Abstract
Using a perfused guinea-pig tracheal tube preparation, we investigated the role of endogenous nitric oxide (NO) in polycation-induced airway hyperreactivity (AHR) to methacholine. Intraluminal (IL) administration of the NO synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; 100 μM) caused a 1.8 fold increase in the maximal contractile response (Emax) to IL methacholine compared to control, without an effect on the pEC50 (−log10 EC50). The polycation poly-L-arginine (100 μg ml−1, IL) similarly enhanced the Emax for methacholine; however, the pEC50 value was also increased, by one log10 unit. L-NAME had no effect on the enhanced methacholine response of poly-L-arginine-treated airways, while the enhanced agonist response was completely normalized by the polyanion heparin (25 u ml−1, IL). In addition, the effect of L-NAME was fully restored in the poly-L-arginine plus heparin treated airways. The results indicate that, in addition to enhanced epithelial permeability, a deficiency of endogenous NO contributes to polycation-induced AHR. The latter finding may represent a novel mechanism of AHR induced by eosinophil-derived cationic proteins in allergic asthma.
Keywords: Nitric oxide, Nω-nitro-L-arginine methyl ester, poly-L-arginine, heparin, methacholine, airway hyperreactivity, tracheal perfusion, guinea-pig
Introduction
Eosinophil-derived cationic polypeptides, particularly major basic protein (MBP), a highly charged protein rich in arginine and lysine residues, have been implicated in the development of airway hyperreactivity (AHR) in allergic asthma (Gleich et al., 1993).
Among the possible mechanisms involved, polycation-induced dysfunction of the airway epithelium appears to play a prominent role. Thus, MBP is highly cytotoxic to airway epithelial cells and induces pathological alterations similar to those found in the airways of asthmatic patients (Motojima et al., 1989). Furthermore, enhanced levels of MBP have been reported in the bronchoalveolar lavage fluid of asthmatic patients, which were correlated with both the degree of epithelial denudation and the severity of AHR (Wardlaw et al., 1988).
MBP has been demonstrated to induce AHR in vivo, an effect that could be closely mimicked by synthetic cationic polypeptides such as poly-L-arginine and poly-L-lysine (Uchida et al., 1993) and inhibited by polyanions such as heparin (Coyle et al., 1993a), indicating the involvement of cationic charge. In an intact guinea-pig tracheal tube preparation in vitro, perfusion of the luminal surface with polycations increased the responsiveness to intraluminally, but not to extraluminally, applied methacholine, directly demonstrating that cationic peptides may indeed induce AHR by alterations of the epithelial layer (Coyle et al., 1993b).
Although overt epithelial damage appeared not to be required for cationic protein-induced AHR (Uchida et al., 1993; Coyle et al., 1993b), a reduced barrier function causing increased airway permeability may be involved (Omari et al., 1993; Hulsmann et al., 1996). In addition, it has been proposed that polycations like MBP may inhibit the agonist-induced release of an epithelium-derived relaxing factor (Flavahan et al., 1988).
One of the epithelium-derived relaxing factors appears to be nitric oxide (NO), which may be generated from agonist-induced activation of constitutive NO synthase (cNOS) isozymes present in the epithelium (Nijkamp et al., 1993; Kobzik et al., 1993). Recently, we have demonstrated that a deficiency of endogenous cNOS-derived NO is involved in allergen-induced AHR after the early asthmatic reaction (De Boer et al., 1996; Schuiling et al., 1998), which may be due to a reduced availability of the substrate L-arginine (Meurs et al., 1998). Since it has been recently reported that polycations, including MBP and poly-L-arginine, may reduce cellular uptake of L-arginine (Hirschmann et al., 1998), we hypothesized that limitation of substrate for cNOS activity induced by eosinophil-derived or synthetic polycationic peptides may cause a reduced contractile agonist-induced NO production and subsequent AHR to these agonists. Using a perfused guinea-pig tracheal tube preparation, this hypothesis was tested by assessing the effects of the nonselective NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) on the airway constriction induced by intraluminal (IL) application of methacholine in untreated and poly-L-arginine-treated airways.
Methods
Tracheal perfusion
Specified pathogen-free Dun-kin Hartley guinea-pigs (Harlan, Heathfield, U.K.), weighing 600–800 g, were used in this study. The animals were killed by a sharp blow on the head and exsanguinated. The tracheas were rapidly removed and placed in Krebs-Henseleit (KH) solution (37°C) of the following composition (mM): NaCl 117.50, KCl 5.60, MgSO4 1.18, CaCl2, 2.50, NaH2PO4 1.28, NaHCO3 25.00, D-glucose 5.50; gassed with 5% CO2 and 95% O2; pH 7.4.
The tracheas were prepared free of serosal connective tissue and cut into two halves of approximately 17 mm before mounting in a perfusion setup, as described previously (De Boer et al., 1996). To this aim, the tracheal preparations were attached at each side to stainless steel perfusion tubes fixed in a Delrin perfusion holder. The holder with the trachea was then placed in a water-jacketed organ bath (37°C) containing 20 ml of gassed KH (the serosal or extraluminal (EL) compartment). The lumen was perfused with recirculating KH from a separate 20 ml bath (mucosal or IL compartment) at a constant flow rate of 18 ml min−1. Two axially centred side-hole catheters connected with pressure transducers (TC-XX, Viggo-Spectramed B.V., Bilthoven, The Netherlands) were situated at the distal and proximal ends of the trachealis to measure hydrostatic pressures (Poutlet and Pinlet, respectively). The signals were fed into a differential amplifier to obtain the difference between the two pressures (ΔP=Pinlet–Poutlet), which was plotted on a flatbed chart recorder. ΔP reflects the resistance of the tracheal segment to perfusion and is a function of the mean diameter of the trachea between the pressure taps (Munakata et al., 1989). The transmural pressure in the trachea was set at 0 cm H2O. At the perfusion flow rate used, a baseline ΔP of 0.1–1.0 cm H2O was measured, depending on the diameter of the preparation.
After a 45 min equilibration period with three washes with fresh KH (both IL and EL), 1 μM isoprenaline was added to the EL compartment for maximal smooth muscle relaxation to assess basal tone. After three washes during at least 30 min, the trachea was exposed to EL 40 mM KCl in KH to obtain a receptor-independent reference response. Subsequently, the preparation was washed four times with KH during 45 min until basal tone was reached and a cumulative concentration response curve was made with IL methacholine. When used, L-NAME (100 μM), poly-L-arginine (100 μg ml−1) and heparin (25 u ml−1) were applied to the IL reservoir, 40 min prior to agonist-addition.
Data analysis
To compensate for differences in ΔP due to variation in resting internal diameter of the preparations used, IL responses of the tracheal tube preparations to methacholine were expressed as a percentage of the response induced by EL administration of 40 mM KCl. The contractile effect of 10 mM methacholine (highest concentration) was defined as Emax (De Boer et al., 1996). Using this Emax, the sensitivity to methacholine was evaluated as pEC50 (−log10 EC50) value. Data are expressed as means±s.e.mean. Statistical analysis was performed using the Student's t-test for unpaired observations. A P value <0.05 was considered statistically significant.
Chemicals
Methacholine chloride, poly-L-arginine hydrochloride (mol wt 5000–15000), heparin sodium and L-Nω-nitro arginine methyl ester were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Results
IL perfusion of the airways with the NOS inhibitor L-NAME (100 μM) caused a significant 1.8 fold increase in the Emax of IL methacholine compared to control (P<0.001), without a significant effect on the pEC50 of the agonist (Figure 1, Table 1). A comparable 1.6 fold (P<0.001) increase in methacholine Emax was caused by the synthetic polycation poly-L-arginine (100 μg ml−1, IL), while the pEC50 was significantly enhanced from 2.86±0.12 to 3.90±0.17 (P<0.001). Very remarkably, L-NAME had no additional effect on the methacholine responsiveness of the poly-L-arginine-treated airways (Figure 1, Table 1).
Figure 1.

Effect of 100 μM L-NAME, 100 μg ml−1 poly-L-arginine, 100 μg ml−1 poly-L-arginine+100 μM L-NAME, 25 u ml−1 heparin, 100 μg ml−1 poly-L-arginine+25 u ml−1 heparin, and 100 μg ml−1 poly-L-arginine+25 u ml−1 heparin+100 μM L-NAME on methacholine-induced constriction of intact perfused guinea-pig tracheae. Methacholine-induced constriction of the perfused airway preparations was measured as an increase in differential pressure (ΔP) at constant flow and normalized to the response of 40 mM KCl (EL). Results are means±s.e.mean of 3–8 experiments.
Table 1.
Effects of (combinations of) L-NAME (100 μM), poly-L-arginine (100 μg ml-1) and heparin (25 u ml-1) on the responsiveness to methacholine of intact perfused guinea-pig tracheae
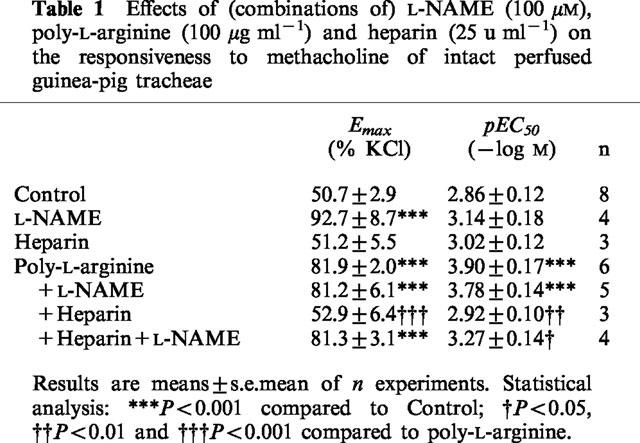
The enhanced responsiveness to methacholine in the presence of poly-L-arginine was completely reversed by coperfusion with the polyanion heparin (25 u ml−1), while heparin by itself had no effect on the methacholine response of untreated airways (Figure 1, Table 1). In addition, the effect of L-NAME was fully restored in the airways treated with poly-L-arginine plus heparin (Figure 1, Table 1).
L-NAME, poly-L-arginine, as well as heparin had no effect on basal airway tone (not shown).
Discussion
This in vitro study demonstrates for the first time that a deficiency of cNOS-derived NO is involved in polycation-induced enhanced airway responsiveness. Thus, using the NOS inhibitor L-NAME, we confirmed the previous finding that methacholine-induced constriction of guinea-pig tracheal tube preparations is functionally antagonized by agonist-induced cNOS-derived NO (Nijkamp et al., 1993; De Boer et al., 1996), while the enhanced airway responsiveness to methacholine in poly-L-arginine perfused airways, which was also noted by others (Coyle et al., 1993b), was insensitive to the NOS inhibitor. The deficiency of NO supports the previous hypothesis of Flavahan et al. (1988) that loss of an epithelium derived relaxing factor may contribute to polycation-induced enhanced contractility. This hypothesis was based on the observation that MBP caused an increase in airway smooth muscle responsiveness in guinea-pig tracheal ring preparations that was dependent on the presence of intact epithelium (Flavahan et al., 1988).
In accordance with previous studies (Coyle et al., 1993b; Omari et al., 1993), we found that both the maximal response and the sensitivity to the contractile agonist were enhanced in the presence of poly-L-arginine. Since inhibition of cNOS by L-NAME in the control airways increased the Emax, but not the sensitivity (pEC50) to methacholine, deficient NOS activity in the poly-L-arginine-treated airways may have contributed to the increased Emax in these airways. The enhanced sensitivity to IL methacholine in the poly-L-arginine-treated airways may reflect an enhanced epithelial permeability for the agonist due to disruption of the diffusion barrier, since this effect can be mimicked by both mechanical (Munakata et al., 1989) and chemical (Coyle et al., 1993b) denudation of the airway epithelium. It should, however, be noted that the increase in pEC50 for methacholine by one log10 unit is approximately one order of magnitude smaller than that observed after denudation (Munakata et al., 1989; Coyle et al., 1993b) and than the difference in pEC50 values of EL and IL applied methacholine (ΔpEC50 (EL-IL)) previously noted in our own study (De Boer et al., 1996), indicating that the epithelial barrier function is only partially lost.
In line with previous observations by Coyle et al. (1993b), the poly-L-arginine-induced AHR to methacholine was effectively blocked by the polyanion heparin, indicating that cationic charge interactions may underly the development of the enhanced agonist responsiveness. This is also true for the reduced NOS activity by the polycation, since the effect to L-NAME was fully restored by coperfusion of poly-L-arginine with heparin.
The present findings are obviously of interest with respect to the mechanism of allergen-induced AHR in vivo. Thus, using a guinea-pig model of allergic asthma, characterized by allergen-induced early and late allergic reactions, airway eosinophilia and AHR after both reactions (Santing et al., 1994), we have recently established that a deficiency of cNOS-derived NO contributes to the increased ex vivo responsiveness of isolated perfused tracheae to methacholine and histamine after the early allergic reaction, at 6 h after inhalational challenge of the animals with ovalbumin aerosol (De Boer et al., 1996). Using the same animal model, a reduced production or effectiveness of endogenous NO was similarly found to contribute to the AHR to histamine in vivo after the early allergic reaction (Schuiling et al., 1998), while evidence for a deficiency of cNOS-derived NO modulating airway reactivity was recently also found in patients with severe asthma (Ricciardolo et al., 1997). Therefore, it can be proposed that polycationic peptides released by activated eosinophils in the inflamed airways may contribute to the deficiency of bronchodilating cNOS-derived NO and subsequent AHR in allergic asthma.
The mechanism of polycation-induced NO deficiency remains to be established. However, one possible mechanism could be polycation-induced inhibition of the cellular uptake of L-arginine by cationic aminoacid transporters, as indicated very recently in rat and guinea-pig alveolar macrophages (Hirschmann et al., 1998).
In conclusion, the present data demonstrate that a deficiency of NO is involved in polycation-induced AHR, which could represent a novel mechanism of AHR induced by eosinophil-derived cationic polypeptides in allergic asthma.
Abbreviations
- AHR
airway hyperreactivity
- cNOS
constitutive nitric oxide synthase
- EL
extraluminal
- Emax
maximal effect
- IL
intraluminal
- KH
Krebs-Henseleit
- L-NAME
Nω-nitro-L-arginine methyl ester
- MBP
major basic protein
- NO
nitric oxide
- ΔP
differential (hydrostatic) pressure
- Pinlet
(hydrostatic) pressure at the inlet
- Poutlet
(hydrostatic) pressure at the outlet
- pEC50
−log10 of the concentration causing 50% of effect
References
- COYLE A.J., ACKERMAN S.J., IRVIN C.G. Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. Am. Rev. Respir. Dis. 1993a;147:896–900. doi: 10.1164/ajrccm/147.4.896. [DOI] [PubMed] [Google Scholar]
- COYLE A.J., MITZNER W., IRVIN C.G. Cationic proteins alter smooth muscle function by an epithelium-dependent mechanism. J. Appl. Physiol. 1993b;74:1761–1768. doi: 10.1152/jappl.1993.74.4.1761. [DOI] [PubMed] [Google Scholar]
- DE BOER J., MEURS H., COERS W., KOOPAL M., BOTTONE A.E., VISSER A.C., TIMENS W., ZAAGSMA J.Deficiency of nitric oxide in allergen-induced airway hyperreactivity to contractile agonists after the early asthmatic reaction Br. J. Pharmacol. 19961161109–1116.An ex vivo study [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAVAHAN N.A., SLIFMAN N.R., GLEICH G.J., VANHOUTTE P.M. Human eosinophil major basic protein causes hyperreactivity of respiratory smooth muscle. Am. Rev. Respir. Dis. 1988;138:685–688. doi: 10.1164/ajrccm/138.3.685. [DOI] [PubMed] [Google Scholar]
- GLEICH G.J., ADOLPHSON C.R., LEIFERMAN K.M. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- HIRSCHMANN J., HEY C., HAMMERMANN J.G., FOLKERTS J.G., NIJKAMP F.P., GLEICH G.J., WESSLER I., RACKÉ K. Inhibition of L-arginine transport in rat and guinea pig alveolar macrophages (AMΦ) by poly-cationic peptides. Br. J. Pharmacol. 1998;123:177P. [Google Scholar]
- HULSMANN A.R., RAATGREEP H.R., DEN HOLLANDER J.C., BAKKER W.H., SAXENA P.R., DE JONGSTE J.C. Permeability of human isolated airways increases after hydrogen peroxide and poly-L-arginine. Am. J. Respir. Crit. Care Med. 1996;153:841–846. doi: 10.1164/ajrccm.153.2.8564141. [DOI] [PubMed] [Google Scholar]
- KOBZIK L., BREDT D.S., LOWENSTEIN C.J., DRAZEN J., GASTON B., SUGARBAKER D., STAMLER J.S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am. J. Respir. Cell. Mol. Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- MEURS H., DE BOER J., DUYVENDAK M., SCHUURMAN F.E., ZAAGSMA J.Role for L-arginine, but not for superoxide anions, in the deficiency of nitric oxide after the allergen-induced early asthmatic reaction in guinea pigs (abstract) Clin. Exp. Allerg 1999(in press) [DOI] [PMC free article] [PubMed]
- MOTOJIMA S., FRIGAS E., LOWGERING D.A., GLEICH G.J. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am. Rev. Respir. Dis. 1989;139:801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- MUNAKATA M., HUANG I., MITZNER W., MENKES H. Protective role of epithelium in the guinea pig airway. J. Appl. Physiol. 1989;66:1547–1552. doi: 10.1152/jappl.1989.66.4.1547. [DOI] [PubMed] [Google Scholar]
- NIJKAMP F.P., VAN DER LINDE H.J., FOLKERTS G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro. Am. Rev. Respir. Dis. 1993;148:727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- OMARI T., SPARROW M.P., CHURCH M.K., HOLGATE S.T., ROBINSON C. A comparison of the effects of polyarginine and stimulated eosinophils on the responsiveness of the bovine isovolumic bronchial segment preparation. Br. J. Pharmacol. 1993;109:553–561. doi: 10.1111/j.1476-5381.1993.tb13606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICCIARDOLO F.L.M., DI MARIA G.U., MISTRETTA A., SAPIENZA M.A., GEPETTI P. Impairment of bronchoprotection by nitric oxide in severe asthma. Lancet. 1997;350:1297–1298. doi: 10.1016/s0140-6736(05)62474-9. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., OLYMULDER C.G., ZAAGSMA J., MEURS H. Relationships among allergen-induced early and late phase airway obstructions, bronchial hyperreactivity, and inflammation in conscious, unrestrained guinea pigs. J. Allergy Clin. Immunol. 1994;93:1021–1030. doi: 10.1016/s0091-6749(94)70051-6. [DOI] [PubMed] [Google Scholar]
- SCHUILING M., ZUIDHOF A.B., BONOUVRIE A.A., VENEMA N., ZAAGSMA J., MEURS H. Role of nitric oxide in the development and partial reversal of allergen-induced airway hyperreactivity in conscious, unrestrained guinea-pigs. Br. J. Pharmacol. 1998;123:1450–1456. doi: 10.1038/sj.bjp.0701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCHIDA D.A., ACKERMAN S.J., COYLE A.J., LARSEN G.L., WELLER P.F., FREED J., IRVIN C.G. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am. Rev. Respir. Dis. 1993;147:982–988. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- WARDLAW A.J., DUNNETTE S., GLEICH G.J., COLLINS J.V., KAY A.B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Am. Rev. Respir. Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]


