Abstract
GR128107 (3-(1-acetyl-3-methyl-piperidine)-5-methoxyindole) has previously been reported to be a competitive melatonin receptor antagonist in blocking melatonin inhibition of [3H]-dopamine release from rabbit retina, a response mediated by the MT2 receptor subtype.
GR128107, like melatonin, induced a rapid (maximum response in 60–90 min) pigment aggregation in a clonal line of Xenopus laevis melanophores. GR128107 behaved as a partial agonist (pEC50 8.58±0.03, n=3) with an Emax of 0.83 (relative to melatonin, pEC50 10.09±0.03, n=3).
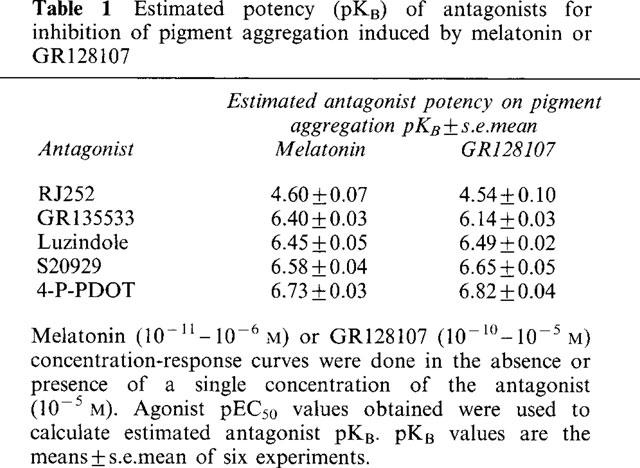
The concentration-response curve for pigment granule aggregation to both melatonin and GR128107 was displaced in a parallel, rightward manner by melatonin receptor antagonists with very similar potencies; estimated pKB RJ252 (against melatonin 4.60/against GR128107 4.54) <GR135533 (6.40/6.14) < Luzindole (6.45/6.49) < S20929 (6.58/6.65) < 4-P-PDOT (6.73/6.85).
Both melatonin- and GR128107-induced pigment granule aggregation was prevented by pre-treatment of melanophores with pertussis toxin (10–1000 ng ml−1).
Prolonged pre-treatment of melanophores with melatonin desensitized the pigment aggregation response to GR128107. In desensitized cells, the maximal aggregation produced by GR128107 was only 0.27±0.01 (n=4) and the pEC50 was reduced (vehicle 8.57±0.12; melatonin pre-treated 7.84±0.09, n=4). The maximal response to melatonin in desensitized melanophores was unchanged but the pEC50 was reduced (vehicle 10.49±0.03; melatonin pre-treated 9.83±0.04, n=4).
These results demonstrate that GR128107 induces pigment granule aggregation in Xenopus melanophores by activating a cell membrane melatonin receptor coupled via a pertussis toxin-sensitive G-protein.
The partial agonist activity of GR128107 in melanophores may be apparent because of the very high density of melatonin receptors in these cells (Bmax 1223 fmol mg protein−1) compared to the low density of sites in rabbit retina (Bmax 3.1 fmol mg protein−1). This suggestion is supported by the finding that GR128107, like melatonin, acted as a full agonist and inhibited forskolin-stimulation of cyclic AMP accumulation in NIH-3T3 cells expressing a high density of human mt1 or MT2 receptors.
Keywords: Melatonin, melatonin receptor subtypes, melatonin agonists, melatonin antagonists, melanophores, pigment aggregation, 2-[125I]-iodomelatonin binding, GR128107
Introduction
The well-established physiological actions of melatonin on seasonal and circadian rhythms (Arendt, 1998; Armstrong, 1989) and vascular reactivity (Ting et al., 1997; Geary et al., 1997) are thought to be mediated by specific, high affinity, G-protein-coupled, cell membrane receptors. In recent years, radioligand binding studies using the radiolabelled melatonin agonist, 2-[125I]-iodomelatonin have identified and characterized these receptors in a number of sites, including the suprachiasmatic nucleus (SCN) of the hypothalamus, the pars tuberalis of the pituitary, the retina and cerebral and tail arteries (for reviews see Morgan et al., 1994; Dubocovich, 1995). Detailed analysis of melatonin binding site distribution indicates a heterogeneous pattern throughout the CNS (Bittman & Weaver, 1990). Two mammalian melatonin receptor subtypes have been identified to date: mt1 and MT2 (Reppert et al., 1994; 1995a). The mRNA for the mt1 subtype is readily detected by Northern analysis and in situ hybridization in the SCN and pars tuberalis (Reppert et al., 1994), and in the human brain mt1 transcripts are widely expressed, although at a low level (Mazzucchelli et al., 1996). The presynaptic melatonin heteroreceptor in rabbit retina inhibiting electrically-evoked [3H]-dopamine release has been shown to be the MT2 subtype (Dubocovich et al., 1997). A third subtype, not detected in mammals, termed the Mel1c has also been cloned from Xenopus laevis (Ebisawa et al., 1994), and is also detected in the chicken and zebra fish (Reppert et al., 1995b).
The finding that melatonin binding sites are more widely distributed in the brain than was first realized, albeit at a low density, suggests that the hormone may have additional roles not yet fully characterized. In fact, melatonin has been reported to have several other actions of which the most well-established is its sleep-promoting ability. A number of studies have shown that pharmacological doses of melatonin produce an acute hypnotic effect in various species (Holmes & Sugden, 1982; Attenburrow et al., 1995), and one group has reported an effect in man with doses which give blood levels within the physiological nighttime range (Dollins et al., 1994). It is not clear if the hypnotic action is related to melatonin's ability to phase shift a circadian rhythm in `sleep-propensity' or an intrinsic sleep-inducing action. It is also claimed that melatonin is an anti-oxidant (Reiter et al., 1995), a stimulator of the immune system (Morrey et al., 1994), an anti-proliferative agent (Hill & Blask, 1988) and has cytoprotective properties against amyloid β protein toxicity (Pappolla et al., 1997). The pharmacology of these responses and the role of the mt1 and MT2 receptors is not known. The lack of potent and specific melatonin receptor antagonists has hampered the characterization of the pharmacology of melatonin (Sugden et al., 1998).
However, GR128107 (3-(-1-acetyl-3-methyl-piperidine) 5-methoxyindole) was recently shown to act as a potent competitive antagonist (pA2 10.2), blocking melatonin-induced inhibition of calcium-dependent [3H]-dopamine release evoked by electrical stimulation in rabbit retina (Dubocovich et al., 1997). Interestingly, in radioligand binding experiments using 2-[125I]-iodomelatonin and recombinant human mt1 and MT2 receptor subtypes, these authors reported that GR128107 was selective for the MT2 subtype (pKi mt1 7, MT2 9.1). In the present study, we have examined the effect of GR128107 in a well-established cellular model of melatonin receptor action, the pigment granule response of Xenopus laevis melanophores. In this model, GR128107 was not an antagonist, rather it acted as a partial agonist, causing movement of pigment granules towards the centre of the cell (pigment aggregation), by activating the melanophore melatonin receptor. Binding experiments on Xenopus melanophore membranes revealed a very high receptor density compared to rabbit retina suggesting that the presence of a considerable receptor reserve in melanophores may allow the partial agonist action of GR128107 to be exposed. GR128107, like melatonin, also inhibited forskolin-stimulated cyclic AMP accumulation in NIH-3T3 cells expressing recombinant human mt1 and MT2 receptor subtypes.
Methods
Cell cultures
Xenopus leavis fibroblast and melanophore clonal cell lines (Daniolos et al., 1990) were provided by Dr M.R. Lerner (University of Texas, Dallas, U.S.A.) and were grown at 24–27°C in the dark without any gaseous exchange. 0.7×Leibovitz medium (L-15) containing 100 i.u. ml−1 penicillin, 0.1 mg ml−1 streptomycin, 4 mM L-glutamine and 10% heat inactivated (56°C for 30 min) fetal calf serum (`Myoclone'; GIBCO/BRL), was conditioned by fibroblasts and used to feed melanophores every 3–5 days. 0.7×L-15 was used to maintain the appropriate osmolarity for amphibian cells. All cell types were maintained as monolayers in flasks (Falcon; Becton Dickinson, Meylan Cedex, France) with 175 cm2 growth area.
NIH-3T3 cells expressing recombinant human mt1 or MT2 receptor subtypes were provided by Dr S.M. Reppert (Harvard Medical School, Boston, Massachusetts, U.S.A.), and were grown in complete Dulbecco's Eagle medium (cDMEM) containing 100 i.u. ml−1 penicillin, 0.1 mg ml−1 streptomycin, 4 mM L-glutamine, 10% foetal bovine serum (Imperial Laboratories, U.K.) and Geneticin (G418; 1 mg ml−1, GIBCO/BRL) in humidified 5% CO2/95% air at 37°C.
Measurement of pigment aggregation
Ninety six-well cell culture plates containing approximately 6–8×103 melanophores per well were used for pigment aggregation experiments. One hour prior to all concentration-response experiments, conditioned medium in each well was aspirated and replaced with 100 μl (for antagonist experiments) or 150 μl (for agonist experiments) 0.7×L-15 medium (containing 1 mg ml−1 bovine albumin, 100 i.u. ml−1 penicillin, and 0.1 mg ml−1 streptomycin). In 0.7×L-15 medium, pigment remained dispersed. The change in distribution of pigment granules within melanophores was quantitated using a Bio-Tek microtitre plate reader (model EL3115, Anachem, Luton, U.K.) by measuring the change in absorbance, (630 nm) before and after drug treatment. The fractional change in absorbance, 1-(Af/Ai) where Ai is the initial absorbance before drug treatment and Af is the final absorbance at a given time after drug treatment was calculated for each concentration of drug tested. All drugs were added from concentrated stock solutions in 50 μl of 0.7×L-15 medium. The final assay volume was 200 μl per well. All drugs were freshly prepared from 10−2 M stock solutions in methanol or DMSO kept at −20°C. The maximal concentration of solvent was 1% v/v which did not cause pigment redistribution in melanophores (data not shown). As light has been shown to cause pigment dispersion (Daniolos et al., 1990), melanophores were kept in the dark at 24–27°C during drug treatment, even though our previous study showed that normal laboratory light caused no significant pigment redistribution or alteration in melatonin potency (Teh & Sugden, 1997). As Xenopus melanophores possess an endogenous serotonin receptor which triggers pigment granule dispersion (Potenza & Lerner, 1992), methiothepin (MTP, 10−6 M), a non-subtype selective serotonin receptor antagonist, was added to ensure these serotonin receptors were not involved in the pigment responses.
Antagonists (10−5 M) were incubated with cells for 60 min before the addition of agonist (either GR128107 or melatonin). None of the antagonists alone caused any pigment movement on the melanophores (data not shown). Concentration-response curves for the GR128107 or melatonin in the absence or presence of antagonists were measured after 90 min of agonist incubation.
Pertussis toxin (PTX) was activated by incubation with dithiothreitol (DTT, 20 mM) in 0.7×L-15 for 20 min at 37°C just before use. Distilled water was treated in the same manner and used as a vehicle. The DTT-activated PTX was diluted with 0.7×L-15 to give three final concentrations of 10 ng ml−1, 100 ng ml−1 and 1 μg ml−1. Melanophores were pre-incubated for 24 h with either vehicle or PTX, and concentration-response curves to GR128107 or melatonin then determined.
For the desensitization experiment, melanophores were incubated in 0.7×L-15 or melatonin (10−9 M) for 72 h. After 72 h, medium was removed and melanophores rapidly washed with 0.7×L-15 and concentration-response curves to melatonin and GR128107 determined.
In one experiment, the degree of pigment aggregation in individual cells was measured. Two 35 mm dishes were seeded with ∼1.7×104 melanophores and the cells cultured for 3 days. Medium was replaced with 0.7×L-15 for 1 h, then melanophores were treated with melatonin (10−9 M) or GR128107 (10−7 M) for 90 min. Before drug treatment a randomly-chosen field of cells in each dish was viewed (10× objective) using a Bio-Rad MRC600 laser scanning confocal microscope attached to a Nikon Diaphot microscope and the image digitally captured. The same field was captured after drug treatment and the images aligned, and the area occupied by pigment granules within individual cells measured before (Areai) and after (Areaf) treatment using the NIH Image software (v.1.59). The fractional change in area occupied by pigment granules (1-[Areaf/Areai]) was calculated for each cell. Two hundred cells were measured for each drug.
Membrane preparation
Confluent melanophores were washed with 0.7×Dulbecco's phosphate buffered saline (PBS) and detached from the flask with trypsin/EDTA (1×, 1–2 min, Sigma). Cells were centrifuged (750×g, 1 min) and lysed in ice-cold buffer A (mM): Tris-HCl 50, pH 7.4, MgCl2 5, containing KCl 45 by 3–5 passes through a 25 gauge hypodermic needle. Cell debris, nuclei and melanosomes were removed by centrifugation (2500×g, 5 min) and the supernatant centrifuged (100,000×g, 30 min) to recover cell membranes. Rabbit retinae were dissected from adult (12–24 month old) New Zealand white rabbits killed by urethane overdose during the daytime (15.00–17.00 h). Membranes were prepared as described previously (Pickering et al., 1996). Each confluent flask of NIH-3T3 cells expressing either human mt1 or MT2 receptor subtypes was washed once with 10 ml PBS, and cells were scraped into 4 ml ice-cold buffer A. Aliquots of NIH-3T3 cell homogenates, Xenopus melanophore and rabbit retina membranes were frozen in liquid nitrogen until used in binding experiments. Protein concentration was determined by a dye-binding method (Bradford, 1976) using bovine serum albumin as standard.
2-[125I]-Iodomelatonin binding assays
For saturation assays, 30 μl of Xenopus melanophore membrane (4 μg protein) or rabbit retina membranes (60 μg protein) were incubated with 20 μl buffer A and 50 μl 2-[125]-iodomelatonin (6.25–800 pM) in the presence of either 1 μl cold melatonin (10−6 M) or 1 μl methanol to define non-specific or total 2-[125I]-iodomelatonin binding, respectively. Competition assays using recombinant human mt1 or MT2 receptors used 17 μg and 32 μg of homogenate protein respectively. Samples were incubated at either 37°C (rabbit retina, mt1, MT2) or 25°C (melanophores) for 90 min. Preliminary studies showed that equilibrium is reached under these conditions (data not shown). Specific binding was calculated by subtracting non-specific binding from total binding. For competition assays, 80–100 pM 2-[125I]-iodomelatonin was used. Binding assays were terminated by the addition of 2 ml ice-cold buffer A to each tube and immediate filtration through 1% v/v polyethylenimine pre-treated glass fibre filters (GF/C, Whatman Ltd., Maidstone, Kent, U.K.). Each tube was then rinsed with a further 2 ml of buffer and each filter washed twice with 5 ml of buffer. 2-[125I]-iodomelatonin trapped on the filter was counted (Cobra II Auto-Gamma, PACKARD). All assays were done on duplicate homogenate or membrane aliquots and were repeated at least three times.
Determination of cyclic AMP
NIH-3T3 cells expressing human mt1 or MT2 receptor subtypes were seeded into 24-well plates and cultured for 48 h until they reached confluence (3–4×104 cells per well). After washing twice with DMEM, IBMX (2.5×10−4 M) was added for 10 min at 37°C before the addition of forskolin (10−5 M) in the absence or presence of melatonin (10−12–10−7 M) or GR128107 (10−10–10−5 M). All drugs were diluted in DMEM and contained IBMX as described above. After 10 min incubation at 37°C, the medium in each well was aspirated and 300 μl ice-cold acetic acid (50 mM) was added and cells were scraped from the plate. The lysate was then collected and boiled for 3 min. All samples were kept at −20°C until cyclic AMP was determined by RIA following acetylation as described previously (Harper & Brooker, 1975; Vanecek et al., 1985).
Data analysis
In pigment aggregation experiments, pEC50±s.e.mean values were determined by curve-fitting individual data points using the four parameter logistic equation:
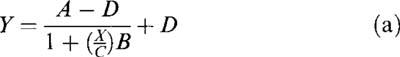 |
where X is the concentration of melatonin or GR128107, Y is the fractional change in absorbance, A is the maximal absorbance in the absence of analogue, B is the slope factor, C is the concentration of the analogue producing half of the maximal response (EC50) and D is the minimal absorbance. The concentration of melatonin or GR128107 inhibiting forskolin-stimulation of cyclic AMP accumulation by 50% (IC50) was also determined using this equation. Antagonist potency (pKB) was estimated by constructing dose-response curves to melatonin or GR128107 in the absence and presence of a single concentration of antagonist. Estimated pKB values were calculated from the equation log (concentration ratio-1)−log [antagonist].
Data from saturation experiments were analysed by non-linear regression using the equation:
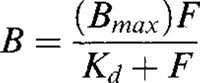 |
where B is the concentration of 2-[125I]-iodomelatonin bound to the receptor, Bmax is the maximal concentration of binding sites, F is the concentration of free 2-[125I]-iodomelatonin and Kd is the equilibrium dissociation constant. IC50 values in competition assays were determined using the four parameter logistic equation and inhibition constants (Ki) for each compound were then calculated.
Materials
Melatonin, DTT and all cell culture media and reagents were purchased from Sigma Chemical Co. (Poole, U.K.) unless indicated. MTP was purchased from ICN (Thame, U.K.), and luzindole (N-acetyl-2-benzyltryptamine) and 4-phenyl-2-propionamidotetralin (4-P-PDOT) from Tocris Cookson (Bristol, U.K.). PTX was purchased from Porton Products (Salisbury, Wilts., U.K.), and 2-[125I]-iodomelatonin and cyclic AMP 2′-O-succinyl [125I]-tyrosine methyl ester (specific activity 2200 Ci mol−1) from DuPont U.K. Ltd. (Stevenage, U.K.). GR128107 (3-(1-acetyl-3-methyl-piperidine)-5-methoxy-indole) and GR135533 (3-(-N-propyl-2-pyrrolidine)-5-methoxyindole) were generously provided by Dr I.J.M. Beresford (Glaxo, Stevenage, U.K.). S20929 (N-[2-napth-1-yl-ethyl]cyclopropyl carboxamide) was generously provided by Institut de Recherches Internationales Servier, France. N-trifluoroacetyl-2,6-dibromotryptamine (RJ252, compound 11d in Garratt et al., 1995) was provided by Prof P.J. Garratt (University College London, England, U.K.).
Results
Characterization of melanophore pigment response to GR128107: Time- and concentration-response relationships
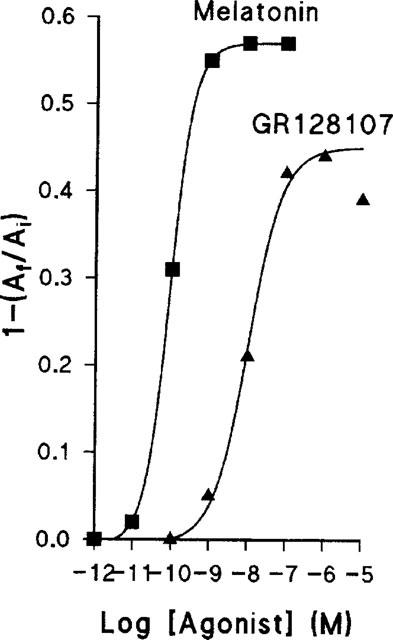
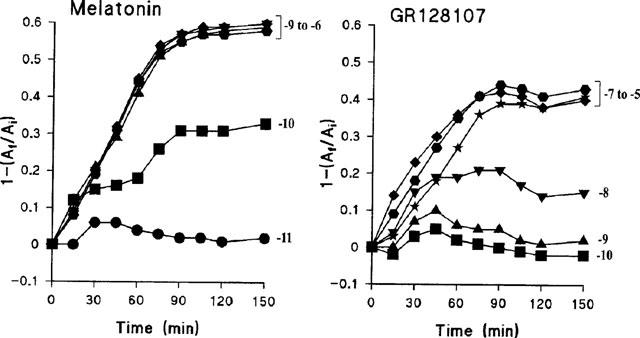
GR128107, like melatonin, produced a time- and concentration-dependent redistribution of melanophore pigment granules toward the cell centre. Figure 1 shows the time-course of pigment aggregation produced by GR128107 and melatonin measured as the fractional change in cell absorbance as described in Methods. Both agonists produced a maximal pigment aggregation by 90 min which was maintained for at least 150 min. Concentration-response curves for melatonin and GR128107 at 90 min gave pEC50 values (means±s.e.mean, n=3) of 10.09±0.02 and 8.58±0.03 respectively (Figure 2). In several experiments, the maximal pigment aggregation in response to GR128107 was slightly, but consistently, less than that to melatonin. GR128107 gave a maximal response (Emax 0.83±0.03 of that produced by melatonin) which was significantly less than that produced by melatonin (P>0.0001, n=11 Student's t-test assuming unequal variances). The result indicates clearly that GR128107 was a partial agonist in this model.
Figure 1.
Time-course of melatonin- and GR128107-induced pigment aggregation in Xenopus laevis melanophores. The extent of pigment granule aggregation was determined at the times indicated by measuring the fractional change in absorbance, 1-(Af/Ai), as described in Methods. Each point represents the mean value obtained from triplicate samples. Error bars are omitted since they were all within the area of the symbols, i.e. s.e.mean all less than 0.02. •, 10−11 M; ▪, 10−10 M; ▴, 10−9 M; ▾, 10−8 M; ⧫, 10−7 M; [filled hexagon], 10−6 M; ★, 10−5 M.
Figure 2.
An example of concentration-response curves for melatonin- and GR128107-induced pigment aggregation. The fractional change in absorbance, 1-(Af/Ai), was measured at 90 min. Each point represents the mean value obtained from triplicate samples. Error bars are omitted since they were all within the area of the symbols i.e. s.e.mean less than 0.02. The pEC50 values (means±s.e.mean, n=3) were: melatonin, 10.09±0.02; GR128107, 8.58±0.03.
To determine if the lower maximum response to GR128107 was due to a reduction in the proportion of melanophores which responded or due to a decrease in the size of the response, aggregation was measured in 200 individual cells. Most melanophores responded to melatonin (10−9 M) with a reduction in pigmented area of between 65–85% (Figure 3). The number of melanophores showing such large responses was reduced with GR128107 (10−7 M) while the number showing a more modest reduction in pigmented area appeared to increase. Very few cells failed to respond to either drug (melatonin, five cells out of 200; GR128107, ten cells out of 200).
Figure 3.
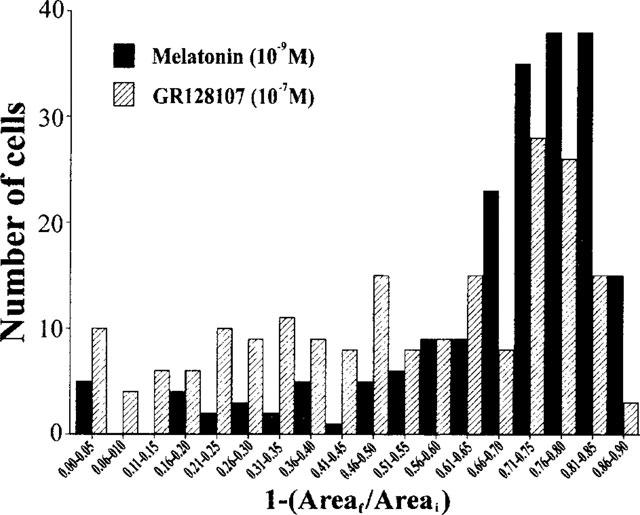
Frequency histogram showing the magnitude of the pigment aggregation response to melatonin (solid bars) and GR128107 (hatched bars) in a population of individual melanophores. The area covered by pigment granules in 200 cells chosen at random was determined before (Areai) and 90 min after (Areaf) treatment with melatonin (10−9 M) or GR128107 (10−7 M). The change in pigment area in each cell was calculated (1-[Areaf/Areai]).
Blockade of GR128107-induced pigment aggregation by melatonin receptor antagonists
Pigment aggregation in response to melatonin and GR128107 was antagonized by several putative melatonin receptor antagonists (Table 1). These included luzindole (N-acetyl 2-benzyltryptamine) which has previously been shown to be a competitive antagonist of melatonin-induced inhibition of electrically evoked [3H]-dopamine release from rabbit retina (Dubocovich, 1988) and S20929, a naphthalenic derivative which has been reported to antagonize melatonin inhibition of forskolin stimulation of cyclic AMP synthesis (Delagrange et al., 1995). Luzindole and S20929 also competitively antagonized the vasoconstrictor effect of melatonin in rat tail artery (Ting et al., 1997). Three other antagonists were tested: RJ252, which was previously shown to antagonize melatonin-induced pigment movement in primary cultures of melanophores (Garratt et al., 1995), and GR135533 and 4-P-PDOT, MT2 selective antagonists which block melatonin inhibition of dopamine release in rabbit retina (Dubocovich et al., 1997). Although few melatonin receptor antagonists have yet been described, these compounds represent examples of each of the different chemical classes of antagonist. All of the antagonists were devoid of any agonist activity in melanophores, as no pigment granule aggregation was observed at the concentration used (10−5 M, data not shown). Despite the narrow potency range of the few antagonists available, the potency order – RJ252<GR135533<Luzindole<S20929<4-P-PDOT – was the same irrespective of the agonist used (melatonin or GR128107). Furthermore, the estimated pKB values were very similar for both agonists (Table 1).
Table 1.
Estimated potency (pKB) of antagonists for inhibition of pigment aggregation induced by melatonin or GR128107
Effect of pertussis toxin on melatonin and GR128107-induced pigment aggregation
Pigment granule aggregation in response to melatonin and GR128107 was blocked by pre-treating melanophores with pertussis toxin, indicating that both agents induce aggregation through receptors linked to a pertussis toxin sensitive, G1-like GTP-binding protein (Figure 4). The response to GR128107 appeared to be more sensitive to pertussis toxin treatment than that of melatonin: for example, a concentration of 10 ng ml−1 of pertussis toxin reduced the maximum aggregation response to melatonin by only 15% but reduced the response to GR128107 by 42%.
Figure 4.
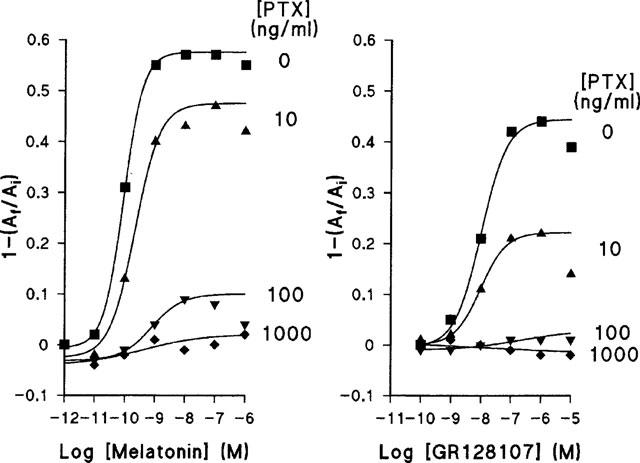
Effect of pertussis toxin (PTX) on the concentration-response curves for melatonin and GR128107. Melanophores were treated for 24 h with PTX or vehicle as described in Methods. Each data point represents a mean of n=4 measurements. s.e.mean values all smaller than the symbols (all less than 0.01).
Receptor desensitization studies
To determine if prolonged treatment of melanophores with melatonin would desensitize the cells to the aggregating action of GR128107, cells were treated with melatonin for 3 days. To ensure continuous and full receptor stimulation a concentration of 10−9 M was used. After 72 h, melatonin was removed, the cells rapidly washed with 0.7×L-15 medium and the pigment aggregation response to melatonin and GR128107 determined. Aggregation responses were compared to cells cultured for 3 days in 0.7×L-15. Both control and melatonin-pretreated cells gave identical maximal aggregation responses to melatonin (Figure 5), but the potency was reduced in those melanophores which had been pretreated with melatonin: mean pEC50±s.e.mean control 10.49±0.03, melatonin pretreated 9.83±0.04 (n=4). The maximal aggregation response to GR128107 was dramatically reduced (Emax 0.27±0.01, n=4) in melatonin-pretreated melanophores, and the potency of GR128107 was also reduced (pEC50 control 8.57±0.12, melatonin pre-treated, 7.84±0.09, n=4).
Figure 5.
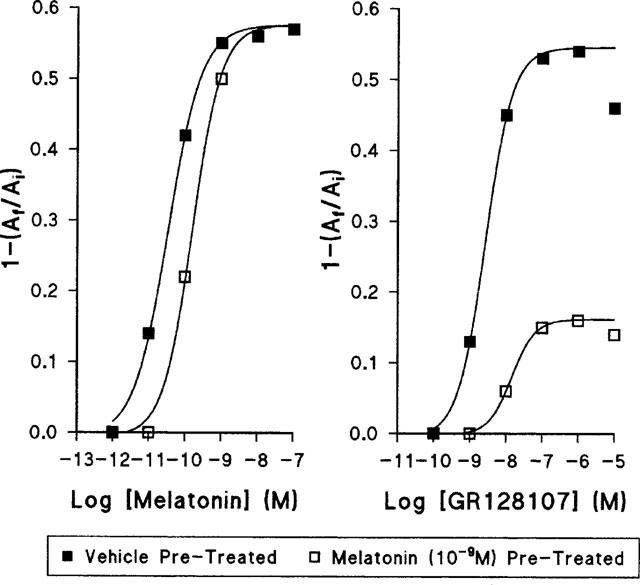
The effect of desensitization of melanophores on the concentration-response curves to melatonin and GR128107. Cells were treated with melatonin (10−9 M, open squares) or vehicle (closed squares) for 72 h as described in Methods, washed and agonist concentration response curves determined. Each data point represents a mean of n=4 measurements. All s.e.mean were less than the area of the symbols (0.02) and hence are omitted.
2-[125I]-Iodomelatonin binding studies
Radioligand binding studies on membranes prepared from Xenopus melanophores showed that these cells express a very high density of high affinity 2[125I]-iodomelatonin binding sites (Kd 62.8±12.5 pM, Bmax 1223±14.3 fmol mg protein−1). Saturation experiments on membranes prepared from rabbit retina showed that the 2-[125I]-iodomelatonin binding sites in this tissue had a similar affinity (Kd 46.8±8.2 pM) but expressed a much lower density of receptors (Bmax 3.1±0.2 fmol mg protein−1). Competition binding assays on the melanophore membranes gave a pKi (mean of duplicate determinations) of 9.38 for melatonin and 7.84 for GR128107.
Radioligand binding assays on homogenates of NIH-3T3 cells expressing recombinant human mt1 and MT2 receptor subtypes were used to determine the subtype selectivity of melatonin and GR128107. In agreement with an earlier report using these subtypes expressed in COS-7 cells (Dubocovich et al., 1997), melatonin showed little subtype selectivity (mean pKi±s.e.mean, n=3: mt1 9.11±0.04; MT2 9.47±0.03). However, GR128107 did show some selectivity for the MT2 subtype (59 fold, mean pK1±s.e.mean, n=3: hmt1 6.85±0.03; MT2 8.62±0.12), but not as much as reported previously (113 fold, Dubocovich et al., 1997).
Inhibition of forskolin-stimulation of cyclic AMP accumulation
Both melatonin and GR128107 inhibited forskolin-stimulated cyclic AMP accumulation in NIH-3T3 cells expressing human melatonin receptors (Bmax mt1 73.8±2.1 fmol mg protein−1; MT2 23.9±0.2 fmol mg protein−1, Figure 6). Maximum inhibition of cyclic AMP was similar for both compounds; approximately 80–90% in NIH-3T3 cells expressing the mt1 subtype and ∼75% in cells expressing the MT2 subtype. The IC50 value (mean±s.e.mean, n=4) for melatonin was 9.29±0.11 at mt1 and 9.75±0.17 at the MT2 cells; for GR128107 the IC50 values were 7.19±0.12 at mt1 and 7.95±0.10 at MT2.
Figure 6.
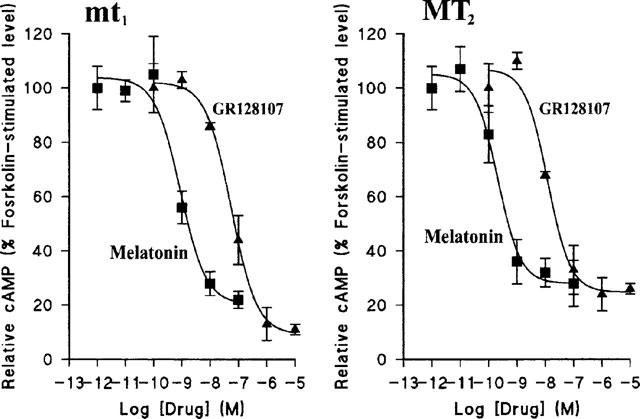
Concentration-dependent inhibition of forskolin-stimulated cyclic AMP accumulation by melatonin and GR128107 in NIH-3T3 cells stably transfected with recombinant human mt1 or MT2-receptors. Cells were stimulated with forskolin (10−5 M, 10 min) in the presence of various concentrations of melatonin (Mel; closed squares) or GR128107 (GR; closed triangles). Cyclic AMP accumulation was determined in duplicate as described in Methods. Each data point represents the mean±s.e.mean of four experiments.
Discussion
We initially tested GR128107 as part of a study correlating the potency of putative receptor antagonists to inhibit the melatonin response in Xenopus melanophore clonal line, with their subtype specificity in radioligand binding assays, to identify the receptor subtype responsible for pigment aggregation. The morphological colour change induced by melatonin triggering the movement of melanosomes toward the cell centre is one of the oldest and best-documented responses to melatonin (see Rollag & Adelman, 1992), but it is not known which receptor subtype mediates this response. GR128107 has previously been shown to be a competitive antagonist at the melatonin heteroreceptor in rabbit retina, which is an MT2 subtype, and was reported to show considerable selectivity (113 fold) for the MT2 over the mt1 subtype in radioligand binding assays (Dubocovich et al., 1997). We were surprised to find therefore that in Xenopus melanophores, GR128107 produced a concentration-related pigment aggregation with a very similar time-course to melatonin. Under the microscope, the appearance of melanophores treated with a maximal concentration of GR128107 was indistinguishable from melatonin-treated melanophores. Pigment movement was readily reversed on washing out GR128107 indicating that the changes in pigment granule distribution it caused were not due to any cytotoxic effects of the drug. Concentration-response curves indicated that GR128107 was a partial agonist. Analysis of the area occupied by pigment granules in individual cells showed that the partial agonism of GR128107 was due to a reduced average response, rather than an increase in the proportion of melanophores failing to respond, which was low for both drugs (2–5%).
There are a number of reasons to suggest that GR128107 induces pigment granule aggregation by activating the melatonin receptor on melanophores. First, the response was blocked by several compounds previously shown to act as melatonin receptor antagonists. Although few melatonin receptor antagonists are yet available and these are incompletely characterized and of modest potency, all five antagonists tested produced a parallel rightward shift in the GR128107 and melatonin concentration-response curves. The potency order for these antagonists using melatonin or GR128107 was identical, despite their narrow range of potencies, and the estimated pKB values were very similar with either agonist. Second, pretreatment of melanophores overnight with pertussis toxin blocked not only the response to melatonin but also that to GR128107. It has been shown previously that the pigment granule aggregation response to melatonin is inhibited by pertussis toxin (White et al., 1987), suggesting a role for a G1/G0 protein. Other cellular responses to melatonin in pars tuberalis, pars distalis and chick retina are also pertussis toxin-sensitive (for review see Morgan et al., 1994). Interestingly, the aggregation response to GR128107 appeared to be more sensitive to pertussis toxin than the response to melatonin, as a low concentration of toxin (10 ng ml−1) produced a more marked reduction in the response to GR128107 than to melatonin. Thirdly, pretreatment of melanophores for a prolonged period (72 h) with a concentration of melatonin which produces maximal aggregation (10−9 M), resulted not only in a desensitization of the response when cells were challenged with fresh melatonin, but also a reduced pigment aggregation response to GR128107. This cross-desensitization suggests that melatonin and GR128107 activate the same receptor. The maximal response to GR128107 (but not to melatonin) was reduced in the melatonin desensitized melanophores, consistent with the observation that GR128107 is a partial agonist since a reduction in the number of melanophore receptors available to mediate the pigment aggregation response would be expected to compromise the response to a partial agonist more readily than that of a full agonist (Kenakin, 1997). Indeed, this may explain why the response to GR128107 was more dramatically reduced by a low dose of pertussis toxin; the low dose of toxin would inactivate a fraction of the G-proteins able to couple to the melatonin receptors on the melanophore cell membrane and this would again inhibit the response to a partial agonist more than a full agonist. Finally, although a number of endogenous receptors have been identified on melanophores, including those for peptides such as melanocyte stimulating hormone, vasoactive intestinal polypeptide, vasotocin and endothelin C as well as a β1-adrenoceptor and a 5-HT receptor (McClintock et al., 1996), activation of all of these provokes a dispersal of pigment granules rather than aggregation, so that its unlikely that GR128107 interacts with them. Indeed, to date the melatonin receptor is the only receptor which has been shown to be linked to pigment granule aggregation in wild-type Xenopus melanophores.
A possible explanation of why GR128107 acts as an agonist in the present experiments, yet is a melatonin receptor antagonist in rabbit retina (Dubocovich et al., 1997), comes from an examination of melatonin binding sites in the two tissues using 2-[125I]-iodomelatonin. High affinity binding (Kd 40–60 pM) was detected in both tissues but the density of sites was dramatically different. In agreement with a previous study, 2-[125I]-iodomelatonin binding density was relatively low in rabbit retina (Bmax∼3 fmol mg protein−1; Bmax∼5 fmol mg protein−1 in Blazynski & Dubocovich, 1991). Receptor expression in melanophores was over 400 fold greater (Bmax∼1220 fmol mg protein−1). The existence of such a large receptor reserve in melanophores may explain why GR128107 acted as a partial agonist in this model yet in retina, where few or no spare receptors may exist, it behaved as an antagonist. For other receptors, reducing density by alkylation (e.g. α-adrenoceptors, Kenakin, 1984a) or down-regulation following chronic stimulation (e.g. β-adrenoceptors, Kenakin & Ferris, 1983) can reduce the response to low efficacy agonists. In tissues with low receptor density or coupling efficiency, partial agonists can act as antagonists; an example is prenalterol, a partial β-adrenoceptor agonist, produces 85% of the response to a full agonist (isoprenaline) in guinea-pig right atria but antagonizes isoprenaline in dog coronary artery (Kenakin, 1984b).
GR12 8107 also acted as an agonist in NIH-3T3 cells expressing recombinant mt1 and MT2 receptor subtypes, causing an inhibition of forskolin-stimulated cyclic AMP accumulation. These cells express an intermediate number of receptors–70–165 fmol mg protein−1 when the data are expressed per mg of membrane protein as for retina and melanophores–considerably less than melanophores but many fold higher than retina. Perhaps such receptor densities are sufficiently high to allow expression of agonist activity. The functional response observed (i.e. agonist or antagonist action) may also depend on other factors including the efficiency of the mechanisms which convert receptor stimulus into a functional response in these different cells and tissues, and the particular end-point measured following receptor activation. However, it is clear that GR128107 can act as an agonist not only on melanophore receptors, where the subtype mediating the response has not been formally defined, but also on mt1 and MT2 subtypes. There is no evidence to support the idea that GR128107 has distinct functional effects on different melatonin receptor subtypes, although the effect of GR128107 on recombinant mel1c receptor subtype has not yet been examined.
Further studies in this and other model systems should allow subtype selective melatonin receptor antagonists to be identified. These will be invaluable in defining the physiological and pathophysiological role of melatonin.
Note added in proof
The nomenclature and classification of melatonin receptors used here has been approved by the Nomenclature Committee of IUPHAR (Dubocovich et al., 1998). `mt1' corresponds to the recombinant melatonin receptor subtype previously known as Mel1a, which has not yet been linked to a specific functional response. `MT2' refers to native receptors, previously known as Mel1b, which have a defined function and pharmacological characteristics similar to the recombinant `mt2'. The Mel1c receptor subtype remains outside of this nomenclature system as it does not appear to be expressed in mammals.
Acknowledgments
We are grateful to Dr Steven Reppert for providing the NIH-3T3 cells expressing human mt1 and MT2 receptor subtypes, and Dr Michael Lerner for supplying the clonal Xenopus melanophore cell line. We thank Dr I.J.M. Beresford (Glaxo), Dr B. Guardiola (Servier) and Professor P.J. Garratt (UCL) for supplying melatonin antagonists, and Dr Kevin Pedley (KCL) for his help with confocal microscopy.
Abbreviations
- DMEM
Dulbecco's modified Eagles medium
- DMSO
dimethyl sulphoxide
- DTT
dithiothreitol
- GR128107
(3-(1-acetyl-3-methyl-piperidine)-5-methoxyindole)
- L-15
Leibovitz medium
- MTP
methiothepin
- PTX
pertussis toxin
- PBS
Dulbecco's phosphate buffered saline
- SCN
suprachiasmatic nucleus
References
- ARENDT J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev. Reprod. 1998;3:13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG S.M. Melatonin and circadian control in mammals. Experientia. 1989;45:932–939. doi: 10.1007/BF01953050. [DOI] [PubMed] [Google Scholar]
- ATTENBURROW M.E.J., DOWLING B.A., SARGENT P.A., SHARPLEY A.L., COWEN P.J. Melatonin phase advances circadian rhythm. Psychopharmacol. 1995;121:503–505. doi: 10.1007/BF02246501. [DOI] [PubMed] [Google Scholar]
- BITTMAN E.L., WEAVER D.R. The distribution of melatonin binding sites in neuroendocrine tissues of the ewe. Biol. Reprod. 1990;43:986–993. doi: 10.1095/biolreprod43.6.986. [DOI] [PubMed] [Google Scholar]
- BLAZYNSKI C., DUBOCOVICH M.L. Localization of 2-[125I]iodomelatonin binding sites in mammalian retina. J.Neurochem. 1991;56:1873–1880. doi: 10.1111/j.1471-4159.1991.tb03443.x. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DANIOLOS A., LERNER A.B., LERNER M.R. Action of light on frog pigment cells in culture. Pigment Cell Res. 1990;3:38–43. doi: 10.1111/j.1600-0749.1990.tb00260.x. [DOI] [PubMed] [Google Scholar]
- DELAGRANGE P., RENARD P., CAIGNARD D.H., GUARDIOLA-LEMAITRE B.Development of melatonin analogs The Pineal Gland and its Hormone 1995New York: Plenum Press; 139–153.ed. Fraschini, F. pp [Google Scholar]
- DOLLINS A.B., ZHDANOVA I.V., WURTMAN R.J., LYNCH H.J., DENG M.H. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOCOVICH M.L. Luzindole (N-0774): a novel melatonin receptor antagonist. J. Pharmacol. Exp. Ther. 1988;246:902–910. [PubMed] [Google Scholar]
- DUBOCOVICH M.L. Melatonin receptors: are there multiple subtypes. TiPS. 1995;16:50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- DUBOCOVICH M.L., CARDINALI D.P., GUARDIOLA-LEMAITRE B., HAGAN R.M., KRAUSE D.N., SUGDEN D., VANHOUTTE P.M., YOCCA F.D. The IUPHAR Compendium of Receptor Characterization and Classification. IUPHAR Media, London, U.K; 1998. Melatonin receptors; pp. 187–193. [Google Scholar]
- DUBOCOVICH M.L., MASANA M.I., IACOB S., SAURI D.M. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:365–375. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- EBISAWA T., KARNE S., LERNER M.R., REPPERT S.M. Expression cloning of a high affinity melatonin receptor from Xenopus dermal melanophores. Proc. Natl. Acad. Sci. U.S.A. 1994;93:6133–6137. doi: 10.1073/pnas.91.13.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRATT P.J., JONES R., TOCHER D.A., SUGDEN D. Mapping the melatonin receptor. 3. Design and synthesis of melatonin agonists and antagonists derived from 2-phenyltryptamine. J. Med. Chem. 1995;38:1132–1139. doi: 10.1021/jm00007a010. [DOI] [PubMed] [Google Scholar]
- GEARY G.G., KRAUSE D.N, DUCKLES S.P. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am. J. Physiol. 1997;273:H1530–H1536. doi: 10.1152/ajpheart.1997.273.3.H1530. [DOI] [PubMed] [Google Scholar]
- HARPER J.F., BROOKER G.J. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2′-O-acetylation by acetic anhydride in aqueous solution. J. Cyclic Nucleotide Res. 1995;1:207–218. [PubMed] [Google Scholar]
- HILL S.M., BLASK D.E. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer. 1988;48:6121–6126. [PubMed] [Google Scholar]
- HOLMES S.W., SUGDEN D. Effects of melatonin on sleep and neurochemistry in the rat. Br. J. Pharmacol. 1982;76:95–101. doi: 10.1111/j.1476-5381.1982.tb09194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P. The relative contribution of affinity and efficacy to agonist activity: organ selectivity of noradrenaline and oxymetazoline with reference to the classification of drug receptors. Br. J. Pharmacol. 1984a;81:131–143. doi: 10.1111/j.1476-5381.1984.tb10753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P. The classification of drugs and drug receptors in isolated tissues. Pharmacol. Rev. 1984b;36:165–222. [PubMed] [Google Scholar]
- KENAKIN T.P. Pharmacologic analysis of drug-receptor interaction. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- KENAKIN T.P., FERRIS R.M. Effects of in vivo β-adrenoceptor down-regulation on cardiac responses to prenalterol and pirbuterol. J. Cardiovasc. Pharmacol. 1983;5:90–97. doi: 10.1097/00005344-198301000-00014. [DOI] [PubMed] [Google Scholar]
- MAZZUCCHELLI C., PANNACCI M., NONNO R., LUCINI V., FRASCHINI F., STANKOV B.M. The melatonin receptor in the human brain; cloning experiments and distribution studies. Mol. Brain Res. 1996;39:117–126. doi: 10.1016/0169-328x(96)00017-4. [DOI] [PubMed] [Google Scholar]
- MCCLINTOCK T.S., RISING J.P., LERNER M.R. Melanophore pigment dispersion responses to agonists show two patterns of sensitivity to inhibitors of cAMP-dependant protein kinase and protein kinase C. J. Cell Physiol. 1996;167:1–7. doi: 10.1002/(SICI)1097-4652(199604)167:1<1::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- MORGAN P.J., BARRETT P., HOWELL H.E., HELLIWELL R. Melatonin receptors: Localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- MORREY K.M., MCLACHLAN J.A., SERKIN C.D., BAKOUCHE O. Activation of human monocytes by the pineal hormone melatonin. J. Immunol. 1994;153:2671–2680. [PubMed] [Google Scholar]
- PAPPOLLA M.A., SOS M., OMAR R.A., BICK R.J., HICKSON-BICK D.L.M., REITER R.J., EFTHIMIOPOULOS S., ROBAKIS N.K. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 1997;17:1683–1690. doi: 10.1523/JNEUROSCI.17-05-01683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKERING H., SWORD S., VONHOFF S., JONES R., SUGDEN D. Analogues of diverse structure are unable to differentiate native melatonin receptors in the chicken retina, pars tuberalis and Xenopus melanophores. Br. J. Pharmacol. 1996;119:379–387. doi: 10.1111/j.1476-5381.1996.tb15997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTENZA M.N., LERNER M.R. A rapid quantitative bioassay for evaluating the effects of ligands upon receptors that modulate cAMP levels in a melanophore cell line. Pigment Cell Res. 1992;5:372–378. doi: 10.1111/j.1600-0749.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- REITER R.J., MELCHIORRI D., SEWERYNEK E., POEGGELER B., BARLOW-WALDEN L., CHUANG J., ORTIZ G.G., ACUNA-CASTROVIEJO D. A review of the evidence supporting melatonin's role as an antioxidant. J. Pineal Res. 1995;18:1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- REPPERT S.M., GODSON C., MAHLE C.D., WEAVER D.R., SLAUGENHAUPT S.A., GUSELLA J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc. Natl. Acad. Sci. U.S.A. 1995a;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPPERT S.M., WEAVER D.R., CASSONE V.M., GODSON C., KOLAKOWSKI L.F., JR Melatonin receptors are for the birds: molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron. 1995b;15:1003–1015. doi: 10.1016/0896-6273(95)90090-x. [DOI] [PubMed] [Google Scholar]
- REPPERT S.M., WEAVER D.R., EBISAWA T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- ROLLAG M.D., ADELMAN M.R. Color change: a non-invasive measure of melatonin action. Animal Rep. Sci. 1992;30:67–89. [Google Scholar]
- SUGDEN D., PICKERING H., TEH M.-T., GARRATT P.J. Melatonin receptor pharmacology: toward subtype specificity. Biol. Cell. 1998;89:29–41. doi: 10.1016/s0248-4900(98)80009-9. [DOI] [PubMed] [Google Scholar]
- TEH M.-T., SUGDEN D. A clonal line of Xenopus laevis melanophores: a model system for evaluating melatonin receptor agonists and antagonists. J. Endocrinol. 1997;155:P56. [Google Scholar]
- TING K.N., DUNN W.R., DAVIES D.J., SUGDEN D., DELAGRANGE P., GUARDIOLA-LEMAITRE B., SCALBERT E., WILSON V.G. Studies on the vasoconstrictor action of melatonin and putative melatonin receptor ligands in the tail artery of juvenile Wistar rats. Br. J. Pharmacol. 1997;122:1299–1306. doi: 10.1038/sj.bjp.0701511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANECEK J., SUGDEN D., WELLER J., KLEIN D.C. Atypical synergistic α1- and β-adrenergic regulation of adenosine 3′-5′-monophosphate and guanosine 3′-5′-monophosphate in rat pinealocytes. Endocrinology. 1985;116:2167–2173. doi: 10.1210/endo-116-6-2167. [DOI] [PubMed] [Google Scholar]
- WHITE B.H., SEKURA R.D., ROLLAG M.D. Pertussis toxin blocks melatonin-induced pigment aggregation in Xenopus dermal melanophores. J. Comp. Physiol. B. 1987;157:153–159. doi: 10.1007/BF00692359. [DOI] [PubMed] [Google Scholar]


