Abstract
Human saphenous veins were used to assess the cooperative participation of adenosine 5-triphosphate (ATP), neuropeptide Y (NPY), and noradrenaline (NA) in the vasomotor responses elicited following electrical depolarization of the perivascular nerve terminals. Rings from recently dissected human biopsies were mounted to record isometric muscular contractions; the motor activity elicited in the circular muscle layer following electrical depolarization (2.5–20 Hz, 50 V, 0.5 msec) were recorded.
Incubation of the biopsies with either 100 nM tetrodotoxin (TTX) or 1 μM guanethidine abolished the vasomotor response elicited by electrical nerve depolarization. The independent application of either ATP or NA to vein rings induced concentration-dependent contractions.
Tissue incubation with 30 μM suramin or 10 nM prazosin produced 10 fold rightward displacements of the α,β-methylene ATP and NA concentration-response curves respectively. NPY contracted a limited number of biopsies, the vasoconstriction elicited was completely blocked by 1 μM BIBP 3226. A 5 min incubation of the biopsies with 10–100 nM NPY synergized, in a concentration-dependent fashion, both the ATP and the ATP analogue-induced contractions. Likewise, tissue preincubation with 10 nM NPY potentiated the vasomotor responses evoked with 20–60 nM NA.
Neither suramin, BIBP 3226, nor prazosin was individually able to significantly modify the derived frequency-tension curves. In contrast, the co-application of 30 μM suramin and 10 nM prazosin or 30 μM suramin and 1 μM BIBP 3226, elicited a significant (P<0.01) downward displacement of the respective frequency-tension curves.
The simultaneous application of the three antagonists–30 μM suramin, 1 μM BIBP 3226 and 10 nM prazosin–caused a significantly greater displacement of the frequency-tension curve than that achieved in experiments using two of these antagonists.
Electrically-evoked vasomotor activity is blocked to a larger extent by tissue incubation with 2.5 μM chloroethylclonidine and 30 μM suramin rather than with 10 nM 5 methyl urapidil and 30 μM suramin. As a result, the α1-adrenoceptor involved in the vasomotor activity has tentatively been associated with the α1B adrenoceptor family subtype.
Results support the physiological role of ATP in sympathetic neurotransmission. The present results are consistent with the working hypothesis that human sympathetic vasomotor reflexes involve the coordinated motor action of ATP, NPY, and NA acting on vascular smooth muscle cells. The present results support the concept of sympathetic co-transmission in the human saphenous vein.
Keywords: Co-transmission, sympathetic nerve activity, ATP receptors, NPY vasomotor effect, noradrenaline vasoconstriction, human saphenous vein
Introduction
The view that the sympathetic nervous system is essentially dependent only on the action of noradrenaline (NA) has been convincingly challenged. During the past decade, extracellular adenosine 5′-triphosphate (ATP) has arisen as an integral component of sympathetic reflexes, acting in conjunction with neuropeptide Y (NPY) and noradrenaline (NA). Hence, the notion of sympathetic co-transmission is firmly rooted (Campbell, 1987; Burnstock, 1990).
Implicit in the concept of co-transmission is the notion that the sympathetic triad–ATP, NPY and NA–must be co-stored, co-released and, in addition, they must act postjunctionally in a coordinated fashion. Two peripheral neuronal systems have been particularly attractive as models to study the physiology of co-transmission. The short axon neurons innervating the rodent vas deferens and the perivascular sympathetic neurons have played a pivotal role in the genesis of our current understanding of co-transmission. There is ample evidence of the co-localization of ATP and NA in the synaptic vesicles of peripheral nerve terminals, where numerous immunocytochemical studies have additionally co-localized NPY in the sympathetic varicosity (Lundberg, 1996). Taking a next step in the characterization of co-transmission, Fried et al. (1986) were the first to demonstrate that NPY is co-stored with NA in the large vesicles of peripheral sympathetic nerve varicosities. The sympathetic triad is also co-released in both the vas deferens neuroeffector junction and the vascular smooth muscle (Kasakov et al., 1988; Ellis & Burnstock, 1990; Torres et al., 1992; Donoso et al., 1997a) where the triad co-operates postjunctionally to produce a motor response (Donoso et al., 1994; 1997b; Lundberg, 1996; Racchi et al., 1997). Lundberg (1996) has comprehensively reviewed the latest contributions to our understanding of sympathetic co-transmission.
Crucial to the elucidation of the co-transmission mechanism is the pharmacological characterization of membrane bound receptors for the sympathetic triad of neurochemicals. The recent cloning of members of the growing family of P2x purinoceptors (Surprenant et al., 1995; Brake & Julius, 1996) has confirmed Burnstock's intriguing proposal for the extracellular role of ATP (Burnstock, 1972; 1976) and the subsequent identification of ATP receptors differing from those activated by adenosine (Burnstock & Kennedy 1985; Valera et al., 1994). Six NPY receptors subtypes have been cloned (Alexander & Peters, 1997; Malmström, 1997). Their pharmacological characterization is being avidly pursued. Specific drug receptor antagonists have played an increasingly important role in the pharmacological characterization of these receptors. Such is the case of suramin, for the purinoceptors, and BIBP 3226, for the NPY Y1 receptor respectively (Dunn & Blakely 1988; Rudolf et al., 1994).
In 1987, Ramme et al., demonstrated that ATP is a neurotransmitter in the jejunal branches of the rabbit mesenteric artery. Around the same time, it became clear that NPY was abundantly localized in peripheral perivascular nerve terminals and linked to the sympathetic control of circulatory homeostasis (Ekblad et al., 1984; Edvinsson et al., 1984; 1985). Despite these fundamental observations, relatively little research has been conducted in vascular smooth muscles to better identify the nature of sympathetic co-transmission and, particularly, to characterize the roles played by ATP and NPY in the physiology of sympathetic reflexes. Moreover, considering the potential clinical application of this concept to the control of the human vascular homeostasis, this concept has been scarcely investigated in isolated human vessels likely due to the limited access to human biopsies. Interesting studies performed in Sweden have demonstrated the presence of NPY Y1 receptors, and its corresponding mRNA, in omental and cerebral arteries, and argued positively in favour for its role in sympathetic co-transmission in human blood vessels (Bergdahl et al., 1996; Nilsson et al., 1996a, 1996b).
Studies using the human saphenous vein have identified the presence of α1 and α2 adrenoceptors (Müller-Schweinitzer, 1984; Steen et al., 1984; Docherty & Hyland 1985; Docherty 1987) and a component of the electrically-evoked motor response resistant to the simultaneous blockade of both α-adrenoceptor subtypes (Pelleg & Burnstock, 1990; Taddei et al., 1990; Stephens et al., 1992). Furthermore, Rump & von Kügelgen (1994) demonstrated the outflow of neuronal ATP following the electrical stimulation of the perivascular nerves in this blood vessel. This finding is a positive indicator of co-transmission in this human tissue. In view of the limited information of co-transmission in human veins, and in an attempt to study the role of ATP as a component of the sympathetic triad, the present investigation utilizes human saphenous vein biopsies as a model blood vessel to pharmacologically examine the functional contributions of ATP, NPY and NA receptors to human vascular homeostasis. These results conform the basis for a more precise understanding of the sympathetic reflexes, showing for the first time that the vasomotor activity elicited by activation of human sympathetic perivascular nerves implies the coordinated action of ATP, NPY and NA.
Methods
Human biopsies and the in vitro mounting of blood vessel rings
Saphenous vein samples were obtained from at least 90 patients undergoing cardiac revascularization surgery at our School of Medicine's Clinical Hospital. Within minutes of resection of the vein, samples were transported in sterile solution from the nearby operating room to our laboratory. The samples were immediately transferred to a container with Krebs-Ringer solution bubbled with 95% O2/5% CO2 and maintained at 37°C. The composition of the Krebs-Ringer buffer is as follows (mM): NaCl 118, KCl 5.4, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 23.8 and glucose 11.1. Ring preparations, 3–4 mm in diameter, were mounted in bath chambers where circular muscle layer contractions were recorded isometrically as previously detailed by Racchi et al. (1997). A basal tension of 1–1.5 g was maintained throughout the experiment.
Experimental protocols
The protocols performed were carried out in accordance with the recommendations of the School of Medicine Ethics Committee, as regulated by the P. Catholic University. The size of the vein biopsy attained was variable, most of the biopsies generated one and only on occasions the tissue sufficed to prepare two rings; the ‘n' indicated in the figures refers to the experiments performed with separate rings assessed in each experimental protocol. Mounted saphenous vein rings under conditions of constant buffer flux, were equilibrated to 1–1.5 g of tension for 1 h. During the incubation period, the buffer was continually changed favouring the washout of any contaminant drug administered to the patient prior to or during the surgical procedure. Then the analytical reference stimulation was carried out with 70 mM potassium chloride and tissue viability was assessed by cumulatively exposing the ring samples to increasing concentrations of NA (0.1 nM–10 μM). Rings that did not respond to the potassium challenge or exhibited less than 0.5 g of tension with the maximal concentration of NA were discarded. The population of rings that had to be discarded summed circa 10% of the total biopsy population.
All rings were transmurally deplorized for 30 s by electrical pulses (70 V, 0.5 ms duration) delivered through platinum electrodes located at opposite sides of the bath chamber. A series of stimuli (2.5, 5, 10 and 20 Hz) were applied sequentially upon complete muscle relaxation, as indicated by a return to the basal tension. For each segment, this stimulation protocol was performed twice under normal buffer conditions to define the control frequency-tension curve.
Studies with tetrodotoxin and guanethidine
To assess the nature of the contractile responses generated by electrical depolarization of the nerve endings present in the biopsies, the frequency-tension curve protocol was performed in biopsies prior to and following a 10 min incubation with 100 nM tetrodotoxin (TTX). Parallel experiments were performed incubating the biopsies for 30 min with 1 μM guanethidine, a drug known to block the vesicular exocytosis of noradrenaline. Results compare the frequency-contraction curve obtained before and after guanethidine exposure.
Participation of purinergic receptors
To examine the participation of P2X purinoceptors in the motor tone of the human saphenous vein, frequency-tension curves were produced before and after a 30 min incubation period in the presence of 30 μM suramin. Using the same incubation protocol, concentration-response curves were created for the synthetic, slowly degraded analogue, α,β-methylene ATP. Exogenous ATP was also utilized, although its pharmacology was not studied in the presence of suramin. The concentration of suramin chosen for the studies involving the electrical depolarization of the perivascular nerves was based on that required to cause a 10 fold rightward displacement of the α,β-methylene ATP concentration-response curve.
Influence of NPY, studies with BIBP 3226
The effect of exogenous applications of 0.1–100 nM NPY was next examined. In view of the limited number of biopsies that responded directly with a vasomotor effect, few experiments could be performed to measure the motor effect elicited by NPY before and 15 min after tissue incubation with 1 μM BIBP 3226. This concentration of the antagonist had been successfully used in blocking the effect of endogenous NPY in biopsies from human mesenteric vessels (Racchi et al., 1997). Frequency-tension protocols were performed before and after a 15 min incubation with 1 μM BIBP 3226 in order to identify the change in the frequency-tension relation caused by this receptor antagonist. The drug was then thoroughly flushed from the system and another frequency-tension curve was generated to assess the reversibility of BIBP 3226.
NPY-induced potentiation of the vasomotor responses induced by either ATP, α,β-methylene ATP and NA
To examine whether tissue preincubation with NPY potentiated the vasomotor responses elicited by ATP or α,β-methylene ATP, an ATP concentration-response protocol was performed prior to and following a 5 min tissue incubation with 10 nM NPY. Separate rings were challenged with 3 μM α,β-methylene ATP in the absence and following a 5 min preincubation with 10, 30 or 100 nM NPY. Likewise, a NA concentration-response protocol was performed in separate biopsies prior to and following a 5 min incubation with 10 nM NPY. In a separate series of experiments, the biopsies were challenged with 20 and 60 nM NA in the absence (control) and following a 5 min incubation with 1–100 nM NPY.
Influence of prazosin and other α1-adrenoceptor antagonists
To determine the participation of the α1-adrenoceptor and its subtypes, frequency-contraction curves were generated in biopsies prior to and 30 min after tissue incubation with 10 nM prazosin. This concentration of the antagonist was chosen for our studies because it displaced the NA concentration-response curve rightward about 10 fold. The same biopsy rings were challenged with NA before and after exposure to this antagonist. In parallel biopsies, the same protocols were performed, except that prazosin was replaced by either 1–10 nM 5-methyl urapidil or 1–10 μM chloroethylclonidine. These agents show relative selectivity for α1A- and α1B-adrenoceptors respectively. Separate ring vessels were incubated with each antagonist for 30 min before generating either the NA concentration-response curve or the frequency-contraction curves.
Combined application of suramin plus prazosin and of suramin and BIBP 3226
Frequency-tension experiments were performed prior to and 30 min following the simultaneous exposure to 30 μM suramin and 10 nM prazosin in order to explore additive effect of purinoceptor and α1-adrenoceptor antagonism. In parallel experiments prazosin was replaced in the antagonist pair by either 10 nM 5-methyl urapidil or by 2.5 μM chloroethylclonidine. Separate protocols were carried out combining 30 μM suramin and 1 μM BIBP 3226 to investigate the effect of simultaneously blocking purinoceptors and NPY-Y1 receptors.
Influence of the simultaneous application of suramin, prazosin, and BIBP 3226
Parallel experiments were designed to assess whether the combined use of all three antagonists caused a blockade greater in magnitude than those observed using combinations of two receptor antagonists. The vein rings were incubated simultaneously with the triad of antagonists (30 μM suramin, plus 10 nM prazosin plus 1 μM BIBP 3226) for 30 min prior to a second frequency-tension curve.
Data analysis
All data is normalized and presented as the percentage of the tension elicited by 70 mM potassium chloride, standard applied at the beginning of every experimental protocol. The tension generated by this standard did not differ significantly throughout each experimental study. Co-variance analysis was applied to the frequency-tension curves, with statistical significance being defined at a P value of less than 0.05. The Student's t-test, and Dunnett's tables to compare multiple groups with a common control was used as appropriate; the Bonferroni-correction was also used when required.
Drug sources and providers
Tetrodotoxin, guanethidine sulphate, noradrenaline hydrochloride, adenosine 5′-triphosphate sodium salt, α,β-methylene ATP lithium salt, and prazosin hydrochloride were purchased from Sigma Chemical Co (St. Louis, MO, U.S.A.). Chloroethylclonidine and 5 methyl urapidil were obtained from RBI (Natick, MA, U.S.A.). Synthetic pNPY was purchased from Peninsula Labs. (Belmont, CA, U.S.A.). Suramin was kindly provided to us by Hoechst, Germany. BIBP 3226 was kindly provided by Dr K. Rudolf, Thomae GmbH (Biberach, Germany).
Results
Sympathetic nature of the contractions elicited by electrical depolarization of the nerve terminals
Electrical depolarization of the perivascular nerve terminals evoked frequency-dependent vasomotor responses. Frequency-tension curves were literary abolished in the presence of 100 nM TTX or 1 μM guanethidine, evidencing the neuronal origin and the sympathetic nature of the nerve-evoked contractions (Figure 1). We frequently observed that the 10 and 20 Hz-induced contractions raise the tissue tension even after ending the 30 s electrical stimulation (Figure 1). This observation might reflect the lag between the delivery of the electrical stimuli and the development of the maximal muscular tension. Control studies repeating twice the frequency-tension curves revealed essentially no difference, ruling out a significant contribution of a winding phenomenon.
Figure 1.
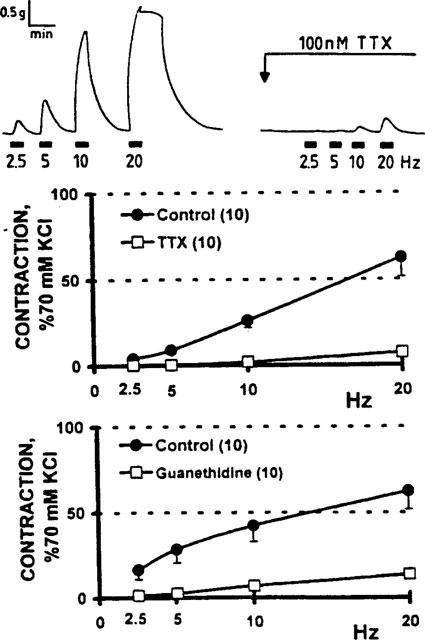
Vasomotor activity elicited by electrical stimulation of the perivascular nerves of human saphenous vein biopsies; blockade by tetrodotoxin and guanethidine. Upper panel: representative recordings of the vasomotor activity of a recently dissected human saphenous vein ring. Reproducible, frequency-dependent contractions were recorded following transmural electrical nerve stimulation; blockade of neurotransmission by 100 nM tetrodotoxin (TTX). Middle panel: frequency-tension curves were generated before and 15 min after tissue incubation with TTX (n=10). Lower panel: separate biopsies following a 30 min tissue incubation with 1 μM guanethidine (n=10). Analysis of variance shows that the curve displacement evoked by both drugs was significant (P<0.001). Symbols represent the mean values; bars, the s.e.mean.
Influence of purinoceptors
The vasomotor effect elicited by ATP was mimicked by α,β-methylene ATP albeit at concentrations almost 1000 fold lower (Figure 2). While 30 μM suramin significantly displaced the α,β-methylene ATP concentration-response curve to the right 17.5 fold (P<0.01), the antagonist was unable to modify the frequency-tension curve evoked by transmural electrical nerve stimulation (Figure 2). The ATP-induced motor responses were not sustained; those generated by the non-hydrolyzable analogue were more stable, although fading was also evident.
Figure 2.
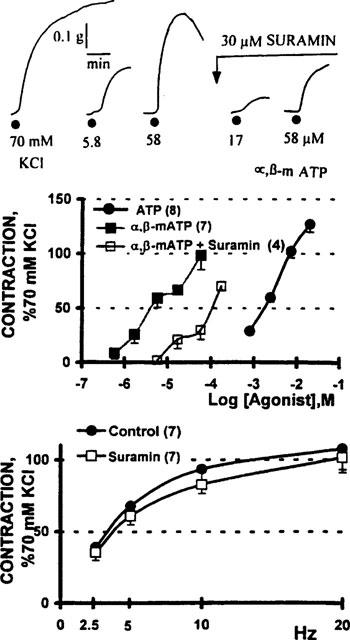
Vasomotor contractions induced by adenosine 5′-triphosphate (ATP) and α,β-methylene ATP (α,β-mATP) in human saphenous vein biopsies. Upper panel: polygraphic record shows the isometric contractions generated by the application of 5.8 and 58 μM α,β-mATP to a human saphenous vein ring; a 5 min tissue incubation with 30 μM suramin blocked the purinergic-induced contractions. Middle panel: comparative potency of α,β-mATP and ATP. Open squares denote the α,β-mATP concentration-response curve obtained in vein rings incubated 5 min with 30 μM suramin (n=4). The suramin induced a 17.5 fold displacement of the α,β-mATP concentration-response curve (P<0.01, Student's t-test). Lower panel: frequency-tension curves obtained prior to and following a 30 min incubation with suramin (n=7). Symbols indicate the mean values; bars, the s.e.mean.
NPY receptors and vascular reactivity
1–100 nM NPY did not consistently produce a vasomotor response. In the few tissues that demonstrated a defined concentration-response relationship (11/92 biopsies), the apparent median effective concentration of NPY was circa 100 nM. Tissue incubation with 1 μM BIBP 3226 abolished the NPY-induced vasoconstriction; however, the frequency- tension curve was refractile to the antagonist (Figure 3).
Figure 3.
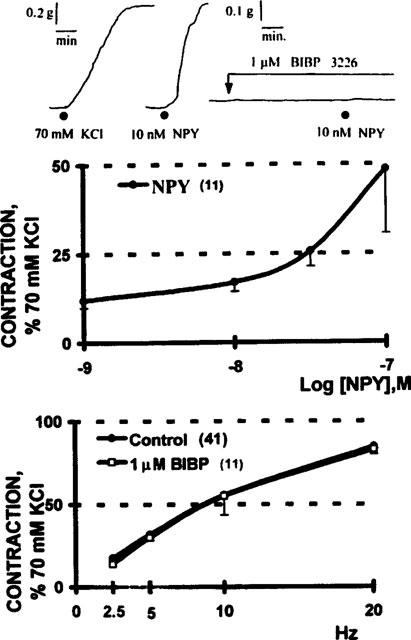
The vasomotor effect of NPY is mediated by NPY Y1 receptors; resistance of the electrically-evoked contractions to antagonism with 1 μM BIBP 3226. Upper panel: a polygraphic recording of a saphenous vein ring that contracted to the application of 10 nM NPY, the contractile effect was totally blocked by 1 μM BIBP 3226. Note the 2 fold difference in calibration between the KCl standard and the response elicited by NPY. Middle panel: in the few of the biopsies that contracted to the application of NPY (11/92), the vasomotor effect was concentration-dependent. Lower panel: 1 μM BIBP 3226 did not modify the frequency-tension curve elicited by electrical depolarization of the perivascular nerve fibres. Symbols indicate the mean values; bars, the s.e.mean.
NPY-induced potentiation of purinergic and adrenergic vasomotor responses
Although 10 nM NPY failed to consistently elicit a vasomotor response per se, it significantly potentiated, in a concentration-dependent fashion, the motor responses evoked by either ATP or α,β-methylene ATP (Figure 4). In the presence of 10 nM NPY, the ATP concentration-response curve appeared to be displaced leftwards. The magnitude of the vasomotor effect elicited by 3 μM α,β-methylene ATP was doubled by 10 nM NPY and tripled in the presence of 100 nM NPY.
Figure 4.
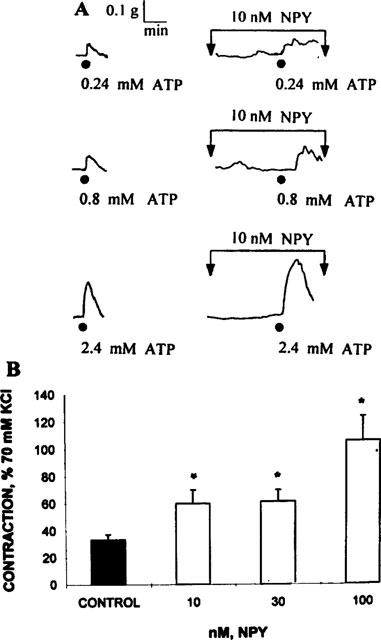
Neuropeptide Y-induced potentiation of the vasocontractile activity of adenosine 5′-triphosphate (ATP) and its non hydrolyzable analogue α,β-methylene ATP (α,β m-ATP). Upper panel: representative polygraphic recording of a protocol showing the contractile responses of a saphenous vein ring stimulated following application of 0.24, 0.8 and 2.4 mM ATP and its synergism following a 5 min incubation with 10 nM NPY. The ATP contractions are not sustained; 10 nM NPY did not elicit a consistent vasomotor response. Lower panel: Vasomotor contractions elicited by a 3 μM α,β-methylene ATP challenge in the absence (black column) and in the presence of 10, 30 and 100 nM NPY (n=4, open columns). Mean values; bars s.e.mean, *P<0.01 (Dunnett's tables, versus control).
Tissue preincubation with 10 nM NPY appeared to displace the NA concentration-response curve to the left. The magnitude of the NPY-induced NA potentiation was dependent not only on the concentration of NPY, but also on the NA concentration. Interestingly, the magnitude of the NPY-induced facilitation was proportionally larger with 20 nM NA than with 60 nM NA (Figure 5), suggesting that the potentiation might be more relevant at the lowest agonist concentration.
Figure 5.
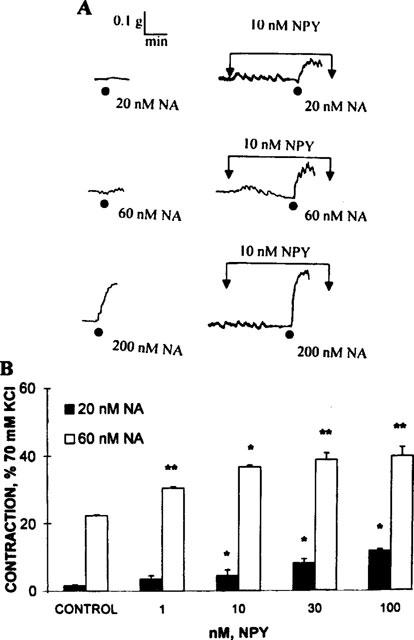
Potentiation of the noradrenaline (NA)-evoked vasomotor responses by 10 nM NPY. Upper panel: polygraphic recording showing a NA concentration-response protocol obtained in a single biopsy in the absence and following 5 min of tissue incubation with 10 nM NPY. While NPY does not elicit per se a vasomotor response, it consistently synergizes the NA-induced vasomotor responses. Lower panel: potentiation of the 20 and 60 nM NA-evoked vasomotor responses in the presence of 1–100 nM NPY. Black columns denote the vasomotor effect elicited by 20 nM NA (n=4), while the open columns denote the potentiation of the 60 nM NA-evoked responses (n=4). Columns denote the mean values, bars the s.e.mean, *P<0.05 value; **P<0.01 (Dunnett's tables for comparison with a single control).
Involvement of α1-adrenoceptors
In contrast to the 6 fold displacement of the NA concentration-response curve (P<0.01) induced by prazosin, the antagonist was essentially unable to modify, to a similar extent, the frequency-tension curve (Figure 6). Furthermore, tissue incubation with either 10 nM 5-methyl urapidil, or 1–10 μM chloroethylclonidine alone did not block the electrically-evoked contractions (Figure 7).
Figure 6.
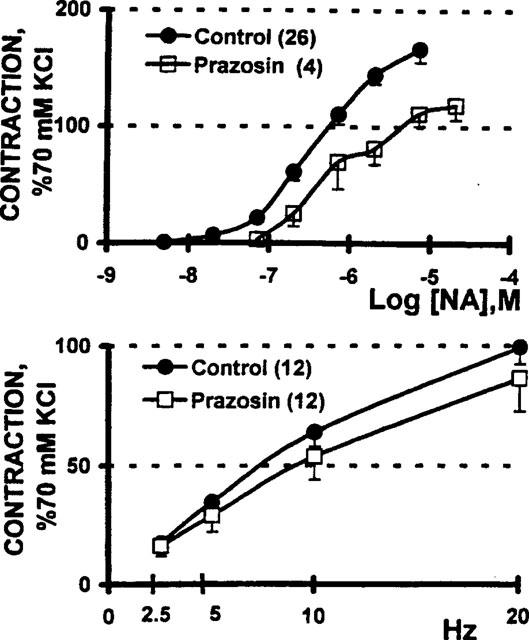
Prazosin blocks the vasomotor contraction elicited by exogenous noradrenaline (NA), but not for contractile effect elicited by electrical depolarization of the perivascular nerve terminals. Upper panel: NA concentration-response curves were generated before (n=26) and after (n=4) a 30 min incubation with 10 nM prazosin; the rightward displacement of the NA concentration-response curve caused a 6 fold increase in the median effective concentration (P<0.01, Student's t-test). Lower panel: tissue incubation with 10 nM prazosin did not significantly modify the frequency-tension curve (n=12). Symbols indicate the mean values; bars, the s.e.mean.
Figure 7.
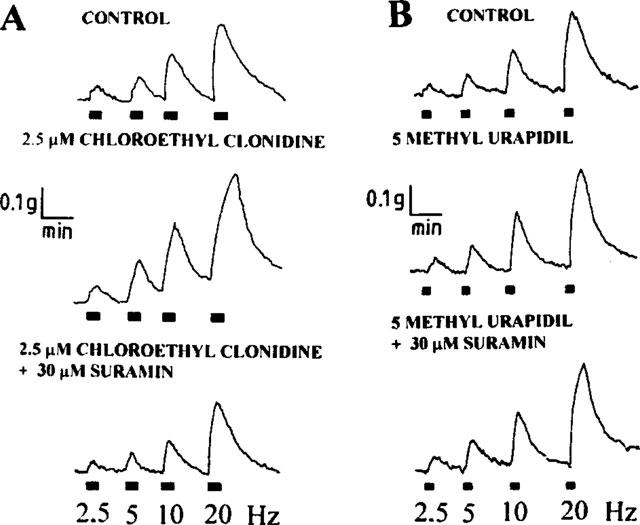
Tentative identification of the nature of the α1-adrenoceptor involved in sympathetic co-transmission. Representative protocols show that tissue incubation with either 2.5 μM chloroethylclonidine (A, an α-1B adrenoceptor blocker) or 10 nM 5 methylurapidil (B, an α-1A adrenoceptor blocker) alone blocked the vasomotor responses evoked by electrical depolarization of the sympathetic perivascular nerve fibres. Chloroethylclonidine evidenced a modest potentiation of the vasomotor responses. However, the simultaneous incubation with 30 μM suramin and 2.5 μM chloroethylclonidine during 30 min (left panel) evidenced a blockade of the vasomotor response effect that was not observed following the incubation with 30 μM suramin and 10 nM 5-methyl urapidil in a parallel vein preparation (right panel).
Combined application of an α1-adrenoceptor and a purinoceptor antagonist, or a purinergic and NPY Y1 antagonist
Simultaneous incubation of the biopsies with either suramin and prazosin, or suramin and BIBP 3226, caused a significant blockade of the sympathetic nerve-evoked vasomotor response (Figure 8). The downward displacement caused by the combination of suramin and prazosin almost halved the vasomotor responses elicited by nerve stimulation (P<0.01). In the case of the suramin and BIBP 3226 antagonism combination, the pair also resulted in a significant displacement of the curve, but the magnitude only reached a P value <0.05. The downwards displacement of the frequency-tension curve by the antagonist pairs demonstrate the tissue sensitivity to the combined antagonist treatment.
Figure 8.
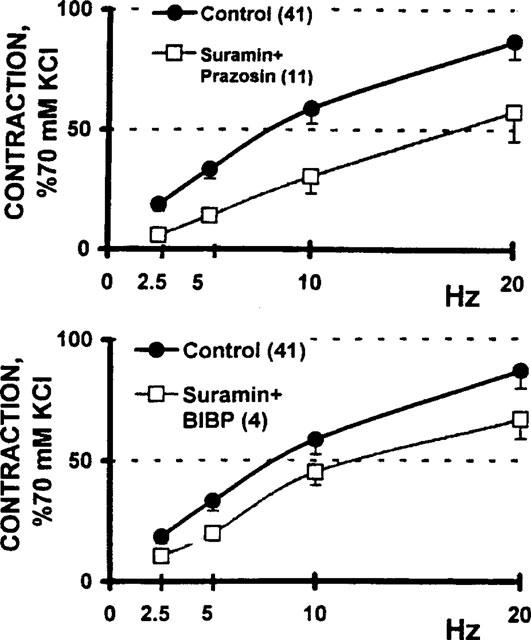
Blockade of the electrically evoked vasomotor responses by a combination of neurotransmitter receptor antagonist drugs. Upper panel: blockade of sympathetic co-transmission following a 30 min tissue incubation with the combination of either 30 μM suramin and 10 nM prazosin (n=11) or, lower panel: 30 μM suramin and 1 μM BIBP 3226 (n=4). Co-variance analysis indicate that the downward displacement caused by the suramin plus prazosin combination reached statistical significance at the P<0.01 level, while the pair suramin plus BIBP 3226 reached a P<0.05.
We assessed next whether the α-1A or the α-1B adrenoceptor, or a combination of both, are involved in the sympathetic neurotransmission of the saphenous vein. It is clear that the combination of chloroethylclonidine plus suramin, is more effective than the combination of 5-methyl urapidil plus suramin (Figure 7), suggesting that the α1B-adrenoceptor might have a predominant role in the sympathetic vasomotor activity of this vascular territory.
Blockade of neurotransmission caused by simultaneous incubation with suramin, prazosin, and BIBP 3226
The downward displacement of the frequency-tension curve elicited by the combined application of all three antagonists was more intense than that elicited by either of the previously tested antagonist pairs (Figure 9, P<0.01). The electrically-evoked vasomotor response elicited by the 2.5–5 Hz stimuli was abolished after incubation with the triple combination of antagonists.
Figure 9.
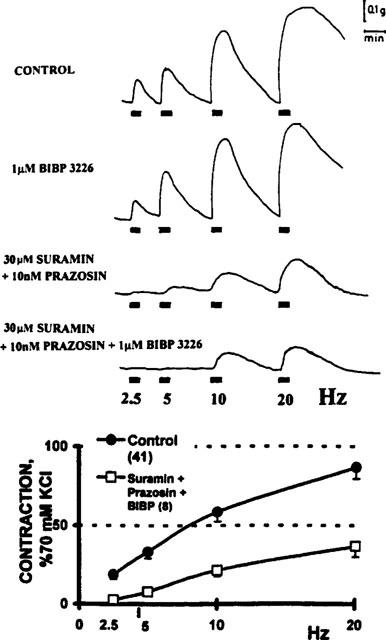
Further blockade of sympathetic neurotransmission by the combination of the three principal antagonists. Upper panel: representative tracing from a typical experiment showing the vasomotor responses elicited following electrical transmural depolarization of the perivascular sympathetic nerve fibres of a human saphenous vein biopsy. Control refers to the recordings obtained prior to drug applications. The tissue was next incubated with 1 μM BIBP 3226 alone and 30 min later a second frequency-tension curve was generated. Immediately thereafter, the biopsy was incubated with a combination of 30 μM suramin and 10 nM prazosin during 30 min and the electrical stimuli were next delivered. Finally, the biopsy was incubated with a combined triple mixture of suramin plus prazosin plus BIBP 3226. Lower panel: The downward displacement of electrically-evoked frequency-tension curve caused by incubation with 30 μM suramin, 10 nM prazosin, and 1 μM BIBP 3226 (n=11) was lower (P<0.05) than that attained by incubation with only two of the three antagonists. Symbols represent the mean values; bars the s.e.mean.
Discussion
The present results indicate that the electrical stimulation of the sympathetic nerves surrounding the human saphenous vein provokes vasomotor responses that involve the combined postjunctional action of extracellular ATP, NPY and NA. Of particular interest in this study is the observation that antagonist combinations (suramin and prazosin; suramin and BIBP; or suramin, BIBP and prazosin) are able to induce a blockade of the electrically-induced vasomotor response that each antagonist individually could not. Recent experiments from our laboratory have shown that purinoceptor blockade with suramin may be successfully replaced by desensitization with two applications of 50 μM α,β-methylene ATP (Huidobro-Toro, 1998). While the paired antagonists provoked partial reduction of the vasomotor response, only in the collective presence of all three antagonists did the electrically-induced response experience a substantial inhibition of the vasomotor effect of the transmitters released, resulting in the complete obliteration of the vasomotor response at stimulation frequencies of 2.5 and 5 Hz. This finding is totally compatible with the competitive nature of the antagonists and the current literature identifying a component of the vasomotor tone of the human saphenous vein resistant to α-adrenoceptor antagonism, (Steen et al., 1984; Taddei et al., 1990; Stephens et al., 1992) and to the combination of α1-adrenoceptor antagonism plus suramin (Rump & von Kügelgen, 1994). A parsimonious interpretation of the present results would suggest that the success of the antagonist combinations is related to reaching a threshold level of receptor antagonism. However, looking beyond this simple additive explanation, we hypothesize that some sort of coordinated physiological synergism must be operating, in which, in addition to the activation of transmitter-specific receptors, there is an integral dynamic interplay between the transmitters, potentiating a response that the combined independent activity of the three can not.
The sympathetic nature of the vasomotor response elicited by electrical stimulation of the perivascular nerves is supported by two findings. First, the response is virtually abolished by 100 nM tetrodotoxin, a classical indicator of the neuronal origin of vasomotor responses. Second, guanethidine, a pharmacological agent that interferes with the exocytosis of sympathetic neurotransmitters (Brock & Cunnane, 1988), reduced markedly the elicited vasomotor response. In further support of this proposal, experiments in progress demonstrate that the electrical depolarization of the nerves surrounding the human saphenous vein releases both NPY and NA to the superfusion media (M.V. Donoso and J.P. Huidobro-Toro, manuscript in preparation). Therefore, it is highly probably that the vasomotor responses elicited by transmural nerve depolarization of the isolated rings are similar in nature to those provoked in vivo, which ultimately result in varying degrees of vasoconstriction.
A proper physiological interpretation of the present results relies heavily on the pharmacodynamics of the antagonists, particularly the receptor selectivity and specificity of these agents. It was of paramount importance to independently characterize the pharmacology of these drugs in this tissue prior to deriving conclusions on the nature of the neurotransmitters involved in the vasomotor responses recorded. For this purpose, suramin, BIBP 3226, and prazosin, as well as 5 methyl urapidil and chloroethyclonidine, were examined as antagonists of their corresponding ligands prior to the evaluation of their efficacy in blocking the said responses. Suramin, a low affinity, competitive antagonist of purinoceptors (Dunn & Blakeley, 1988) has offered insights into our understanding of the role of ATP in the sympathetic transmission, despite its known effect as inhibitor of enzymes, including ecto ATPases (Voogd et al., 1993). Recent protocols carried out in our laboratory have desensitized the purinoceptors, following repeated applications of 50 μM α,β-methylene ATP, as an additional procedure to inhibit ATP-induced vasomotor responses (Huidobro-Toro, 1998). Likewise, BIBP 3226, a competitive, high-affinity and selective NPY Y1 receptor antagonist (Rudolf et al., 1994; Doods et al., 1995; Lundberg & Modin, 1995; Malmström & Lundberg, 1995; Mezzano et al., 1998) has been an invaluable tool in the characterization of NPY as a sympathetic co-transmitter or neuromodulator in peripheral vascular neuroeffector junctions. The implications that some NPY antagonists may have partial agonist properties in other NPY receptor subtypes is an additional problem that cannot be ignored at present.
ATP is an efficacious but not a potent agonist to contract the human saphenous vein. However, its non-hydrolyzable analogue, α,β-methylene ATP, is about 1000 fold more potent. This finding could indicate that the vascular smooth muscle must be rich in ecto ATPases. If ATP is expected to act as an excitatory transmitter in a vascular neuroeffector junction, one would anticipate that this compound would be easily degraded by such enzymes. The use of the ecto ATPase inhibitor, FPL 67156, demonstrates that this is the case. In the presence of this drug, the concentration-response curve for ATP in rabbit ear biopsies is displaced to the left in a concentration-dependent fashion (Crack et al., 1995), arguing that ATP must be very rapidly hydrolyzed. Our studies in the human saphenous vein extend the study of Rump & von Kügelgen (1994), who were the first to study the activation of P2X purinoceptors in human vascular biopsies. Experiments are pending to assess whether the ATP-induced responses in biopsies treated with an ecto ATPase inhibitor are sustained.
The present results do not permit an exact characterization of the P2X receptor present in the human saphenous vein. However, based on the observation that α,β-methylene ATP is markedly more potent than ATP plus the finding of desensitization, the present results suggest the possible involvement of P2X1 or P2X3 receptors subtypes (Brake & Julius 1996). However, Cario-Toumaniantz et al. (1998) based on electrophysiological and molecular biology protocols, demonstrated that the P2X7 receptor subtype is abundantly expressed in human saphenous veins. Therefore, multiple P2X receptors might be expected in vascular smooth muscles. Not all vascular territories, demonstrate equal sensitivity to ATP and structurally related analogues. Recent results from our laboratory show that human mammary artery rings, but not mammary vein rings, are markedly resistant to ATP and analogues (Huidobro-Toro, 1998). These results may be interpreted to indicate that ATP receptors are not evenly distributed along the vascular tree, and that various P2X receptor subtypes might be present in the vascular smooth muscle cells.
The present results related to NPY, are of interest. Most of the known studies emphasize that NPY acts indirectly, potentiating, for example, the vasomotor effect of NA (Wahlestedt et al., 1985) or ATP (Westfall et al., 1995). This is also the case with the human saphenous vein. While in the vast majority of the biopsies we studied, 1–10 nM NPY never elicited a defined motor response per se, this concentration is however sufficient to evoke an increased magnitude of the vasomotor response elicited by ATP, α,β-methylene ATP and NA. These observations extend previous experimental data obtained in rodent biopsies (Edvinsson et al., 1984; Wahlestedt et al., 1985) and some limited cases of human blood vessels, to include now the human saphenous vein. As a result, in spite of an apparently minimal contraction induced by NPY through the Y1 receptor, this agonist by virtue of its synergistic effect becomes an efficient participant of sympathetic co-transmission. Although the extension of the project precluded a detailed investigation of the mechanism underlying the potentiation, the data presented allows the suggestion that NPY causes a parallel leftward shift of the ATP and NA concentration-response curves. Therefore, even minute amounts of NPY released at the vascular neuroeffector junction markedly magnifies the vascular sympathetic response. These results emphasize some physiological implications of co-transmission in the control of human vascular homeostasis, revealing the synergism exerted by NPY on the sensitivity of NA and ATP as sympathetic co-transmitters. In further support of this notion, the simultaneous administration of ATP plus NA elicited a clear synergistic response (Huidobro-Toro, 1998), implying the physiological advantage of co-transmission.
Few in vitro studies with human blood vessels have shown that NPY is a direct vasocontractile agonist, except in arteries from the cerebral and temporal circulation (Edvinsson 1985; Jansen et al., 1992; Abounader et al., 1995; Nilsson et al., 1996a). As observed in the dog saphenous vein (Pheng et al., 1997), the human equivalent does indeed demonstrate vasoconstriction upon direct application of NPY, but the number of responding tissues represents only about 10% of the biopsies studied. Although a precise explanation of this variance is lacking, local receptor distribution may play a role. In our study, the rare and erratic appearance of a direct vasomotor effect precluded a thorough pharmacodynamic study. In the few biopsies that exhibited a direct vasomotor effect of NPY, the peptide although potent, resulted less efficacious as a vasoconstrictor compared to the standard of KCl. In contrast to its activity in peripheral blood vessels, NPY is known to contract cerebral mammalian arteries including those of humans (Abounader et al., 1995). In these vessels, as in the saphenous vein, the vasomotor effect of NPY is evoked through the activation of NPY Y1 receptors, as evidenced by its selective BIBP 3226 blockade. Whether subtypes of NPY receptors are present in the vascular system awaits further research. An interesting additional remark pertinent to the discussion should focus on the discrepancies between the effectiveness of NPY in vivo and in vitro preparations. It has been suggested that a vascular tone is required for a direct effect of NPY in certain blood vessels (Grundemar et al., 1992; Cortés et al., 1999).
A challenging aspect of this project was to tentatively explore the nature of the α1-adrenoceptors localized in the human saphenous vein. The relative selectivity of drugs such as 5 methyl urapidil and chloroethylclonidine has been used as a classical criteria for the pharmacodynamical classification of the α1-adrenoceptors (Minneman et al., 1988; Han et al., 1990), into 1A- and 1B-adrenoceptors respectively. However, recent data indicates that the situation is more complex than anticipated since chloroethylclonidine also acts presynaptically and possibly also at postjunctional α2-adrenoceptors. Recently, Guimaraes et al. (1997) demonstrated that chloroethylclonidine acts additionally on prejunctional adrenoceptors in the canine saphenous vein. Perhaps the present observation that chloroethylclonidine alone augments the magnitude of the electrically evoked vasomotor responses in human saphenous vein biopsies could be due to its antagonist action at a prejunctional α2-adrenoceptor. Independent of these experimental caveats that remain to be resolved, the present observations are compatible, at least tentatively, with the conclusion that the human saphenous vein contains a larger population of α1B-adrenoceptors. Consonant with this interpretation, chloroethylclonidine is more effective as an antagonist of the vasomotor effect of exogenous NA than 5 methyl urapidil.
The present results present an integral picture of the coordinated action of ATP, NPY and NA operating in the physiology of the human vascular neuroeffector junction. Of particular interest is the observation that in the human saphenous vein NPY may either promote a direct vasomotor effect or it synergizes the vasomotor activity of ATP and NA. Thereby, the human vascular system appears to be a sensitive bioassay for small concentrations of NPY. Thus, NPY plays a critical role as an integral component of the sympathetic triad in the regulation of the vascular tone through sympathetic stimulation. Further research should focus on the pharmacodynamics of NPY in human arteries as compared to the present study restricted to the saphenous vein.
It is important to assess whether the present observations, based on excised vascular segments maintained in vitro, can be applied to the physiology of the sympathetic control of human blood vessel homeostasis. Along these lines, the recent study of Donoso et al. (1997b) demonstrated that the combined application of antagonists is capable of greatly blocking the vasomotor activity provoked by the stimulation of sympathetic nerves regulating the flow in the arterial mesenteric bed. These results provide hope for linking the present in vitro results to more generalized in vivo applications in humans. As such, the present study offers, for the first time, evidence of functional co-transmission in the sympathetic nerves of the human saphenous vein.
To assess the real physiological implications of these findings, two lines of investigation appear enticing. It would be of interest to mimic the effects evoked by electrical stimulation of the perivascular nerve using the neurotransmitters which compose the sympathetic triad. The present results demonstrate that low concentrations of NPY potentiate the vasomotor activities of NA and ATP, thus indicating the system's synergistic character. The second challenge is the study of co-transmission in arteries, particularly in resistance human vessels. Experiments in progress using the human mammary artery and its corresponding vein, show that essentially the same principles described in the saphenous vein apply to these vessels, as well as to the human mesenteric artery and vein (Racchi et al., 1997).
In summary, the present results indicate that the stimulation of the perivascular sympathetic nerves of the human saphenous vein results in vasomotor responses that involves the postjunctional action of extracellular ATP, in conjunction with NPY and NA. A careful therapeutic analysis of this information might predict that the combined use of purinergic, NPYergic and α-adrenergic antagonists might uncover a novel approach to the treatment of vascular pathologies including hypertension.
Acknowledgments
Thanks are expressed to R. Miranda for conducting the NPY-induced synergism experiments and for assisting with the design of some of the figures. This work was partially funded by FONDECYT grant 1980966 and Cátedra Presidencial en Ciencias 1995, awarded to the senior investigator.
Abbreviations
- ATP
adenosine 5′-triphosphate
- BIBP 3226
(R)-N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide
- NA
noradrenaline
- NPY
neuropeptide Y
- TTX
tetrodotoxin
References
- ABOUNADER R., VILLEMURE J.G., HAMEL E. Characterization of neuropeptide Y receptors in human cerebral arteries with selective agonists and the new Y1 antagonist BIBP 3226. Br. J. Pharmacol. 1995;116:2245–2250. doi: 10.1111/j.1476-5381.1995.tb15060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., PETERS J.A.1997 receptor and ion channel nomenclature supplement Trends in Pharmacol. Sci. 1997suppl.8th edition [PubMed]
- BERGDAHL A., NILSSON T., CANTERA L., NILSSON L., SUN X.Y., HEDNER T., ERLINGE D., VALDEMARSON S., EDVINSSON L. Neuropeptide Y potentiates noradrenaline-induced contraction through the neuropeptide Y Y1 receptor. Eur. J. Pharmacol. 1996;316:59–64. doi: 10.1016/s0014-2999(96)00636-x. [DOI] [PubMed] [Google Scholar]
- BRAKE A.J., JULIUS D. Signaling by extracellular nucleotides. Annu. Rev. Cell Dev. Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- BROCK J.A., CUNNANE T.C. Studies on the mode of action of bretylium and guanethidine in post-ganglionic sympathetic nerves. Naunyn-Schmiedeberg's Arch. Pharmacol. 1988;338:504–509. doi: 10.1007/BF00179321. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- BURNSTOCK G. Do some nerve cells release more than one transmitter. Neuroscience. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Co-transmission. Arch. Int Pharmacodyn. 1990;304:7–33. [PubMed] [Google Scholar]
- BURNSTOCK G., KENNEDY C. Is there a basis for distinguishing two types of P2-purinoceptors. Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- CAMPBELL G. Co-transmission. Ann. Rev. Pharmacol. 1987;27:51–70. doi: 10.1146/annurev.pa.27.040187.000411. [DOI] [PubMed] [Google Scholar]
- CARIO-TOUMANIANTZ C., LOIRAND G., LADOUX A., PACAUD P. P2X7 Receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ. Res. 1998;83:196–203. doi: 10.1161/01.res.83.2.196. [DOI] [PubMed] [Google Scholar]
- CORTES V., DONOSO M.V., BROWN N., FANJUL R., LOPEZ C., FOURNIER A., HUIDOBRO-TORO J.P.A novel mode of neuropeptide Y vasoconstriction: the requirement of a primer and activation of non-Y1/non-Y2NPY receptors highlight sympathetic co-transmission J. Pharmacol. Exp. Ther. 1999. in press [PubMed]
- CRACK B.E., POLLARD C.E., BEUKEN M.W., ROBERTS S.M., HUNT S.F., INGALL A.H., MCKECHNIE K.C.W., IJZERMAN A.P., LEFF P. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br. J. Pharmacol. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCHERTY J.R. The use of the human saphenous vein in pharmacology. Trends Pharmacol. Sci. 1987;8:358–361. [Google Scholar]
- DOCHERTY J.R., HYLAND L. Evidence for neuro-effector transmission through postjunctional α2-adrenoceptors in human saphenous vein. Br. J. Pharmacol. 1985;84:573–576. doi: 10.1111/j.1476-5381.1985.tb12942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONOSO M.V., BATES F., MONTIEL J., HUIDOBRO-TORO J.P. Adenosine 5′-triphosphate, the neurotransmitter in the prostatic portion of the longitudinal muscle layer of the rat vas deferens. Neurosc. Lett. 1994;169:59–62. doi: 10.1016/0304-3940(94)90356-5. [DOI] [PubMed] [Google Scholar]
- DONOSO M.V., BROWN N., CARRASCO C., CORTES V., FOURNIER A., HUIDOBRO-TORO J.P. Stimulation of the sympathetic mesenteric artery nerves releases neuropeptide Y potentiating the vasomotor activity of noradrenaline. J. Neurochem. 1997a;69:1048–1059. doi: 10.1046/j.1471-4159.1997.69031048.x. [DOI] [PubMed] [Google Scholar]
- DONOSO M.V., STEINER M., HUIDOBRO-TORO J.P. BIBP 3226, suramin and prazosin identify neuropeptide Y, adenosine 5′-triphosphate and noradrenaline as sympathetic co-transmitters in the rat arterial mesenteric bed. J. Pharmacol. Exp. Ther. 1997b;282:691–698. [PubMed] [Google Scholar]
- DOODS, WIENEN W., ENTZEROTH H., RUDOLF K., EBERLEIN W., ENGELL W., WIELAND H.A. Pharmacological characterization of the selective non-peptide neuropeptide Y-Y1 receptor antagonist BIBP 3226. J. Pharmacol. Exp. Ther. 1995;275:136–142. [PubMed] [Google Scholar]
- DUNN P.M., BLAKELEY A.G.H. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br. J. Pharmacol. 1988;93:243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDVINSSON L. Characterization of the contractile effect of neuropeptide Y in feline cerebral arteries. Acta Physiol. Scand. 1985;125:33–41. doi: 10.1111/j.1748-1716.1985.tb07690.x. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., EKBLAD E., HAKANSSON R., WAHLESTEDT K. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br. J. Pharmacol. 1984;83:519–525. doi: 10.1111/j.1476-5381.1984.tb16516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKBLAD E., EDVINSSON L., WAHLESTEDT K., UDDMAN R., HAKANSON R. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular fibres. Regul. Pept. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- ELLIS J., BURNSTOCK G. Neuropeptide Y modulation of sympathetic co-transmission in the guinea pig vas deferens. Br. J. Pharmacol. 1990;100:457–462. doi: 10.1111/j.1476-5381.1990.tb15828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED G., TERENIUS L., BRODIN E., EFENDIC S., DOCKRAY G., FAHRENKRUG J., GOLDSTEIN M., HOKFELT T. Neuropeptide Y, enkephalin, and noradrenaline co-exist in sympathetic neurons innervating the spleen. Cell Tissue Res. 1986;243:495–508. doi: 10.1007/BF00218056. [DOI] [PubMed] [Google Scholar]
- GRUNDEMAR L., MORNER E., HOGESTATT E., WAHLESTEDT C., HAKANSON R. Characterization of vascular neuropeptide Y receptors. Br. J. Pharmacol. 1992;105:45–50. doi: 10.1111/j.1476-5381.1992.tb14208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIMARAES S., PAIVA M., PEREIRA O. α-Adrenoceptor-mediated prejunctional effects of chloroethylclonidine in the canine saphenous vein. J. Pharmacol. Exp. Ther. 1997;282:1326–1330. [PubMed] [Google Scholar]
- HAN C., LI J., MINNEMAN K.P. Subtypes of alfa1-adrenoceptors in rat blood vessels. Eur. J. Pharmacol. 1990;190:97–104. doi: 10.1016/0014-2999(90)94116-f. [DOI] [PubMed] [Google Scholar]
- HUIDOBRO-TORO J.P. Sympathetic co-transmission in human blood vessels. Acta Physiol. Therap. Latinam. 1998;48 Suppl.:12–13. [Google Scholar]
- JANSEN I., UDDMAN R., EKMAN R., OLESEN J., OTTOSON A., EDVINSSON L. Distribution and effects of neuropeptide Y, vasoactive intestinal peptide, substance P, and calcitonin gene-related peptide in human middle meningeal arteries: comparison with cerebral and temporal arteries. Peptides. 1992;13:527–536. doi: 10.1016/0196-9781(92)90084-g. [DOI] [PubMed] [Google Scholar]
- KASAKOV L., ELLIS J., KIRKPATRICK K., MILNER P., BURNSTOCK G. Direct evidence for the concomitant release of noradrenaline, adenosine 5′-triphosphate and neuropeptide Y from sympathetic nerves supplying the guinea pig vas deferens. J. Auton. Nervous System. 1988;22:75–82. doi: 10.1016/0165-1838(88)90156-7. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of co-transmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Revs. 1996;48:113–178. [PubMed] [Google Scholar]
- LUNDBERG J.M., MODIN A. Inhibition of sympathetic vasoconstriction in pigs in vivo by the neuropeptide Y Y1 receptor antagonist BIBP 3226. Br. J. Pharmacol. 1995;116:2971–2982. doi: 10.1111/j.1476-5381.1995.tb15952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMSTROM E. Neuropeptide Y Y1 mechanisms in sympathetic vascular control. Acta Physiol. Scand. 1997;160 Suppl.:1–55. [PubMed] [Google Scholar]
- MALMSTROM R., LUNDBERG J.M. Endogenous NPY acting on Y1 receptors account for major part of the sympathetic vasoconstriction on guinea pig vena cava: evidence using BIBP 3226 and 3435. Eur. J. Pharmacol. 1995;294:661–668. doi: 10.1016/0014-2999(95)00606-0. [DOI] [PubMed] [Google Scholar]
- MEZZANO V., DONOSO M.V., CAPURRO D., HUIDOBRO-TORO J.P. Increased neuropeptide Y pressor activity in Goldblatt hypertensive rats; in vivo studies with BIBP 3226. Peptides. 1998;19:1227–1232. doi: 10.1016/s0196-9781(98)00031-x. [DOI] [PubMed] [Google Scholar]
- MINNEMAN K.P., HAN C., ABEL P.W. Comparison of α1-adrenergic receptor subtypes distinguished by chloroethylclonidine and WB 4101. Mol. Pharmacol. 1988;33:509–514. [PubMed] [Google Scholar]
- MULLER-SCHWEINITZER E. Alpha-adrenoceptors, 5-hydroxytryptamine receptors and the action of dihydroergotamine in human venous preparations obtained during saphectomy procedures for varicose veins. Naunyn-Schmied. Arch. Pharmacol. 1984;327:299–303. doi: 10.1007/BF00506240. [DOI] [PubMed] [Google Scholar]
- NILSSON T., CANTERA L., EDVINSSON L. Presence of neuropeptide Y Y1 receptors mediating vasoconstriction in human cerebral arteries. Neurosc. Lett. 1996a;203:1–5. doi: 10.1016/0304-3940(96)12315-6. [DOI] [PubMed] [Google Scholar]
- NILSSON T., YOU J., SUN X.Y., HEDNER T., EDVINSSON L. Characterization of neuropeptide Y receptors mediating contraction, potentiation and inhibition of relaxation. Blood Press. 1996b;5:164–175. doi: 10.3109/08037059609062125. [DOI] [PubMed] [Google Scholar]
- PELLEG A., BURNSTOCK G. Physiological importance of ATP released from nerve terminals and its degradation to adenosine in humans. Circulation. 1990;82:2269–2272. doi: 10.1161/01.cir.82.6.2269. [DOI] [PubMed] [Google Scholar]
- PHENG L.H., FOURNIER A., DUMONT Y., QUIRION R., REGOLI D. The dog saphenous vein: a sensitive and selective preparation for the Y2 receptor of neuropeptide Y. Eur. J. Pharmacol. 1997;327:163–167. doi: 10.1016/s0014-2999(97)89656-2. [DOI] [PubMed] [Google Scholar]
- RACCHI H., SCHLIEM A., DONOSO M.V., RAHMER A., ZUNIGA A., GUZMAN S., RUDOLF K., HUIDOBRO-TORO J.P. Neuropeptide Y Y1 receptors are involved in the vasoconstriction caused by human sympathetic nerve stimulation. Eur. J. Pharmacol. 1997;329:79–83. doi: 10.1016/s0014-2999(97)00160-x. [DOI] [PubMed] [Google Scholar]
- RAMME D., REGENOLD J.T., SARKE K., BUSSE R., ILLES P. Identification of ATP as a neuroeffector transmitter in jejunal branches of the rabbit mesenteric artery. Naunyn. Schmiedeberg's Arch. Pharmacol. 1987;336:267–273. doi: 10.1007/BF00172677. [DOI] [PubMed] [Google Scholar]
- RUDOLF K., EBERLEIN W., ENGEL W., WIELAND H.A., WILLIM K.D., ENTZEROTH M., WIENEN W., BECK-SICKIMGER A.G., DOODS H.N. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP 3226. Eur. J. Pharmacol. 1994;271:R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- RUMP L.C., VON KÜGELGEN I. A study of ATP as a sympathetic co-transmitter in the human saphenous vein. Br. J. Pharmacol. 1994;111:65–72. doi: 10.1111/j.1476-5381.1994.tb14024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEEN S., SJÖBERG T., SKÄRBY T., NORGREN L., ANDERSSON K.E. The postjunctional α-adrenoceptors of the human saphenous vein. Acta Pharmacol. Toxicol. 1984;55:351–357. doi: 10.1111/j.1600-0773.1984.tb01994.x. [DOI] [PubMed] [Google Scholar]
- STEPHENS N., BUND S.J., FARAGHER E.B., HEAGERTY A.M. Neurotransmission in human resistance arteries: contribution of α1- and α2-adrenoceptors but not P2 purinoceptors. J. Vasc. Res. 1992;29:347–352. doi: 10.1159/000158950. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., BUELL G., NORTH A. P2x receptors bring new structures to ligand-gated ion channels. Trends in Neurosc. 1995;18:224–230. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- TADDEI S., PEDRINELLI R., SALVETTI A. Sympathetic nervous system-dependent vasoconstriction in humans. Evidence for mechanistic role of endogenous purine compounds. Circulation. 1990;82:2061–2067. doi: 10.1161/01.cir.82.6.2061. [DOI] [PubMed] [Google Scholar]
- TORRES G., BITRAN M., HUIDOBRO-TORO J.P. Co-release of neuropeptide Y and noradrenaline from the sympathetic nerves supplying the rat vas deferens: influence of calcium and stimulation intensity. Neurosc. Lett. 1992;148:39–42. doi: 10.1016/0304-3940(92)90799-d. [DOI] [PubMed] [Google Scholar]
- VALERA S., HUSSY N., EVANS R.J., ADAMI N., NORTH R.A., SUPRENANT A., BUELL G.N. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;271:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- VOOGD T.E., VANSTERKENBURG E.L.M., WILTING J., JANSSEN L.H.M. Recent research on the biological activity of suramin. Pharmacol. Revs. 1993;45:177–203. [PubMed] [Google Scholar]
- WAHLESTEDT K., EDVINSSON L., EKBLAD E., HAKANSON R. Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J. Pharmacol. Exp. Ther. 1985;234:735–741. [PubMed] [Google Scholar]
- WESTFALL T., YANG C.L., CURFMAN-FALVEY M. Neuropeptide Y-ATP interactions in the vascular sympathetic neuroeffector junction. J. Cardiovasc. Pharmacol. 1995;26:682–687. doi: 10.1097/00005344-199511000-00002. [DOI] [PubMed] [Google Scholar]


