and SHRSP was 18±2.1 mV (n=6) and 12±1.3 mV (n=11), respectively; the difference between these values being significant (P<0.01). It has been ascertained in concentration-response experiment that ACh of 1 μM induced maximal hyperpolarization in smooth muscle of the mesenteric artery both from WKY and SHRSP.
Figure 5.
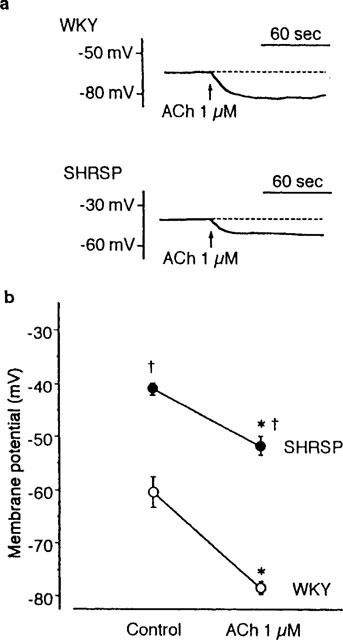
Effect of acetylcholine (ACh) on the membrane potential of the smooth muscle of the rat mesenteric artery. Changes in the membrane potential were measured in the presence of noradrenaline (5 μM), L-NOARG (100 μM) and indomethacin (10 μM). (a) A typical membrane potential recording induced by 1 μM ACh in preparations from WKY and SHRSP. (b) The mean membrane potentials in the preparations from WKY and SHRSP in the absence and presence of ACh. The number of preparations was 23 and 43, respectively for WKY and SHRSP in the absence of ACh (Control), and 6 and 11, respectively for WKY and SHRSP in the presence of ACh (ACh 1 μM). Asterisks indicate significant difference from respective control value (P<0.01) and daggers indicate significant difference from the value of WKY (P<0.01). Note the smaller hyperpolarization induced by ACh in the preparations from SHRSP.
Discussion
In the mesenteric arteries of SHR, ACh-induced relaxation is known to be impaired (Watt & Thurston, 1989; Jameson et al., 1993; Li & Bukoski, 1993; Takase et al., 1994; Fujii et al., 1992) as in other blood vessels (see Lüscher & Vanhoutte, 1986; Winquist, 1988). A similar result has been reported in the mesenteric artery of SHRSP (Diedrich et al., 1990), as we confirmed in this study. The impaired relaxation is associated with a contractile response, especially at high concentrations of ACh (Diedrich et al., 1990). It has been shown that impaired relaxation of mesenteric artery of SHR and SHRSP is improved by treatment with indomethacin or meclofenamate, which are known to block the cyclo-oxygenase pathway of the arachidonic acid cascade (Watt & Thurston, 1989; Jameson et al., 1993; Li & Bukoski, 1993; Takase et al., 1994; Diedrich et al., 1990; Fujii et al., 1992). We confirmed this in this study. Thus, impaired endothelium-dependent relaxation in the mesenteric artery can be explained by the co-release of EDCF which is thought to be a product of the arachidonic acid cascade via the cyclo-oxygenase pathway (Mizuno et al., 1982). This EDCF would be thromboxane A2 and/or prostaglandin H2 as in other blood vessels of SHRSP (Auch-Schwelk et al., 1990; Ito et al., 1991; Mayhan, 1992), since SQ29,548, a blocker of thromboxane A2 and prostaglandin H2 receptors, augmented the maximal relaxation.
However, reduced NO production has also been reported in cultured aortic endothelial cells of SHRSP (Malinski et al., 1993; Grunfeld et al., 1995). Grunfeld et al. (1995) explained that the reduction is brought about by scavenging NO by excessively produced superoxide anion. In the present experiment with mesenteric artery, it was indicated that the release of NO was also reduced in the preparation from SHRSP, since the effect of L-NOARG was smaller in this preparation when compared with that in the preparation from WKY. The inhibition of endothelium-dependent relaxation via the inhibition of NO synthesis agrees with result from previous reports (Li et al., 1994; Fujii et al., 1992), but differs from the findings of Li & Bukoski (1993) who showed that L-NOARG had no effect on the endothelium-dependent relaxation induced by ACh in the mesenteric artery of WKY, although they observed also that ACh-induced relaxation was attenuated in the mesenteric artery of SHR. The cause of this discrepancy is uncertain, but our results indicate that a reduction in NO synthesis, even in preparations from WKY, impairs of endothelium-dependent relaxation in a fashion similar to that in preparations from SHRSP. The tendency to reverse the relaxation appeared in the preparation from WKY in the presence of L-NOARG, may indicate some interaction between NO and EDCF as has been suggested by Auch-Schwelk et al. (1992). The effect of methylene blue, which showed a similar effect as L-NOARG on the ACh-induced relaxation, may be explained mainly by the inhibition of cyclic GMP production in the smooth muscle, although inhibition of NO synthesis (Mayer et al., 1993) or production of oxygen-derived free radicals which inactivates NO (Wolin et al., 1990) may also be involved.
We also showed that the relaxation induced by ACh was inhibited only partially by L-NOARG. The inability of L-NOARG to block the endothelium-dependent relaxation induced by ACh has also been reported in the mesenteric artery of the rat (Nagao et al., 1992; Parsons et al., 1994). This indicates that a factor other than NO is also involved in the relaxation (Li et al., 1994). One factor which may be involved in the relaxation, especially in the presence of L-NOARG, is EDHF (Fujii et al., 1992; McPherson & Angus, 1991; Chen & Suzuki, 1989; Chen et al., 1988; Garland & McPherson, 1992; Fujii et al., 1993; Waldron & Garland, 1994). We showed in the present experiment that ACh induced hyperpolarization of the smooth muscle membranes of the mesenteric arteries of both WKY and SHRSP in the presence of noradrenaline, L-NOARG and indomethacin. It has been known that NO does not cause hyperpolarization of the membranes of the mesenteric artery in the presence of noradrenaline (Garland & McPherson, 1992). Moreover, the hyperpolarization induced by ACh has been reported not to be blocked by methylene blue or L-NOARG (Fujii et al., 1992; Garland & McPherson, 1992), indicating the involvement of an EDHF other than NO in ACh-induced hyperpolarization. Thus, we concluded that an EDHF other than NO is involved in the ACh-induced relaxation of the mesenteric arteries of WKY and SHRSP.
In our experiments, the relaxation induced by ACh in the presence of L-NOARG and indomethacin was markedly attenuated by TEA or by increasing the K+ concentration in the incubation medium. These results are similar to those from preparations from SHR (Li et al., 1994; Fujii et al., 1993), and suggest that the relaxation remaining in the presence of L-NOARG is caused by hyperpolarization of the smooth muscle membrane due to an increased K+ conductance. However, the possible blockage of EDHF release caused by inhibiting the hyperpolarization of the endothelial cell membrane can not be ruled out (Demirel et al., 1994).
Li et al., (1994) reported that EDHF is involved in the ACh-induced relaxation of the resistance arteries of WKY but not SHR. A similar conclusion was made by Adeagbo & Triggle (1993) in the perfused mesenteric artery. In this study we showed that relaxation induced by K+ channel activation due to the release of EDHF is impaired in the mesenteric artery of SHRSP. Similar results have recently been reported in endothelium-dependent relaxation in SHR (Kahonen et al., 1995). This may be caused by the impaired release of EDHF, as has been proposed in the mesenteric artery of SHR (Fujii et al., 1992). Mechanism of the marked difference in membrane potential in mesenteric artery of SHRSP in the presence of NA under condition treated with L-NOARG and indomethacin would be explained by the smaller resting membrane potential (Fujii et al., 1992) and higher sensitivity to NA (Kuriyama & Suzuki, 1978). The hyperpolarization by ACh was much still smaller in preparations from SHRSP than in those from WKY, regardless the membrane potential much far from K+ equivalent potential. In addition, it has been reported that the hyperpolarization by K+ channel opener was not different in the mesenteric arteries from WKY and SHR (Fujii et al., 1992), indicating that K+ channel itself was not different between these preparations. Thus, the reduction of the release of EDHF in the mesenteric artery of SHRSP was also supported by membrane potential recording.
The hyperpolarization of smooth muscle membranes that causes relaxation may be brought about by activating both ATP-activated (K+ATP) and/or Ca-activated K+ channels (K+Ca). Hyperpolarization due to the former has been shown in a number of blood vessels (Standen et al., 1989). In contrast, it was shown in the present experiment with rat mesenteric arteries that relaxation was blocked in the presence of apamin and ChTX, both of which are known to block K+Ca, but not by glibenclamide, which blocks K+ATP. It has recently been shown that ACh-induced hyperpolarization of small arterial smooth muscle results from activation of both ChTX-sensitive and apamin-sensitive K+ channels (Hashitani & Suzuki, 1997). Thus, it can be concluded that the relaxation in the presence of both L-NOARG and indomethacin in WKY and SHRSP mesenteric arteries is brought about by hyperpolarization due to activation of both the small and intermediate K+Ca conductance in these preparations, although possibility of the involvement of voltage-dependent K+ channel (Zygmunt et al., 1997) remained to be investigated. The proportion of the involvement of these channels may differ between smooth muscles from WKY and SHRSP, since the effect of apamin and ChTX affected the relaxation differently.
In conclusion, endothelium-dependent relaxation in the mesenteric artery of SHRSP was impaired when compared with that of WKY. All of decreased release of NO and EDHF, and the increased release of indomethacin-sensitive EDCF are involved in impairing relaxation. Present data suggests that the reduced release of NO can potentiate the release or the actions of EDCF, which impairs relaxation. It was also suggested that EDHF exerts is action through the activation of Ca2+-sensitive K+ channel.
References
- ADEAGBO A.S.O., TRIGGLE C.R. Varying extracellular [K+]: A functional approach to separating EDHF and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J. Cardiovasc. Pharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- AUCH-SCHWELK W., KATUSIC Z.S., VANHOUTTE P.M. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contraction. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- AUCH-SCHWELK W., KATUSIC Z.S., VANHOUTTE P.M. Nitric oxide inactivates endothelium derived contracting factor in the rat aorta. Hypertension. 1992;19:442–445. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle. J. Physiol. 1989;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H., WESTON A.H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br. J. Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMIREL E., RUSKO J., LASKEY R.E., ADAMS D.J., VAN BREEMEN C. TEA inhibits ACh-induced EDRF release: Endothelial Ca2+-dependent K+ channels contribute to vascular tone. Am. J. Physiol. 1994;267:H1135–H1141. doi: 10.1152/ajpheart.1994.267.3.H1135. [DOI] [PubMed] [Google Scholar]
- DIEDRICH D., YANG Z., BUHLER F.R., LUSCHER T.F. Impaired endothelium-dependent relaxations in hypertensive resistance arteries involve cyclooxygenase pathway. Am. J. Physiol. 1990;258:H445–H451. doi: 10.1152/ajpheart.1990.258.2.H445. [DOI] [PubMed] [Google Scholar]
- FUJII K., OHMORI S., TOMINAGA M., ABE I., TAKATA Y., OHYA Y., KOBAYASHI K., FUJISHIMA M. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am. J. Physiol. 1993;265:H509–H516. doi: 10.1152/ajpheart.1993.265.2.H509. [DOI] [PubMed] [Google Scholar]
- FUJII K., TOMINAGA M., OHMORI S., KOBAYASHI K., KOGA T., TAKATA Y., FUJISHIMA M. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circulation Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., McPHERSON G.A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat mesenteric artery. Br. J. Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNFELD S., HAMILTON C.A., MESAROS S., McCLAIN S.W., DOMINICZAK A.F., BOHR D.F., MALINSKI T. Role of superoxide in the depressed nitric oxide production by the endothelium of genetically hypertensive rats. Hypertension. 1995;26:854–857. doi: 10.1161/01.hyp.26.6.854. [DOI] [PubMed] [Google Scholar]
- HASHITANI H., SUZUKI H. K+ channels which contribute to the acetylcholine-induced hyperpolarization in smooth muscle of the guinea-pig submucosal arteriole. J. Physiol. 1997;501:319–329. doi: 10.1111/j.1469-7793.1997.319bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWA J., GHIBAUDI L., WILLIAMS P., CHATTERJEE M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am. J. Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- ITO T., KATO T., IWAMA Y., MURAMATSU M., SHIMIZU K., ASANO H., OKUMURA K., HASHIMOTO H., SATAKE T. Prostaglandin H2 as an endothelium-derived contracting factor and its interaction with endothelium-derived nitric oxide. J. Hypertension. 1991;9:729–736. doi: 10.1097/00004872-199108000-00006. [DOI] [PubMed] [Google Scholar]
- JAMESON M., DAI F.-U., LUSCHER T., SKOPEC J., DIEDRICH A., DIEDRICH D. Endothelium-derived contracting factors in resistance arteries of young spontaneously hypertensive rats before development of overt hypertension. Hypertension. 1993;21:280–288. doi: 10.1161/01.hyp.21.3.280. [DOI] [PubMed] [Google Scholar]
- KAHONEN M., MAKYNEN H., WU X., ARVOLA P., PORSTI I. Endothelial function in spontaneously hypertensive rats: influence of quinapril treatment. Br. J. Pharmacol. 1995;115:859–867. doi: 10.1111/j.1476-5381.1995.tb15012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H., SUZUKI H. Electrical property and chemical sensitivity of vascular smooth muscles in normatensive and spontaneously hypertensive rats. J. Physiol. 1978;285:409–424. doi: 10.1113/jphysiol.1978.sp012579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J., BIAN K., BUKOSKI R.D. A non-cyclo-oxygenase, non-nitric oxide relaxing factor is present in resistance arteries of normotensive but not spontaneously hypertensive rats. Am. J. Med. Sci. 1994;307:7–14. doi: 10.1097/00000441-199401000-00002. [DOI] [PubMed] [Google Scholar]
- LI J., BUKOSKI R.D. Endothelium-dependent relaxation of hypertensive resistance arteries is not impaired under all conditions. Circulation Res. 1993;72:290–296. doi: 10.1161/01.res.72.2.290. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F., VANHOUTTE P.M. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rats. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- MALINSKI T., KAPTURCZAK M., DAYHARSH J., BOHR D. Nitric oxide synthase activity in genetic hypertension. Biochem. Biophys. Res. Commun. 1993;194:654–658. doi: 10.1006/bbrc.1993.1871. [DOI] [PubMed] [Google Scholar]
- MAYER B., BRUNNER F., SCHMIDT K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- MAYHAN W.G. Role of prostaglandin H2-thromboxane A2 in response of arterioles during chronic hypertension. Am. J. Physiol. 1992;262:H539–H543. doi: 10.1152/ajpheart.1992.262.2.H539. [DOI] [PubMed] [Google Scholar]
- McPHERSON G.A., ANGUS J.A. Evidence that acetylcholine-mediated hyper-polarization of the rat mesenteric artery does not involve the K+ channel opened by cromakalim. Br. J. Pharmacol. 1991;103:1184–1190. doi: 10.1111/j.1476-5381.1991.tb12321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZUNO K., YAMAMOTO S., LANDS W.E.M. Effects of non-steroidal anti-inflammatory drugs on fatty acid cyclooxygenase and prostaglandin hydroperoxidase activities. Prostaglandins. 1982;23:743–757. [PubMed] [Google Scholar]
- MOORE P.K., AL-SWAYEH O.A., CHONG N.W.S., EVANS R.A., GIBSON A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br. J. Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAO T., ILLIANO S., VANHOUTTE P.M. Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-L-arginine in rats. Am. J. Physiol. 1992;263:H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., YAMORI Y., NAGAOKA A. Establishment of the stroke-prone spontaneously hypertensive rat (SHR) Circulation Res. 1974;34–35 Suppl:143–153. [Google Scholar]
- PALMER R.M.J., REES D.D., ASHTON D.S., MOMCADA S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem. Biophys. Res. Commun. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- PARSONS S.J.W., HILL A., WALDRON G.J., PLANE F., GARLAND C.J. The relative importance of nitric oxide and nitric oxide-independent mechanisms in acetylcholine-evoked dilatation of the rat mesenteric bed. Br. J. Physiol. 1994;113:1275–1280. doi: 10.1111/j.1476-5381.1994.tb17136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON P., VANHOUTTE P.M. Vasodilator and vasoconstrictor substances produced by the endothelium. Rev. Physiol. Biochem. Pharmacol. 1993;122:1–67. doi: 10.1007/BFb0035273. [DOI] [PubMed] [Google Scholar]
- STANDEN N.B., QUAYLE J.M., DAVIS N.W., BRAYDEN J.E., HUANG Y., NELSON T.M. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- SUNANO S., OSUGI S., KANEKO K., YAMAMOTO K., SHIMAMURA K. Effects of chronic treatment with SQ 29852 on spontaneous smooth muscle tone and endothelium-dependent relaxation in aorta of stroke- prone spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1992;19:602–609. doi: 10.1097/00005344-199204000-00018. [DOI] [PubMed] [Google Scholar]
- SUNANO S., OSUGI S., SHIMAMURA K. Blood pressure and impairment of endothelium-dependent relaxation in spontaneously hypertensive rats. Experientia. 1989;45:705–708. doi: 10.1007/BF01974563. [DOI] [PubMed] [Google Scholar]
- TAKASE H., DOHI Y., KOJIMA M., SATO K. Changes in the endothelial cyclooxygenase pathway in resistance arteries of spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1994;23:329–330. [PubMed] [Google Scholar]
- WALDRON G.J., GARLAND C.J. Contribution of both nitric oxide and change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br. J. Pharmacol. 1994;112:831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATT P.A.C., THURSTON H. Endothelium-dependent relaxation in resistance vessels from the spontaneously hypertensive rats. J. Hypertension. 1989;7:661–666. doi: 10.1097/00004872-198908000-00010. [DOI] [PubMed] [Google Scholar]
- WINQUIST R.J.Endothelium-dependent relaxations in hypertensive blood vessels Relaxing and contracting factors 1988Clifton, NJ: Humana Press; 473–494.ed. Vanhoutte, P.M. pp [Google Scholar]
- WOLIN M.S., CHERRY P.D., RODENBURG J.M., MESSINA E.J., KALEY G. Methylene blue inhibits vasodilation of skeletal muscle arterioles to acetylcholine and nitric oxide via the extracellular generation of superoxide anion. J. Pharmacol. Exp. Ther. 1990;254:872–876. [PubMed] [Google Scholar]
- ZYGMUNT P.M., EDWARDS G., WESTON A.H., LARSSON B., HOGESTATT E.D. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of rat hepatic artery. Br. J. Pharmacol. 1997;121:141–149. doi: 10.1038/sj.bjp.0701108. [DOI] [PMC free article] [PubMed] [Google Scholar]


