Abstract
The use of pulmonary exogenous surfactant as a vehicle for intratracheally administered antibiotics to improve local antimicrobial therapy has been proposed. The present study investigated lung clearance rates in the rat of intratracheally instilled technetium labelled tobramycin with and without the addition of surfactant to the antibiotic solution.
The influence of surfactant on 99mTc-tobramycin lung clearance rates was studied dynamically with a gamma-camera in anaesthetized spontaneously breathing animals and in mechanically ventilated animals.
The results show that instillation of 99mTc-tobramycin with use of surfactant as vehicle significantly increases 99mTc-tobramycin lung clearance compared to instillation of 99mTc-tobramycin solution alone (P=0.006 between the two spontaneously breathing groups of animals and P=0.02 between the two ventilated groups of animals, ANOVA for repeated time measurements). The half life (t½) of composite clearance curves in spontaneous breathing animals was 147 min for animals receiving 99mTc-tobramycin versus 61 min for animals receiving 99mTc-tobramycin with surfactant. In mechanically ventilated animals this was 163 min versus 51 min, respectively.
It is concluded that exogenous surfactant, used as vehicle for intratracheally instilled 99mTc-tobramycin, increases lung clearance rate of 99mTc-tobramycin in rats.
Keywords: Pulmonary surfactant, surfactant as carrier, 99mTc-tobramycin, lung clearance, local antimicrobial therapy
Introduction
The use of exogenous pulmonary surfactant as a carrier or vehicle for intratracheally administered antibiotics to enhance the efficacy of local antimicrobial therapy in pneumonia has been proposed (Kharasch et al., 1991; Lachmann & Gommers, 1993; Schäfer, 1991; Van 't Veen et al., 1995; 1996a, 1996b). The expected advantages of this new delivery method are twofold. First, the surfactant instillation itself is potentially therapeutic in pneumonia: it promotes re-expansion of atelectatic areas and can correct impaired lung function and gas exchange in pneumonia (for review see Gommers & Lachmann, 1993; Van 't Veen et al., 1997). Second, it is expected that together with the pulmonary surfactant a high appropriate antibiotic dose can be delivered to the peripheral lung regions and even into atelectatic lung regions (Kharasch et al., 1991). It is believed that in pneumonia, selective delivery of antibiotics to the lung parenchmya increases their local effectiveness and decreases the risk of systemic side effects from potentially toxic agents such as aminoglycosides (Ramsey et al., 1993; Touw et al., 1995).
We recently demonstrated the in vivo efficacy of pulmonary surfactant as a vehicle for intratracheally administered tobramycin in mice with a respiratory infection with Klebsiella pneumonia. Survival rates of infected animals were significantly improved after tracheal instillation of a surfactant-tobramycin mixture compared with instillation of tobramycin alone (Van 't Veen et al., 1996b). Explanations for the observed difference in survival remained speculative and included an inherent effect of the exogenous surfactant instillation and/or an increased intrapulmonary spreading of the tobramycin when instilled with surfactant as vehicle. Preliminary results have shown that spreading of intratracheally instilled technetium labelled tobramycin (99mTc-tobramycin) within infected rat lungs was increased when pulmonary surfactant was added to the 99mTc-tobramycin solution (Lachmann et al., 1997).
To further explore the use of surfactant as a vehicle for antibiotics and seek to explain the earlier observed in vivo efficacy (Van 't Veen et al., 1996b), the present study investigated lung clearance of intratracheally instilled 99mTc-tobramycin with and without the use of surfactant as vehicle. Lung clearance of 99mTc-tobramycin was dynamically studied in healthy rats using sequential gamma-camera images. To study the effect of mechanical ventilation on lung clearance rates, lung clearance was studied in both spontaneously breathing animals and in mechanically ventilated animals.
Methods
Antibiotic labelling
Tobramycin was labelled with technetium using a method previously described for gentamicin (Al-Kourishi, 1988). Briefly, 10 mg tobramycin sulphate (Sigma Chemical Co, St. Louis, MO, U.S.A.) was dissolved in 100 μl H2O while flushing with nitrogen. Stannous chloride dihydrate (Merck, Darmstadt, Germany) was dissolved in 0.5 M HCl solution (previously flushed with nitrogen) to a solution of 0.1 mg ml−1. The following substances were added to the tobramycin solution under continuous nitrogen flushing: 100 μl 0.1 mg ml−1 SnCl2.2H2O solution in 0.5 M HCl; 250 μl 0.2 M NaOH solution; 450 μl 0.2 M NaHCO3 solution (pH=8.3); 100 μl Na99mTcO4, activity 100 Mbq. The reaction was allowed to proceed for 60 min. To estimate the amount of free, unbound 99mTcO4−, a sample was taken at the end of the reaction time and placed on a silica gel thin layer chromotagraphy (TLC, Silica gel 60F254, Merck, Darmstadt, Germany) and run in an 85% methanol/water system. Distribution of activity over a TLC-strip was counted using a gamma counter (Wallac 1480, Wallac oy, Finland).
Surfactant
A freeze-dried natural surfactant preparation was used, isolated from porcine lungs as previously described (Gommers et al., 1993). It consisted of approximately 90–95% phospholipids, 1% hydrophobic proteins (surfactant-proteins B and C) and 1% free fatty acids, the remainder being other lipids such as cholesterol and glyceride. There was no surfactant-protein A in this surfactant preparation. Surfactant powder was added to the antibiotic solution and shaken by hand to a final concentration of 25 mg ml−1.
Animal preparation
All animal experiments were approved by the institutional board for animal care. Care and handling were in accordance with the European Community guidelines for animal experimentation (86/609/EEG). In all experiments male Sprague Dawley rats (180–220 g, Iffa Credo, Brussels, Belgium) were used and food and water were given ad libitum.
Animals were anaesthetized with a combination of 0.315 mg ml−1 fentanyl citrate and 10 mg ml−1 fluanisone injected intramuscularly (dose 0.5 ml kg−1 h−1, Hypnorm®, Janssen Pharmaceuticals, Tilburg, The Netherlands) and 5 mg ml−1 midazolam injected subcutaneously (dose 1 ml kg−1 h−1, Dormicum®, Roche, Mijdrecht, The Netherlands). Animals received an intravenous line inserted in the arcus pedis, through which saline was administered (5 ml kg−1 h−1) to maintain fluid balance.
In addition, animals subjected to mechanical ventilation were tracheotomized after which the trachea was cannulated with a metal tube. Ventilated animals received pancuronium bromide (0.5 mg kg−1, intramuscularly) every hour to maintain muscle relaxation. Animals were mechanically ventilated using a Servo Ventilator 900C (Siemens-Elema, Solna, Sweden) at the following settings: pressure-controlled ventilation, frequency=30 breaths per min, peak airway pressure =14 cmH2O, positive end expiratory pressure (PEEP)= 3 cmH2O, inspiratory/expiratory ratio=1 : 2 and 21% oxygen. These ventilatory settings were maintained throughout the study period. At the end of the study, animals were killed with an overdose of midazolam injected intravenously.
Lung clearance studies
Lung clearance of intratracheally instilled 99mTc-tobramycin or 99mTc-tobramycin-surfactant was studied in 16 rats, randomly assigned to four groups of four animals each:
Group 1 Spontaneously breathing, intratracheal 99mTc-tobramycin: volume=1 ml kg−1, concentration=10 mg tobramycin ml−1.
Group 2 Spontaneously breathing, intratracheal 99mTc-tobramycin-surfactant: volume=1 ml kg−1, concentrations=10 mg tobramycin+25 mg surfactant ml−1.
Group 3 Mechanically ventilated, intratracheal 99mTc-tobramycin: volume=1 ml kg−1, concentration=10 mg tobramycin ml−1.
Group 4 Mechanically ventilated, intratracheal 99mTc-tobramycin-surfactant: volume=1 ml kg−1, concentration=10 mg tobramycin+25 mg surfactant ml−1.
Intratracheal instillation in spontaneously breathing animals was achieved by inserting a metal tube 2 cm into the trache via direct laryngoscopy. A catheter, connected to a 1 ml syringe was inserted through the tube and extended 1 cm beyond the tip of the tube. Subsequently, 1 ml kg−1 99mTc-tobramycin or 99mTc-tobramycin-surfactant was instilled. The cannula and tube were immediately removed after instillation.
For intratracheal instillation in mechanically ventilated animals, animals were disconnected from the ventilator and a catheter connected to a 1 ml syringe was inserted into the trachea cannula extending 1 cm beyond the tip of the cannula for instillation of 1 ml kg−1 99mTc-tobramycin or 99mTc-tobramycin-surfactant.
Before instillation of the solution, animals were placed in position on a gamma camera (ROTA II, Siemens) equipped with a LEAP collimator and using a 20% window around the 99mTc peak. 99mTc-tobramycin or 99mTc-tobramycin-surfactant was injected intratracheally at time zero and acquisition was started immediately thereafter. Data were stored in a dedicated computer (Hermes, Nuclear Diagnostics, Stockholm, Sweden). Dynamic acquisition took place in groups 1 and 2 for a total of 300 min; 30 frames of 10 min, 256×256 matrix and in groups 3 and 4 for a total of 180 min; 10 frames of 2 min, 8 frames of 5 min and 12 frames of 10 min, 256×256 matrix.
Data analyses
The 99mTc-tobramycin clearance measurements were analysed by drawing a region of interest over both lungs and generating a time-activity curve, corrected for radioactive decay over time. No background corrections were made. Differences between animals in injected activity at t=0 min were corrected by dividing counts per minute (c.p.m.) At t=x with c.p.m. at t=0 for each rat. Corrected data of the activity per lung region over time were log-transformed for equal distribution and analysed for statistical significance by ANOVA for repeated time measurements using the SAS statistical package (SAS Inc, Cary, U.S.A.).
A mean composite time activity curve was generated for each group on which mono-exponential and bi-exponential functions were fitted, using the curve-fit function of GraphPad Prism version 2.00 (GraphPad Software Incorporated, CA, U.S.A.). With this program, equations were compared for statistically significant best fit using the F-test, P⩽0.05, two tailed.
The amount of activity present in the lung as a percentage of the total injected dose was calculated at t=0, 30, 180 and 300 min. Differences between groups were analysed using a one-way ANOVA with a Student-Newman-Keuls post hoc test. Differences were considered significant when P⩽0.05, two-tailed.
Results
Figure 1 shows representative images made with the gamma-camera at several time points after injection of the radioactive tobramycin label. After endotracheal instillation, radioactivity was instantly distributed within both lungs resulting in a gamma-camera image anatomically corresponding with lungs and trachea. Shortly after endotracheal instillation radioactivity appeared outside the lung region and accumulated, in particular, in regions corresponding to the kidney, bladder and to a lesser degree in stomach, liver and gut. Increased radioactivity was not observed in the thyroid or salivary glands.
Figure 1.
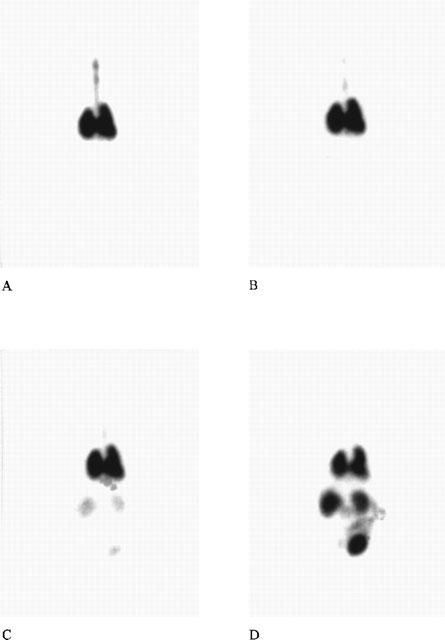
Gamma-camera scans from a representative animal at t=5 min (A), 20 min (B), 60 min (C) and 300 min (D) after endotracheal instillation of 99mTc-tobramycin–surfactant. The degree of darkening reflects the amount of activity present. After intratracheal instillation the activity is distributed through both lungs within minutes. The scans B–D show a time-related increase in activity localized primarily in the kidneys and bladder.
The composite time activity data for 99mTc-tobramycin instilled with or without surfactant as vehicle are shown in Figure 2A for spontaneously breathing animals and in Figure 2B for mechanically ventilated animals. The curves represent the mono-exponential function, best fitted on the composite data using the following equation:
Where t=time (min), k=the clearance rate (min−1) and Platgeau and Span are constants. The constants Plateau, Span and k for the best fitted curve of the respective groups are shown in Table 1, including the calculated half life (T½½) of the radioactive tracer in the lung and the coefficient of determination (r2) of the curve. T½ (min) of the fraction of the radioactive tracer that is mono-exponentially cleared from the lung is calculated using the following equation:
 |
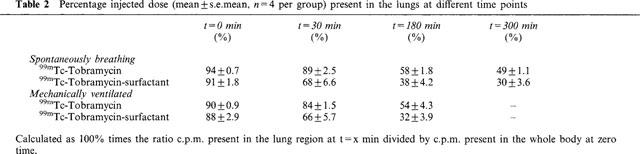
Lung clearance of the 99mTc-tobramycin was significantly increased when surfactant was used as vehicle, both in spontaneously breathing animals (P=0.006, ANOVA repeated time measurements) and mechanically ventilated animals; (P=0.02, ANOVA repeated time measurements). No significant differences in lung clearance were found between spontaneously breathing animals and ventilated animals receiving 99mTc-tobramycin with surfactant as vehicle, or similarly receiving 99mTc-tobramycin only (P>0.05, ANOVA repeated time measurements). Table 2 gives the percentage of the total injected dose present in the lungs at t=0, 30, 180 and 300 min after intratracheal instillation as measured with the gamma-camera. Percentage of the injected dose present in the lungs was significantly decreased in the tobramycin-surfactant group compared to the tobramycin group from t=0.5 h onwards both in the spontaneously breathing animals and the ventilated animals (P⩽0.05, one-way ANOVA with Students-Newman-Keuls post hoc test). No significant differences were found at time zero.
Figure 2.
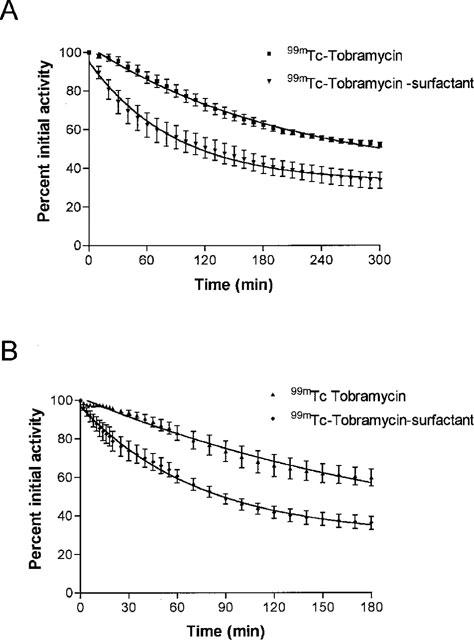
(A and B) Average activity (mean±s.e.mean, n=4 per group) present over the lung region as a percentage of the activity present over the lung region at t=0 plotted against time for the four studied groups. (A) shows spontaneously breathing animals and (B) shows mechanically ventilated animals. The curve represents the mono-exponential function fitted to the data of which the parameters are shown in Table 1.
Table 1.
Characteristics of the curves fitted on the composite time activity data
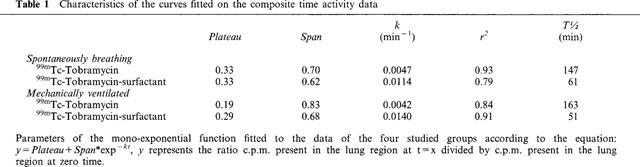
Table 2.
Percentage injected dose (mean±s.e.mean, n=4 per group) present in the lungs at different time points
Discussion
The aim of the present study was to quantify the influence of pulmonary surfactant as vehicle on lung clearance of endotracheally instilled tobramycin in healthy rats. The results show that after endotracheal instillation of 99mTc-tobramycin, lung clearance rate of the radiotracer was significantly increased when surfactant was added to the 99mTc-tobramycin solution.
The use of technetium labelled tobramycin enabled an accurate quantification of the amount of tobramycin present in the lung and sequential analysis within one subject. The labelling method was similar to that described for gentamicin (Al-Kouraishi 1988). The labelling procedure could cause formation of impurities such as 99mTc-pertechnetate (99mTcO4−) and hydrolyzed-reduced technetium (99mTcO2). Both of these molecules have different clearance characteristics to tobramycin and could, thus, result in false interpretations of the tobramycin lung clearance. 99mTcO2 is colloidal and unable to pass the alveolar-capillary barrier (O'Brodovich et al., 1986). In contrast 99mTcO4− clears quickly from the lung; in patients, the T½ of aerosolized pertechnetate is approximately 7 min (Kotzerke et al., 1996). 99mTcO4− accumulates in the thyroid gland, the gastric mucosa, and the salivary glands (O'Brodovich et al., 1986). Tobramycin is primarily cleared from the circulation by renal filtration and would, therefore, accumulate in the kidneys and bladder after entering the systemic circulation (Israel et al., 1976). Al-Kouraishi (1988) estimated the percentage 99mTcO2 to be 6–10% and the percentage 99mTcO4− to be 1% after labelling gentamicin with 99mTc. Control studies (data not shown) in our laboratory by means of chromatographic assays in vitro and biodistribution after intravenous injection in rats in vivo showed the quality of the tobramycin label to be comparable to that reported for gentamicin. It is, therefore, concluded that the formation of 99mTcO2 and 99mTcO4− is not relevant in the present study.
According to the monoexponential curves best fitted on the composite time activity data, in each group a percentage of the intratracheally instilled activity was not cleared from the lung region; that is, equal to the value for plateaus in Table 1×100%. Although speculative, the percentage not cleared according to this mono-exponential curve may represent intracellular 99mTc-tobramycin, macromolecular bounded 99mTc-tobramycin, 99mTc-tobramycin present in the trachea tube and/or 99mTcO2. Most likely a longer study period would reveal a bi-exponential clearance curve.
After intratracheal instillation, 99mTc-tobramycin can potentially be cleared from the lungs via the upper airways, lymphatics or vascular compartment, or it may remain intracellular within the lung parenchyma. However, based on its molecular size it can generally be expected that once 99mTc-tobramycin has reached the distal airways after intratracheal instillation, systemic absorption is the primary route for lung clearance. In the current study, although not actually measured, systemic absorption can readily be assumed based on the high lung clearance rates and the accumulation of radioactivity in primarily the kidneys and bladder.
The assumption that systemic absorption is the main clearance route of tobramycin after distal pulmonary deposition is supported by two earlier studies on pharmacokinetics of aerosolized tobramycin in cystic fibrosis patients (Cooney et al., 1994; Touw et al., 1997). In the study by Touw et al. (1997) it was demonstrated that the systemic availability, calculated from urinary output, ranged from 6.0–27.4% of the total inhaled tobramycin dose. Further, Cooney et al. (1994) studied tobramycin serum concentrations after inhalation and found a mean systemic availability (±s.d.) of 9.13±3.82%. These percentages for systemic availability are similar to the reported percentage of the aerosolized drug that is actually deposited in the lung after inhalation, i.e. about 10% (Ilowite et al., 1987; Mukhopadhyay et al., 1994), suggesting that the systemic availability is a close reflection of the deposited pulmonary dose.
In the current study the T½ of 99mTc-tobramycin in the lung ranged from 51–163 min. Valcke et al. (1990) reported a T½ of 126 min of tobramycin concentrations in alveolar lining fluid after aerosol inhalation by spontaneously breathing healthy rats. This is comparable to the 147 min found in our study in the spontaneously breathing animals receiving 99mTc-tobramycin only. In patients, Cooney et al. (1994) found a mean absorption time of aerosolized tobramycin across the alveoli to vary widely from 15–150 min.
The results of the current study demonstrate that clearance rates are increased more than 2 fold, when the 99mTc-tobramycin solution is instilled with surfactant as vehicle. No significant difference was found between mechanically ventilated animals and spontaneously breathing animals receiving the same intratracheal instillation: 99mTc-tobramycin or 99mTc-tobramycin-surfactant, respectively. Clearance rate of a solute from airways is dependent on a great number of factors related to the properties of both the solute and the lung. For instance, molecular size and charge of the solute, epithelial integrity, available regional surface area and intra-alveolar pressures (for review see O'Doherty & Peters, 1997). As clearance rate is influenced by so many factors it is difficult to define with certainty the mechanism that causes the current findings.
One plausible explanation for the observed increase in lung clearance rate of 99mTc-tobramycin after instillation with surfactant compared to instillation without surfactant, would be an increased alveolar deposition of the tracer and an increased exposed surface area within the lung. It has been shown that radioaerosol clearance rates from the larger airways, bronchi, trachea and nasal epithelia are significantly slower than that of the alveolar-capillary membrane (Greiff et al., 1990; Cheema et al., 1988). In addition, transfer rate of hydrophilic solution across the alveolar-capillary barrier is dependent on passive diffusion through intracellular junctions; increased exposed surface area and thus an increased number of intracellular junctions, will lead to increased clearance rates (O'Doherty & Peters, 1997).
These explanations agree with previous studies in which a more peripheral and more homogenous pulmonary distribution of an intratracheally instilled radiotracer (Kharasch et al., 1991; Lachmann et al., 1997) or adenoviral vectors (Katkin et al., 1997) was demonstrated when surfactant was used as vehicle in comparison to saline. Unfortunately, a more peripheral and homogenous distribution could not be quantified in the current study due to a too low resolution of the images for this purpose.
It should be noted that the current findings are in contrast with two earlier studies (Davis et al., 1994; Smith et al., 1995). Davis et al. (1994) found that surfactant instillation in healthy adult rats 30 min prior to intratracheal instillation of recombinant super oxide dismutase (rhSOD) resulted in significantly increased rhSOD lung concentrations 24 h later. It was speculated that rhSOD became entrapped in the lipid bilayer of surfactant which could cause a decrease in lung clearance. However, this association would appear to be weak since subsequent in vitro studies showed that rhSOD could easily be separated from the surfactant by sedimentation (Davis et al., 1994). Smith et al. (1995) reported a delayed absorption of furosemide from the lungs when surfactant, as compared to saline, was used as a vehicle for intratracheal delivery in healthy guinea-pigs. It was speculated that the delayed absorption of furosemide when instilled with surfactant as vehicle resulted from binding of furosemide to surfactant proteins or to liposome formation (Smith et al., 1995).
Explanations for the discrepancy between these two studies (Davis et al., 1994; Smith et al., 1995) and the present results are speculative and may relate to methodological differences between the studies as well as to differences in the agents used. For instance, in the study by Davis et al. (1994), no difference in pulmonary distribution of the tracheally instilled rhSOD is to be expected between the two treatment groups because rhSOD was instilled using saline as vehicle in both groups. This contrasts with our study, in which a difference in pulmonary distribution between the groups using surfactant or buffer as vehicle is expected and in turn provides a plausible explanation for the difference in lung clearance rates. The differing results demonstrate, however, the complexity of pulmonary clearance rates of intratracheally instilled solutes and highlights the need for further study.
The combined administration of surfactant and antimicrobial agents to the lungs is considered to be an effective and innovative approach in the treatment of severe pulmonary infection. However, actual data on efficiency or pharmacokinetics of this delivery method is still limited. The current study was an effort to further clarify the pharmacokinetic characteristics of intratracheally instilled tobramycin using surfactant as vehicle. The major concern that emerges from the current results is the high clearance rate after endotracheal instillation in both groups and therefore the risk of systemic side effects due to high systemic levels. In this point it seems that the use of surfactant as vehicle for antibiotic delivery to the lung defeats its object in preventing systemic side effects when achieving the first objective, namely appropriate doses at the site of infection.
In conclusion, the present study has demonstrated an increased clearance rate of intratracheally instilled technetium labelled tobramycin solution when surfactant was added to the solution. This increase in clearance might well be explained by a more peripheral distribution of the 99mTc-tobramycin within the lung when surfactant is added to the solution, as has been observed in earlier studies on surfactant as a vehicle. More studies are needed to further clarify the pharmacokinetic interactions and to determine the clinical relevance of these findings.
Acknowledgments
The authors thank Mr E.R. Hendrik for technical assistance and Mrs L. Visser-Isles for English language editing.
Abbreviations
- rhSOD
recombinant super oxide dismutase
- TLC
thin layer chromotagraphy
References
- AL-KOURAISHI S.H. Labelling and quality control of gentamycin with 99mTc and biodistribution. J. Radioanal. Nucl. Chem. 1988;125:203–211. [Google Scholar]
- CHEEMA M.S., GROTH S., MARRIOTT C. Binding and diffusion characteristics of 14C EDTA and 99mTc-DTPA in respiratory tract mucus glycoprotein from patients with chronic bronchitis. Thorax. 1988;43:669–673. doi: 10.1136/thx.43.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COONEY G.F., LUM B.L., TOMASELLI M., FIEL S.B. Absolute bioavailability and absorption characteristics of aerosolized tobramycin in adults with cystic fibrosis. J. Clin. Pharmacol. 1994;34:255–259. doi: 10.1002/j.1552-4604.1994.tb03995.x. [DOI] [PubMed] [Google Scholar]
- DAVIS J.M., ROSENFELD W.N., KOO H.-C., GONENNE A. Pharmacologic interactions of exogenous lung surfactant and recombinant human Cu/Zn superoxide dismutase. Pediatr. Res. 1994;35:37–40. doi: 10.1203/00006450-199401000-00009. [DOI] [PubMed] [Google Scholar]
- GOMMERS D., LACHMANN B. Surfactant therapy: does it have a role in adults. Clin. Intensive Care. 1993;4:284–295. [Google Scholar]
- GOMMERS D., VILSTRUP C., BOS J.A.H., LARSSON A., WERNER O., HANNAPPEL E., LACHMANN B. Exogenous surfactant therapy increases static lung compliance and cannot be assessed by measurements of dynamic compliance alone. Crit. Care Med. 1993;21:567–574. doi: 10.1097/00003246-199304000-00019. [DOI] [PubMed] [Google Scholar]
- GREIFF I., WOLLMER P., ERJEFALT I., PIPKORN U., PERSSON C.G.A. Clearance of 99mTc-DTPA from guinea pig nasal, tracheobronchial, and bronchoalveolar airways. Thorax. 1990;45:841–845. doi: 10.1136/thx.45.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILOWITE J.S., GORVOY J.D., SMALDONE G.C. Quantitative deposition of aerosolized gentamicin in cystic fibrosis. Am. Rev. Respir. Dis. 1987;136:1445–1449. doi: 10.1164/ajrccm/136.6.1445. [DOI] [PubMed] [Google Scholar]
- ISRAEL K.S., WELLES J.S., BLACK H.R. Aspects of the pharmacology and toxicology of tobramycin in animals and humans. J. Infect. Dis. 1976;S134:97–103. doi: 10.1093/infdis/134.supplement_1.s97. [DOI] [PubMed] [Google Scholar]
- KATKIN J.P., HUSSER R.C., LANGSTON C., WELTY S.E. Exogenous surfactant enhances the delivery of recombinant adenoviral vectors to the lung. Hum. Gene Ther. 1997;8:171–185. doi: 10.1089/hum.1997.8.2-171. [DOI] [PubMed] [Google Scholar]
- KHARASCH V.S., SWEENEY T.D., FREDBERG J., LEHR J., DAMOKOSH A.I., AVERY M.E., BRAIN J.D. Pulmonary surfactant as a vehicle for intratracheal delivery of technetium sulfur colloid and pentamidine in hamster lungs. Am. Rev. Respir. Dis. 1991;144:909–913. doi: 10.1164/ajrccm/144.4.909. [DOI] [PubMed] [Google Scholar]
- KOTZERKE J., VAN DER HOFF J., BURCHERT W., WAGNER T.O.F., EMTER M., HUNDESHAGEN H. A compartmental model for alveolar clearance of pertechnegas. J. Nucl. Med. 1996;37:2066–2071. [PubMed] [Google Scholar]
- LACHMANN B., GOMMERS D. Is it rational to treat pneumonia with exogenous surfactant. Eur. Respir. J. 1993;6:1437–1438. [PubMed] [Google Scholar]
- LACHMANN B., VAN 'T VEEN A., GOMMERS D., WOLLMER P. Lung distribution of tracheally administered 99mTc-tobramycin in pneumonia: effect of pulmonary surfactant as vehicle. Am. J. Resp. Crit. Care Med. 1997;155:A108. [Google Scholar]
- MUKHOPADHYAY S., STADDON G.E., EASTMAN C., PALMER M., RHYS DAVIES E., CARSWELL F. The quantitative distribution of nebulized antibiotic in the lung in cystic fibrosis. Respir. Med. 1994;88:203–211. doi: 10.1016/s0954-6111(05)80348-8. [DOI] [PubMed] [Google Scholar]
- O'BRODOVICH H., COATES G., MARRIN M. Effect of inspiratory resistance and PEEP on 99mTc-DTPA clearance. J. Appl. Physiol. 1986;60:1461–1465. doi: 10.1152/jappl.1986.60.5.1461. [DOI] [PubMed] [Google Scholar]
- O'DOHERTY M.J., PETERS A.M. Pulmonary technetium 99m diethylene triamine penta-acetic acid aerosol clearance as an index of lung injury. Eur. J. Nucl. Med. 1997;24:81–87. doi: 10.1007/BF01728316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMSEY B., DORKIN H.L., EISENBERG J.D., GIBSON R.L., HARWOOD I.R., KRAVITZ R.M. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N. Engl. J. Med. 1993;328:1740–1746. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- SCHÄFER K.P.Molecular aspects of lung surfactant proteins and their use as pulmonal carriers Drug targeting and delivery 1991London: Ellis Horwood Ltd; 155–166.ed. Junginger, H.J. pp [Google Scholar]
- SMITH S.A., PILLERS D.M., GILHOOLY J.T., WALL M.A., OLSEN G.D. Furosemide pharmacokinetics following intratracheal instillation in the guinea pig. Biol. Neonate. 1995;68:191–199. doi: 10.1159/000244237. [DOI] [PubMed] [Google Scholar]
- TOUW D.J., BRIMICOMBE R.W., HODSON M.E., HEIJERMAN H.G.M., BAKKER W. Inhalation of antibiotics in cystic fibrosis. Eur. Respir. J. 1995;8:1594–1604. [PubMed] [Google Scholar]
- TOUW D.J., JACOBS F.A.H., BRIMICOMBE R.W., HEIJERMAN H.G.M., BAKKER W., BREIMER D.D. Pharmacokinetics of aerosolized tobramycin in adult patients with cystic fibrosis. Antimicrob. Agents Chemother. 1997;41:184–187. doi: 10.1128/aac.41.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALCKE Y., PAUWELS R., VAN DER STRAETEN M. The penetration of aminoglycosides into the alveolar lining fluid of rats. Am. Rev. Respir. Dis. 1990;142:1099–1103. doi: 10.1164/ajrccm/142.5.1099. [DOI] [PubMed] [Google Scholar]
- VAN 'T VEEN A., GOMMERS D., LACHMANN B.Rationale for surfactant therapy in pneumonia Yearbook of Intensive Care and Emergency Medicine 1997Berlin: Springer; 638–653.ed. Vincent, J.L. pp [Google Scholar]
- VAN 'T VEEN A., GOMMERS D., MOUTON J.W., KLUYTMANS J.A.J.W., KRIJT E.J., LACHMANN B. Exogenous pulmonary surfactant as a drug delivering agent; influence of antibiotics on surfactant activity. Br. J. Pharmacol. 1996a;118:595–598. doi: 10.1111/j.1476-5381.1996.tb15442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN 'T VEEN A., MOUTON J.W., GOMMERS D., KLUYTMANS J.A.J.W., DEKKERS P., LACHMANN B. Influence of pulmonary surfactant on in vitro bactericidal activities of amoxicillin, ceftazidime, and tobramycin. Antimicrob. Agents Chemother. 1995;39:329–333. doi: 10.1128/aac.39.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN 'T VEEN A., MOUTON J.W., GOMMERS D., LACHMANN B. Pulmonary surfactant as vehicle for intratracheally instilled tobramycin in mice infected with Klebsiella pneumoniae. Br. J. Pharmacol. 1996b;119:1145–1148. doi: 10.1111/j.1476-5381.1996.tb16016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


