Abstract
The present study was performed to investigate the ability of the multidrug resistance protein (MRP1) to transport different cationic substrates in comparison with MDR1-P-glycoprotein (MDR1). Transport studies were performed with isolated membrane vesicles from in vitro selected multidrug resistant cell lines overexpressing MDR1 (A2780AD) or MRP1 (GLC4/Adr) and a MRP1-transfected cell line (S1(MRP)).
As substrates we used 3H-labelled derivatives of the hydrophilic monoquaternary cation N-(4′,4′-azo-n-pentyl)-21-deoxy-ajmalinium (APDA), the basic drug vincristine and the more hydrophobic basic drug daunorubicin. All three are known MDR1-substrates.
MRP1 did not mediate transport of these substrates per se. In the presence of reduced glutathione (GSH), there was an ATP-dependent uptake of vincristine and daunorubicin, but not of APDA, into GLC4/Adr and S1(MRP) membrane vesicles which could be inhibited by the MRP1-inhibitor MK571.
ATP- and GSH-dependent transport of daunorubicin and vincristine into GLC4/Adr membrane vesicles was inhibited by the MRP1-specific monoclonal antibody QCRL-3.
MRP1-mediated daunorubicin transport rates were dependent on the concentration of GSH and were maximal at concentrations ⩾10 mM. The apparent KM value for GSH was 2.7 mM. Transport of daunorubicin in the presence of 10 mM GSH was inhibited by MK571 with an IC50 of 0.4 μM.
In conclusion, these results demonstrate that MRP1 transports vincristine and daunorubicin in an ATP- and GSH-dependent manner. APDA is not a substrate for MRP1.
Keywords: Drug resistance, ABC transporter, MDR1, MRP1, ATP-dependent drug transport, GSH, daunorubicin, vincristine, APDA
Introduction
Human multidrug resistance is frequently associated with the overexpression of the MDR1-P-glycoprotein (MDR1) (Juliano & Ling, 1976) and/or the multidrug resistance protein (MRP1) (Cole et al., 1992), two members of the ATP binding cassette (ABC) transporter superfamily (Higgins, 1992). Although they share only 15% amino acid sequence homology, both proteins confer resistance to a broad range of natural product drugs in drug selected cell lines as well as in transfected cells (Loe et al., 1996c; Broxterman et al., 1995; Gottesman & Pastan, 1993). Currently, it is generally accepted that MDR1 functions as an ATP-dependent transport protein capable of reducing intracellular levels of various natural product drugs (Borst & Schinkel, 1997; Germann, 1996; Ruetz & Gros, 1994a). Most of these drugs, e.g. vinca alkaloids, anthracyclines, epipodophillotoxins, actinomycin D and paclitaxel, have unrelated chemical structures but share the property of being hydrophobic compounds.
MRP1 was first identified in tumour cells with a multidrug resistant phenotype without overexpression of MDR1 (Cole et al., 1992). Although MRP1 is able to confer resistance to drugs which are MDR1 substrates, the substrate specificity of MRP1 seems different from that of MDR1. Transport studies with membrane vesicles isolated from MRP1 overexpressing cells, either in vitro selected or transfected, revealed that MRP1 is a transporter of multivalent organic anions, preferentially glutathione S-conjugates (GS S-conjugates), (Barnouin et al., 1998; Loe et al., 1996b; 1997; Jedlitschky et al., 1996; Müller et al., 1994b), but also of sulphate conjugates (Jedlitschky et al., 1996) and glucuronides (Jedlitschky et al., 1996; Loe et al., 1996a). Also oxidized glutathione (GSSG), complexes of reduced glutathione (GSH) with arsenite (Zaman et al., 1995) and unmodified compounds in the presence of GSH (Loe et al., 1996b; 1997) are MRP1 substrates. In view of its substrate specificity and the ubiquitous expression of MRP1 in human tissues (Zaman et al., 1993) and blood cells (Burger et al., 1994), the putative physiological role of MRP1 seems to be cellular extrusion of metabolites of GSH-dependent detoxification reactions (Müller et al., 1996).
The molecular basis of the drug specificity of MRP1 is not fully understood. Two models currently exist describing the drug transport mechanism of MRP1. First, MRP1 may be a transporter of drug conjugates (e.g. GS S-conjugates and glucuronide conjugates) (Priebe et al., 1998; Jedlitschky et al., 1996; Ishikawa et al., 1995), however, stable GS S-conjugates of vincristine and daunorubicin could not be detected in HPLC-analysed media from MRP1 transfected cells (Zaman et al., 1995). Moreover, there is no evidence that the chemotherapeutic agents to which MRP1 confers resistance, are substrates for GSH- or glucuronic acid conjugation (for review see O'Brien & Tew, 1996; Tew, 1994). Secondly, MRP1 may transport unmodified natural product drugs in the presence of GSH. For example, it has been demonstrated that unmodified vincristine is transported by MRP1 but only in the presence of physiological concentrations of GSH (Loe et al., 1996b). Furthermore, resistance to vinca alkaloids and anthracyclines is reversed by GSH depletion in MRP1-overexpressing cells, but not in cells overexpressing MDR1 (Zaman et al., 1995; Versantvoort et al., 1995). Therefore, GSH appears to play an important role in MRP1-mediated drug resistance. In this study we compared MDR1 and MRP1 with respect to their ability to transport different classes of cationic drugs and investigated the role of GSH in MRP1-mediated transport. We hypothesized that the hydrophobic basic drug daunorubicin has not necessarily to be conjugated with GSH to be a MRP1 substrate (Priebe et al., 1998; Ishikawa et al., 1995), but is transported by MRP1 in the presence of GSH. We used vincristine as a previously demonstrated MRP1 substrate (Loe et al., 1996b) to characterize our experimental system. Furthermore, experiments were performed with the permanently positively charged monoquaternary cationic derivate of the anti-arrhythmic drug ajmaline (N-(4′, 4′-azo-n-pentyl)-21-deoxy-ajmalinium, APDA) to study the ability of MRP1 to mediate the transport of more hydrophilic cations which are excellent MDR1 substrates in vitro.
Methods
Chemicals
N - ( 4′,4′-azo-n-pentyl ) - 21 - deoxy - [ 21 -3H ] -ajmalinium [3H]-(APDA) (46 GBq mmol−1) (Müller et al., 1994a), was kindly provided by Dr G. Kurz (University of Freiburg, Germany), [G-3H]-vincristine (226 GBq mmol−1) was obtained from Amersham (Little Chalfont, U.K.) and [14,15,19,20-3H(N)]-leukotriene C4 ([3H]-LTC4) (4884 GBq mmol−1) and [G-3H]-daunorubicin (46.62 GBq mmol−1) were from NEN (Boston, MA, U.S.A.). Benzonase, grade I protease free, was from Merck (Darmstadt, Germany). ATP, creatine phosphate and creatine kinase were purchased from Boehringer (Mannheim, Germany). The MRP1 inhibitor MK571 was kindly provided by Dr A.W. Ford-Hutchinson (Merck-Frosst, Pointe Claire-Dorval, Quebec, Canada) and the MDR1 inhibitor PSC833 was a kind gift of Novartis (Basel, Switzerland). The monoclonal antibody (mAb) QCRL-3, directed against MRP1, was obtained from Signet Laboratories (Dedham, MA, U.S.A.). β,γ-Methyleneadenosine 5′-triphosphate (AMP-PCP), GSH, the vacuolar H+-ATPase (V-type ATPase) inhibitor bafilomycin A1 (Baf) and all other chemicals were from Sigma (St. Louis, MO, U.S.A.).
Cell culture
Culture procedures for the human GLC4 small cell lung cancer line and its doxorubicin selected multidrug resistant counterpart GLC4/Adr have been described previously (Zijlstra et al., 1987). Culture of the non-small cell lung cancer cell line SW1573/S1, further designated as S1, and of its MRP1 transfected subline S1(MRP) was performed as reported (Zaman et al., 1994) only with stepwise increase of the Geneticin concentration to 0.4 mg ml−1 (Gibco, Paisley, U.K.). The human ovarian tumour cell line A2780 was cultured in RPMI1640 medium, supplemented with 10% foetal calf serum (FCS), (Gibco). The multidrug resistant A2780AD line was isolated from the A2780 line by a multistep selection with doxorubicin and maintained in RPMI1640 medium supplemented with 10% FCS as described (Rogan et al., 1984).
Preparation of membrane vesicles
Membrane vesicles were prepared as described previously (Müller et al., 1994b) with minor modifications. Briefly, cells were harvested, washed with phosphate-buffered saline (PBS, mM: NaCl 137, KCl 2.7, Na2HPO4 10.1, KH2PO4 1.8, pH 7.4) and centrifuged at 180×g, for 10 min at 4°C. The resulting pellet was diluted 40 fold in hypotonic buffer (1 mM NaHCO3, pH 7.4) and stirred gently in the presence of 100 U Benzonase for 1 h. The cell lysate was centrifuged at 100,000×g for 30 min at 4°C and the remaining pellet was resuspended in 5 ml isotonic TS buffer (10 mM Tris/250 mM sucrose, pH 7.4) and homogenized with a Dounce B homogenizer in the presence of 100 U Benzonase. The crude membrane fraction was layered on top of a 38% (w/v) sucrose solution in 10 mM Tris (pH 7.4) and centrifuged at 280,000×g for 1 h at 4°C in a swing out rotor. The interface layer was collected, diluted to 25 ml in TS buffer and centrifuged at 100,000×g for 30 min at 4°C. The resulting pellet was resuspended in 300–500 μl isotonic buffer. Vesicles were formed by passing the suspension 20 times through a 25 gauge needle. The membrane vesicles were snap frozen in liquid nitrogen and stored at −80°C. Protein content was measured by a Bradford-based Biorad protein assay (Biorad laboratories, Hercules, CA, U.S.A.).
Immunodetection of MRP1 and MDR1
Ten μg protein of each membrane preparation was separated on a SDS/7.5% polyacrylamide gel and transferred to nitrocellulose (Amersham) by electroblotting. MRP1 was detected by the rat monoclonal MRPr1 (1 : 500), kindly provided by Dr R. Scheper, (Free University, Amsterdam, the Netherlands). MDR1 was detected by the monoclonal antibody C219 (1 : 500), (Centocor, Malvern, MA, U.S.A.). MRP2 protein levels were analysed with the monoclonal antibody M2 III-5, a kind gift of Dr R. Oude Elferink (Academic Medical Centre, Amsterdam, the Netherlands). Primary antibodies were visualized by enhanced chemiluminescence (ECL), (Pierce, Rockford, IL, U.S.A.).
Transport studies
Uptake of [3H]-APDA (300 nM), [3H]-vincristine (300 nM) and [3H]-daunorubicin (600 nM) into membrane vesicles was measured by a rapid filtration technique. Membrane vesicles (50 μg protein) were rapidly thawed and added to the buffer system containing 4 mM ATP or AMP-PCP, 10 mM MgCl2, 10 mM creatine phosphate, 100 μg ml−1 creatine kinase, 10 mM Tris pH 7.4 and 250 mM sucrose. After 1 min prewarming at 37°C, the substrate was added (110 μl final volume). At indicated time points, samples (25 μl) were taken and diluted in 1 ml ice cold stopsolution (PBS). These solutions were subsequently filtered through OE66 cellulose acetate filters, pore size 0.2 μm (Schleicher & Schuell, Dassel, Germany), presoaked in PBS. Filters were rinsed with 5 ml PBS/0.05% Tween 20 followed by 5 ml PBS. After rinsing, the filters were air dried and radioactivity was counted with a liquid scintillation counter. Experiments with [3H]-daunorubicin were performed similarly, except that the PBS stopsolution contained 1 mM ethidium bromide, the filters were presoaked in PBS/1 mM ethidium bromide and the filters were first washed by 5 ml PBS/1 mM ethidium bromide, followed by PBS/0.05% Tween 20 and 5 ml PBS. This method reduced the background binding of [3H]-daunorubicin with 70–80% and resulted in a signal to noise ratio of 10 : 2–3. Experiments with [3H]-LTC4 (1.5 nM) were performed with 10 μg protein, NC45 nitrocellulose filters, pore size 0.45 μm (Schleicher & Schuell), and 10 mM Tris/250 mM sucrose buffer (pH 7.4) instead of PBS. If required, PSC833, MK571, GSH and/or Baf were added together with the membrane vesicles to the buffer system.
Time course experiments of [3H]-daunorubicin (600 nM) uptake were carried out with membrane vesicles from GLC4/Adr cells (100 μg protein in 220 μl reaction volume) in the presence of 1 μM Baf and in the presence or the absence of 5 mM GSH. At indicated time points, samples of 25 μl were taken and treated as described above. Aspecific binding of [3H]-daunorubicin was measured at 4°C in the presence of 4 mM ATP and 5 mM GSH and subtracted from all values obtained at 37°C.
Uptake experiments in the presence of the MRP1-specific monoclonal antibody (mAb) QCRL-3 or the control mAb directed against the T-cell marker CD3 (a kind gift of Dr B.J. Kroesen, University Hospital Groningen) were performed for 1 min as described above. The mAbs were added together with the membrane vesicles to the buffer system.
All transport data are presented as the difference of the values measured in the presence of ATP and in the presence of AMP-PCP. The non-hydrolyzable ATP analogue AMP-PCP did not support uptake, demonstrating that ATP hydrolysis was required.
Results
Immunoblot analysis of MRP1 and MDR1
The levels of MRP1 and MDR1 in membrane subfractions were analysed by immunoblotting. Increased levels of the 190 kDa MRP1 (Müller et al., 1994b; Roelofsen et al., 1997) were detected in membranes from GLC4/Adr and MRP1-transfected S1(MRP) cells, while membranes isolated from A2780, A2780AD, GLC4 and S1 cells exhibited lower levels (Figure 1). MDR1 was found to be overexpressed in membranes isolated from A2780AD cells. No expression of MDR1 was found in membrane preparations from the other cell lines. MRP2 was not detected in membranes from any of these cell lines (data not shown).
Figure 1.
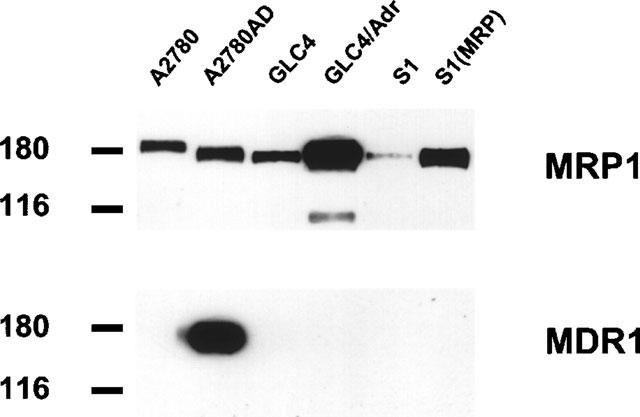
Immunoblot analysis of isolated membrane subfractions. Membrane proteins (10 μg) were resolved on 7.5% SDS–PAGE and transferred to nitrocellulose by electroblotting. Protein levels were analysed with monoclonal antibodies raised against MRP1 (MRPr1) and MDR1 (C219). Primary antibodies were visualized by enhanced chemiluminescence. Sizes of molecular weight markers are indicated in kDa.
ATP-dependent uptake of cationic drugs in membrane vesicles from MDR1- and MRP1-overexpressing cells
Transport properties of MDR1 and MRP1 were compared with two hydrophobic basic drugs ([3H]-vincristine and [3H]-daunorubicin) and the more hydrophilic cation ([3H]-APDA). Figure 2a shows ATP-dependent uptake of [3H]-APDA, [3H]-vincristine and [3H]-daunorubicin into membrane vesicles from A2780 and A2780AD cells. ATP-dependent uptake of all three substrates into A2780AD membrane vesicles was increased compared to A2780 membrane vesicles. This uptake was inhibited by the MDR1 inhibitor PSC833. These results indicate that the ATP-dependent transport of [3H]-APDA, [3H]-vincristine and [3H]-daunorubicin into membrane vesicles from A2780AD is mediated by MDR1.
Figure 2.
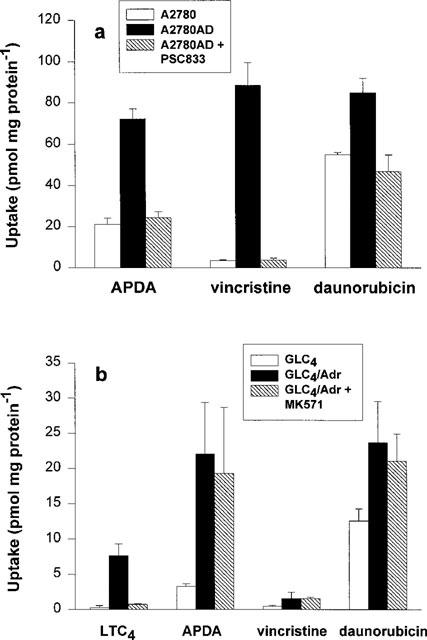
ATP-dependent uptake of cationic drugs into membrane vesicles from MDR1- and MRP1-overexpressing cells. Uptake of [3H]-APDA (300 nM), [3H]-vincristine (300 nM) and [3H]-daunorubicin (300 nM) into membrane vesicles (50 μg) from A2780 and A2780AD cells was measured during 5 min as described under ‘experimental procedures'. Uptake into A2780AD membrane vesicles was also measured in the presence of 1 μM of the MDR1 inhibitor PSC833 (a). Similar experiments were performed with membrane vesicles from GLC4 and from GLC4/Adr cells either in the absence or the presence of 5 μM of the MRP1 inhibitor MK571 (b). [3H]-LTC4 was used as control substrate for MRP1-mediated transport. Data shown are means ±s.d. from at least three experiments with at least triplicate determinations.
Under the same conditions, transport studies were performed with membrane vesicles from GLC4 cells and the MRP1-overexpressing GLC4/Adr cell line (Figure 2b). The cysteinyl-leukotriene LTC4 was used as a control substrate for MRP1-mediated transport. ATP-dependent transport of [3H]-LTC4 into membrane vesicles from GLC4/Adr cells was 16 fold higher than the uptake into GLC4 membrane vesicles. This was inhibited by the MRP1 inhibitor MK571. In the presence of ATP, uptake of [3H]-APDA and [3H]-daunorubicin into GLC4/Adr membrane vesicles was significantly higher than into GLC4 membrane vesicles. [3H]-vincristine uptake was only modestly higher. In contrast to [3H]-LTC4, uptake of these three cationic compounds into GLC4/Adr membrane vesicles was not inhibited by MK571. The uptake of [3H]-APDA, [3H]-vincristine and [3H]-daunorubicin into membrane vesicles from S1(MRP) and S1 cells was similar. Furthermore, uptake of all three substrates into S1(MRP) membrane vesicles was not inhibited by MK571 (data not shown). These results indicate that the uptake of [3H]-APDA, [3H]-vincristine and [3H]-daunorubicin per se into membrane vesicles from MRP1-overexpressing cells, although ATP-dependent, is not mediated by MRP1.
MRP1-mediated transport of [3H]-vincristine and [3H]-daunorubicin but not of [3H]-APDA in the presence of GSH
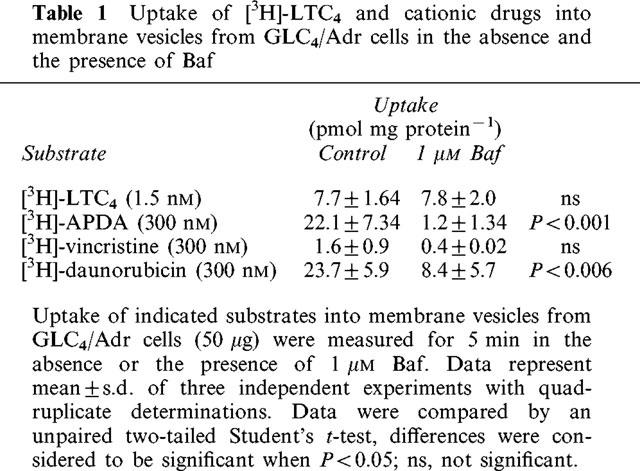
Next, we investigated the GSH-dependency of MRP1-mediated transport. The above described non-MRP1-mediated uptake of the monoquaternary cation [3H]-APDA and the weak base [3H]-daunorubicin into GLC4/Adr membrane vesicles (Figure 2b) might be driven by a proton gradient. To eliminate this uptake we used the vacuolar H+-ATPase (V-type ATPase) inhibitor bafilomycin A1 (Baf) to block the potential involvement of proton gradient generating V-type ATPases. Uptake of [3H]-APDA and [3H]-daunorubicin into GLC4/Adr membrane vesicles in the presence of 1 μM Baf was reduced to 5 and 35% of the control values, respectively, while [3H]-vincristine uptake was only slightly inhibited. In addition, Baf did not alter [3H]-LTC4 uptake (Table 1). These results indicate that Baf reduces non-MRP1-mediated uptake of cationic compounds into GLC4/Adr membrane vesicles without affecting the transport activity of MRP1. Therefore, all experiments to investigate the role of GSH in MRP1-mediated cation transport were performed in the presence of 1 μM Baf.
Table 1.
Uptake of [3H]-LTC4 and cationic drugs into membrane vesicles from GLC4/Adr cells in the absence and the presence of Baf
GSH (5 mM) did not affect the uptake [3H]-APDA into GLC4/Adr membrane vesicles (Figure 3a). However, the uptake of [3H]-vincristine and [3H]-daunorubicin, in the presence of ATP, was stimulated by GSH. This stimulated uptake was inhibited by MK571. With membrane vesicles from S1(MRP) cells, similar results were obtained (Figure 3b). The levels of GSH-mediated uptake of [3H]-vincristine and [3H]-daunorubicin into GLC4/Adr and into S1(MRP) membrane vesicles correlated with levels of MRP1 protein expression (Figure 1). These data show that MRP1-mediated ATP-dependent transport of daunorubicin appears to be GSH-dependent. To gain more insight into the initial uptake phase, a 2 min time course of [3H]-daunorubicin uptake was measured (Figure 4). Because of a relatively low overexpression of MRP1 in S1(MRP) membrane vesicles, GLC4/Adr membrane vesicles were used for these experiments. In the presence of 5 mM GSH, the uptake of [3H]-daunorubicin was linear up to 1 min. After 3 min, a steady state uptake of about 30 pmol mg protein−1 was reached which did not significantly change during the next 7 min (data not shown). The non-hydrolyzable ATP analogue AMP-PCP did not stimulate uptake, indicating that GSH-dependent uptake of [3H]-daunorubicin requires ATP hydrolysis. MK571 (2 μM) completely inhibited the GSH-dependent [3H]-daunorubicin uptake.
Figure 3.
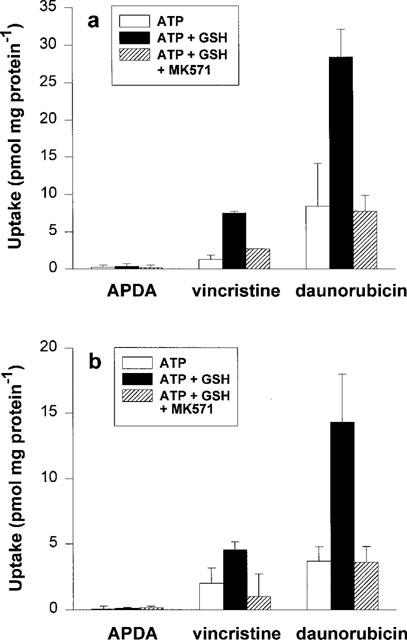
ATP-dependent uptake of cationic drugs in the presence of GSH. (a) represents uptake during 5 min of [3H]-APDA (300 nM), [3H]-vincristine (300 nM) and [3H]-daunorubicin (600 nM) into membrane vesicles prepared from GLC4/Adr cells in the absence and the presence of 5 mM GSH. Uptake was also measured in the presence of 5 μM MK571. (b) represents the same experiments with MRP1-transfected S1 (MRP) cells. All experiments were performed in the presence of 1 μM Baf. Data represent mean±s.d. from at least two experiments with quadruplicate determinations.
Figure 4.
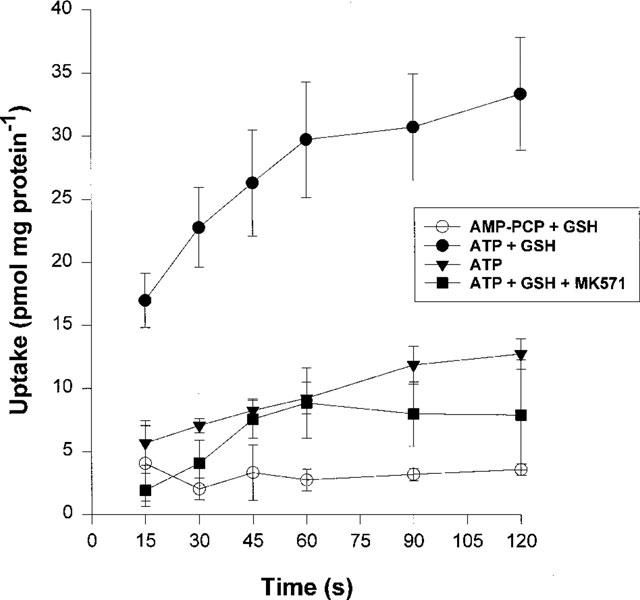
Time course of MRP1-mediated daunorubicin transport. Initial uptake of [3H]-daunorubicin (600 nM) into GLC4/Adr membrane vesicles (100 μg protein, 220 μl final volume) was followed during 2 min with different conditions. All experiments were performed in the presence of 1 μM Baf. GSH and MK571 were added to a final concentration of 5 mM and 2 μM, respectively. Data points are means±s.d. from at least four independent experiments with quadruplicate measurements. Experiments were performed with two different batches of membrane vesicles.
Inhibition of [3H]-vincristine, [3H]-daunorubicin and [3H]-LTC4 transport with the monoclonal antibody (mAb) QCRL-3
To demonstrate that transport of [3H]-daunorubicin and [3H]-vincristine is specifically mediated by MRP1, uptake of both cations into GLC4/Adr membrane vesicles was measured in the presence of the mAb QCRL-3 which recognizes a conformation dependent MRP1-epitope (Hipfner et al., 1994). [3H]-LTC4 was used as positive control for QCRL-3 inhibition. Results are presented in Figure 5. Transport of all three substrates was inhibited by QCRL-3 with IC50's of 2, 20 and 12 μg ml−1 (0.23, 2.3 and 1.4 μg mAb per 50 μg protein) respectively for [3H]-daunorubicin, [3H]-vincristine and [3H]-LTC4. A control mAb directed against the T-cell marker CD3 with an identical isotype as QCRL-3 did not affect [3H]-LTC4 transport and only slightly inhibited transport of [3H]-daunorubicin and [3H]-vincristine (less than 15% at the highest QCRL-3 concentration, data not shown).
Figure 5.
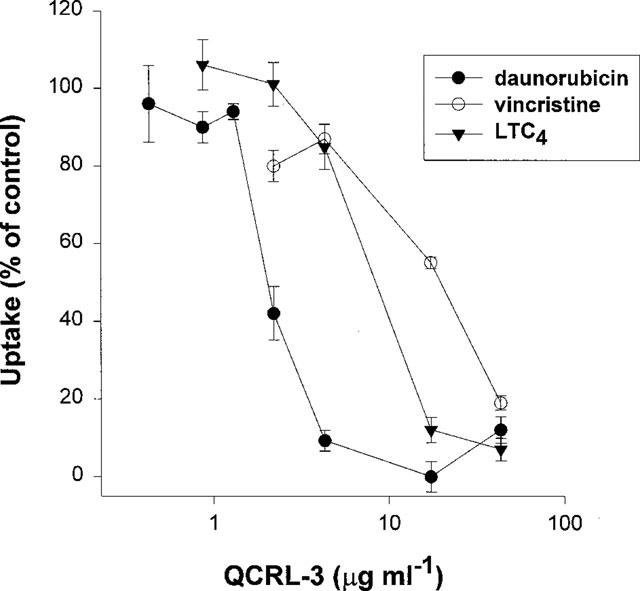
Inhibition of MRP1-mediated transport by the mAb QCRL-3. Uptake of [3H]-daunorubicin (600 nM), [3H]-vincristine (300 nM) into GLC4/Adr membrane vesicles (50 μg) was measured for 1 min in the presence of 1 μM Baf, 5 mM GSH and increasing concentrations of the MRP1 specific mAb QCRL-3. [3H]-LTC4 (1.5 nM) was used as control for MRP1 inhibition by QCRL-3. Data are plotted as percentages of control. Uptake rates of control experiments were 19, 4.3 and 3.9 pmol mg protein−1 min−1, respectively, for [3H]-daunorubicin, [3H]-vincristine and [3H]-LTC4. Data points represent means±s.e.mean of triplicate measurements in a single experiment.
Dependency of MRP1-mediated daunorubicin transport on GSH concentration
The effect of different GSH concentrations on MRP1-mediated daunorubicin transport was investigated with GLC4/Adr membrane vesicles. Figure 6 shows that [3H]-daunorubicin transport was stimulated by increased concentrations of GSH with a maximum of 10 mM after which a steady state level was reached. The KM value was determined to be 2.7 mM.
Figure 6.
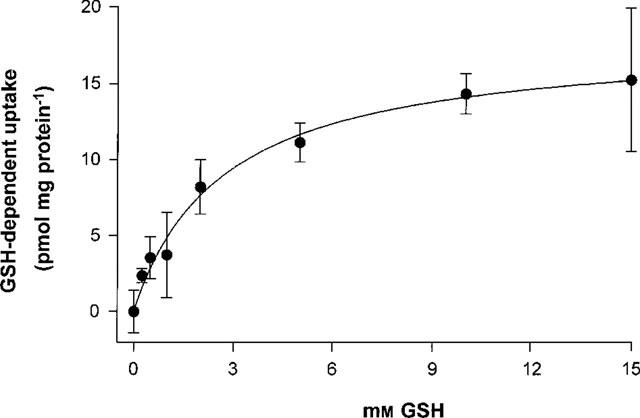
Dependency of MRP1-mediated daunorubicin transport on the GSH concentration. Uptake of [3H]-daunorubicin into GLC4/Adr membrane vesicles was measured for 1 min as described at Figure 5 in the presence of different GSH concentrations. Data points represent the difference between ATP-dependent uptake and ATP-dependent uptake in the presence of GSH. Data points are means±s.e.mean of at least triplicate measurements in a single experiment. Two additional experiments showed the same results. Curve fitting and calculation of the KM> value was performed with the Graphpad Prism™ program. The initial ATP-dependent uptake of daunorubicin in the absence of GSH was 9.8±1.4 pmol mg protein−1.
Determination of IC50 value of MK571 for MRP1-mediated daunorubicin transport
The inhibitory effect of MK571 on MRP1-mediated dauno-rubicin transport was measured in the presence of 10 mM GSH. MK571 effectively inhibited GSH-stimulated [3H]-daunorubicin transport in a dose-dependent manner with an IC50 of 0.4 μM (Figure 7).
Figure 7.
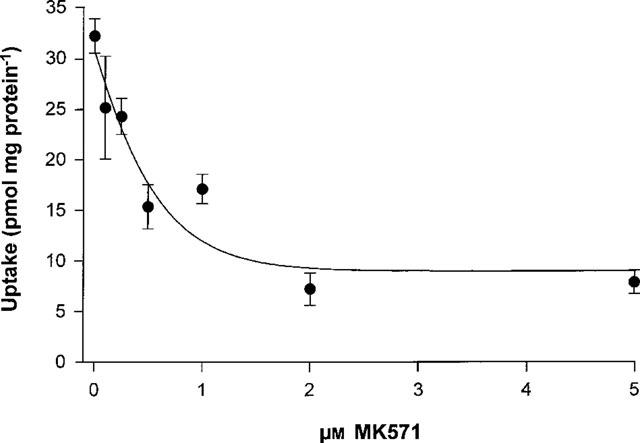
Determination of IC50 for MK571 of GSH-dependent daunorubicin transport by MRPI. Uptake of [3H]-daunorubicin into GLC4/Adr membrane vesicles was measured in the presence of 10 mM GSH with different concentrations of MK571 as described at Figure 5. Data points represent means±s.e.mean of triplicate measurements in a single experiment. The experiment was performed twice with similar results. Curve fitting and calculation of the IC50 value was performed with the Graphpad Prism™ program.
Discussion
MDR1 and MRP1, two distantly related members of the large superfamily of ABC-transporter proteins, are both able to confer resistance of cells against a broad spectrum of natural product drugs (Loe et al., 1996c; Broxterman et al., 1995; Gottesman & Pastan, 1993). MDR1-related multidrug resistance is most likely due to the ATP-dependent extrusion of unmodified drugs from the cells mediated by the MDR1 protein (Dong et al., 1996; Urbatsch et al., 1995; Sharom et al., 1995; Shapiro & Ling, 1994; Ruetz & Gros, 1994b; Horio et al., 1988). Also MRP1-mediated multidrug resistance depends on the transport capacity of MRP1, but the mechanism by which this protein mediates drug transport appears to differ from MDR1. Most MRP1 substrates are organic compounds that need to be conjugated with e.g. GSH before being transported. Also some organic cationic drugs appear to be MRP1 substrates. Although these substrates are not known to form GS S-conjugates, it has been shown that the presence of GSH is required for MRP1-associated resistance against the cytotoxicity of these drugs (Versantvoort et al., 1995; Zaman et al., 1995). This suggests that for MRP1-mediated extrusion of these cations, GSH is required without a need for conjugation. This prompted us to investigate the role of GSH in MRP1-mediated organic cation transport.
We have used three cationic substrates: two more hydrophobic basic drugs [3H]-vincristine and [3H]-daunorubicin and the permanently positively charged organic cation [3H]-APDA. These drugs are substrates for MDR1 as demonstrated by us in this study and by others previously (Müller et al., 1994a; Tamai & Safa, 1990; Kamimoto et al., 1989). To test the ability of MRP1 to transport these substrates in vitro we have used membrane vesicles prepared from the GLC4/Adr cell line. This cell line was used for two reasons. First, GLC4/Adr exhibits a very high overexpression of MRP1. This high level could not be found in MRP1-transfected cell lines such as S1(MRP) or even not in Spodoptera frugiperda or Trichoplusia ni (High Five™) insect cells, overexpressing MRP1 by using the baculovirus expression system (Renes et al., unpublished data). Furthermore, it has been demonstrated recently that GLC4/Adr cells exclusively overexpress MRP1 but not the isoforms MRP2, MRP3, MRP4 and MRP5 (Kool et al., 1997).
We showed that [3H]-APDA and [3H]-daunorubicin, and to a much lower extent [3H]-vincristine, were taken up in membrane vesicles from GLC4/Adr cells in the presence of ATP but not in the presence of AMP-PCP. However, this uptake was not inhibited by the leukotriene D4 receptor antagonist and MRP1-inhibitor MK571 (Gekeler et al., 1995; Jones et al., 1989). This is a clear indication that MRP1, in contrast to MDR1, is not directly involved in ATP-dependent transport of these cationic substrates. We have recently demonstrated that GLC4/Adr cells have a more pronounced Golgi apparatus and contain more intracellular vesicular structures than GLC4 cells (van Luyn et al., 1998). We therefore speculated that addition of ATP might activate V-type ATPases that are present in membranes of certain intracellular organelles. These V-type ATPases generate a proton-gradient by hydrolyzing ATP and are involved in acidification of intracellular compartments of the endocytotic and exocytotic pathway (for review see Gluck et al., 1996; Mellman et al., 1986). The crude membrane preparations we used contain plasma membranes as well as intracellular organelles, including acidic vesicular structures which could be responsible for the MRP1-independent uptake of cations. The macrolide antibiotic bafilomycin A1 (Baf) is known to function as a specific inhibitor for V-type ATPases (Bowman et al., 1988). Addition of Baf blocked ATP-stimulated uptake of the cations into GLC4/Adr membrane vesicles but it did not influence MRP1-mediated transport of [3H]-LTC4. Therefore, Baf was used in all further experiments.
Several reports have suggested an important role of GSH in MRP1-mediated drug efflux from cells with a multidrug resistant phenotype (O'Brien & Tew, 1996 and references therein). Experiments with MRP1-transfected cells showed that depletion of GSH results in a complete reversal of resistance to vinca alkaloids and anthracyclines (Zaman et al., 1995). Furthermore, MRP1 mediates vincristine transport only in the presence of GSH (Loe et al., 1996b, this study). Reducing agents such as 2-mercaptoethanol, dithiothreitol and L-cysteine, have been tested but none of these could increase uptake of [3H]-vincristine in membrane vesicles from MRP1 transfected cells (Loe et al., 1996b) indicating that it is not the reducing capacity of GSH that is responsible for this effect. GSH itself seems to play an essential role in MRP1-mediated transport of hydrophobic drugs and our findings support this. In this study we show that in the presence of physiological concentrations of GSH, the hydrophobic basic drug dauno-rubicin is transported by MRP1 in an ATP-dependent manner. The rate of MRP1-mediated daunorubicin transport is dependent on the concentration of GSH. This implies that changes in the intracellular GSH concentration will have a marked effect on the MRP1-mediated daunorubicin transport from drug resistant cells.
The specificity of MRP1-mediated cation transport, in particular of daunorubicin, was demonstrated by the MRP1-specific mAb QCRL-3 (Loe et al., 1996b; 1997) and by MK571 inhibition. QCRL-3 inhibited MRP1-mediated transport of daunorubicin in the presence of GSH better than LTC4 transport. One explanation for this effect may be a difference in binding sites for hydrophobic cations and for anions at MRP1. The competitive inhibitor MK571 inhibited MRP1-mediated GSH-dependent daunorubicin transport in a dose-dependent manner with an IC50 value of 0.4 μM. This is similar to what has been reported for LTC4-inhibition (Leier et al., 1994). The difference between MRP1 inhibition by QCRL-3 and by MK571 can be explained by the difference in binding of each of these compounds to MRP1.
The mechanism by which GSH facilitates MRP1-mediated transport of hydrophobic cationic drugs has not yet been fully elucidated. There is no evidence for conjugation of GSH to drugs to which MRP1 confers resistance (O'Brien & Tew, 1996; Zaman et al., 1995). There are indications that MRP1 mediates GSH-transport (Rappa et al., 1997; Zaman et al., 1995), and may function as a co-transporter for GSH and the drug. Thus GSH may be a low affinity substrate for MRP1. From experiments using the vanadate-trapping technique it has been suggested that GSH as well as anticancer drugs directly interact with MRP1 (Taguchi et al., 1997). Transport of anionic MRP1-substrates such as GSH- and glucuronide-conjugates are inhibited by hydrophobic (cationic) vinca alkaloids (Loe et al., 1996b; Müller et al., 1994b) and anthracyclines (Loe et al., 1996b). One hypothesis explaining these results is that MRP1 may contain two binding sites: one for hydrophobic compounds and one for hydrophilic compounds. This would allow a similar binding of GSH and the hydrophobic drug as well as binding of hydrophobic compounds conjugated to GSH, glucuronate or sulphate. Further studies are needed to address this important issue.
In conclusion, we showed that in addition to vincristine MRP1-mediated transport of the unmodified anticancer drug daunorubicin is dependent on GSH. APDA is not a substrate for MRP1.
Acknowledgments
This study is supported by grant RUG 95-1007 from the Dutch Cancer Society. Hans Koning (University Hospital Groningen) is acknowledged for technical assistance. We thank Dr A.W. Ford-Hutchinson for providing MK571 and Dr G. Kurz for the gift of [3H]-APDA. In addition we thank Drs R. Scheper, R. Oude Elferink and B.J. Kroesen for providing monoclonal antibodies and Dr H.W. van Veen (Dept. Microbiology, University of Groningen) for critical comments on the manuscript.
Abbreviations
- ABC transporter
ATP binding cassette transporter
- AMP-PCP
β,γ-methyleneadenosine 5′-triphosphate
- [3H]-APDA
N-(4′,4′-azo-n-pentyl)-21-deoxy-[21-3H]ajmalinium
- Baf
bafilomycin A1
- FCS
foetal calf serum
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GS S-conjugates
glutathione S-conjugates
- LTC4
leukotriene C4
- MDR1
MDR1-P-glycoprotein
- MRP
multidrug resistance protein
- PBS
phosphate-buffered saline
- TS
tris/sucrose
- V-type ATPase
vacuolar H+-ATPase
References
- BARNOUIN K., LEIER I., JEDLITSCHKY G., POURTIER-MANZANEDO A., KÖNIG J., LEHMAN W-D., KEPPLER D. Multidrug resistance protein-mediated transport of chlorambucil and melphalan conjugated to glutathione. Br. J. Cancer. 1998;77:201–209. doi: 10.1038/bjc.1998.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORST P., SCHINKEL A.H. Genetic dissection of the function of mammalian P-glycoproteins. Trends Genet. 1997;13:217–221. doi: 10.1016/S0168-9525(97)01112-8. [DOI] [PubMed] [Google Scholar]
- BOWMAN E.J., SIEBERS A., ALTENDORF K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROXTERMAN H.J., GIACCONE G., LANKELMA J. MRP and other drug transport-related resistance to natural product agents. Curr. Opin. Oncol. 1995;7:532–540. doi: 10.1097/00001622-199511000-00011. [DOI] [PubMed] [Google Scholar]
- BURGER H., NOOTER K., ZAMAN G.J.R., SONNEVELD P., VAN WINGERDEN K.E., OOSTRUM R.G., STOTER G. Expression of the multidrug resistance-associated protein (MRP) in acute and chronic leukemias. Leukemia. 1994;8:990–997. [PubMed] [Google Scholar]
- COLE S.P.C., BHARDWAJ G., GERLACH J.H., MACKIE J.E., GRANT C.E., ALMQUIST K.C., STEWART A.J., KURZ E.U., DUNCAN A.M., DEELEY R.G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- DONG M., PENIN F., BAGGETTO L.G. Efficient purification and reconstitution of P-glycoprotein for functional and structural studies. J. Biol. Chem. 1996;271:28875–28883. doi: 10.1074/jbc.271.46.28875. [DOI] [PubMed] [Google Scholar]
- GEKELER V., ISE W., SANDERS H.K., ULRICH W., BECK J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- GERMANN U.A. P-glycoprotein – A mediator of multidrug resistance in tumour cells. Eur. J. Cancer. 1996;32A:927–944. doi: 10.1016/0959-8049(96)00057-3. [DOI] [PubMed] [Google Scholar]
- GLUCK S.L., UNDERHILL D.M., IYORI M., HOLLIDAY L.S., KOSTROMINOVA T.Y., LEE B.S. Physiology and biochemistry of the kidney vacuolar H+-ATPase. Annu. Rev. Physiol. 1996;58:427–445. doi: 10.1146/annurev.ph.58.030196.002235. [DOI] [PubMed] [Google Scholar]
- GOTTESMAN M.M., PASTAN I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- HIGGINS C.F. ABC transporters: from microorganism to man. Annu. Rev. Cell Biol. 1992;67:113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- HIPFNER D.R., GAULDIE S.D., DEELEY R.G., COLE S.P.C. Detection of the Mr 190,000 multidrug resistance protein, MRP, with monoclonal antibodies. Cancer Res. 1994;54:5788–5792. [PubMed] [Google Scholar]
- HORIO M., GOTTESMAN M.M., PASTAN I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKAWA T., AKIMARU K., KUO M.T., PRIEBE W., SUZUKI M. How does the MRP/GS-X pump export doxorubicin. J. Natl. Cancer Inst. 1995;87:1639–1640. doi: 10.1093/jnci/87.21.1639. [DOI] [PubMed] [Google Scholar]
- JEDLITSCHKY G., LEIER I., BUCHHOLZ U., BARNOUIN K., KURZ G., KEPPLER D. Transport of glutathione, glucuronate and sulphate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988–994. [PubMed] [Google Scholar]
- JONES T.R., ZAMBONI R., BELLEY M., CHAMPION E., CHARETTE L., FORD-HUTCHINSON A.W., FRENETTE R., GAUTHIER J., LEGER S., MASSON P., MCFARLENE C.S., PIECHUTA H., ROKAH J., WILLIAMS H., YOUNG R.N., DEHAVEN R.N., PONG S.S. Pharmacology of L-660,711 (MK-571): a novel potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1989;67:17–28. doi: 10.1139/y89-004. [DOI] [PubMed] [Google Scholar]
- JULIANO R.L., LING V. A surface glycoprotein modulating drug permeability in chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- KAMIMOTO Y., GATMAITAN Z., HSU J., ARIAS I.M. The function of Gp170, the multidrug resistance gene product in rat liver canalicular membrane vesicles. J. Biol. Chem. 1989;264:11693–11698. [PubMed] [Google Scholar]
- KOOL M., DE HAAS M., SCHEFFER G.L., SCHEPER R.J., VAN EIJK M.J.T., JUIJN J.A., BAAS F., BORST P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- LEIER I., JEDLITSCHKY G., BUCHHOLZ U., COLE S.P.C., DEELEY R.G., KEPPLER D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994;269:27807–27810. [PubMed] [Google Scholar]
- LOE D.W., ALMQUIST K.C., COLE S.P.C., DEELEY R.G. ATP-dependent 17β-estradiol 17-(β-D-glucuronide) transport by multidrug resistance protein (MRP) J. Biol. Chem. 1996a;271:9683–9689. doi: 10.1074/jbc.271.16.9683. [DOI] [PubMed] [Google Scholar]
- LOE D.W., ALMQUIST K.C., DEELEY R.G., COLE S.P.C. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. J. Biol. Chem. 1996b;271:9675–9682. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- LOE D.W., DEELEY R.G., COLE S.P.C. Biology of the multidrug resistance-associated protein, MRP. Eur. J. Cancer. 1996c;32A:945–957. doi: 10.1016/0959-8049(96)00046-9. [DOI] [PubMed] [Google Scholar]
- LOE D.W., STEWART R.K., MASSEY T.E., DEELEY R.G., COLE S.P.C. ATP-dependent transport of Aflatoxin B1 and its glutathione conjugates by the product of the multidrug resistance protein (MRP) gene. Mol. Pharmacol. 1997;51:1034–1041. doi: 10.1124/mol.51.6.1034. [DOI] [PubMed] [Google Scholar]
- MELLMAN I., FUCHS R., HELENIUS A. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- MÜLLER M., MAYER R., HERO U., KEPPLER D. ATP-dependent transport of amphiphilic cations across the hepatocyte canalicular membrane mediated by mdr1 P-glycoprotein. FEBS. Lett. 1994a;343:168–172. doi: 10.1016/0014-5793(94)80312-9. [DOI] [PubMed] [Google Scholar]
- MÜLLER M., MEIJER C., ZAMAN G.J.R., BORST P., SCHEPER R.J., MULDER N.H., DE VRIES E.G.E., JANSEN P.L.M. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc. Natl. Acad. Sci. U.S.A. 1994b;91:13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜLLER M., ROELOFSEN H., JANSEN P.L.M. Secretion of organic anions by hepatocytes: involvement of homologues of the multidrug resistance protein. Semin. Liver Dis. 1996;16:211–220. doi: 10.1055/s-2007-1007233. [DOI] [PubMed] [Google Scholar]
- O'BRIEN M.L., TEW K.D. Glutathione and related enzymes in multidrug resistance. Eur. J. Cancer. 1996;32A:967–978. doi: 10.1016/0959-8049(96)00051-2. [DOI] [PubMed] [Google Scholar]
- PRIEBE W., KRAWCZYK M., KUO M.T., YAMANE Y., SAVARAJ N., ISHIKAWA T. Doxorubicin- and daunorubicin-glutathione conjugates, but not unconjugated drugs, competitively inhibit leukotriene C4 transport mediated by MRP/GS-X pump. Biochem. Biophys. Res. Commun. 1998;247:859–863. doi: 10.1006/bbrc.1998.8887. [DOI] [PubMed] [Google Scholar]
- RAPPA G., LORICO A., FLAVELL R.A., SARTORELLI A.C. Evidence that the multidrug resistance protein (MRP) functions as a co-transporter for glutathione and natural product drugs. Cancer Res. 1997;57:5232–5237. [PubMed] [Google Scholar]
- ROELOFSEN H., VOS T.A., SCHIPPERS I.J., KUIPERS F., KONING H., MOSHAGE H., JANSEN P.L.M., MÜLLER M. Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocyte-derived cells. Gastroenterology. 1997;112:511–521. doi: 10.1053/gast.1997.v112.pm9024305. [DOI] [PubMed] [Google Scholar]
- ROGAN A.M., HAMILTON T.C., YOUNG R.C., KLECKER R.W., OZOLS R.F. Reversal of adriamycin resistance by verapamil in human ovarium cancer. Science. 1984;224:994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- RUETZ S., GROS P. A mechanism for P-glycoprotein action in multidrug resistance: are we there yet. Trends Pharmacol. Sci. 1994a;15:260–263. doi: 10.1016/0165-6147(94)90322-0. [DOI] [PubMed] [Google Scholar]
- RUETZ S., GROS P. Functional expression of P-glycoproteins in secretory vesicles. J. Biol. Chem. 1994b;269:12277–12284. [PubMed] [Google Scholar]
- SHAPIRO A.B., LING V. ATPase activity of purified and reconstituted P-glycoprotein from chinese hamster ovary cells. J. Biol. Chem. 1994;269:3745–3754. [PubMed] [Google Scholar]
- SHAROM F.J., YU X., CHU J.W.K., DOIGE C.A. Characterization of the ATPase activity of P-glycoprotein from multidrug resistant chinese hamster ovary cells. Biochem. J. 1995;308:381–390. doi: 10.1042/bj3080381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGUCHI Y., YOSHIDA A., TAKADA Y., KOMANO T., UEDA K. Anti-cancer drugs and glutathione stimulate vanadate-induced trapping of nucleotide on multidrug resistance-associated protein (MRP) FEBS Lett. 1997;401:11–14. doi: 10.1016/s0014-5793(96)01421-4. [DOI] [PubMed] [Google Scholar]
- TAMAI I., SAFA A.R. Competitive interaction of cyclosporins with the vinca alkaloid-binding site of P-glycoprotein in multidrug-resistant cells. J. Biol. Chem. 1990;265:16509–16513. [PubMed] [Google Scholar]
- TEW K.D. Glutathione associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- URBATSCH I.L., SANKARAN B., WEBER J., SENIOR A.E. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J. Biol. Chem. 1995;270:19383–19390. doi: 10.1074/jbc.270.33.19383. [DOI] [PubMed] [Google Scholar]
- VAN LUYN M.J.A., MÜLLER M., RENES J., MEIJER C., SCHEPER R.J., NIENHUIS E.F., MULDER N.H., JANSEN P.L.M., DE VRIES E.G.E. Transport of glutathione conjugates into secretory vesicles is mediated by the multidrug-resistance protein 1. Int. J. Cancer. 1998;76:55–62. doi: 10.1002/(sici)1097-0215(19980330)76:1<55::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- VERSANTVOORT C.H.M., BROXTERMAN H.J., BAGRIJ T., SCHEPER R.J., TWENTYMAN P.R. Regulation by glutathione of drug transport in multidrug-resistant human lung tumour cell lines overexpressing multidrug resistance-associated protein. Br. J. Cancer. 1995;72:82–89. doi: 10.1038/bjc.1995.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMAN G.J.R., FLENS M.J., VAN LEUSDEN M.R., DE HAAS M., MULDER H.S., LANKELMA J., PINEDO H.M., SCHEPER R.J., BAAS F., BROXTERMAN H.J., BORST P. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMAN G.J.R., LANKELMA J., VAN TELLINGEN O., BEIJNEN J., DEKKER H., PAULUSMA C., OUDE ELFERINK R.P.J., BAAS F., BORST P. Role of glutathione in the export of compounds from cells by the multidrug-associated protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMAN G.J.R., VERSANTVOORT C.H., SMIT J.J., EIJDEMS E.W., DE HAAS M., SMITH A.J., BROXTERMAN H.J., MULDER N.H., DE VRIES E.G.E., BAAS F., BORST P. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993;53:1747–1750. [PubMed] [Google Scholar]
- ZIJLSTRA J.G., DE VRIES E.G.E., MULDER N.H. Multifactorial drug resistance in an adriamycin resistant human small cell lung carcinoma cell line. Cancer Res. 1987;47:1780–1784. [PubMed] [Google Scholar]


