Abstract
The possible existence of a β3-adrenergic receptor (β3-AR) in the human detrusor muscle was investigated by in vitro functional studies and analysis of mRNA expression.
Isoprenaline, noradrenaline and adrenaline each produced a concentration-dependent relaxation of the human detrusor. The rank order for their relaxing potencies was isoprenaline (pD2 6.37±0.07) ⩾ noradrenaline (pD2 6.07±0.12) ⩾ adrenaline (pD2 5.88±0.11).
Neither dobutamine (β1- and β2-AR agonist) nor procaterol (β2-AR agonist) produced any significant relaxation at concentrations up to 10−5 M. BRL37344A, CL316243 and CGP-12177A (β3-AR agonists), relaxed the preparations significantly at concentrations higher than 10−6 M. The pD2 values for BRL37344A, CL316243 and CGP-12177A were 6.42±0.25, 5.53±0.09 and 5.74±0.14, respectively.
CGP-20712A (10−7–10−5 M), a β1-AR antagonist, did not affect the isoprenaline-induced relaxation. On the other hand, ICI-118,551, a β2-AR antagonist, produced a rightward parallel shift of the concentration-relaxation curve for isoprenaline only at the highest concentration used (10−5 M) and its pKB value was 5.71±0.19. Moreover, SR58894A (10−7–10−5 M), a β3-AR antagonist, caused a rightward shift of the concentration-relaxation curve for isoprenaline in a concentration-dependent manner. The pA2 value and slope obtained from Schild plots were 6.24±0.20 and 0.68±0.31.
The β1-, β2- and β3-AR mRNAs were all positively expressed in detrusor smooth muscle preparations in a reverse transcription polymerase chain reaction assay.
In conclusion, the present results provide the first evidence for the existence of the β3-AR subtype in the human detrusor. They also suggest that the relaxation induced by adrenergic stimulation of the human detrusor is mediated mainly through β3-AR activation.
Keywords: Human bladder, β-adrenoceptor subtypes, functional analysis, mRNA analysis
Introduction
There is much evidence indicating that, at least in animals, activation of the sympathetic nervous system contributes to urine storage by relaxing the detrusor muscle via activation of β-adrenoceptors (β-ARs; see review by Andersson, 1993). Although β-ARs were originally subclassified into β1- and β2-subtypes (Lands et al., 1967a,1967b), another subtype, the β3-subtype, has since been reported (Emorine et al., 1989; 1994). Furthermore, it has been known for some years that there are species differences in the subtypes of β-ARs involved in the relaxation of the detrusor (Elmér, 1974; Nergårdh et al., 1977; Anderson & Marks, 1984; Levin et al., 1988; Li et al., 1992; Goepel et al., 1997; Seguchi et al., 1998). Recently, we reported that part of the relaxation induced by adrenergic stimulation in the rat and canine detrusor was mediated by β3-AR (Yamazaki et al., 1998).
In humans, although the β-ARs present in the detrusor muscle have been shown to have functional characteristics typical of neither β1- nor β2-ARs (Nergårdh et al., 1977; Larsen, 1979), receptor-binding studies carried out using selective radio-ligands have indicated a predominance of the β2-subtype (Levin et al., 1988). It is still unclear whether β3-ARs are present in the human detrusor and, if they are, what function they perform. In the present study, we set out to determine which β-AR subtypes are involved in the relaxation of the human detrusor that occurs on adrenergic stimulation. We were particularly interested in β3-ARs, and we used in vitro functional studies and mRNA analysis. Some of the results have been presented in a preliminary communication (Igawa et al., 1998).
Methods
Patients and specimens
The study involved 56 patients (44 men and 12 women; aged 66.2±1.5, range 23–82 years) undergoing open pelvic surgery at Shinshu University Hospital, 38 for bladder carcinoma, eight for renal pelvic or ureteral carcinoma, two for prostatic carcinoma, three for bladder stone, three for benign prostatic hyperplasia, and two for vesico-ureteral reflex. On the basis of preoperative urodynamic studies and neurological examinations, all patients were judged to have normal bladder function. None of the patients had diseases known to interfere with the β-AR system or had received medication known to interfere with that system. General anaesthesia was induced with a short-acting barbiturate and was maintained with fentanyl and a mixture of oxygen, nitrous oxide and isoflurane. Written informed consent was obtained from all patients before their operation. The study was approved by the Ethics Committee of Shinshu University School of Medicine.
All specimens were taken from macroscopically normal tissue in the anterior or posterior wall of the bladder body via a longitudinal incision. In all cases, except when total cystectomy was performed for bladder carcinoma, specimens were obtained from the margin of the longitudinal incision in the anterior bladder wall during the operation itself. For functional studies, the specimens were placed immediately after excision in pre-oxygenated Krebs solution (for composition see below) at 4°C and transported to the laboratory. The specimens for RNA analysis were frozen immediately after excision and stored in liquid nitrogen.
Functional studies
After the mucosa and adventitia had been removed, detrusor muscle strips measuring approximately 10×5×3 mm were isolated. Each preparation was suspended in a 10 ml organ bath containing Krebs solution; this was maintained at 37°C and continuously gassed with a mixture of 95% oxygen and 5% carbon dioxide. One end of each strip was connected to a force-displacement transducer (SB-1T, Nihon-Kohden, Tokyo, Japan) and changes in muscle tension were measured and recorded on a pen-writing oscillograph (Rectigraph 8S, NEC Sanei, Tokyo, Japan). The preparation was gradually stretched until a stable tension of 10 mN was obtained. Concentration-response curves for β-AR agonists were obtained by cumulative addition of the appropriate drug to the bathing fluid. Each preparation was used in only one experiment, i.e. to obtain one concentration-curve for one of the agonists. To test the antagonistic potency of β-AR antagonists against isoprenaline, one of the antagonists was added to the bath 30 min before the addition of isoprenaline. Concentration-response curves for isoprenaline were thus obtained in the presence of the antagonist. All experiments were conducted in the presence of 10−6 M phentolamine, an α-adrenoceptor antagonist.
Analysis of functional data
The results are expressed as means±s.e.mean. The relaxing effect of each agonist is expressed as a percentage of the maximal relaxation induced by 10−5 M forskolin, which was used as a reference drug. The pD2 value, which is the negative logarithm of the EC50 value, was calculated for each agonist from its concentration-relaxation curve. The pA2 value for each antagonist, as defined by Arunlakshana & Schild (1959), was obtained from linear regression analysis of the plot of mean values of log (CR-1) vs the negative log of the antagonist concentration. When a parallel rightward shift of the concentration-relaxation curve was observed only at the highest concentration of antagonist used, the pKB value (the negative logarithm of (antagonist concentration/CR-1)) was calculated from the pD2 values in the presence (at the highest concentration) and absence of antagonist. Statistical analysis was performed using a Student's two-tailed t-test. A probability level of less than 0.05 was accepted as significant.
mRNA analysis
All procedures were carried out according to the manufacturer's instructions unless otherwise specified. Total RNA was extracted from human detrusor smooth muscle (approximate 1 g) using TRIzol® (Gibco-BRL, Rockville, MD, U.S.A.). The total RNA preparations were digested by RQ1 RNase-free DNase (Promega, Madison, WI, U.S.A.) to remove contaminating genomic DNA. The amount of the total RNA was determined by a spectrophotometer (DU-640, Beckman Instruments Inc., Fullerton, CA, U.S.A.).
Primers (Sawady Technology Inc., Tokyo, Japan) for β1-, β2-, and β3-AR were used according to a previous report (Krief et al., 1993) and primers for glyceraldehyde-3-phosphate dehydrogenase (G3PDH), as an internal standard, were designed in base on a DNA sequence (Tso et al., 1985). The sequences of these primers are shown in Table 1. The reverse transcription-polymerase chain reaction (RT-PCR) was carried out using Titan™ one tube RT-PCR system (Boehringer-Mannheim, Mannheim, Germany). The PCR mixtures consisted of 1×the RT-PCR buffer (containing MgCl2 (1.5 mM) and dimethyl sulphoxide (DMSO); Boehringer-Mannheim), dNTPs (0.2 mM), 0.4 μM each of PCR primers, DTT (5 mM), 5 U RNasin® RNase inhibitor (Promega, U.S.A.), 1 μl enzyme mix (expand™ high fidelity enzyme mix and avian myeloblastosis virus (AMV)-reverse transcriptase) and 1 μg total RNA, in a volume of 50 μl. cDNA synthesis and PCR amplification was continuously performed without opening the reaction tubes in a thermal cycler (Gene Amp® 2400, PE Applied-Biosystems, Foster City, CA, U.S.A.).
Table 1.
Oligonucleotides used as RT-PCR primers

cDNA synthesis was performed at 50°C for 30 min with reverse transcriptase and PCR primer. Following initial heating of samples at 94°C for 2 min, 10 cycles were performed, which consisted of denaturation (30 s at 94°C), annealing (30 s at 58°C) and elongation (1 min at 68°C). Furthermore, the PCR amplification was repeated for 25 cycles for β1-, β2- and β3-AR and for 14 cycles for G3PDH in the same manner except that the elongation time was added 5 s for each cycle (These numbers of repeated cycles and the elongation times were determined by results of pilot experiments).
A portion (10 μl) of PCR-products were visualized by electrophoresis of 3% LO3 agarose gels (TAKARA-Shuzo, Ohtsu, Japan) with 0.5 μg ml−1 ethidium bromide (Gibco-BRL). To identify these PCR-products, direct sequencing of PCR-products was performed according to the dideoxy chain termination method. The PCR-products were labelled by Big-dye terminator (PE Applied-Biosystems) using the sense and anti-sense PCR primers, and analysed by DNA sequencer (ABI PRISM™ 310, PE Applied-Biosystems).
Drugs and solutions
The following drugs were used; (±)-isoprenaline hydrochloride, forskolin (Wako Pure Chemical, Osaka, Japan), (±)-dobutamine hydrochloride, BRL37344A ((±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]propyl]phenoxy]-acetic acid sodium), (±)-CGP-12177A hydrochloride ((±)-4-(3-t-butylamino-2-hydroxypropoxy) benzimidazol-2-one hydrochloride), ICI-118,551 hydrochloride (erythro-(±)-1-(7-methylindan - 4 - yloxy ) - 3 - isopropylaminobutan -2 - ol hydrochloride) (Funakoshi, Tokyo, Japan), procaterol hydrochloride (Sigma Chemical, St. Louis, MO, U.S.A.), (±)-noradrenaline (Sankyo, Tokyo, Japan), (−)-adrenaline (Daiichi, Tokyo, Japan), phentolamine mesylate (Ciba-Geigy, Basel, Switzerland) and dimethyl sulphoxide (DMSO) (Nacalai tesque, Kyoto, Japan). CL316243((R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]propyl]- 1,3-benzodioxole-2,2-dicarboxylate), CGP-20712A (2-hydroxy-5(2-((2-hydroxy-3-(4-((1-methyl-4-trifluoromethyl)1H-imidazole-2-yl)-phenoxy)propyl) amino) ethoxy)-benzamide monomethane sulphonate) and SR58894A (3-(2-allylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2 S)-2-propanol hydrochloride) were synthesized in our laboratories (Kissei, Hotaka, Japan). The drugs were dissolved as follows: forskolin, in 100% DMSO; the other drugs, in distilled water. The solutions were prepared on the day of the experiment and kept in dark vessels to minimize light-induced degradation. Subsequent dilutions of the drugs were prepared in distilled water. The reported concentrations are the calculated final concentrations in the bath solution. The Krebs solution used had the following composition (mM): NaCl 118.1, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25.0, KH2PO4, 1.2 and glucose 11.1 (pH 7.4).
Results
Relaxation responses to β-AR agonists
A distinct relaxation of the human detrusor preparation was produced by forskolin (10−5 M), the tension decreasing to 47.3±2.4% (n=60) of the initial tension. Isoprenaline (10−10–10−4 M), a non-selective β-AR agonist, produced relaxation in a concentration-dependent manner (Figure 1). The maximal effect, which was observed at a concentration of 10−4 M, averaged 78.7±1.4% (n=29) of the forskolin (10−5 M)-induced maximal relaxation. Both noradrenaline (10−10–10−4 M) and adrenaline (10−10–10−4 M) also relaxed the preparations in a concentration- dependent manner. The rank order for the relaxing activity of these drugs was isoprenaline ⩾ noradrenaline ⩾ adrenaline, the pD2 values being 6.37±0.07 (n=29), 6.07±0.12 (n=6) and 5.88±0.11 (n=6), respectively (Figure 2). The difference in pD2 value between isoprenaline and adrenaline was statistically significant (P<0.05). However, there was no statistically significant difference in pD2 value between isoprenaline and noradrenaline, or between noradrenaline and adrenaline.
Figure 1.

Representative recording of the effect of isoprenaline on resting tension in a human detrusor preparation.
Figure 2.

Effects of isoprenaline, noradrenaline, adrenaline, dobutamine, and procaterol on resting tension in human detrusor preparations. All experiments were performed in the presence of 10−6 M phentolamine. Each point represents the mean±s.e.mean of 6–29 experiments. Data are expressed as a percentage of the maximal relaxation induced by 10−5 M forskolin.
On the other hand, neither dobutamine (10−10–10−4 M), which stimulates both β1- and β2-ARs, nor procaterol (10−10–10−4 M), a selective β2-AR agonist, produced any significant relaxation at concentrations up to 10−5 M (Figure 2). When applied at 10−4 M, dobutamine and procaterol produced relaxing effects that were the equivalent of 46.2±3.4% (n=8) and 34.2±5.2% (n=11), respectively, of the forskolin (10−5 M)-induced relaxation. However, neither of these effects reached a maximum at a concentration of 10−4 M and so the pD2 values were not determined.
Both BRL37344A (10−10–10−4 M) and CL316243 (10−10–10−4 M), selective β3-AR agonists, and CGP-12177A (10−10–10−4 M), a selective β3-AR partial agonist and β1-/β2-AR antagonist, relaxed the preparation when applied at concentrations greater than 10−6 M (Figure 3). The pD2 values (and maximal relaxation at 10−4 M) were 6.42±0.25 (47.4±4.5%; n=7) for BRL37344A, 5.53±0.09 (43.7±6.4%; n=7) for CL316243 and 5.74±0.14 (33.3±4.2%; n=11) for CGP-12177A, respectively.
Figure 3.

Effects of isoprenaline, BRL37344A, CL316243 and CGP-12177A on resting tension in human detrusor preparations. All experiments were performed in the presence of 10−6 M phentolamine. Each point represents the mean±s.e.mean of 7–29 experiments. Data are expressed as a percentage of the maximal relaxation induced by 10−5 M forskolin.
Effect of β-AR antagonists on the isoprenaline-induced relaxation
CGP-20712A (10−7–10−5 M; n=5–6), a selective β1-AR antagonist, failed to affect the concentration-relaxation curve for isoprenaline (Figure 4a). At concentrations from 10−7 M to 3×10−6 M, ICI-118,551 (n=6–7), a selective β2-AR antagonist, did not affect the relaxation induced by isoprenaline. However, at 10−5 M ICI-118,551 produced a rightward parallel shift of the isoprenaline concentration-response curve (Figure 4b). The pKB value determined by using 10−5 M ICI-118,551 was 5.71±0.19.
Figure 4.

Effects of CGP-20712A (a) and ICI-118,551 (b) on the isoprenaline-induced relaxation of human detrusor muscle preparations. (a) Concentration-response relationships for isoprenaline, either alone or in the presence of CGP-20712A 10−7 M, 3×10−7 M, 10−6 M, 3×10−6 M or 10−5 M. Each point represents the mean±s.e.mean (n=5–6). (b) Concentration-response relationships for isoprenaline, either alone or in the presence of ICI-118,551 10−7 M, 3×10−7 M, 10−6 M, 3×10−6 M, or 10−5 M. Each point represents the mean±s.e.mean (n=6–7). All experiments were carried out in the presence of 10−6 M phentolamine. Data are expressed as a percentage of the maximal relaxation induced by 10−5 M forskolin.
In the presence of both CGP-20712A (10−7 M) and ICI-118,551 (10−7 M), on the other hand, addition of SR58894A (10−7–10−5 M; n=7–12), a selective β3-AR antagonist, caused a rightward shift of the concentration-relaxation curve for isoprenaline in a concentration-dependent manner (Figure 5a). A Schild plot analysis yielded a pA2 value of 6.24±0.20 and a slope of 0.68±0.31 (Figure 5b). The slope did not differ from unity for this antagonist.
Figure 5.
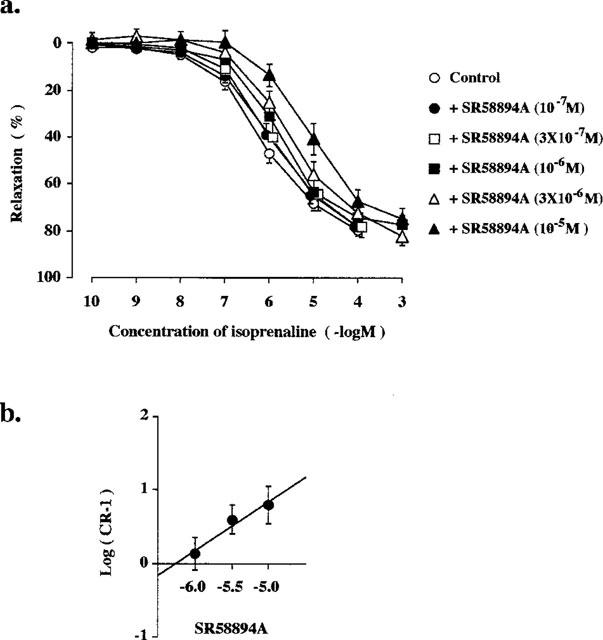
Effect of SR58894A on the isoprenaline-induced relaxation of human detrusor muscle preparations. All experiments were carried out in the presence of CGP-20712A (10−7 M), ICI-118,551 (10−7 M) and phentolamine (10−6 M). (a) Concentration-response relationships for isoprenaline, either alone or in the presence of SR58894A 10−7 M, 3×10−7 M, 10−6 M, 3×10−6 M or 10−5 M. Each point represents the mean±s.e.mean (n=7–12). Data are expressed as a percentage of the maximal relaxation induced by 10−5 M forskolin. (b) Schild plot for the inhibition of the isoprenaline-induced relaxation produced by SR58894A.
Expression of β-AR mRNA in human detrusor
RT-PCR amplification was carried out using specific primers corresponding to β1-, β2- and β3-AR sequences and, as a template, mRNA obtained from human detrusor preparations from three patients. As shown in Figure 6, PCR products for β1-, β2- and β3-AR were detected identically in all the preparations and the expected size of PCR products for β1-, β2- and β3-AR were 265, 329 and 314 bp, respectively. The PCR product for G3PDH, an internal standard, was detected also in each preparations obtained from all the three patients and its expected size was 452. The sequences of the PCR products analysed by DNA sequencer were identified with their reported sequences as regards the detectable signals.
Figure 6.

Detection of β1-, β2- and β3-AR mRNA in human detrusor tissue by RT-PCR. Detrusor preparations were obtained from three different patients (#1, #2 and #3). PCR amplification for β1- (lanes 2–4), β2- (lanes 5–7), β3-AR (lanes 8–10) and G3PDH (lanes 11–13) were carried out for 35, 35, 35 and 24 cycles, respectively. Expected size of PCR-products for β1-, β2-, β3- AR and G3PDH were 265, 329, 314 and 452, respectively. Lanes 1 and 14 show 100 bp DNA ladder (Gibco-BRL).
Discussion
The present study, combining functional and molecular biological investigations, provides evidence for the existence of β3-AR in the human detrusor. It further suggests that the major β-AR subtype involved in the relaxation of the human detrusor smooth muscle observed on adrenergic stimulation is neither the β1- nor the β2-AR, but most probably the β3-AR.
First, we examined the relative potencies with which endogenous and synthetic catecholamines relaxed the human detrusor muscle. The rank order of potency for the three catecholamines producing β-AR-mediated responses has been reported to be isoprenaline > noradrenaline > adrenaline for β1- and β3-ARs, but isoprenaline > adrenaline > noradrenaline for β2-AR (Lands et al., 1967a; Emorine et al., 1989). In the present study, the rank order of potency in relaxing human detrusor was isoprenaline ⩾ noradrenaline ⩾ adrenaline. Although the pD2 value for noradrenaline was greater than that for adrenaline, the difference was not statistically significant. Thus, no difinitive conclusions in terms of a functional predominance of either subtype of β-AR in the human detrusor can be drawn.
Second, we tested the potencies with which selective agonists for the β-AR subtypes relaxed the human detrusor muscle. Neither dobutamine, which stimulates both β1- and β2-ARs (Ozaki et al., 1982; Ruffolo et al., 1984; Ruffolo, 1987; Aikawa et al., 1996), nor procaterol, a β2-AR agonist, produced any significant relaxation at up to 10−5 M. In fact, the concentration at which dobutamine and procaterol did induce a relaxing effect in our preparation was around 10−4 M. At this concentration, it is doubtful that the drugs stimulated any β-AR subtype selectively.
We then examined the relaxing effects of the selective β3-AR agonists, BRL37344A (Oriowo et al., 1996), CL316243 (Bloom et al., 1992) and CGP-12177A. CGP-12177A has been reported to be a partial agonist for the β3-AR and an antagonist for β1- and β2-ARs (Kaumann, 1996). These β3-AR agonists were more potent in relaxing our preparations than either dobutamine or procaterol. Thus, these findings suggest that the β3-AR is functionally predominant over β1- and β2-ARs in the human detrusor. Although the β3-AR agonists, BRL37344A and CL316243, produced relaxations in the human detrusor at concentrations over 10−6 M, the maximal relaxations induced by these β3-AR agonists were only half of the maximal relaxation induced by isoprenaline. This suggests that other β-ARs than the β3-AR may coexist and contribute to the relaxation of the human detrusor.
Confirmation of the predominant role of the β3-AR in the human detrusor was obtained in our investigation of the potencies with which several β-AR antagonists counteracted the isoprenaline-induced relaxation of our preparations. In fact, CGP-20712A, a selective β1-AR antagonist, did not affect the isoprenaline-induced relaxation. Moreover, ICI-118,551, a selective β2-AR antagonist, shifted the concentration-relaxation curve for isoprenaline only at a high concentration (10−5 M). But, the shift was parallel and its pKB value (5.71) was comparable to the pA2 value of 5.31 for the antagonist in antagonizing isoprenaline-induced relaxation of rat oesophageal muscularis mucosae, which is known to be mediated predominantly through β3-ARs (De Boer et al., 1993) and the pKB value of <5.5 for rat cardiac putative β4-AR (Kaumann, 1997). This suggests that the functional β-ARs in the human detrusor belong to neither the β1- nor β2-AR subtypes, but probably to some other subtype (β3- or β4).
We then studied the antagonistic activity of SR58894A, a recently developed β3-AR selective antagonist (Manara et al., 1996), against the isoprenaline-induced relaxation. In the presence of 10−7 M CGP-20712A (β1-AR antagonist) and 10−7 M ICI-118,551 (β2-AR antagonist), SR58894A counteracted the isoprenaline-induced relaxation of the human detrusor. This finding provides functional evidence for β3-ARs in the human detrusor. It has been reported that bupranolol, a non-selective β-AR antagonist, has an antagonistic action at β1- and β2-ARs at low concentrations (nM), whereas at higher concentrations (μM) it also affects the β3-AR (Kaumann, 1989; Koike et al., 1995). In a preliminary study (Igawa et al., 1998), pretreatment with bupranolol at 10−7, 10−6 and 10−5 M produced an apparent rightward shift of the concentration-response curve for isoprenaline. Its pA2 value for this effect was 7.27, which is comparable to the pA2 values (about 7.3–7.5) for β3-AR reported by Langin et al. (1991). Thus, the findings with bupranolol further support the view that the relaxation induced by adrenergic stimulation in the human detrusor is mediated mainly by β3-ARs, rather than by β1- or β2-ARs. However, the slope for SR58894A, obtained from Schild plots, was 0.68. This suggests that other β-ARs, possibly the putative β4-AR (Kaumann, 1997) and/or atypical β-ARs, may coexist and play a functional role in the relaxation of the human detrusor. Indeed, the presence of atypical β-ARs has been postulated in human colonic smooth muscle (De Ponti et al., 1996). Further studies are needed to determine whether such ARs exist also in the human detrusor.
To obtain a biological correlate of the pharmacological evidence for a functional β3-AR in the human detrusor, we used a PCR assay to study the expression of the mRNA for each of the various β-AR genes. In fact, the mRNAs for all three β-AR subtypes were demonstrated in the human detrusor in the present study. The DNA sequencer analysis performed subsequently confirmed that each sequence of these PCR products was identified with its reported sequence. Although the distribution of the β3-AR mRNA has been investigated in a variety of human tissues, such as white fat, gall bladder, small intestine, stomach and prostate (Krief et al., 1993; Berkowitz et al., 1995), to our knowledge, this is the first report of a distribution of β3-AR mRNA in the human urinary bladder. A putative β4-AR has been reported in the human heart (Kaumann, 1997). If β4-AR cDNA is isolated and its DNA sequence is determined, more detailed analysis of the expression of β-AR would be possible.
Taken together, the results of the present pharmacological study and those of the mRNA analysis indicate the presence of the β3-AR in the human detrusor. They further suggest that the relaxation of the human detrusor produced by adrenergic stimulation is mediated mainly by the β3-AR.
Acknowledgments
This work was supported by the Swedish Medical Research Council (grant no 6837).
Abbreviations
- AMV
avian myeloblastosis virus
- β-AR
β-adrenoceptor
- DMSO
dimethyl sulphoxide
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- RT-PCR
reverse transcription-polymerase chain reaction
References
- AIKAWA J., FUKAZAWA M., ISHIKAWA M., MOROI M., NAMIKI A., YAMAGUCHI T. Vascular smooth muscle relaxation by α-adrenoceptor blocking action of dobutamine in isolated rabbit aorta. J. Cardiovasc. Pharmacol. 1996;27:33–36. doi: 10.1097/00005344-199601000-00006. [DOI] [PubMed] [Google Scholar]
- ANDERSON G.F., MARKS B.H. Beta adrenoreceptors in the rabbit bladder detrusor muscle. J. Pharmacol. Exp. Ther. 1984;228:283–286. [PubMed] [Google Scholar]
- ANDERSSON K.-E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–52. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERKOWITZ D.E., NARDONE N.A., SMILEY R.M., PRICE D.T., KREUTTER D.K., FREMEAU R.T., SCHWINN D.A. Distribution of β3-adrenoceptor mRNA in human tissues. Eur. J. Pharmacol. 1995;289:223–228. doi: 10.1016/0922-4106(95)90098-5. [DOI] [PubMed] [Google Scholar]
- BLOOM J.D., DUTIA M.D., JOHNSON B.D., WISSNER A., BURNS M.G., LARGIS E.E., DOLAN J.A., CLAUS T.H. Disodium (R,R)-5-[2-[[2-(3-Chlorophenyl)-2-hydroxyethyl]-amino] propyl]-1,3-benzodioxole 2,2-dicarboxylate (CL316,243). A potent β-adrenergic agonist virtually specific for β3 receptors. A promising antidiabetic and antibesity agent. J. Med. Chem. 1992;35:3081–3084. doi: 10.1021/jm00094a025. [DOI] [PubMed] [Google Scholar]
- DE BOER R.E.P., BROUWER F., ZAAGSMA J. The β-adrenoceptors mediating relaxation of rat oesophageal muscularis mucosae are predominantly of the β3-, but also of the β2-subtype. Br. J. Pharmacol. 1993;110:442–446. doi: 10.1111/j.1476-5381.1993.tb13830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PONTI F., GIBELLI G., CROCI T., ARCIDIACO M., CREMA F., MANARA L. Functional evidence of atypical β3-adrenoceptors in the human colon using the β3-selective adrenoceptor antagonist, SR 59230A. Br. J. Pharmacol. 1996;117:1374–1376. doi: 10.1111/j.1476-5381.1996.tb15294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMÉR M. Inhibitory β-adrenoceptors in the urinary bladder of the rat. Life Sci. 1974;15:273–280. doi: 10.1016/0024-3205(74)90217-3. [DOI] [PubMed] [Google Scholar]
- EMORINE L.J., BLIN N., STROSBERG A.D. The human β3-adrenoceptor: the search for a physiological function. Trends Pharmacol. Sci. 1994;15:3–7. doi: 10.1016/0165-6147(94)90118-x. [DOI] [PubMed] [Google Scholar]
- EMORINE L.J., MARULLO S., BRIEND-SUREN M.M., PATEY G., TATE K., DELAVIER-KLUTCHKO C., STROSBERG A.D. Molecular characterization of the human β3-adrenergic receptor. Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- GOEPEL M., WITTMANN A., RUBBEN H., MICHEL M.C. Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol. Res. 1997;25:199–206. doi: 10.1007/BF00941983. [DOI] [PubMed] [Google Scholar]
- IGAWA Y., YAMAZAKI Y., TAKEDA H., AKAHANE M., AJISAWA Y., YONEYAMA T., NISHIZAWA O. Possible β3-adrenoceptor-mediated relaxation of the human detrusor. Acta. Physiol. Scand. 1998;164:117–118. doi: 10.1046/j.1365-201X.1998.00406.x. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Is there a third heart β-adrenoceptor. Trends Pharmacol. Sci. 1989;10:316–320. doi: 10.1016/0165-6147(89)90065-5. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. (−)-CGP 12177-induced increase of human atrial contraction through a putative third β-adrenoceptor. Br. J. Pharmacol. 1996;117:93–98. doi: 10.1111/j.1476-5381.1996.tb15159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J. Four β-adrenoceptor subtypes in the mammalian heart. Trends Pharmacol. Sci. 1997;18:70–76. doi: 10.1016/s0165-6147(96)01033-4. [DOI] [PubMed] [Google Scholar]
- KOIKE K., HORINOUCHI T., TAKAYANAGI I. Possible mechanisms of β-adrenoceptor-mediated relaxation induced by noradrenaline in guinea pig taenia caecum. Eur. J. Pharmacol. 1995;279:159–163. doi: 10.1016/0014-2999(95)00147-d. [DOI] [PubMed] [Google Scholar]
- KRIEF S., LÖNNQVIST F., RAIMBAULT S., BAUDE B., SPRONSEN A.V., ARNER P., STRÖSBERG A.D., RICQUIER D., EMORINE L.J. Tissue distribution of β3-adrenergic receptor mRNA in man. J. Clin. Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS A.M., ARNOLD A., MCAULIFF J.P., LUDUENA F.P., BROWN T.G., JR Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967a;214:597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- LANDS A.M., LUDUENA F.P., BUZZO H.J. Differentiation of receptors responsive to isoproterenol. Life Sci. 1967b;6:2241–2249. doi: 10.1016/0024-3205(67)90031-8. [DOI] [PubMed] [Google Scholar]
- LANGIN D., PORTILLO M.P., SAULNIER-BLACHE J.-S., LAFONTAN M. Coexistence of three β-adrenoceptor subtypes in white fat cells of various mammalian species. Eur. J. Pharmacol. 1991;199:291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- LARSEN J.-J. α- and β-adrenoceptors in the detrusor muscle and bladder base of the pig and β-adrenoceptors in the detrusor muscle of man. Br. J. Pharmacol. 1979;65:215–222. doi: 10.1111/j.1476-5381.1979.tb07821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN R.M., RUGGIERI M.R., WEIN A.J. Identification of receptor subtypes in the rabbit and human urinary bladder by selective radio-ligand binding. J. Urol. 1988;139:844–848. doi: 10.1016/s0022-5347(17)42659-0. [DOI] [PubMed] [Google Scholar]
- LI J.H., YASAY G.D., KAU S.T. β-Adrenoceptor subtypes in the detrusor of guinea-pig urinary bladder. Pharmacology. 1992;44:13–18. doi: 10.1159/000138868. [DOI] [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., FUR G.L. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NERGÅRDH A., BORÉUS L.O., NAGLO A.-S. Characterization of the adrenergic beta-receptor in the urinary bladder of man and cat. Acta. Pharmacol. Toxicol. 1977;40:14–21. doi: 10.1111/j.1600-0773.1977.tb02049.x. [DOI] [PubMed] [Google Scholar]
- ORIOWO M.A., CHAPMAN H., KIRKHAM D.M., SENNITT M.V., RUFFOLO R.R., JR, CAWTHORNE M.A. The selectivity in vitro of the stereoisomers of the beta-3 adrenoceptor agonist BRL37344. J. Pharmacol. Exp. Thera. 1996;277:22–27. [PubMed] [Google Scholar]
- OZAKI N., KAWAKITA S., TODA N. Effects of dobutamine on isolated canine cerebral, coronary, mesenteric, and renal arteries. J. Cardiovasc. Pharmacol. 1982;4:456–461. doi: 10.1097/00005344-198205000-00017. [DOI] [PubMed] [Google Scholar]
- RUFFOLO R.R. The pharmacology of dobutamine. Am. J. Med. Sci. 1987;294:244–248. doi: 10.1097/00000441-198710000-00005. [DOI] [PubMed] [Google Scholar]
- RUFFOLO R.R., JR, MESSICK K., HORNG J.S. Interactions of the three inotropic agents, ASL-7022, dobutamine, and dopamine, with α- and β-adrenoceptors in vitro. Naynyn-Schmiedeberg's Arch. Pharmacol. 1984;326:317–326. doi: 10.1007/BF00501436. [DOI] [PubMed] [Google Scholar]
- SEGUCHI H., NISHIMURA J., ZHOU Y., NIIRO N., KUMAZAWA J., KANAIDE H. Expression of β3-adrenoceptors in rat detrusor smooth muscle. J. Urol. 1998;159:2197–2201. doi: 10.1016/S0022-5347(01)63305-6. [DOI] [PubMed] [Google Scholar]
- TSO J.Y., SUN X.H., KAO T.H., REECE K.S., WU R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: geomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAZAKI Y., TAKEDA H., AKAHANE M., IGAWA Y., NISHIZAWA O., AJISAWA Y. Species differences in the distribution of β-adrenoceptor subtypes in bladder smooth muscle. Br. J. Pharmacol. 1998;124:593–599. doi: 10.1038/sj.bjp.0701870. [DOI] [PMC free article] [PubMed] [Google Scholar]


