Abstract
Resveratrol, naringenin and naringin are naturally occurring flavonoids in grapes and grapefruits. The anti-inflammatory effects of these flavonoids have been well documented, but the mechanism is poorly characterized. High concentration of NO are produced by inducible NO synthase (iNOS) in inflammation, and the prevention of the expression of iNOS may be an important anti-inflammatory mechanism. In this study, the effects of these flavonoids on the induction of NO synthase (NOS) in RAW 264.7 cells activated with bacterial lipopolysaccharide (LPS, 50 ng ml−1) were investigated.
Resveratrol was found strongly to inhibit NO generation in activated macrophages, as measured by the amount of nitrite released into the culture medium, and resveratrol strongly reduced the amount of cytosolic iNOS protein and steady state mRNA levels. However, the inhibitory abilities of naringenin were lower, and the inhibitory abilities of naringin were almost negligible.
In electrophoretic mobility shift assays, the activation of NFκB induced by LPS for 1 h was inhibited by resveratrol (30 μM). Furthermore, in immunoblotting analysis, cells treated with LPS plus resveratrol showed an inhibition of phosphorylation as well as degradation of IκBα, and a reduced nuclear content of NFκB subunits.
The flavonoids may be of value for inhibiting the enhanced expression of iNOS in inflammation through down-regulation of NFκB binding activity.
Keywords: Resveratrol, flavonoids, inducible NO synthase, NFκB, RAW 264.7 monocyte/macrophages
Introduction
Nitric oxide (NO) has a role in mediating macrophage cytotoxicity, regulating blood pressure, and in neurotransmission. NO is synthesized in vivo from L-arginine by nitric oxide synthase (NOS) with NADPH and oxygen as substrates. Molecular cloning and sequencing analysis has revealed that there are at least three main types of NOS isoforms. Two Ca2+/calmodulin-dependent isoforms are constitutively expressed in the endothelium of blood vessel and the neuron of the brain. These isoforms synthesize small amounts of NO in response to various agonists that increase intracellular Ca2+. The high output isoform, inducible-NOS (iNOS), is expressed in various cell types following its transcriptional induction (Nathan & Xie, 1994). Among the most important stimuli for induction of iNOS is bacterial endotoxic lipopolysaccharide (LPS) (Stuehr & Marletta, 1985; Ding et al., 1988). Low concentrations of NO produced by iNOS are likely to contribute much of the antimicrobial activity of macrophages against certain bacterial pathogens. However, high concentrations of NO and its derivatives, such as peroxynitrite and nitrogen dioxide, are found to play important roles in inflammation and in the multistage processes of carcinogenesis (Ohshima & Bartsch, 1994; Halliwell, 1994).
The promoter region of the mouse gene for iNOS has been characterized (Weisz et al., 1994). Several binding sites for transcription factors have been identified in the promoter region of the iNOS gene including NFκB, ISRE, IRF-1 and Oct (Lowenstein et al., 1993; Xie et al., 1993; Kamijo et al., 1994; Martin et al., 1994; Goldring et al., 1996). Of these transcription factors, only the activation of NFκB has been shown to mediate the enhanced expression of the iNOS gene in macrophages exposed to LPS (Xie et al., 1994). The NFκB is an inducible transcription factor originally identified as a hetrodimeric complex consisting of a 50 kDa subunit (p50) and a 65 kDa subunit (p65). A common feature of the regulation of transcription factors belonging to the Rel family is their sequestration in the cytoplasm as inactive complexes with a class of inhibitory molecules known as IκB (Baeuerle & Baltimore, 1996).
Resveratrol is a phytoalexin found in grapes and other plants. The flavonoid naringin is the abundant natural product from grapefruits and related citrus species. The aglycone, naringenin is readily formed from naringin after dietary intake in humans. It is conceivable that resveratrol possesses many biological activities that favour protection against atherosclerosis, including antioxidant activity, modulation of hepatic apolipoprotein and lipid synthesis, inhibition of platelet aggregation as well as the production of anti-atherogenic eicosanoids by human platelet and neutrophils (Soleas et al., 1997). In addition, a cancer chemotherapeutic activity of resveratrol has also been described (Jang et al., 1997). Naringenin is present in grapefruits mainly as its glycosylated form, naringin. Many biological functions of naringenin and naringin have been studied, including anti-inflammatory, antioxidative (Limasset et al., 1993), antimutagenic (Calomme et al., 1996) and anticarcinogenic effects (So et al., 1996). The anti-inflammatory and cancer-preventing characteristics of these flavonoids have been well documented. Nevertheless, how these effects are produced by these flavonoids is not well characterized.
In this study, we have examined the effects of these flavonoids on NO generation, cytosolic iNOS protein, steady state mRNA levels, and gene promoter activity through the translocation of transcription factor NFκB to nucleus. Taken together, these findings indicated that resveratrol could protect against endotoxin-induced inflammation by preventing the activation of NFκB.
Methods
Reagents
LPS (Escherichia coli 0127: E8), naringin, naringenin and resveratrol were purchased from Sigma Chemical (St Louis, MO, U.S.A.). Isotopes were obtained from Amersham (Arlington Heights, IL, U.S.A.). Polynucleotide kinase and oligo (dT)18 were obtained from Pharmacia (Piscataway, NJ, U.S.A.).
Cell culture
RAW 264.7 cells were cultured in RPMI-1640 (without phenol red) supplement with 10% endotoxin-free, heat-inactivated, foetal calf serum (GIBCO, Grand Island, NY, U.S.A.), supplemented with 100 units ml−1 penicillin, and 100 μg ml−1 streptomycin. When the cells reached a density of 2–3×106 cells ml−1, they were activated by incubation in medium containing E. coli LPS (50 ng ml−1). Various concentrations of test compounds dissolved in DMSO were added together with LPS.
Nitrite assay
The nitrite concentration in the culture medium was measured as an indicator of NO production using the Griess reaction (Kim et al., 1995). One hundred microliters of each supernatant was mixed with the same volume of Griess reagent (1% sulphanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in water) and the absorbance of the mixture, at 550 nm, was determined with an enzyme-linked immunosorbent assay plate reader (Dynatech MR-7000; Dynatech Labs, Chantilly, VA, U.S.A.).
Western blots
Total cellular extract was prepared using radio-immunoprecipitation assay buffer (in mM): Tris-HCl (pH 7.4) 50, NaCl 150, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulphate, 1% aprotinin). Total protein (for iNOS and α-tubulin), cytosolic fractions (for Sp1, IκBα, c-Re1, p65 and p50) or nuclear fractions (for Sp1,c-Rel, p65 and p50) containing 30–50 μg of protein were separated on sodium dodecyl sulphate-polyacrylamide minigels (8% for iNOS, Sp1 and 10% for IκBα, c-Rel, p65 and p50) and transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA, U.S.A.). The membranes were incubated overnight at 37°C with 10% bovine serum albumin in phosphate-buffered saline to block nonspecific immunoglobulins and then incubated with macNOS monoclonal antibody (Transduction Laboratories, Lexington, KY, U.S.A.), anti-IκBα, -c-Rel, -p65, -p50 or -Sp1 polyclonal antibodies (Santa Cruz Biochemicals, Santa Cruz, CA, U.S.A.), anti-phospho (Ser32)-specific IκBα (New England Biolabs, Bevery, MA, U.S.A.), or anti-α-tubulin monoclonal antibody (Oncogene Science, Cambridge, U.K.). iNOS, IκBα, p65, p50, c-Rel, Sp1 and α-tubulin protein were detected by chemiluminescence (ECL, Amersham), or by incubation with the coloregenic substrates: nitro blue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) as suggested by the manufacture (Sigma Chemical Co.).
RT–PCR and Northern blot
Following stimulation with LPS for 5 h, cells were washed in ice-cold PBS and total RNA was isolated by acid guanidinium thiocyanate-phenol-chloroform extraction (Chomczynski & Sacchi, 1987). Total RNA (5 μg) was converted to cDNA with 1 μM oligo(dT)18, 0.5 mM concentration of each dNTP, Tris-HCl (pH 8.3) (50 mM), KCl (75 mM), MgCl2, (3 mM), 1 unit μl−1 RNase inhibitor, and 10 units μl−1 Moloney murine leukaemia virus reverse transcriptase at 42°C for 1.5 h. PCR of the cDNA was performed in a final volume of 25 μl containing all four dNTPs (each at 200 μM), KCl (500 mM), MgCl2 (15 mM), 0.1% gelatin, 50 units ml−1 Super Taq DNA polymerase, and each primer at 0.4 μM. The amplification cycles were 95°C for 30 s, 65°C for 45 s, and 72°C for 2 min. The PCR products were separated by electrophoresis on a 1.8% agarose gel after 35 cycles (497-bp iNOS fragment; 983-bp G3PDH fragment) and visualized by ethidiume bromide staining. Amplification of G3PDH served as a control for sample loading and integrity. PCR was performed on the cDNA using the following sense and antisense primers, respectively; iNOS: CCCTTCCGAAGTTTCTGGCAGCAGC and GGCTGTCAGAGAGCCTCGTGGCTTTGG; G3PDH: TGAAGGTCGGTGTGAACGGATTTGGC and CATGTAGGCCATGAGGTCCACCAC. Total RNA (25 μg) was denatured with formaldehyde/formamide and incubated at 65°C for 15 min, size-fractioned on 1.2% formaldehyde-containing agarose, and transferred onto Hybond-N nylon membrane (Amershan Corp., Arlington Heights, IL, U.S.A.) in 20×standard saline citrate (3 M sodium chloride and 0.3 M sodium citrate pH 7.0). The blotted membrane was hybridized with iNOS fragment, which was labelled with 32P by using a Random Primer Labelling kit (Amersham). After hybridization, the membrane was washed, dried and autoradiographed with Kodak X-ray film (Rochester, NY, U.S.A.). After hybridization with iNOS-specific probe, the blot was stripped and reprobed with a probe for GAPDH cDNA as a control (Lin & Lin, 1997).
Preparation of extracts and electrophoretic mobility shift assay
Nuclear and cytoplasmic extracts were prepared according to a modified method of Chen et al., 1995. At the end of the culture, the cells were suspended in hypotonic buffer A (in mM): HEPES (pH 7.6) 10, KCl 10, EDTA 0.1, DTT 1, phenylmethylsulphonyl fluoride 0.5 for 10 min on ice and vortexed for 10 s. Nuclei were pelleted by centrifugation at 12,000×g for 20 s. The supernatants containing cytosolic proteins were collected. A pellet containing nuclei was suspended in buffer C (in mM): HEPES (pH 7.6) 20, EDTA 1, DTT 1, phenylmethylsulphonyl fluoride 0.5, 25% glycerol, 0.4 M NaCl, for 30 min on ice. The supernatants containing nuclear proteins were collected by centrifugation at 12,000×g for 20 min and stored at −70°C. For the electrophoretic mobility shift assay, 5 μg of each nuclear extract was mixed with the labelled double-stranded NFκB oligonucleotide, 5′-AGTTGAGGGGACTTTCCCAGGC-3′, and incubated at room temperature for 20 min. The incubation mixture included 1 μg of poly (dI-dC) in a binding buffer (in mM) HEPES (pH 7.9) 25, EDTA 0.5, DTT 0.5 NaCl 50, 1% Nonidet P-40, 5% glycerol. The DNA/protein complex was electrophoresed on 4.5% nondenaturing polyacrylamide gels in 0.5×Tris/borate/EDTA buffer (Tris 0.0445 M, borate 0.0445 M, EDTA 0.001 M). The specificity of binding was also examined by competition with the unlabelled oligonucleotide. Radioactive bands were detected by autoradiography and the bands were cut, solubilized and counted in a beta scintillation counter (Packard Co.).
Results
Inhibition of NO generation by flavonoids
To investigate the anti-inflammatory effects of the three flavonoids shown in Figure 1, they were tested for their ability to inhibit NO generation in LPS-activated macrophages. The concentration-response curves (Figure 2) were determined 18 h after the flavonoids and LPS had been added to the medium. Resveratrol was found significantly to reduce NO generation, whereas naringenin showed less inhibitory effect over the same concentration range, and the inhibitory ability of naringin was almost negligible. The flavonoids did not interfere the Griess reaction.
Figure 1.
Structures of resveratrol, naringin and naringenin.
Figure 2.
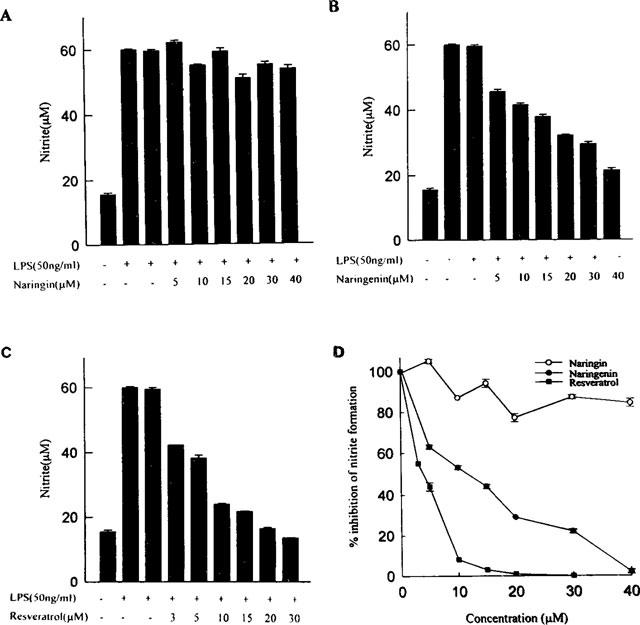
Effects of various concentrations of resveratrol, naringin and naringenin on nitrite release into the culture medium of activated macrophages. RAW 264.7 cells were treated with or without LPS (50 ng ml−1) and various flavonoids or DMSO (0.03%) solvent ((A) naringin; (B) naringenin; (C) resveratrol) for 18 h. At the end of the incubation time, the culture medium was collected for nitrite assay. Each data point is mean±s.e.mean for three determinations. (D) Rates of nitrite release were measured in activated macrophages in the presence of the indicated concentrations of various flavonoids, and the data was normalized (LPS=100%).
Inhibition of iNOS protein
Resveratrol, naringin and naringenin were studied for their effect on iNOS protein in macrophages activated with LPS. Inhibition of iNOS protein by these compounds was detected in concentration-dependent manner (Figure 3). The inhibitory activity of naringenin and naringin was less than that of resveratrol. The flavonoid concentrations inhibiting iNOS protein was similar to those for reduction of nitrite formation.
Figure 3.
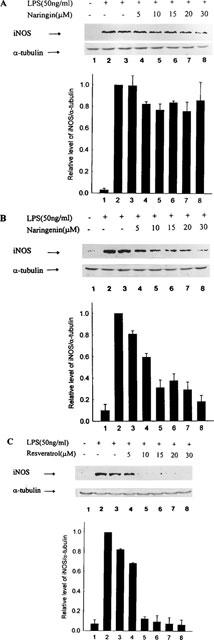
Immuno-blotting analysis of inducible NO systhase in activated macrophages with various flavonoids or solvent only at 18 h ((A) naringin; (B) naringenin; (C) resveratrol). At the end of the incubation time, the total protein was extracted for iNOS protein and α-tubulin analysis by using iNOS and α-tubulin-specific antibodies as described in Methods. Quantification of the iNOS protein expression was performed by densitometric analysis (IS-1000 Digital System) of the immunoblot. Data are expressed as the means±s.e.mean of the ratio of maximal protein expression observed with LPS as determined by three independent experiments. The ratio of iNOS to α-tubulin protein expression observed with LPS alone is set at 1. The relative level was calculated as the ratio of iNOS to α-tubulin protein expression, which was performed by densitometric analysis of the immunoblot.
Effects of flavonoids on iNOS gene expression
In order to investigate whether the suppression of iNOS activity by flavonoids was due to reduced iNOS mRNA, a RT–PCR analysis for total mRNA samples extracted from RAW 264.7 cells was carried out. The amplification of cDNA with primers specific for mouse iNOS and GAPDH (as control gene) is shown in Figure 4A. The results indicate that significantly lower levels of iNOS mRNA is expressed in macrophages activated by LPS in the presence of flavonoids than in their absence. Similar levels were obtained from Northern blot analysis of specific iNOS mRNA in cell extracts (Figure 4C). Coincubation of macrophages with LPS plus resveratrol caused almost complete suppression of iNOS mRNA after 5 h induction, and weaker suppression was found in the presence of LPS plus naringenin. Naringin had no effect on LPS-induced iNOS mRNA expression.
Figure 4.
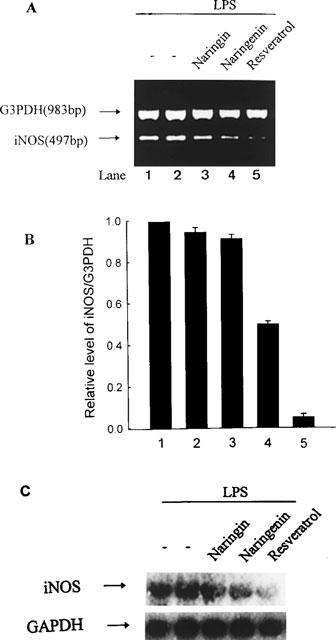
RT–PCR analysis of the expression of iNOS mRNA. (A) RAW 264.7 cells were treated with no flavonoid (lane 1), DMSO (0.03%, lane 2), naringin (30 μM, lane 3), naringenin (30 μM, lane 4), or resveratrol (30 μM, lane 5) before stimulation with LPS (50 ng ml−1) for 5 h. Total RNA was extracted from treated cells and the iNOS mRNA expression was determinated as described in Methods. G3PDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Quantification of the iNOS RNA expression was performed by densitometric analysis (IS-1000 Digital System) of the RT–PCR analysis. Data are expressed as the means±s.e.mean of the ratio of maximal RNA expression observed with LPS in three independent experiments. The ratio of iNOS to G3PDH RNA expression observed with LPS alone is set at 1. The relative level was calculated as the ratio of iNOS to G3PDH RNA expression. (C) Total RNA was extracted from treated cells and assayed for iNOS mRNA expression by Northern blot analysis. Blots were hybridized to 32P-labelled iNOS probe as described in Methods. Signals for GAPDH mRNA for each lane are shown as controls.
Inhibition of flavonoids on LPS-induced nuclear protein with NFκB binding activity
Deletion and mutational analyses have demonstrated that the transcription factor NFκB is involved in the activation of iNOS by LPS. To investigate if the flavonoids selectively inhibited activation of NFκB, analysis of NFκB binding activity by gel mobility shift assay was performed. As shown in Figure 5A, the induction of specific NFκB binding activity by LPS was significantly inhibited by resveratrol (30 μM). On the other hand, inhibition by naringenin was lower, and naringin was almost inactive. The addition of excess unlabelled consensus oligonucleotide completely prevented the band shifts, demonstrating the specificity of the protein/DNA interaction (Figure 5B).
Figure 5.
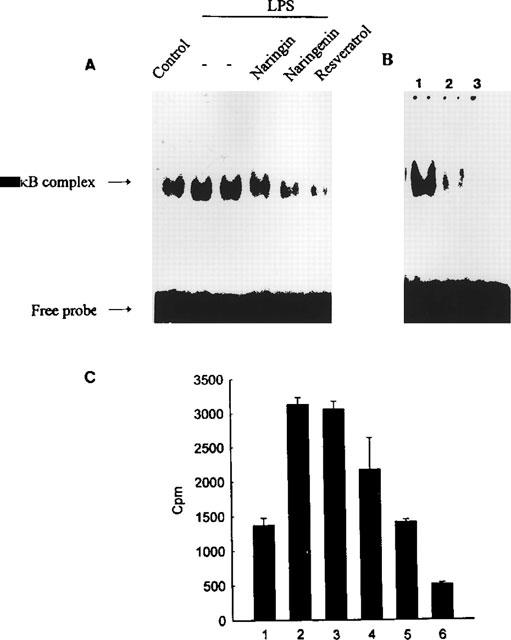
Electrophoretic mobility shift assay using a 5′-end-labelled consensus oligonucleotide for NFκB binding and nuclear extracts from RAW 264.7 cells. (A) RAW 264.7 cells were treated with LPS (50 ng ml−1) without or with different flavonoids (30 μM) or DMSO (0.03%) solvent and then incubated for 1 h. (B) shows competition assay for the identification of NFκB-binding specificity. Lane 1: NFκB complex in LPS-treated RAW 264.7 cells; lane 2: addition of a 25 fold molar excess of the nonradioactive NFκB before adding the [32P]-NFκB element; lane 3: addition of a 50 fold molar excess of the nonradioactive NFκB before adding the [32P]-NFκB element. (C) the actual radioactivity (c.p.m) present in respective bands. Data are expressed as the means±s.e.mean of the radioactivity (c.p.m.) in three independent experiments.
Effect of flavonoids on the phosphorylation and degradation of IκBα
To determine whether the inhibitory action of these flavonoids was due to their effect on the phosphorylation and degradation of IκBα, the phosphorylated and cytoplasmic levels of IκBα protein were examined by immunoblot analysis. After 30 min activation of macrophages by LPS, the serine-phosphorylated IκBα protein was detected by Ser32-phospho-specific IκBα antibody. The results are illustrated in Figure 6A. Resveratrol had a strong ability to inhibit LPS-induced IκBα phosphorylation. In order to confirm the involvement of flavonoids in the prevention of the degradation of IκBα protein, immunoblot analysis of IκBα protein was performed. Figure 6B shows that the cytosolic IκBα protein was detected after 1 h activation, and the inhibitory pattern for resveratrol was similar to the pattern of inhibition of IκBα phosphorylation. The inhibitory effect of naringenin was lower, and the inhibitory effect of naringin was negligible.
Figure 6.
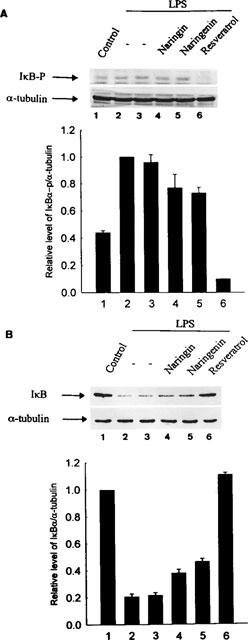
The inhibition by flavonoids of LPS-mediated IκBα phosphorylation and degradation. RAW 264.7 cells were treated with LPS (50 ng ml−1) without or with different flavonoids (30 μM) or DMSO (0.03%) solvent and then incubated for 30 min or 1 h. Cytosolic fractions were prepared and analysed for the content of IκBα protein by Western blot. (A) After 30 min activation, the phosphorylated IκBα was detected by Ser32-phospho-specific antibody. Quantification of the phosphorylated IκBα protein expression was performed by densitometric analysis (IS-1000 Digital System) of the Western blot. Data are expressed as the means±s.e.mean of the ratio of maximal phosphorylated IκBα observed with LPS in three independent experiments. The ratio of phosphorylated IκBα to α-tubulin protein expression observed with LPS alone is set at 1. The relative level was calculated as the ratio of phosphorylated IκBα to α-tubulin protein expression. (B) The content of IκBα protein was detected after 1 h activation. Quantification of the IκBα protein expression was performed by densitometric analysis (IS-1000 Digital System) of the Western blot. Data are expressed as the means ±s.e.mean of the ratio of maximal IκBα protein expression in three independent experiments. The ratio of IκBα to α-tubulin protein expression observed with control is set at 1. The relative level was calculated as the ratio of IκBα to α-tubulin protein expression.
Reduction of nuclear NFκB level by various flavonoids
The above results suggested that flavonoids could reduce iNOS expression by blocking iNOS promoter activation. Since activation of transcription factor NFκB is necessary for iNOS induction, we tested if flavonoids perturbed the distribution of NFκB subunits (c-Rel, p65 and p50) as assessed by nuclear accumulation. As shown in Figure 7, coincubation with LPS plus resveratrol decreased the NFκB proteins in nucleus. Sp1, a nuclear protein was used as control to confirm that there was no contamination with nuclear proteins during extraction of the cytosolic fraction. These results suggest that inhibition of NO production by flavonoids occurs via blocking the phosphorylation as well as degradation of IκB protein; thus preventing the translocation of NFκB protein, and finally suppressing NFκB activation in the nucleus.
Figure 7.
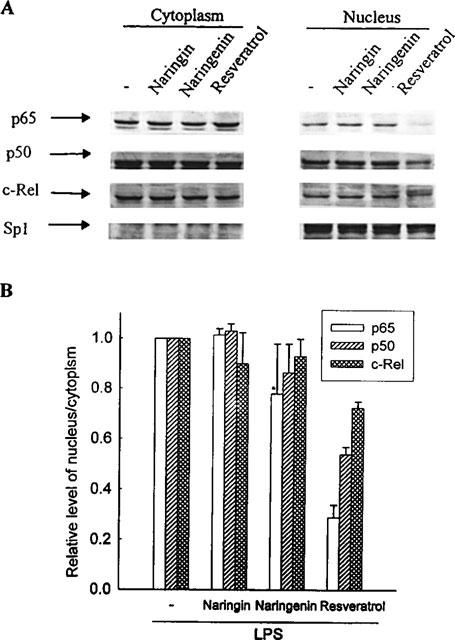
Flavonoids reduced nuclear NFκB levels. RAW 264.7 cells were treated with LPS (50 ng ml−1) without or with different flavonoids (30 μM) and then incubated for 1 h. (A) Cytosolic and nuclear fractions were prepared and analysed for the content of c-Rel, p65, p50 and Sp1 proteins. (B) Quantification of the NFκB (c-Rel, p65 and p50) protein expression was performed by densitometric analysis (IS-1000 Digital System) of the Western blot. Data are expressed as the means±s.e.mean of the ratio of maximal NFκB nuclear translocation observed with LPS in three independent experiments. The ratio of NFκB nuclear translocation observed with LPS is set at 1. The relative level was calculated as the ratio of nuclear to cytoplasmic NFκB.
Discussion
Flavonoids occur ubiquitously in the plant kingdom and are common components of the human diet. The flavonoids exhibit a wide structural diversity; more than 4000 different flavonoids have been identified from various plants. Flavonoids have been shown to have structually-dependent, highly specific effects on a variety of enzymes and are able to interfere with numerous cellular processes, including growth and differentiation (Brandi, 1992). Resveratrol (3,5,4′-trihydroxystilbene) is one of the stilbene family. Naringin (naringenin 7-hesperidoside) is the glycosylated form of naringenin. Here we demonstrated that resveratrol strongly inhibited the induction of iNOS in RAW 264.7 cells activated with LPS with an IC50 of 5 μM. Naringenin had less inhibitory activity, and naringin was almost devoid of an inhibitory effect. It is difficult to deduce a structure-activity relationship from these compounds, but it may be noted that naringin is the only compound with a rhamnglucoside group at the 7 position. In addition, our laboratory has observed that tea polyphenols (10 μM) significantly reduce iNOS protein (Lin & Lin, 1997). The tea polyphenols also have an hydroxyl group at the 5 and 7 positions. This suggests that the hydroxy groups of resveratrol and naringenin may be important for the inhibitory activity on iNOS induction. Based on these findings, it seems that those compounds with neighbouring hydroxyl moieties had the most potent anti-inflammatory property, and glycosylation of OH groups reduced this activity.
Mammals are in permanent contact with Gram-negative bacteria and LPS (Schletter et al., 1995). Low doses of LPS are considered beneficial for the host. On the other hand, the presence of a large amount of LPS leads to dramatic pathophysiological reactions such as fever, leukopenia, hypotension and multi-organ failure. LPS stimulates host cells (mainly monocytes/macrophages but also endothelial cells, smooth muscle cells and neutrophils) to produce and release NO by induction of iNOS protein. The iNOS isoform can produce high, persistent concentrations of NO on induction with endotoxin alone or in combination with cytokines in many cell types. It is expressed in the resting state in other cells, potentially resulting in cytotoxicity, tissue damage, or DNA damage. Here we showed that resveratrol inhibits the expression of iNOS. Thus, resveratrol may act as protectant against the effects of agents which stimulate iNOS induction.
Activation of NFκB is necessary for LPS induction of the iNOS promoter (Xie et al., 1994). NFκB is composed mainly of two proteins: p50 and p65. In its unstimulated form, NFκB is present in the cytosol bound to the inhibitory protein, IκB. After stimulation of cells by a variety of agents, IκB becomes phosphorylated and this triggers a proteolytic degradation of IκB. Serine phosphorylation of IκB is sufficient for efficient degradation. On the other hand, stoichiometric phosphorylation of IκB on tyrosine 42 dose not cause a subsequent proteolytic degradation of the IκB but, apparently, is sufficient to release IκB from NFκB and hence activate NFκB (Imbert et al., 1996). Our results showed that resveratrol reduces iNOS expression by blocking transcription of its gene, a conclusion supported by the observation that it reduced steady state iNOS mRNA levels, promoter activity (as assessed by gel mobility assay), and nuclear accumulation of NFκB subunits. The mechanisms by which resveratrol can interfere with the activation of NFκB are not clear. One possibility is that resveratrol could interact with ankyrin domains present in IκB, because the phosphorylation of IκB was inhibited by resveratrol. Such an interaction could conceivably hinder IκB phosphorylation and subsequent dissociation of NFκB.
However, whether resveratrol physically interacts with IκB remains to be determined. Cells treated with LPS could generate ROIs by inducing NADPH oxidase activity (Bastian & Hibbs, 1994). We also found that hydroxy radicals can activate protein tyrosine kinase (Lee et al., 1996). Furthermore, resveratrol has been found to possess potent protein kinase inhibitory activity and antioxidant activity (Miller & Rice-Evans, 1995; Chen et al., 1990; Jayatilake et al., 1993). A role for protein tyrosine kinase and ROIs has been implicated in NFκB activation (Bastian & Hibbs, 1994; Baldwin, 1996). Therefore, we postulate that resveratrol might inhibit the activation of NFκB through inhibiting the LPS-induced phosphorylation and degradation of IκBα. It is possible that the anti-inflammatory and anti-cancer properties of the flavonoids may be mediated, at least in part, by inhibition of iNOS expression through down-regulation of NFκB binding activity.
Acknowledgments
We would like to thank Dr K. F. Fork for the gift of resveratrol. This study was supported by grants from the National Science Council [NSC 87-2316-B-002-011] and the National Health Research Institute [DOH87-HR-403].
Abbreviations
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IκB
inhibitor κB
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NFκB
nuclear factor-κB
- NO
nitric oxide
- PCR
polymerase chain reaction
- ROI
reactive oxygen intermediate
- RT
reverse transcription
References
- BAEUERLE P.A., BALTIMORE D. NFκB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- BALDWIN A.S., JR The NFκB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- BASTIAN N.R., HIBBS J.B., JR Assembly and regulation of NADPH oxidase and nitric oxide synthase. Curr. Opin. Immunol. 1994;6:131–139. doi: 10.1016/0952-7915(94)90044-2. [DOI] [PubMed] [Google Scholar]
- BRANDI M.I. Flavonoids: biochemical effects and therapeutic applications. Bone Miner. 1992;19:S3–S14. doi: 10.1016/0169-6009(92)90861-7. [DOI] [PubMed] [Google Scholar]
- CALOMME M., PIETERS L., VLIETINCK A., BERGHE D.M. Inhibition of bacterial mutagenesis by citrus flavonoids. Planta Medica. 1996;62:222–226. doi: 10.1055/s-2006-957864. [DOI] [PubMed] [Google Scholar]
- CHEN F., KUHN D.C., SUN S.C., GAYDOS L.J., DEMERS L.M. Dependence and reversal of nitric oxide production on NF-κB in silica and lipopolysaccharide-induced macrophages. Biochem. Biophys. Res. Commun. 1995;214:839–846. doi: 10.1006/bbrc.1995.2363. [DOI] [PubMed] [Google Scholar]
- CHEN Y.T., ZHANG R.L., JIA Z.J., JU Y. Flavonoids as superoxide scavenger and antioxidants. Free Rad. Biol. Med. 1990;9:19–21. doi: 10.1016/0891-5849(90)90045-k. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI & SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DING A.H., NATHAN C.F., STUEHR D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J. Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- GOLDRING C.E.P., REVENEAU S., ALGARTE M., JEANNIN J.-F. In vivo footprinting of the mouse inducible nitric oxide synthase gene: inducible protein occupation of numerous sites including Oct and NF-IL6. Nucleic Acid Res. 1996;24:1682–1687. doi: 10.1093/nar/24.9.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994;344:721–725. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- IMBERT V., RUPEC R.A., LIVOLSI A., PAHL H.L., TRAENCKNER B.M., MUELLER-DIECKMANN C., FARAHIFAR D., ROSSI B., AUBERGER P., BAEUERLE P.A., PEYRON J. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- JANG M., CAI L., UDEANI G.O., SLOWING K.V., THOMAS C.F., BEECHER W.W., FONG H.H.S., FARNSWORTH N.R., KINGHORN A.D., MEHTA R.G., MOON R.C., PEZZUTO J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;75:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- JAYATILAKE G.S., JAYASURIYA H., LEE E.S., KOONCHANOK N.M., GEAHLEN R.L., ASHENDEL C.L., McLAUGHLIN J.L., CHANG C.J. Kinase inhibitors from polygonum cuspidatum. J. Nat. Prod. 1993;56:1805–1810. doi: 10.1021/np50100a021. [DOI] [PubMed] [Google Scholar]
- KAMIJO R., HARADA H., MATSUYAMA T., BOSLAND M., GERECITANO J., SHAPIRO D., LE J., KOH S.I., KIMURA T., GREEN S.J., MAK T.W., TANIGUCHI T., VILCEK J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1613–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- KIM H., LEE H.S., CHANG K.T., KO T.H., BAEK K.J., KWON N.S. Chloromethyl ketones block induction of nitric oxide synthase in murine macrophages by preventing activation of nuclear factor-κB. J. Immunol. 1995;154:4741–4748. [PubMed] [Google Scholar]
- LEE S.F., HUANG Y.T., WU W.S., LIN J.K. Induction of c-Jun protooncogene expression by hydrogen peroxide through hydroxyl radical generation and p60Src tyrosine kinase activation. Free Rad. Biol. Med. 1996;21:437–448. doi: 10.1016/0891-5849(96)00040-8. [DOI] [PubMed] [Google Scholar]
- LIMASSET B., DOUCEN C.L., DORE J.C., OJASOO T., DAMON M., PAULET A.C. Effects of flavonoids on the release of reactive oxygen species by stimulated human neutrophils. Biochem. Pharmacol. 1993;46:1257–1271. doi: 10.1016/0006-2952(93)90476-d. [DOI] [PubMed] [Google Scholar]
- LIN Y.L., LIN J.K. (−)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-κB. Mol. Pharmacol. 1997;52:465–472. [PubMed] [Google Scholar]
- LOWENSTEIN C.J., ALLEY E.W., RAVAL P., SNOWMAN A.M., SNYDER S.H., RUSSELL S.W., MURPHY A.W. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon γ and lipopolysccharide. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E., NATHAN C., XIE Q.-W. Role of interferon regulatory factor 1 in iduction of nitric oxide synthase. J. Exp. Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER N.J., RICE-EVANS C.A. Antioxidant activity of resveratrol in red wine. Clin. Chem. 1995;41:1789. [PubMed] [Google Scholar]
- NATHAN C., XIE Q.-W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- OHSHIMA H., BARTSCH H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat. Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- SCHLETTER J., HEINE H., ULMER A.J., RIETSCHEL E.T. Molecular mechanisms of endotoxin activity. Ach. Microbiol. 1995;164:383–389. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- SO F.V., GUTHRIE N., CHAMBERS A.F., MOUSSA M., CARROLL K.K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr. Cancer. 1996;26:167–181. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- SOLEAS G.J., DIAMANDIS E.P., GOLDBERG D.M. Resveratrol: a molecule whose time has come? And gone. Clin. Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- STUEHR D.J., MARLETTA M.A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISZ A., OGUCHI S., CICATIELLO L., ESUMI H. Dual mechanism for the control of inducible-type NO synthase gene expression in macrophages during activation by interferon-γ and bacterial lipopolysaccharide. J. Biol. Chem. 1994;269:8324–8333. [PubMed] [Google Scholar]
- XIE Q.-W., KASHIWABARA Y., NATHAN C. Role of transcription factor NFκB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- XIE Q.-W., WHISNANT R., NATHAN C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J. Exp. Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]


