Abstract
The aim of the present investigation was to characterize and determine the tissue location of the adenosine receptors present in the rat ileum using a method that detects drug action on the cholinergic nerves innervating the longitudinal and circular muscles.
The non-selective adenosine agonist, NECA (10 and 100 nM) caused significant concentration-related reductions in the circular muscle responses to transmural stimulation over the frequency range of 2.5–40 Hz, but did not affect the responses of the longitudinal muscle, nor did it reduce the muscle responses of the guinea-pig ileum.
The affinity order of antagonists at inhibiting the effect of NECA on the circular muscle was: CPDPX>8-PT>DMPX with apparent pA2 values of 9.31, 7.54 and 5.63 respectively. CPDPX (10–100 nM) caused parallel displacements of the concentration-effect curves to CPA with a pKb value of 9.15 and Schild slope of 1.03.
The agonists previously tested in the rat jejunum peristaltic reflex preparation were also shown to inhibit responses of the rat ileum in the following decreasing order of potency: CPA>NECA>2-CADO>R-PIA>S-PIA>>PAA. In addition, CHA and CCPA were also potent agonists. NECA (100 nM) and CPA (32 nM) did not inhibit carbachol (1 μM)-induced tone of tissues pre-treated with TTX (1 μM).
In conclusion, the rat ileum contains inhibitory A1 adenosine receptors situated on cholinergic nerve endings innervating the circular muscle.
Keywords: Adenosine receptors, adenosine agonists, adenosine antagonists, cholinergic nerves, rat ileum, circular muscle, CPDPX, CPA, NECA, 8-PT
Introduction
Extracellular adenosine is formed as a result of several processes in the mammalian body, such as from rapidly metabolizing tissues, hypoxia or by conversion of ATP released from purinergic nerves (see White, 1991 for review). The actions of adenosine are exerted at P1 purinoceptors, as opposed to receptors for ATP designated P2 by Burnstock (1978). P1 receptors have been further classified on the basis of operational, transductional and molecular properties into A1, A2A, A2B and A3 adenosine receptors (see Palmer & Stiles, 1995; Alexander & Peters, 1998 for reviews).
Functional studies have revealed that adenosine and its analogues exert a variety of effects in different regions of the intestine. For example, early studies showed that adenosine inhibited neuroeffector transmission in the guinea-pig ileum (Small & Weston, 1979; Hayashi et al., 1978; Collier & Tucker, 1983) mainly by activating prejunctional A1-receptors resulting in decreased acetylcholine release, but also by an inhibitory post-junctional effect mediated by A2-receptors (Gustafsson et al., 1985).
Later, studies of the rat intestinal tract showed that adenosine agonists induced direct relaxation of the longitudinal muscles of the duodenum, ileum and colon and contraction of the muscularis mucosae in these regions. The adenosine receptors responsible for mediating contraction in the duodenum were identified as A2B and A1 in the ileum and colon (Bailey et al., 1992; Nicholls & Hourani, 1997; Nicholls et al., 1996). The situation was more complex in the longitudinal muscle where relaxations were shown to involve A1 and A2B receptors in the duodenum, A1 in the ileum and A2 in the colon (Bailey & Hourani 1992; Nicholls & Hourani 1997; Nicholls et al., 1996). The presence of [3H]-CPDPX binding sites in the duodenum and colon generally correlated with these findings (Peachey et al., 1994).
The finding that the non-selective adenosine agonists, 5′-N-ethylcarboxamidoadenosine (NECA), displayed antidiarrhoeal activity in rats undergoing morphine withdrawal (Dionyssopoulos et al., 1992) led to a study that identified both antisecretory and antiperistaltic actions of NECA in the small intestine (Coupar & Hancock, 1994). Further studies established that adenosine agonists acted on A2B receptors to inhibit fluid secretion induced by vasoactive intestinal peptide in the jejunum of anaesthetized rats (Hancock & Coupar, 1995a) and A1 receptors were identified as those responsible for the inhibitory actions of adenosine agonists on peristalsis in the rat isolated jejunum (Hancock & Coupar, 1995b). The antiperistaltic action was assumed to be neural, partly on the basis that the EC50 of NECA in the rat jejunum peristaltic reflex preparation was similar to its value in the transmurally-stimulated guinea-pig ileum preparation (25.4 vs 11.2 nM). However, it was concluded that adenosine analogues may act proximal to the final cholinergic neurone in the peristaltic reflex arc, because NECA failed to affect transmurally stimulated cholinergic contractions of the rat jejunum longitudinal muscle (Coupar & Hancock, 1994; Hancock & Coupar, 1995b).
Therefore, the aim of the present investigation was to determine the tissue location of the A1 adenosine receptors in the small intestine. This has been undertaken using a method devised in our laboratory which makes it possible to examine the effect of drugs on the nerves that control both longitudinal and circular muscles of the small intestine (Coupar & Liu, 1996). The majority of experiments were performed on the ileum, since this region had not been investigated in detail previously, but activity of NECA on the jejunum was confirmed also. Some experiments were also performed on the ileum from the guinea-pig, since we reported recently a difference in sensitivities towards α2-adrenoceptor agonists between these two species (Liu & Coupar, 1997).
Methods
The method of measuring the effects of drugs on the nerves that supply the longitudinal and circular muscles of rat ileum follows that described in detail by Coupar & Liu (1996). Salient features of the method are as follows.
Hooded Wistar rats (250–350 g) of either sex were stunned by a blow to the head and killed by exsanguination. A section of ileum (10 cm proximal to the caecum) was excised and rinsed with 5 ml of Krebs-Henseleit solution containing (mM): NaCl, 118; KCl, 4.7; NaHCO3, 25; KH2PO4, 1.2; CaCl2, 2.5; MgSO4, 1.2; D-(+)-glucose, 11. One end of the segment was threaded over an intraluminal electrode made of platinum wire. The electrode assembly with attached tissue was mounted vertically in a 30 ml jacketed organ bath containing Krebs-Henseleit solution maintained at 37°C and continuously gassed with 95% oxygen-5% carbon dioxide. The unattached end of the segment was trimmed and a cannula filled with Krebs-Henseleit solution was tied into position such that the tissue length was 3–4 cm. The opposite end of the cannula was connected to a pressure transducer (Gould P23ID) to monitor intraluminal pressure as an indirect measurement of circular muscle contraction. The segment was next connected to an isometric force displacement transducer (Grass FT03C) to measure longitudinal muscle tension changes. The parameters were recorded on a Grass model 79D polygraph. A Grass S48 stimulator supplied current to the intraluminal electrode and the second platinum plate electrode, which was positioned parallel to the intestine in the bathing solution. The segment was loaded with an initial tension of 1 g, left to equilibrate for 35 min and then partially filled with 0.15 ml of Krebs-Henseleit solution from a 1 ml syringe attached to the pressure transducer via a 3-way tap. Procedures involving transmural electrical stimulation or drug application were started 10 min after filling the segment. Segments of guinea-pig ileum were prepared and set up in the same way as described for rat ileum.
Transmural stimulation was applied to the tissue as square wave pulses of 1 ms duration in 8 s trains at frequencies of 10 Hz. This frequency was selected because it elicits maximal tension increase of the longitudinal muscle and a maximal increase in intraluminal pressure, which enabled accurate measurement of the inhibitory effects of the α-adrenoceptor agonists in a previous study (Coupar & Liu, 1996). Trains of pulses were delivered 3 min apart at supramaximal voltage.
Cumulative concentration-effect relationships were established to the adenosine agonists using contact times of 3 min for each concentration. Antagonists were incubated with the tissues for 20 min before commencing addition of agonists. Each tissue was exposed to only one agonist or agonist/antagonist combination.
In one series of experiments tissues were incubated for 10 min with 1 μM tetrodotoxin (TTX) followed by 1 μM of carbachol, which induced an increase in intraluminal pressure equivalent to 62.2±9.9% of the pre-TTX control response to 10 Hz. One group was then exposed to NECA and the other to CPA to test for possible direct inhibitory activity on the circular muscle.
Drugs
Carbachol chloride was purchased from Sigma Chemical Co. (St. Louis, U.S.A.) and tetrodotoxin from Calbiochem (TTX, La Jolla, U.S.A.).
Adenosine agonists
2-chloroadenosine (CADO), 2-chloro-N6-cyclopentyladenosine (CCPA), N6-cyclohexyladenosine (CHA), N6-cyclopentyladenosine (CPA), 5′-N-ethylcarboxamidoadenosine (NECA), 2-phenylaminoadenosine (PAA, CV 1808), R(−) N6-(2-phenylisopropyl)adenosine (R-PIA), S(+) N6-(2-phenylisopropyl)adenosine (S-PIA). All were obtained from RBI, Natick, U.S.A. and were dissolved in 0.9% w v−1 saline except PAA, which was dissolved in 6% w v−1 ethanol in saline before further dilution in saline.
Adenosine antagonists
8-cyclopentyl-1,3-dipropylxanthine (CPDPX), 3,7-dimethyl-1-propargylxanthine (DMPX), 8-phenyltheophylline (8-PT) were obtained from RBI, Natick, U.S.A. Stock solutions of CPDPX and 8-PT were made up in distilled water containing 0.1% v v−1 DMSO/1 M NaOH (1 : 0.75) as initial solvent. DMPX was dissolved in 0.9% w v−1 saline.
All drugs were freshly prepared except TTX which was diluted to 1 mM in buffer and frozen.
Data analysis
The responses of the tissues to electrical stimulation at different frequencies were normalized to a percentage of the response occurring at 10 Hz which was set at 100%. Frequency-response and concentration-effect curves were compared for statistical differences using 2-way ANOVA.
The quantitative values of the responses elicited by electrical stimulation at the set frequency of 10 Hz in the presence of agonist concentrations were expressed as percentage reduction compared to two pre-drug control responses in terms of arithmetic means±s.e.mean. The potencies of agonists were expressed as geometric mean EC50 values with 95% confidence intervals (CI) relative to individual maximum responses (Emax). These parameters were calculated and sigmoidal concentration-effect curves plotted by fitting a four-parameter logistic equation to the data points. The equation is:
where A is the agonist concentration, a is the basal value, b is the vertical range, c is the pEC50, and d is the mid-point slope (Lew, 1995).
The affinities of the antagonists were expressed as either apparent pA2 values or, in the case of CPDPX, a pKb value. Apparent pA2 values were calculated from the relationship:
where CR is the concentration-ratio of agonist used in the presence and absence of antagonist (B). The errors associated with agonist concentration-ratios were estimated by using log forms (−log EC50=pEC50):
The pKb value of CPDPX using the selective A1 agonist CPA was calculated as described by Lew & Angus (1995) by applying non-linear regression analysis from the relationship:
where B is the antagonist concentration, n the Schild slope and c a constant equal to control EC50/Kb.
The number of tissues used is indicated by n and all calculations and graphics were performed using the computer program Graph Pad Prism (Graph Pad Software Inc., San Diego, U.S.A.). Differences were considered statistically significant when P<0.05.
Results
The typical response to transmural electrical stimulation was a fast increase in longitudinal muscle tension followed by an increase in intraluminal pressure (indicative of circular muscle contraction) after a latency of approximately 4 s and reached its maximal response within 8 s. The longitudinal muscle began relaxing during the period of stimulation and was followed by a relatively large and long-lasting after-contraction, which was not seen in the intraluminal pressure recording (Figure 1). The responses of both muscle layers were frequency-dependent, small responses of both occurred at 2.5 Hz and the frequencies generating the greatest pressures were 10 and 20 Hz (Figure 2). The actual responses to a set frequency of 10 Hz were increases in longitudinal muscle tension and intraluminal pressure of 2.80±0.16 g (n=9) and 9.98±0.53 mmHg (n=9) respectively.
Figure 1.
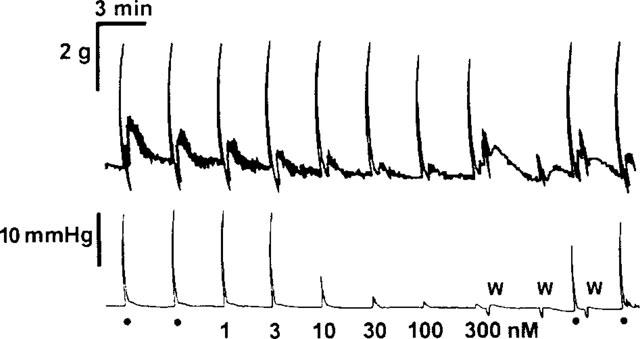
(Top) trace showing isometric longitudinal muscle responses and (bottom) increases in intraluminal pressure as an index of circular muscle contraction in a segment of rat ileum. Responses were induced using transmural stimulation at 1 ms pulse duration, 10 Hz in trains of 8 s, delivered every 3 min. Two control periods of stimulation are shown on the left (•), followed by responses in the presence of cumulatively increasing concentrations of NECA (1–300 nM), which reduced responses of the circular muscle but not the longitudinal. Three replacements of the bath fluid (W) restored responses to pre-NECA control values. Bars indicate the response and time scales.
Figure 2.
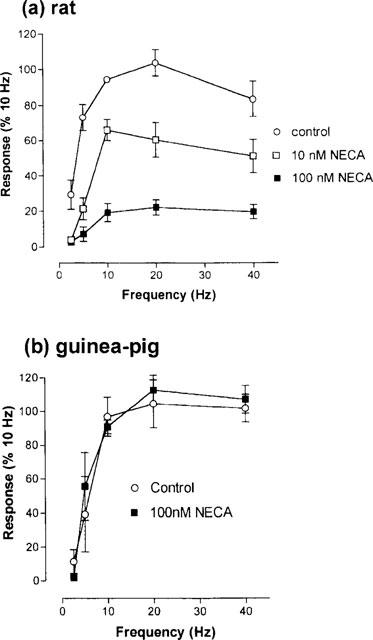
Contractile responses of (a) rat and (b) guinea-pig ileum to frequency-related transmural stimulation of the circular muscle and suppression of them in rat, but not guinea-pig tissues by NECA. The effects are expressed as a percentage of the amplitude of the pressure elicited by 10 Hz stimulation before the frequency-related cycle started. Symbols represent the means of the values obtained from 15 rat preparations (n=5 for each of the three treatments) and nine guinea-pig preparations (n=4 control, n=5 NECA) and bars show the s.e.mean.
NECA at concentrations of 10 and 100 nM caused significant concentration-related reductions in the pressure responses over the range of 2.5–40 Hz (P<0.0001, n=15, two-way ANOVA, Figure 2a). In contrast, responses of the guinea-pig ileum circular muscle to transmural stimulation over the same range were not affected by NECA at 100 nM, the frequency-response curves being superimposable (P>0.05, n=9, two-way ANOVA (Figure 2b).
NECA (1 nM–1 μM) caused a concentration-related inhibition of the circular muscle response of the rat ileum without significantly affecting the response of the longitudinal muscle at the standard frequency of 10 Hz transmural stimulation (Figure 3). The EC50 and Emax values of NECA shown in Table 2 were not significantly different to corresponding values of 13 nM (95% CI, 6.51–25.97) and 99.54% (95% CI, 87.22–111.9) respectively, in segments of rat jejunum (P>0.05).
Figure 3.
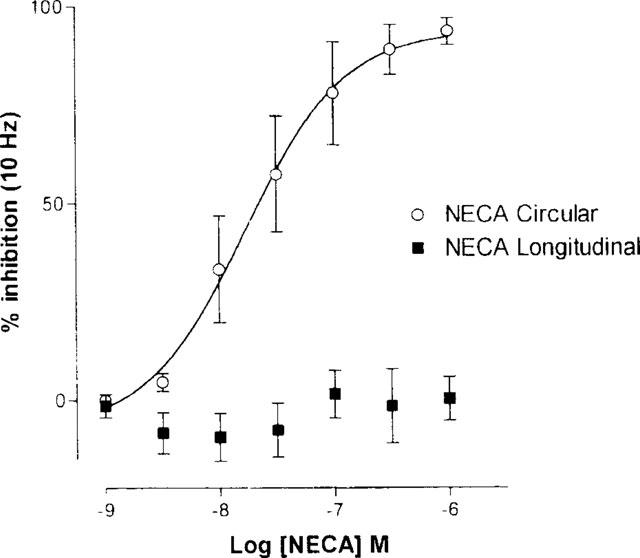
Mean cumulative concentration-response curves for the inhibition of transmurally-stimulated (1 ms pulse duration, 10 Hz for 8 s) increase of the intraluminal pressure and contraction of the longitudinal muscle of the rat ileum by NECA. Responses are plotted as percentage inhibition of the control amplitude of the tension (for longitudinal muscle) or pressure (for circular muscle). Bars indicate the s.e.means (n=6 for longitudinal muscle, n=5 for circular muscle).
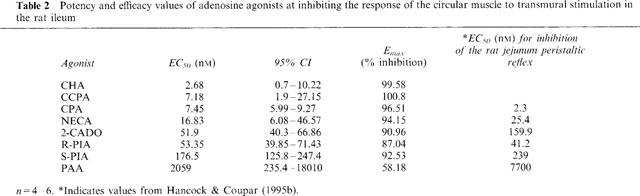
Table 2.
Potency and efficacy values of adenosine agonists at inhibiting the response of the circular muscle to transmural stimulation in the rat ileum
Antagonists
The affinity order of antagonists on the circular muscle response was: CPDPX>8-PT>DMPX. Single concentrations caused parallel rightward displacements of the concentration-effect curve to NECA without suppression of Emax values. The calculated apparent pA2 values from the concentration ratios are shown in Table 1.
Table 1.
Affinity values of adenosine agonists at inhibiting the effect of NECA on transmural stimulation in the rat ileum circular muscle
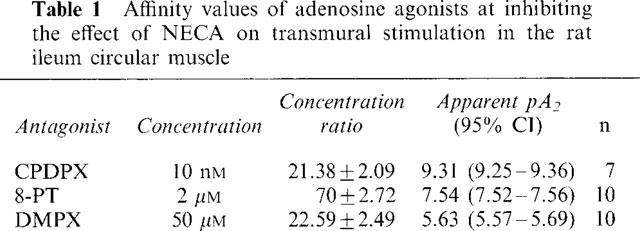
CPDPX (3.2–100 nM) also caused parallel displacements of the concentration-effect curves to CPA (Figure 4). Analysis of the decrease in pEC50 values caused by increasing concentrations of CPDPX showed that the data fitted the non linear equation best with slope fixed at unity giving a pKb value of 9.15 (95% CI 9.40–8.90, n=24). The Schild slope calculated from the variable slope analysis was 1.03.
Figure 4.
Displacement of the cumulative concentration-effect curve to CPA by CPDPX at four different concentrations (CPA in the absence of CPDPX=control). (Insert) Non-linear regression plot of the reduction in the resultant pEC50 values caused by the increasing concentrations of CPDPX (n=24).
Agonists
The adenosine agonists previously tested in the rat jejunum peristaltic reflex preparation were retested on the rat ileum stimulated at the standard frequency of 10 Hz. Like NECA, they did not influence the responses of the longitudinal muscle (P>0.05, n=4–6 for each agonist, ANOVA) and all but PAA induced marked inhibitory responses of the circular muscle at their maximally-effective concentrations. Pairwise comparisons of concentration-effect curves by 2-way ANOVA showed the following rank order of potency: CPA>NECA>2-CADO>R-PIA>S-PIA>>PAA (Table 2, Figure 5).
Figure 5.
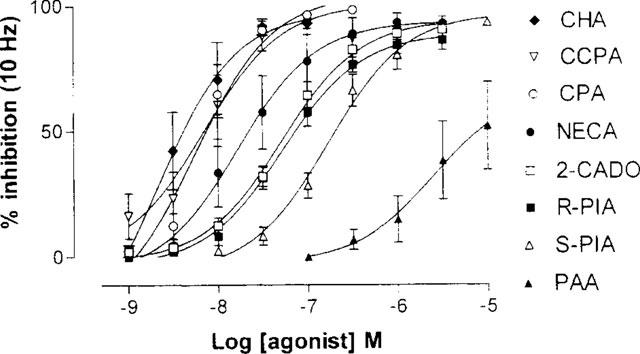
Cumulative concentration-effect curves to adenosine agonists for inhibition of circular muscle contraction (increase in intraluminal pressure) in response to transmural stimulation (10 Hz for 8 s). n=4–6 for each agonist.
In addition to the agonists previously tested in the rat jejunum peristaltic reflex preparation, two other agonists were shown to be potent agonists in the ileum. These were CHA and CCPA with EC50 and Emax values shown in Table 2. The order of potency of these agonists relative to the most potent, CPA, in the previous series of experiments was: CHA⩾CCPA⩾CPA (two-way ANOVA, P>0.05).
Near maximally-effective concentrations of NECA (100 nM) and CPA (32 nM) on the response of the circular muscle to transmural stimulation at 10 Hz were without effect on tone induced by carbachol (1 μM) in tissues pretreated with TTX (1 μM, 10 min) (n=3 for NECA, n=4 for CPA).
Discussion
This study shows that the rat ileum contains inhibitory A1 adenosine receptors situated on cholinergic nerves innervating the circular muscle. This extends our previous finding that A1 receptors are functionally involved in suppressing the peristaltic reflex in the jejunum of the rat (Hancock & Coupar, 1995b).
Functional location
The present study used a method that enables simultaneous investigation of drug activity on the responses of both longitudinal and circular muscles of the small intestine. Transmural electrical stimulation causes activation of cholinergic nerve endings (Coupar & Liu, 1996) and has been used previously to demonstrate the presence of inhibitory α2D-adrenoceptors located on cholinergic nerve endings supplying the circular muscle and post-synaptic α1-adrenoceptors located on both longitudinal and circular layers of the rat ileum (Liu & Coupar, 1997). It has been well established from studies using the guinea-pig ileum that adenosine agonists inhibit the release of acetylcholine evoked at conventional low frequencies of transmural stimulation (Gintzler & Musacchio 1975; Sawynock & Jhamandas 1976) via an action mainly at pre-synaptic A1 adenosine receptors, but supplemented by action at post-junctional A2 receptors (Gustafsson et al., 1985). More recently, Nicholls & Hourani (1997) have identified post-synaptic inhibitory adenosine receptors in the longitudinal muscle layer of the rat ileum. In their experiments adenosine, NECA and CPA relaxed carbachol-contracted preparations via an A1 receptor population. However, the results from the present experiments eliminate the possibility of a post-synaptic site of action of the adenosine agonists in suppressing the response of the circular muscle to nerve stimulation. This was established on the basis that neither NECA nor CPA reduced carbachol-induced tone of the circular muscle in preparations pre-treated with TTX. Hence, the adenosine receptors could be located on pre- and/or post-ganglionic cholinergic neurones, since both of these nerves make a significant contribution to the circular muscle response in the presently used method. This was concluded on the basis that ganglionic blockade with hexamethonium inhibited the circular muscle response to 10 Hz stimulation by 73.2%, while atropine blocked the response (Coupar & Liu, 1996). The present results showing the full agonists caused near maximal inhibitions of the response to nerve stimulation is an indication that A1 receptors are located on both pre- and post-ganglionic neurones.
It is of interest that the responses of the longitudinal muscle to nerve stimulation in the present series of experiments were not affected by any of the adenosine agonists. This is probably due to the different concentrations required to produce inhibition of neural as opposed to smooth muscle activity. This is apparent by comparing the potency values of NECA, which are EC50 values of 16.83 nM (present experiments) vs >1.5 μM (Nicholls & Hourani, 1997) respectively. Since the A1 receptor is the site of action in both tissues of the rat ileum, its difference in sensitivity to agonists is presumably the result of differences in coupling, receptor reserve or other such operational characteristics.
Other findings of the present study were that NECA inhibited the responses of the rat ileum circular muscle to stimulation over a wide range of frequencies from 2.5 to above the maximally-effective frequencies of 10–20 Hz, but neither muscle layers of the guinea-pig ileum preparation set up in identical conditions was affected by NECA. It has been noted previously that the longitudinal muscle response of the guinea-pig ileum to NECA is apparent at frequencies well below 2.5 Hz. In one study, for example, the EC50 of NECA measured at 0.1 Hz was 11.2 nM, but this concentration did not affect the contraction induced by 10 Hz (Coupar & Hancock, 1994). The neuro-inhibition of the guinea-pig ileum preparation by adenosine agonists is mediated via A1 receptors (Tomaru et al., 1995), but there is a relative lack of information concerning sensitivities of other isolated tissues containing this neural receptor to different frequencies. One study to address this has shown that the pre-junctional action of A1 agonists in reducing field-stimulated noradrenaline release in the rabbit isolated iris/ciliary body preparation is ineffective at 5 Hz and maximally effective at frequencies between 20–30 Hz (Crosson & Gray, 1997).
Receptor characterization
The criteria of antagonist affinities and agonist order of potency were used to verify that the adenosine receptors present in the rat ileum were the same as those previously characterized in the jejunum as the A1 subtype. Hence, CPDPX was selected in this study since it has a 740 fold greater affinity at A1 vs A2 receptors in rat brain membranes (Bruns et al., 1987) and is also selective in functional studies of rat tissues (Haleen et al., 1987). The apparent pA2 value of CPDPX against the non-selective agonist NECA in the rat ileum was shown to be 9.31, which was similar to the value of 9.47 using the same agonist in the rat jejunum peristaltic reflex preparation (Hancock & Coupar 1995b). As in the jejunum, CPDPX also blocked the effect of the A1-selective agonist CPA, but with the additional information that the action was competitive. Hence CPDPX (3.2–100 nM) produced rightward shifts in the CPA concentration-effect curve with a pKb value of 9.15 (95% CI 9.40–8.90) and a Schild slope of 1.03. The corresponding value for the rat jejunum is an apparent pA2 value of 9.37 (95% CI 9.01–9.73) which is not statistically different from the present pKb value (Hancock & Coupar 1995b). It has been shown recently that the binding affinities of antagonists, such as CPDPX, are species-dependent with decreasing affinities for brain membranes prepared from rat>mouse>guinea-pig>human. Consequently, it is relevant to compare the present apparent pA2/pKb values of CPDPX with literature values derived from functional studies of rat tissues containing the A1 receptor subclass. Such comparisons reveal similar values of 9.6 for ileum (Nicholls & Hourani 1997), 9.8 for duodenum (Nicholls et al., 1996), 9.53 for colonic muscularis mucosae (Reeves et al., 1993), 9.8 for brain cortex (von Kugelgen et al., 1994) and 9.44 for heart (Haleen et al., 1987).
Another criterion used in this study to differentiate between A1 and A2 receptors was the relative affinities of CPDPX and 8-PT. A difference is evident in isolated tissues where 8-PT is non-selective for A1 and A2 receptors, whilst CPDPX has a 30–50 fold greater affinity for A1, but is equi-effective with 8-PT at A2 receptors (Collis et al., 1989). In this study, CPDPX was significantly more effective at inhibiting NECA-evoked responses than 8-PT, as revealed by their apparent pA2 values of 9.31 and 7.54 respectively. This value for 8-PT is similar to the apparent pA2 of 7.26 previously reported by us for the rat jejunum (Hancock & Coupar 1995b).
DMPX is also a relatively non-selective antagonist, showing slight preference for A2 vs A1 receptors in both binding (affinity ratios, A1/A2 of 7.5 and 4.1, Daly et al., 1986; Seale et al., 1988) and functional experiments (Ukena et al., 1986; Choi et al., 1988) using rats. The apparent pA2 of DMPX against NECA in the present series of experiments was 5.63 giving an antagonist affinity order of: CPDPX>8-PT>DMPX.
Xanthine antagonists are also useful in discounting the possibility of an A3 adenosine receptor population being present in the rat ileum, because they have extremely low affinity at A3 receptors. For example, the pKi values of CPDPX and 8-PT at A3 sites in rat brain membranes are both <4 (Van Galen et al., 1994).
Agonist potency orders are useful in discriminating the presence of different adenosine receptors in isolated tissues. Hence, agonists have been used in this study to confirm the existence of A1 receptors in the rat ileum as revealed by the use of antagonists. It was shown that the order of potency of agonists in reducing circular muscle contraction in response to transmural stimulation was CPA>NECA>2-CADO>R-PIA>S-PIA>>PAA, which is the same as that previously shown for inhibition of peristalsis, except that the order of 2-CADO and R-PIA are reversed (Hancock & Coupar 1995b). However, although the concentration-effect curves of the agonists were statistically different, their EC50 values were not in the ileum. The order is also similar to that used as a framework by Collis & Hourani (1993) for characterizing adenosine receptor subtypes in functional studies where CPA >R-PIA=CHA=>NECA>2- CADO >S-PIA >PAA indicates the presence of A1 receptors. One discrepancy though, is that the rank orders of CHA and CPA in this study are the reverse of their order in the general framework. Also the position of R-PIA in the rank order is lower in the ileum compared to the order in the A1 classification. However, R-PIA was still more potent than S-PIA in this study as it is at A1, A2A and A2B subtypes of the framework. It is worth noting that the order NECA>R-PIA>S-PIA, as was found in the ileum, has also been obtained in guinea-pig atria, a tissue considered to have A1 receptors (Ford & Broadley, 1997). It is possible that the imperfect correlation between agonist affinity order found in the present study and the framework are due to the general difficulties encountered in using agonists to characterize receptors. Hence, differences in receptor reserve or degree of coupling between tissues leads to different potency values. When the potencies are similar, as is the case in this study, different orders between tissues are more likely to occur.
As a result of this problem, CCPA was included in the present study as it displays high affinity (Ki=0.4 nM) and selectivity (nearly 10,000 fold) for A1 vs A2 receptors in binding studies of rat brain (Lohse 1988; Klotz et al., 1989). As such, the high potency value of CCPA obtained in the present study (EC50=7.18 nM) is concordant with an EC50 value of 8.2 nM in rat atrium, which contains an A1 receptor population (Conti et al., 1993). CHA was also included in the present study, because it is considered to be an A1 agonist with a similar binding and functional profile to CPA in rat CNS tissues (Williams et al., 1986; Maemoto et al., 1997; Nakamura et al., 1997). As expected, the potencies of the two structurally similar agonists were the same in the rat ileum, as indicated by their EC50 values.
Functional relevance
It has been suggested by Tomaru et al. (1994) and Suzuki et al. (1995) that endogenous adenosine has a physiological role in the digestive tract partly on the basis that CPDPX increased defecation in conscious rats. It was further suggested that the colon was the site of adenosine release since CPDPX and another A1 antagonist, KF20274, did not affect gastric emptying or small intestinal propulsion (Suzuki et al., 1995). Indeed, our previous results led us to conclude that endogenous adenosine is not released in the rat jejunum to inhibit either peristalsis or fluid secretion (Hancock & Coupar, 1995a,1995b). Nevertheless, the identification of A1 receptors on cholinergic neurones innervating the circular muscle of the rat ileum and the high potency of A1 agonists as identified in this study raises the possibility that adenosine is involved in decreasing intestinal motility during high metabolic demand and/or in certain pathological conditions.
References
- ALEXANDER S., PETERS J. Receptor & ion channel nomenclature supplement. Trends Pharmacol. Sci. 1998;9:7–8. [Google Scholar]
- BAILEY S.J., HICKMAN D., HOURANI S.M.O. Characterisation of the P1-purinoreceptors mediating contraction of the rat colon muscularis mucosae. Br. J. Pharmacol. 1992;105:400–404. doi: 10.1111/j.1476-5381.1992.tb14265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY S.J., HOURANI S.M.O. Effects of purines on the longitudinal muscle of the rat colon. Br. J. Pharmacol. 1992;105:885–892. doi: 10.1111/j.1476-5381.1992.tb09073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNS R.F., FERGUS J.H., BADGER E.W., BRISTOL J.A., SANTAY L.A., HARTMAN J.D., HAYS S.J., HUANG C.C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedeberg's Arch. Pharmacol. 1987;335:59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.A basis for distinguishing two types of purinergic receptor Cell Membrane Receptors for Drugs and Hormones 1978New York: Raven Press; 107–118.eds. Bolis L & Straub, R.W. [Google Scholar]
- CHOI O.H., SHAMIN M.T., PADGETT W.L., DALY J.W. Caffeine and theophylline analogues: correlation of behavioural effects with activity as adenosine receptor antagonists and as phosphodiesterase inhibitors. Life. Sci. 1988;43:387–398. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- COLLIER H.O.J., TUCKER J.F. Novel form of drug dependence on adenosine in the guinea-pig ileum. Nature. 1983;302:618–621. doi: 10.1038/302618a0. [DOI] [PubMed] [Google Scholar]
- COLLIS M.G., HOURANI S.M.O. Adenosine receptor subtypes. Trends Pharmacol. Sci. 1993;14:360–366. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- COLLIS M.G., STOGGALL S.M., MARTIN F.M. Apparent affinity of 1,3-dipropyl-8-cyclopentyladenosine for adenosine A1 and A2 receptors in isolated tissues from guinea-pigs. Br. J. Pharmacol. 1989;97:1274–1278. doi: 10.1111/j.1476-5381.1989.tb12589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI A., MONOPOLI A., GAMBA M., BOREA P.A., ONGINI E. Effects of selective A1 and A2 adenosine receptor agonists on cardiovascular tissues. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:108–112. doi: 10.1007/BF00168545. [DOI] [PubMed] [Google Scholar]
- COUPAR I.M., HANCOCK D.L. The adenosine agonist NECA inhibits intestinal secretion and peristalis. J. Pharm. Pharmacol. 1994;46:801–804. doi: 10.1111/j.2042-7158.1994.tb03733.x. [DOI] [PubMed] [Google Scholar]
- COUPAR I.M., LIU L. A simple method for measuring the effects of drugs on intestinal longitudinal and circular muscle. J. Pharmacol. Toxicol. Methods. 1996;36:147–154. doi: 10.1016/s1056-8719(96)00110-4. [DOI] [PubMed] [Google Scholar]
- CROSSON C.E., GRAY T. Response to prejunctional adenosine receptors is dependent on stimulus frequency. Curr. Eye Res. 1997;16:359–364. doi: 10.1076/ceyr.16.4.359.10690. [DOI] [PubMed] [Google Scholar]
- DALY J.W., PADGETT W.L., SHAMIM M.T. Analogues of caffeine and theophylline: effect of structural alterations on affinity at adenosine receptors. J. Med. Chem. 1986;25:1305–1308. doi: 10.1021/jm00157a035. [DOI] [PubMed] [Google Scholar]
- DIONYSSOPOULOS T., HOPE W., COUPAR I.M. Effect of adenosine analogues on the expression of opiate withdrawal in rats. Pharmacol. Biochem. Behav. 1992;42:201–206. doi: 10.1016/0091-3057(92)90516-i. [DOI] [PubMed] [Google Scholar]
- FORD W.R., BROADLEY K.J. Functional classification of P1-purinoceptors in guinea-pig left and right atria: anomalous characteristics of antagonism by cyclopentyltheophylline. Naunyn Schmiedeberg's Arch. Pharmacol. 1997;355:759–766. doi: 10.1007/pl00005010. [DOI] [PubMed] [Google Scholar]
- GINTZLER A.R., MUSACCHIO J.M. Interactions of morphine, adenosine, adenosine triphosphate and phosphodiesterase inhibitors on the field-stimulated guinea-pig ileum. J. Pharmacol. Exp. Ther. 1975;194:575–582. [PubMed] [Google Scholar]
- GUSTAFSSON L.E., WIKLUND N.P., LUNDIN J., HEDQVIST P. Characterization of pre- and post-junctional adenosine receptors in the guinea-pig ileum. Acta Physiol. Scand. 1985;123:195–203. doi: 10.1111/j.1748-1716.1985.tb07578.x. [DOI] [PubMed] [Google Scholar]
- HALEEN S.J., STEFFEN R.P., HAMILTON H.W. PD116,948, a highly selective A1-adenosine receptor antagonist. Life Sci. 1987;40:555–561. doi: 10.1016/0024-3205(87)90369-9. [DOI] [PubMed] [Google Scholar]
- HANCOCK D.L., COUPAR I.M. Functional characterisation of the adenosine receptor mediating inhibition of intestinal secretion. Br. J. Pharmacol. 1995a;114:152–156. doi: 10.1111/j.1476-5381.1995.tb14919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK D.L., COUPAR I.M. Functional characterisation of the adenosine receptor mediating inhibition of peristalsis in the rat jejunum. Br. J. Pharmacol. 1995b;114:739–744. doi: 10.1111/j.1476-5381.1995.tb14995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI E., MORI M., YAMADA S., KUNITOMO M. Effects of purine compounds on cholinergic nerves. Specificity of adenosine and related compounds on acetylcholine release in electrically stimulated guinea-pig ileum. Eur. J. Pharmacol. 1978;48:297–307. doi: 10.1016/0014-2999(78)90088-2. [DOI] [PubMed] [Google Scholar]
- KLOTZ K-N., LOHSE M.J., SCHWABE U., CRISTALLI G., VITTORI S., GRIFANTINI M. 2-Chloro-N6-[3H]cyclopentyladenosine ([3H]CCPA)–a high affinity agonist radioligand for A1 adenosine receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1989;340:679–683. doi: 10.1007/BF00717744. [DOI] [PubMed] [Google Scholar]
- LEW M.J. Extended concentration-response curves used to reflect full agonist efficacies and receptor occupancy-response coupling ranges. Br. J. Pharmacol. 1995;115:745–749. doi: 10.1111/j.1476-5381.1995.tb14996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEW M.J., ANGUS J.A. Analysis of competitive agonist-antagonist interactions by nonlinear regression. Trends Pharmacol. Sci. 1995;16:328–37. doi: 10.1016/s0165-6147(00)89066-5. [DOI] [PubMed] [Google Scholar]
- LIU L., COUPAR I.M. Characterisation of pre- and post-synaptic α-adrenoceptors in modulation of the rat ileum longitudinal and circular muscle activities. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:248–256. doi: 10.1007/pl00005048. [DOI] [PubMed] [Google Scholar]
- LOHSE M.J., KLOTZ K.-N., SCHWABE U., CRISTALLI G., VITTORI S., GRIFANTINI M. 2-Chloro-N6-cyclopentyladenosine: A highly selective agonist at A1 adenosine receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1988;337:687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- MAEMOTO T., FINLAYSON K., OLVERMAN A., KAHANE A., HORTON R.W., BUTCHER S.P. Species differences in brain adenosine A1 receptor pharmacology revealed by the use of xanthine and pyrazolopyridine based antagonists. Br. J. Pharmacol. 1997;122:1202–1208. doi: 10.1038/sj.bjp.0701465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA I., OHTA Y., KEMMOTSU O. Characterisation of adenosine receptors mediating spinal sensory transmission related to nociceptive information in the rat. Anesthiology. 1997;87:577–584. doi: 10.1097/00000542-199709000-00018. [DOI] [PubMed] [Google Scholar]
- NICHOLLS J., BROWNHILL V.R., HOURANI S.M.O. Characterisation of P1-purinoceptors on rat isolated duodenum longitudinal muscle and muscularis mucosae. Br. J. Pharmacol. 1996;117:170–174. doi: 10.1111/j.1476-5381.1996.tb15170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLLS J., HOURANI S.M.O. Characterisation of adenosine receptors on rat ileum, ileal longitudinal muscle and muscularis mucosae. Eur. J. Pharmacol. 1997;338:143–150. doi: 10.1016/s0014-2999(97)81942-5. [DOI] [PubMed] [Google Scholar]
- PALMER T.M., STILES G.L. Review: Neurotransmitter Receptors VII–Adenosine Receptors. Neuropharmacology. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- PEACHEY J.A., HOURANI S.M.O., KITCHEN I. The binding of 1,3-[3H]-dipropyl-8-cyclopentylxanthine to adenosine A1-receptors in rat smooth muscle preparations. Br. J. Pharmacol. 1994;113:1249–1256. doi: 10.1111/j.1476-5381.1994.tb17132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES J.J., COATES J., JARVIS J.E., SHEEHAM M.J., STRONG P. Characterisation of the adenosine receptor mediating contraction in rat colonic muscularis mucosae. Br. J. Pharmacol. 1993;110:1255–1259. doi: 10.1111/j.1476-5381.1993.tb13950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYNOCK J., JHAMANDAS K.H. Inhibition of acetylcholine release from cholinergic nerves by adenosine, adenine nucleotides and morphine: antagonism by theophylline. J. Pharmacol. Exp. Ther. 1976;197:379–390. [PubMed] [Google Scholar]
- SEALE T.W., ABLA K.A., SHAMIM M.T., CARNEY J.M., DALY J.W. 3,7-dimethyl-1-proargylxanthine: a potent and selective in vivo antagonist of adenosine analogs. Life Sci. 1988;43:1671–1684. doi: 10.1016/0024-3205(88)90478-x. [DOI] [PubMed] [Google Scholar]
- SMALL R.C., WESTON A.H. Theophylline antagonizes some effects of purines in the intestine but not those of intramural inhibitory nerve stimulation. Br. J. Pharmacol. 1979;67:301–308. doi: 10.1111/j.1476-5381.1979.tb08680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI M., TOMARU A., KISHIBAYASHI N., KARASAWA A. Effects of the adenosine A1-receptor antagonist on defecation, small intestinal propulsion and gastric emptying in rats. Jap. J. Pharmacol. 1995;68:119–123. doi: 10.1254/jjp.68.119. [DOI] [PubMed] [Google Scholar]
- TOMARU A., INA Y., KISHIBAYASHI N., KARASAWA A. Excitation and inhibition via adenosine receptors of the twitch responses to electrical stimulation in isolated guinea-pig ileum. Jpn. J. Pharmacol. 1995;69:429–433. doi: 10.1254/jjp.69.429. [DOI] [PubMed] [Google Scholar]
- TOMARU A., ISHII A., KISHIBAYASHI N., SHIMADA J., SUZAKI F., KARASAWA A. Possible physiological role of endogenous adenosine in defecation in rats. Eur. J. Pharmacol. 1994;264:91–94. doi: 10.1016/0014-2999(94)90641-6. [DOI] [PubMed] [Google Scholar]
- UKENA D., SHAMIM M.T., PADGETT W., DALY J.W. Analogs of caffeine: antagonists with selectivity for A2 adenosine receptors. Life Sci. 1986;39:743–750. doi: 10.1016/0024-3205(86)90023-8. [DOI] [PubMed] [Google Scholar]
- VAN GALEN P.J.M., VAN BERGEN A.H., GALLO-RODRIGUEZ C., MELMAN N., OLAM M., IJZERMAN A.P., STILES G.L., JACOBSON K.A. A binding site model and structure activity relationships for the rat A3-adenosine receptor. Mol. Pharmacol. 1994;45:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- VON KUGELGEN I., SPATH L., STARKE K. Evidence for P2-purinoceptor-mediated inhibition of noradrenaline release in rat brain cortex. Br. J. Pharmacol. 1994;113:815–822. doi: 10.1111/j.1476-5381.1994.tb17066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE T.D.Role of ATP and adenosine in the autonomic nervous system Novel Peripheral Neurotransmitters. Sect. 135 of Int. Encycl. Pharmacol. Ther. 1991New York: Pergamon Press; 9–64.ed. Bell, C. pp [Google Scholar]
- WILLIAMS M., BRAUNWALDER A., ERICKSON T.J. Evaluation of the binding of the A-1 selective adenosine radioligand, cyclopentyladenosine (CPA), to rat brain tissue. Naunyn Schmiedeberg's Arch. Pharmacol. 1986;332:179–183. doi: 10.1007/BF00511410. [DOI] [PubMed] [Google Scholar]


