Abstract
We characterized the responses of murine airways and pulmonary vessels to a variety of endogenous mediators in the isolated perfused and ventilated mouse lung (IPL) and compared them with those in precision-cut lung slices.
Airways: The EC50 (μM) for contractions of airways in IPL/slices was methacholine (Mch), 6.1/1.5>serotonin, 0.7/2.0>U46619 (TP-receptor agonist), 0.1/0.06>endothelin-1, 0.1/0.05. In the IPL, maximum increase in airway resistance (RL) was 0.6, 0.4, 0.8 and 11 cmH2O s ml−1, respectively. Adenosine (⩽1 mM), bombesin (⩽100 μM), histamine (⩽10 mM), LTC4 (⩽1 μM), PAF (0.25 μM) and substance P (⩽100 μM) had only weak effects (<5% of Mch) on RL.
Vessels: The EC50 (μM) for vasoconstriction in the IPL was LTC4, 0.06>U46619, 0.05<endothelin-1, 0.02. The maximum increase in pulmonary artery pressure (PAP) was 11, 41 and 48 cmH2O, respectively. At 250 nM, the activity of PAF was comparable to that of LTC4. At 100 μM only, substance P caused a largely variable increase in PAP. Serotonin, adenosine, bombesin, histamine and Mch had no or only very small effects on PAP.
Hyperresponsiveness: In both the IPL and slices, U46619 in subthreshold concentrations (10 nM) reduced the EC50 to 0.6 μM. In the IPL, U46619 raised the maximum airway response to Mch 5 fold and the maximum PAF-induced vasoconstriction 4 fold.
Conclusion: Murine precision-cut lung slices maintain important characteristics of the whole organ. The maximum reagibility of murine airways to endogenous mediators is serotonin<Mch <U46619<ET-1. The reagibility of the murine pulmonary vasculature is serotonin<LTC4≈PAF <U46619<ET-1. The airway and vessel hyperreactivity induced by U46619 raises the possibility that thromboxane contributes directly to airway hyperresponsiveness in various experimental and clinical settings.
Keywords: Vasoconstriction, bronchoconstriction, mouse, perfused lung, precision-cut lung slices, lung explant, hyperresponsiveness, thromboxane, methacholine, endothelin
Introduction
In the past, the limited size of mice has also limited physiological measurements in this species. However, parallel to the advances in molecular biology and cloning techniques as well as in our knowledge of immune reactions, interest in physiological measurements in mice has grown. Therefore, as a model to study physiological lung functions, a set-up for the isolated perfused mouse lung has recently been developed (von Bethmann et al., 1998). This model offers the unique opportunity to simultaneously assess airway and pulmonary vascular physiology in mice. It allows to continuously monitor pulmonary artery pressure, tidal volume, pulmonary compliance and pulmonary resistance. For further investigations it will be essential to know the responses of the mouse lung to fundamental endogenous stimuli of the respiratory tract. Such data are essential as a basis for further investigations in areas such as asthma or hyperreactivity reactions and in addition will allow to compare the sensitivity of murine lungs to those from other species.
So far, only one study has addressed a similar problem in mice (Martin et al., 1988). In that study, which has been performed in vivo, the bronchoconstrictor responses to a variety of agents were investigated. Of the agents examined, only methacholine (Mch) and serotonin provoked bronchoconstriction. However, in this in vivo study the vascular responses to these agents were not examined. In perfused mouse lungs, however, it is possible to study both airway and vascular responses. Other advantages of perfused lungs include the possibility to study pulmonary reactions in the absence of systemic side effects or interactions with other organs.
Another possibility to study pulmonary responses in mice is the use of precision-cut lung slices, where airway responses can be followed under the microscope (Martin et al., 1996). This method combines a number of advantages: (i) the microanatomy is maintained, (ii) it requires cell culture conditions, (iii) but still allows to study functional responses of small and large airways, and (iv) it saves animals since from one lung up to 30 slices can be obtained. So far, however, this technique has not been applied to measure airway responses in murine lungs.
In the present study, we have characterized vascular and airway responses of isolated perfused mouse lungs and of precision-cut mouse lung slices to the following agents: adenosine, bombesin, endothelin-1 (ET-1), histamine, leukotriene C4 (LTC4), Mch, platelet activating factor (PAF), serotonin, substance P and the TP-receptor agonist U46619. In addition, we demonstrated that activation of the thromboxane receptor induces pulmonary hyperresponsiveness (AHR) towards itself as well as towards Mch and PAF.
Methods
Materials
Female Balbc mice (20–25 g) obtained from Charles River (Sulzfeld, Germany) were used as lung donors. Pentobarbitone sodium (Nembutal®) was purchased from the Wirtschaftsgenossenschaft Deutscher Tierärzte (Hannover, Germany); bovine albumin low endotoxin grade (fraction V) from Serva (Heidelberg, Germany); methacholine (Mch), serotonin, substance P, platelet activating factor (PAF), bombesin and histamine from Sigma (Deisenhofen, Germany); U46619 and leukotriene C4 from Cayman (Ann Arbor, MI, U.S.A.); endothelin-1 from Boehringer Mannheim (Mannheim, Germany).
Isolated perfused mouse lung preparation (IPL)
The mouse lungs were prepared and perfused essentially as described recently (von Bethmann et al., 1998). Briefly, lungs were perfused in a non-recirculating fashion through the pulmonary artery at a constant flow of 1 ml min−1 resulting in a pulmonary artery pressure of 2–3 cmH2O. As a perfusion medium we used RPMI 1640 medium lacking phenol red (37°C) that contained 4% low endotoxin grade albumin. The lungs were ventilated by negative pressure (−3 to −9 cmH2O) with 90 breaths min−1 and a tidal volume of about 200 μl. Every 5 min a hyperinflation (−20 cmH2O) was performed. Artificial thorax chamber pressure was measured with a differential pressure transducer (Validyne DP 45-24), and air flow velocity with a pneumotachograph tube connected to a differential pressure transducer (Validyne DP 45-15). The lungs respired humidified air. The arterial pressure was continuously monitored by means of a pressure transducer (Isotec Healthdyne) which was connected with the cannula ending in the pulmonary artery. All data were transmitted to a computer and analysed by the Pulmodyn software (Hugo Sachs Elektronik, March Hugstetten, Germany). For lung mechanics, the data were analysed by applying the following formula: P=V·C−1 + RL·dV·dt−1, where P is chamber pressure, C pulmonary compliance, V tidal volume and RL airway resistance. After 60 min, mean tidal volume was 0.21±0.02 ml (n=61), mean airway resistance 0.23± 0.08 cmH2O s ml−1, and mean pulmonary artery pressure 2.9±1.4 cmH2O. The measured airway resistance was corrected for the resistance of the pneumotachometer and the tracheal cannula of 0.6 cmH2O s ml−1.
Design of the experiments with perfused mouse lungs
After preparation, the lungs were perfused for 45 min without any treatment in order to obtain a baseline. When cumulative concentrations of Mch, bombesin, histamine, adenosine, serotonin or substance P were administered, each concentration was given for 20 min followed by intermission periods of 15 min, in which lungs were perfused with buffer only. In another set of experiments, lungs were perfused with 10 nM U46619 for 30 min and then, with U46169 still being infused, Mch in increasing concentrations (cumulative) or PAF were added. When given as bolus, Mch was injected in a volume of 10 μl directly into the catheter leading to the pulmonary artery.
Precision-cut mouse lung slices
The precision-cut lung slices were prepared and handled in a similar fashion as recently described for rat lungs (Martin et al., 1996). The animals were anaesthetized (60 mg kg−1 pentobarbitone sodium), the trachea was cannulated and the animals were exsanguinated by cutting the vena cava inferior. Immediately after a small vertical cut into the diaphragm to collapse the lungs, agarose solution (0.75%, 48 ml kg−1, 37°C) was instilled into the airways. To solidify the agarose and thereby the lungs, the whole animal was then cooled with ice for 10 min. Afterwards the lungs and heart were removed en bloc from the thoracic cavity. The lung lobes were prepared and embedded in 3% agarose solution. After cooling for 5 min, the embedded lung lobes were cut with a Krumdieck tissue slicer (Alabama Research and Development, Munford, AL, U.S.A.) into 220-μm-thick slices. The slices were transferred into cell culture dishes and incubated in an EBSS salt solution, containing HEPES, amino acids and vitamins for 12 h. During the first 4 h the medium was changed every 30 min.
To analyse contractions of single airways, single slices were moved into a custom made incubation chamber. The design of this chamber which allows to warm, incubate and manipulate the slices under the microscope has been detailed elsewhere (Martin et al., 1996). The airways were imaged and digitized with a digital video camera (Visitron 1300, Visitron Systems, Munich, Germany) controlled by the image analysis software Metamorph (Universal Imaging Incorporation, West Chester, U.S.A.). One image of 2.28 mm2 was represented by 1280×1024 pixels and stored at a rate of at least two pictures a minute on the hard disc of the computer. The stored images were analysed by the image analysis program OPTIMAS 6.0 (Optimas Corporation Bothell, Washington, U.S.A.). The luminal area was taken as the area enclosed by the epithelial luminal border and was quantified after setting the appropriate threshold. Control airway area was defined as 100%. To quantify the bronchoconstriction, the data were expressed as per cent area of the control area. Finally, the area under the curve (AUC) of the decrease in luminal area over time was calculated (GraphPad Prism 2.01, San Diego, U.S.A.).
Design of the experiments with the precision-cut lung slices
After preincubation for 10 min with 1 ml of MEM the first image was acquired. The airway area obtained from this first image served as the reference area (100%). The buffer in the incubation chamber (1 ml) was exchanged with buffer that contained the agents to be studied. The airway was imaged at least every 30 s during the incubation time of 10, 15 or 30 min. The following concentrations were used: U46619 10−9–10−5 M, Mch 10−8–10−4 M, serotonin 10−8–10−4 M, and ET-1 10−10–10−6 M. Mch and serotonin were tested with increasing concentrations in the same slice (cumulative) while with U46619 and ET-1 we used a new slice for each concentration.
Statistics
EC50 values were calculated in Prism 2 (GraphPad, San Diego, CA, U.S.A.) in two different ways. We used either the peak values or, alternatively, the area under the curve (AUC; calculated in GraphPad Prism by the polygon method) for a 20 min period of continuous perfusion with the agents. The former parameter is indicative of the maximum smooth muscle contraction, while the latter parameter integrates the whole extent of airway or vascular contractions.
Results
Isolated perfused mouse lung
At first, we investigated the possibility to perform cumulative concentration-response curves. Therefore, each compound was perfused at an intermediary effective concentration twice in the same lung (Figure 1). A comparable response during the first and second perfusion was only obtained with Mch (Figure 1A). U46619 produced a stronger increase in airway resistance when given a second time (Figure 1C). At the second infusion, serotonin gave similar results in airway responses (Figure 1E), but a smaller increase in PAP (Figure 1F). Perfusion with LTC4 resulted in a stronger PAP increase during the second perfusion (Figure 1H). The ET-1-induced rise in airway resistance and PAP was strong, protracted and largely irreversible (Figure 1I and J).
Figure 1.
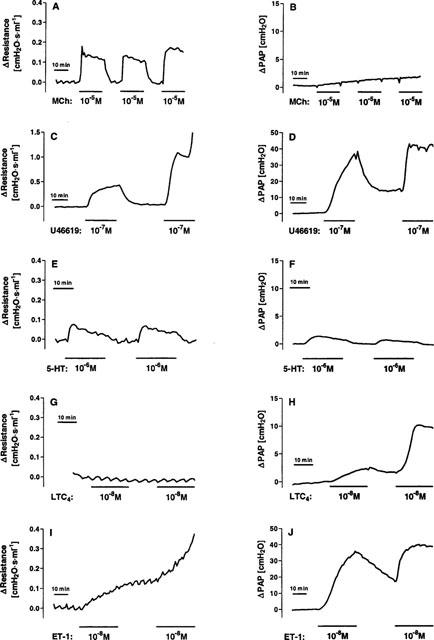
Airway resistance (left column) and mean pulmonary artery pressure (PAP, right column) in response to repetitive perfusion with methacholine (A and B), the thromboxane receptor agonist U46619 (C and D), serotonin (E and F), leukotriene C4 (G and H) or endothelin-1 (I and J). Methacholine (Mch, 10 μM), U46619 (0.1 μM), serotonin (5-HT, 1 μM) or leukotriene C4 (LTC4, 10 nM) were perfused for the time indicated by the black horizontal bars, i.e. 20 min or 30 min for ET-1. The airway resistance and the mean pulmonary artery pressure are expressed in relative terms as the change induced by the agonist.
Thus, only with Mch and serotonin (at least for the airways) cumulative concentration-response curves within a single lung as illustrated in Figure 2 were possible. All the other agents had to be tested at one concentration in a single lung as demonstrated for U46619 and ET-1 in Figure 3. The results of our experiments are summarized in Figure 4 and Table 1. As the different mediators showed great differences in the time-course of the pressor responses elicited, we analysed the peak values as well as the time integral (area under the curve, AUC) of the responses during a perfusion period of 20 min.
Figure 2.
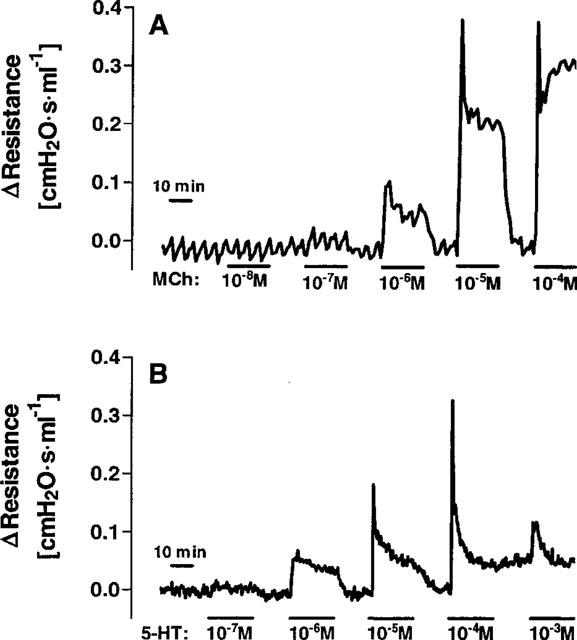
Cumulative concentration response curves of changes in airway resistance in lungs perfused with methacholine (A) or serotonin (B). Methacholine (Mch) and serotonin (5-HT) were perfused for 20 min at each concentration. The airway resistance is expressed in relative terms as the change induced by the agonists.
Figure 3.
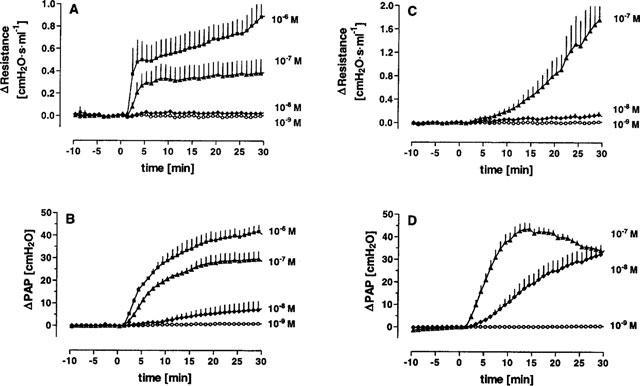
Concentration dependent increase in airway resistance and mean pulmonary artery pressure (PAP) by U46619 (A and B) and ET-1 (C and D). Since both mediators induced hyperresponsiveness when given repetitively to the same lung, each concentration was tested in a separate lung. The time courses of airway resistance (A and C) and mean pulmonary artery pressure (B and D) in response to different concentrations of U46619 and ET-1 (n=3 at each concentration) are shown in this figure. Data are given as means±s.e.mean.
Figure 4.
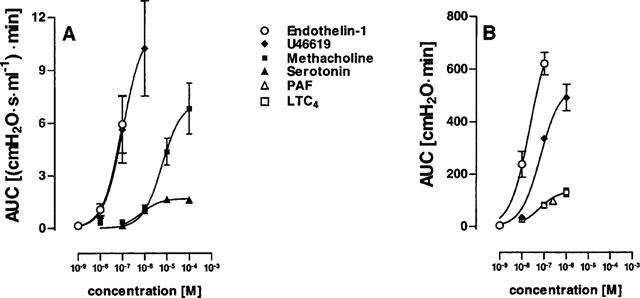
Airway (A) and vascular (B) responses of murine lungs to various mediators. Cumulative concentration-response experiments were carried out for methacholine (n=5) and serotonin (n=3). In these experiments, lungs were perfused for 20 min with each concentration, with intermission periods of 15 min, in which lungs were perfused with buffer only. For U46619 (n=3 for each concentration), leukotriene C4 (n=3 for each concentration) and endothelin-1 (n=3 for each concentration), each concentration was perfused for 30 min in separate lungs. Concentration-response curves were calculated from the area under the curve (AUC), i.e. the time integral of changes in airway resistance (A) or pulmonary artery pressure (B) within 20 min. For platelet activating factor (PAF, n=3) only one concentration, i.e. 250 nM, was tested. Data are given as means±s.e.mean.
Table 1.
EC50 values and maximum responses for airway and vascular responses
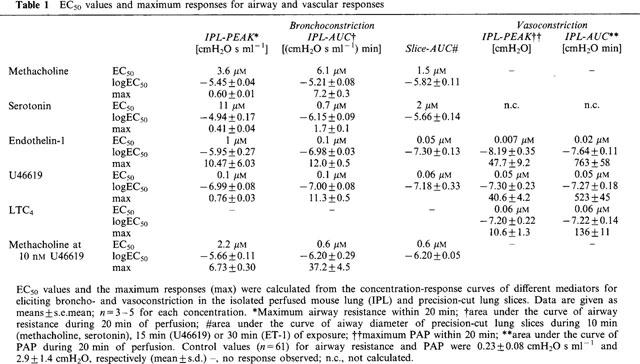
Methacholine (Mch)
Continuous infusion of Mch always resulted in a bi-phasic airway response (Figure 2); the first phase was characterized by a sharp peak in airway resistance, which during the second phase plateaued at a lower level. The EC50 value calculated for Mch was 6.1 μM when the AUC and 3.6 μM when the peak bronchoconstriction was analysed. This response of murine lungs to continuous Mch exposure for 20 min was compared to conditions where Mch was given as a bolus injection directly into the cannula leading to the pulmonary artery. In the latter condition, only the peak response occurred and the amount required to produce a half-maximum increase in airway resistance was 8 nmol (data not shown). The maximum response was comparable during bolus injection and continuous perfusion of Mch (not shown).
U46619, PAF and LTC4
Of these phospholipid mediators, U46619 was the most potent and increased both RL (EC50 0.1 μM) and PAP (EC50 0.05 μM). Perfusion with 1 μM LTC4 did induce only very small alterations in airway resistance (<5% of that induced by 1 μM Mch), but a moderate increase in pulmonary artery pressure (EC50 0.06 μM). PAF was tested at the concentration of 250 nM only and at this concentration behaved similar as LTC4. Of note, if LTC4 and PAF were offered in buffer lacking albumin, both compounds at concentrations of 1 μM and 250 nM, respectively, elicited a small increase in airway resistance by about 0.2 cmH2O s ml−1 (not shown).
Serotonin
At least for the airway response (Figure 1E), cumulative concentration-response curves with serotonin were feasible (Figure 2C). Serotonin elicited a bi-phasic broncho-constriction with a sharp peak during the first 10 s of perfusion, which was soon followed by a phase of fading airway resistance (Figure 2B). Analysing the AUC and the peak values, produced markedly different results in the case of serotonin: the calculated EC50 values were 0.7 and 11 μM, respectively (Table 1). The peak response to serotonin was comparable to methacholine, while the response was weaker if expressed as AUC. Interestingly, at the highest concentration tested, i.e. 1 mM, the response to serotonin was much weaker compared to 0.01 or 0.1 mM, which is in line with previous data (Martin et al., 1988). In addition to bronchoconstriction, serotonin also increased pulmonary artery pressure. However, this response was quite small, i.e. at a serotonin concentration of 1 μM pulmonary artery pressure was increased by 2.2±0.9 cmH2O. The facts that at higher concentrations, no stronger response was obtained and that giving the same concentration of serotonin twice produced a smaller response the second time (Figure 1F), suggests that the vascular effects of serotonin are liable to desensitization.
Endothelin-1
ET-1 elicited both strong airway and vascular responses (Figures 3d and 4). Perfusion with 100 nM ET-1 resulted in a slowly developing bronchoconstriction with a maximum increase of 1.7 cmH2O s ml−1 in airway resistance after 30 min of perfusion, while smaller concentrations were almost ineffective. The EC50 values were 0.1 μM as calculated from the AUC and 1 μM when peak bronchoconstriction was analysed. For PAP, calculated EC50 values were 0.007 and 0.023 μM for peak and AUC data, respectively.
Adenosine, bombesin, histamine and substance P
No or only very small responses were observed with adenosine, bombesin or histamine up to concentrations of 1 mM, 100 μM and 10 mM, respectively (data not shown). Substance P induced vasoconstriction with strongly varying extent, however only at the highest concentration tested, i.e. 100 μM (not shown).
Precision-cut lung slices
We investigated Mch, serotonin, thromboxane and endothelin in a model where agents can be directly applied to murine airways, i.e. precision-cut lung slices. Viable slices of pulmonary tissue (about 220 μm thick) were placed under a microscope and exposed to varying concentrations of agonists for times from 10–30 min. Digital image analysis was used to quantify the diameter of the airway lumen. The studies with Mch and serotonin were done in a cumulative fashion, whereas the concentration-response curve to ET-1 and U46619 was obtained by using a new slice for each single concentration (Figure 5). The following EC50 values were obtained by analysing the AUC data: Mch 1.1 μM, serotonin 2 μM, ET-1 0.05 μM and U46619 0.06 μM (Table 1). Interestingly, as seen in the perfused lung (see above), the airway response to serotonin was weaker in the slices at higher concentrations (⩾100 μM) than at lower concentrations (data not shown).
Figure 5.
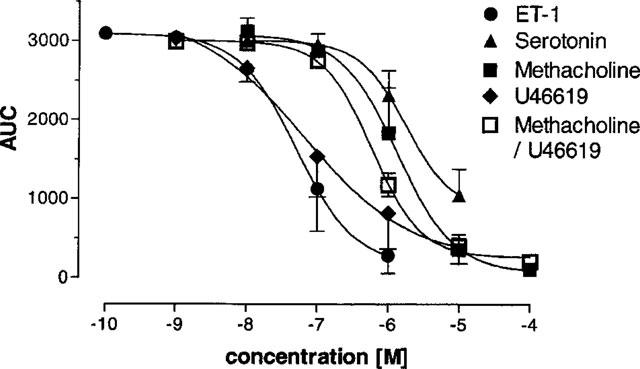
Concentration-response curves of murine airways to various mediators in precision-cut lung slices. Precision-cut lung slices were placed under a microscope and incubated with varying concentrations of methacholine, serotonin, U46619, ET-1 or methacholine/1 nM U46619. For methacholine and serotonin cumulative concentration-response curves were performed; for U46619 and ET-1 for each concentration a new slice was used. A single airway was focused and the size of this airway was followed over time. The AUC of the airway size over time was plotted against the agonist concentration. Data are means±s.d. from 3–6 single slices.
Airway hyperresponsiveness (AHR)
Since we had observed that U46619 caused AHR to itself (Figure 1C), we were interested to study whether U46619 also caused heterologous sensitization, in this case sensitization against Mch. Therefore, lungs were treated with a bolus of 10 nmol Mch (this dose is about the EC50, see above) 15 min before and 15 min after perfusion with different concentrations of U46619. In this set of experiments AHR was only observed at the highest U46616 concentration used, i.e. 1 μM (Figure 6).
Figure 6.
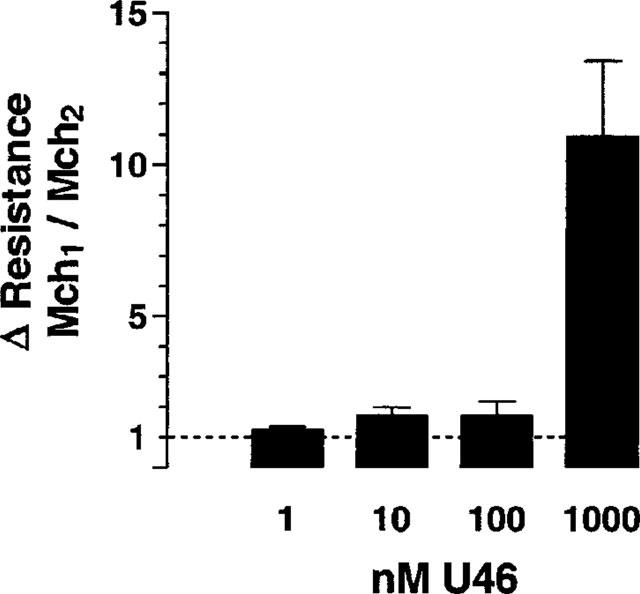
U46619-induced AHR to methacholine. A bolus of 10 nmol methacholine was given 15 min before (Mch1) and 15 min after (Mch2) perfusion with different concentrations of U46619 for 30 min (n=3 for each concentration). The change in airway resistance in response to Mch was recorded. The data are expressed as the ratio Mch1/Mch2, i.e. a ratio of 1 means that the responses before and after U46619 were similar. Data are given as means±s.e.mean.
In a separate set of experiments, lungs were perfused with 10 nM U46619, a concentration that had only marginal effects on airway resistance itself (Figure 3A). While being perfused with U46619, these lungs were treated with increasing concentrations of Mch. In the presence of 10 nM U46619, the bronchoconstriction to Mch was greatly exacerbated, i.e. the EC50 value was shifted from 6 μM in the absence to 0.6 μM in the presence of U46619 (based on AUC). In addition, the maximum increase in airway resistance was greater (Figure 7) and the peak value of bronchoconstriction was raised from 0.6 to 6.7 cmH2O s ml−1 (Table 1). In precision-cut lung slices, the EC50 of Mch-induced airway contraction in the presence of 1 nM (not shown) or 10 nM U46619 was 0.6 μM (Figure 5, Table 1).
Figure 7.
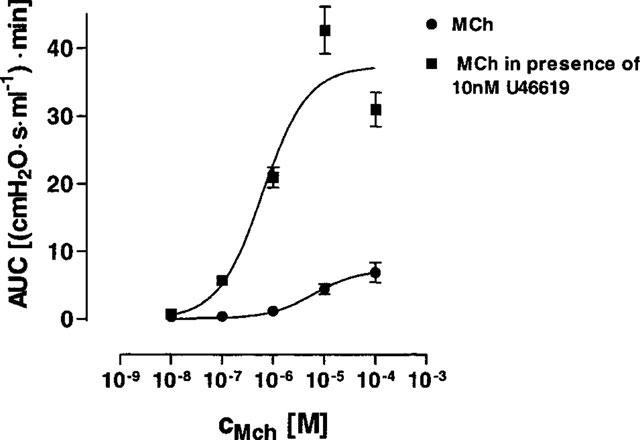
U46619-induced AHR to methacholine. Cumulative concentrations of methacholine were perfused in the absence (n=5) or presence (n=4) of 10 nM U46619. Data are given as mean (AUC of the airway resistance)±s.e.mean.
Vascular hyperresponsiveness
We also investigated whether the presence of U46619 exacerbated the response to PAF. Figure 8 shows that perfusion of PAF in lungs pre-treated with 10 nM U46619, caused a significant increase in the vascular response to PAF. The rise in PAP elicited by PAF as analysed by the AUC data was elevated from 98±17 cmH2O min (n=3) in the absence to 346±25 cmH2O min (n=3) in the presence of U46619.
Figure 8.
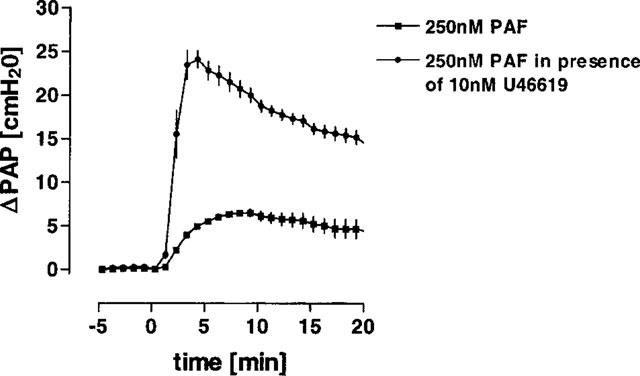
U46619-induced vascular hyperresponsiveness to PAF. 250 nM PAF were perfused in the absence (n=3) or presence (n=3) of 10 nM U46619. Data are given as the mean of the pulmonary artery pressure±s.e.mean.
Discussion
This study presents basic information on murine airways and pulmonary vessels. It shows how murine lungs respond to a variety of endogenous mediators. Only few previous studies have investigated airway (e.g. Martin et al., 1988; Larsen et al., 1992; Manzini, 1992; Brusselle et al., 1995; Garssen et al., 1994; Kips et al., 1996; Hamelmann et al., 1997) or pulmonary vascular (Steudel et al., 1997) responses in mice. Most of these studies have only analysed AHR towards Mch. In view of the promising prospects of genetically engineered mice, information about physiological responses in mice will become increasingly important.
To our knowledge, this is the first study of concentration-response relationships of murine pulmonary vessels in the intact organ. Of the endogenous mediators examined, ET-1, U46619, LTC4 and PAF increased pulmonary artery pressure, even though the action of the latter two agents was weak. Only two substances were highly active on both airways and vessels, i.e. U46619 and ET-1. The finding that the EC50 values for U46619- and ET-1-induced vasoconstriction were lower than the ones for bronchoconstriction, can probably be explained by dilution of U46619 or ET-1 on their way from the vessels to their receptors on airway smooth muscles. This conclusion is drawn from our studies with precision-cut lung slices. In this model, it is possible to study comparatively small airways (110–220 μM luminal diameter) under conditions where diffusion from the vascular site plays no role. In the slice model, we found that the EC50 values for the bronchoconstriction induced by ET-1 and U46619 were close to the ones found for the vasoconstriction in perfused mouse lungs. Thus, the receptors on airways and vessels appear to be similarly sensitive to both mediators.
In general, it appears as if murine airways are less reactive than those of other species. All of the compounds tested here have been shown to be potent smooth muscle contracting agents in rats, rabbits or guinea-pigs. However, in murine lungs strong airway effects were only observed with ET-1, Mch serotonin and the thromboxane receptor agonist U46619. The activity to other well-known mediators such as LTC4, PAF, and histamine was weak or even absent as in the case of adenosine and bombesin. Possibly, the presence of albumin in our perfusion buffer could have suppressed the airway responses to LTC4 (Iacopino et al., 1984) or PAF (Lichey et al., 1984). However, in perfused mouse lungs the response achieved in albumin-free buffer was still quite small. This is in contrast to the perfused rat lung, where LTC4 and PAF are potent bronchoconstrictors also in the presence of 2% albumin (Uhlig & Wollin, 1994; Uhlig et al., 1994; Uhlig & Wendel, 1995). However, the weak response of murine airways to LTC4 and PAF in the absence of albumin still leaves the possibility that murine lungs may react on in situ generated LTC4 and PAF rather than on blood-borne mediators.
Our data confirm and extend those presented by Martin et al. (1988). In line with their data obtained in vivo, we found that murine airways are responsive to Mch and to serotonin, while they are largely resistant to LTC4, PAF, substance P or histamine. They did not investigate thromboxane, ET-1, adenosine or bombesin. A limitation of both studies was that relaxant effects of the agents studied would have gone unnoticed, since pre-contracted tissue were not investigated. For instance, it was reported that in mice substance P may act as a bronchodilator (Manzini, 1992). In another in vivo study, Bruselle et al. (1995) compared the effect of i.v. administration of serotonin and carbachol in Balb/c mice. Analysing the peak increase in RL they noticed that both agents were nearly equally potent. Based on analysis of the peak responses in the IPL or of the contraction of lung slices, here we obtained the same result, i.e. serotonin and Mch were equally effective. However, if we analysed the bronchoconstriction in terms of the AUC, i.e. the duration of the response, the action of serotonin was weaker than that of Mch. Taken together, our data show that important properties of the native organ are maintained in perfused lungs as well as in lung slices.
It is also of interest to compare the data from perfused lungs and lung slices to another in vitro preparation that has been used to study murine airways, i.e. isolated tracheal rings. In this model, the EC50 value for Mch-induced contraction is comparable to our data, i.e. 0.4 μM. However, the maximum response to serotonin is only a quarter of that of Mch and the EC50 is 5000 μM (Garssen et al., 1990). Thus at least with respect serotonin, perfused lungs and lung slices more closely resemble the in vivo situation than do tracheal rings.
The high sensitivity of murine lungs to thromboxane seems a little surprising in view of the scarcity of data available on thromboxane in mice. Recently, however, it was suggested that thromboxane contributes to allergen-induced inflammation in mice (Shi et al., 1998). Besides being a potent pressor agent, thromboxane also caused AHR towards itself as well as towards Mch and PAF. The AHR induced by U46619 was characterized by an aggravated bronchoconstriction as well as by a left shift of the concentration-response curve. The AHR was very pronounced in the presence of U46619 (Figure 6), but it did not appear to last very long, as suggested by the data shown in Figure 5. This is in line with the conclusion drawn by Sato et al. in a study with guinea-pigs (Sato et al., 1996). Thromboxane has been implicated in the AHR caused by antigen (Devillier & Bessard, 1997), PAF (Nagase et al., 1997), endotoxin (Arimura et al., 1993) and interleukin-8 (Xiu et al., 1995). The mechanism of this phenomenon is largely unknown and some authors have speculated that inflammation may be involved (Devillier & Bessard, 1997). However, our finding that this process is also possible in blood-free perfused lungs and in precision-cut lung slices suggests that the mechanism of the thromboxane-induced AHR is unrelated to inflammation and may be a property of thromboxane itself. Whether thromboxane also contributes to asthmatic unspecific AHR in humans remains to be shown, although its extent seems to be limited (Devillier & Bessard, 1997; Kurosawa, 1995). Recently, however, it was suggested that in a subpopulation of asthma patients thromboxane might play a more prominent role (Shi et al., 1998).
In addition to the induction of AHR, U46619 also primed vessels for PAF-induced vasoconstriction. To our knowledge, a direct priming effect of thromboxane on vascular contraction has not been described before, although some previous studies could be explained by such a mechanism. For instance, Salzer & McCall (1990) observed that perfusion of rabbit lungs with endotoxin caused thromboxane release that was associated with vascular hyperreactivity towards PAF. The vascular and airway hyperresponsiveness induced by U46619 shows that hyperresponsiveness can in principle be induced in murine perfused lungs and in precision-cut lung slices. One important limitation of both methods, however, is that one may inadvertently wash away important soluble factors. Of note, Larsen et al. (1994) have shown that tracheas prepared from immunized mice are hyperresponsive to electrical field stimulation, but not to Mch. Clearly, the possibility to study hyperresponsiveness ex vivo or even in vitro requires further study.
Finally, this study has also addressed a number of technical issues that are of importance when investigating physiological lung functions: (1) Cumulative dose-response curves appear to be rarely feasible due to either sensitization or desensitization. (2) During i.v. application or perfusion in isolated lungs, the agonist concentration that finally reaches the airways is lower than the concentration in the pulmonary artery that acts on vascular smooth muscles. (3) Bolus injections into the pulmonary artery result in very high local concentrations as exemplified in this study with Mch. Here, a bolus of 8 nmol corresponded to a concentration of 3.6 μM. (4) Calculation of EC50 values can be based either on the peak responses or the integrated time response (AUC). Since the peak response may be very short-lived, AUC data may be better suited to estimate the impact of agents for the organ or the organism in vivo. (5) Precision-cut lung slices offer a novel way to examine airway responses in mice. The advantage of this model is that one lung yields up to 30 slices in which important parts of the lung anatomy are still preserved. Our study has demonstrated that precision-cut lung slices maintain most of the properties of perfused lungs and of the organ in vivo; even the AHR induced by U46619 could be reproduced in the slices. In our study we noticed only two differences: the response to serotonin was stronger in the precision-cut lung slices and LTC4 failed to give the same (weak) response on airways it did in lungs perfused without albumin.
In summary, of many endogenous mediators studied endothelin-1, methacholine, serotonin and thromboxane execute the strongest pressor responses in murine lungs. On the other hand, murine airways and pulmonary vessels do not or only weakly respond to a variety of inflammatory mediators, namely leukotriene C4, platelet activating factor, substance P, adenosine or histamine, all of which are thought to contribute to the pathophysiology of human asthma and other inflammatory lung diseases. However, important features of such diseases, i.e. bronchoconstriction, airway hyperresponsiveness, pulmonary hypertension and vascular hyperreactivity can be demonstrated in murine perfused lungs.
Acknowledgments
The present study was supported by the Deutsche Forschungsgemeinschaft Grant DFG Uh 88/2-1.
Abbreviations
- 5-HT
serotonin
- AHR
airway hyperreactivity
- AUC
area under the curve
- ET-1
endothelin-1
- IPL
isolated perfused lung
- LTC4
leukotriene C4
- MCH
methacholine
- PAF
platelet activating factor
- PAP
pulmonary artery pressure
- RL
airway resistance
- SP
substance P
References
- ARIMURA A., ASANUMA F., YAGI H., KUROSAWA A., HARADA M. Involvement of thromboxane A2 in bronchial hyperresponsiveness but not lung inflammation induced by bacterial lipopolysaccharide. Eur. J. Pharmacol. 1993;231:13–21. doi: 10.1016/0014-2999(93)90678-b. [DOI] [PubMed] [Google Scholar]
- BRUSSELLE G., KIPS J., JOOS G., BLUETHMANN H., PAUWELS R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am. J. Respir. Cell Mol. Biol. 1995;12:254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- DEVILLIER P., BESSARD G. Thromboxane A2 and related prostaglandins in airways. Fundam. Clin. Pharmacol. 1997;11:2–18. doi: 10.1111/j.1472-8206.1997.tb00163.x. [DOI] [PubMed] [Google Scholar]
- GARSSEN J., NIJKAMP F.P., VAN VUGT E., VAN DER VLIET H., VAN LOVEREN H. T cell-derived antigen binding molecules play a role in the induction of airway hperresponsiveness. Am. J. Respir. Crit. Care Med. 1994;150:1528–1538. doi: 10.1164/ajrccm.150.6.7952611. [DOI] [PubMed] [Google Scholar]
- GARSSEN J., VAN LOVEREN H., VAN DER VLIET H., NIJKAMP F.P. An isometric method to study respiratory smooth muscle responses in mice. J. Pharm. Meth. 1990;24:209–217. doi: 10.1016/0160-5402(90)90031-f. [DOI] [PubMed] [Google Scholar]
- HAMELMANN E., SCHWARZE J., TAKEDA K., OSHIBA A., LARSEN G.L., IRVIN C.G., GEFLAND E.W. Non-invasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- IACOPINO V.J., COMPTON S., FITZPATRICK T., RAMWELL P., ROSE J., KOT P. Responses to leukotriene C4 in the perfused rat lung. J. Pharmacol. Exp. Therap. 1984;229:654–657. [PubMed] [Google Scholar]
- KIPS J.C., BRUSELLE G.J., JOOS G.F., PELEMAN R.A., TAVERNIER J.H., DEVOS R.R., PAUWELS R.A. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 1996;153:535–539. doi: 10.1164/ajrccm.153.2.8564093. [DOI] [PubMed] [Google Scholar]
- KUROSAWA M. Role of thromboxane A2 synthase inhibitors in the treatment of patients with bronchial asthma. Clin. Therap. 1995;17:2–11. doi: 10.1016/0149-2918(95)80002-6. [DOI] [PubMed] [Google Scholar]
- LARSEN G.L., RENZ H., LOADER J.E., BRADLEY K.L., GELFAND E.W. Airway responses to electrical field stimulation in sensitized inbred mice. J. Clin. Invest. 1992;89:747–752. doi: 10.1172/JCI115651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHEY J., FRIEDERICH T., FRANKE J., NIGAM S., PRIESNITZ M., OEFF K. Pressure effects and uptake of platelet-activating factor in isolated rat lung. J. Appl. Physiol. 1984;57:1039–1044. doi: 10.1152/jappl.1984.57.4.1039. [DOI] [PubMed] [Google Scholar]
- MANZINI S. Bronchodilation by tachykinins and capsaicin in the mouse main bronchus. Br. J. Pharmacol. 1992;105:968–972. doi: 10.1111/j.1476-5381.1992.tb09086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN C., UHLIG S., ULLRICH V. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur. Respir. J. 1996;9:2479–2487. doi: 10.1183/09031936.96.09122479. [DOI] [PubMed] [Google Scholar]
- MARTIN T.R., GERARD N.P., GALLI S.J., DRAZEN J.M. Pulmonary responses to bronchoconstrictor responses in the mouse. J. Appl. Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- NAGASE T., ISHII S., KATAYAMA H., FUKUCHI Y., OUCHI Y., SHIMIZU T. Airway responsiveness in transgenic mice overexpressing platelet-activating factor receptor. Roles of thromboxanes and leukotrienes. Am. J. Respir. Crit. Care Med. 1997;156:1621–1627. doi: 10.1164/ajrccm.156.5.9703016. [DOI] [PubMed] [Google Scholar]
- SALZER W.L., MCCALL C.E. Primed stimulation of isolated perfused rabbit lung by endotoxin and platelet activating factor induces enhanced production of thromboxane and lung injury. J. Clin. Invest. 1990;85:1135–1143. doi: 10.1172/JCI114545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO T., IWAMA T., SHIKADA K., TANAKA S. Airway hyperresponsiveness to acetylcholine induced by aerosolized arachidonic acid metabolites in guinea-pigs. Clin. Exp. Allergy. 1996;26:957–963. [PubMed] [Google Scholar]
- SHI H., YOKOYAMA A., KOHNO N., HIRASAWA Y., KONDO K., SAKAI K., HIWADA K. Effect of thromboxane A2 inhibitors on allergic pulmonary inflammation in mice. Eur. Respir. J. 1998;11:624–629. [PubMed] [Google Scholar]
- STEUDEL W., ICHINOSE F., HUANG P.L., HURFORD W.E., JONES R.C., BEVAN J.A., FISHMAN M.C., ZAPOL W.M. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ. Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- UHLIG S., WENDEL A.Lipid mediators in perfused lung Interdisziplinäre Aspekte der Pneumologie 1995Stuttgart: Georg Thieme Verlag; 66–74.eds. Von Wichert, P. & Siegenthaler, W. pp [Google Scholar]
- UHLIG S., WOLLIN L. An improved setup for the isolated perfused rat lung. J. Pharm. Tox. Meth. 1994;31:85–94. doi: 10.1016/1056-8719(94)90047-7. [DOI] [PubMed] [Google Scholar]
- UHLIG S., WOLLIN L., WENDEL A. Contributions of thromboxane and leukotrienes to platelet-activating factor-induced impairment of lung function in the rat. J. Appl. Physiol. 1994;77:262–269. doi: 10.1152/jappl.1994.77.1.262. [DOI] [PubMed] [Google Scholar]
- VON BETHMANN A.N., BRASCH F., NÜSING R., VOGT K., VOLK D., MÜLLER K.-M., WENDEL A., UHLIG S. Hyperventilation induces release of cytokines from perfused mouse lung. Am. J. Respir. Crit. Care Med. 1998;157:263–272. doi: 10.1164/ajrccm.157.1.9608052. [DOI] [PubMed] [Google Scholar]
- XIU Q., FUJIMURA M., NOMURA M., SAITO M., MATSUDA T., AKAO N., KONDO K., MATSUSHIMA K. Bronchial hyperresponsiveness and airway neutrophil accumulation induced by interleukin-8 and the effect of the thromboxane A2 antagonist S-1452 in guinea-pigs. Clin. Exp. Allergy. 1995;25:51–59. doi: 10.1111/j.1365-2222.1995.tb01002.x. [DOI] [PubMed] [Google Scholar]


