Abstract
The class Ic anti-arrhythmic agent, flecainide is known to inhibit the transient outward K current (Ito) selectively in human atrium. We studied the effects of propafenone, another class Ic anti-arrhythmic agent, on K currents in human atrial myocytes using a whole-cell voltage-clamp method.
Propafenone inhibited both Ito and the sustained or ultra-rapid delayed rectifier K current (Isus or Ikur) evoked by depolarization pulses. The concentration for half-maximal inhibition (IC50) was 4.9 μM for Ito and 8.6 μM for Isus. Propafenone blocked Ito and Isus in a voltage- and use-independent fashion and accelerated the inactivation time constant of Ito [from 28.3 to 6.7 ms at 10 μM propafenone].
The steady-state inactivation curve for Ito was unaffected by propafenone. Propafenone did not affect the initial current at depolarizing potentials, but it did produce a block that increased as a function of time after depolarization (time constant of 3.4 ms). This suggests that propafenone preferentially blocked Ito in the open state.
Propafenone had no significant effect on the rate at which Ito recovered from inactivation at −80 mV suggesting that propafenone dissociates rapidly from the channel.
The steady-state activation curve for Isus was not affected by propafenone. Propafenone slowed the time course of the onset of the Isus tail current. This suggests that propafenone blocked Isus in the open state.
The present results suggest that, unlike flecainide, propafenone blocks both Ito and Isus in human atrial myocytes in the open state at clinically relevant concentrations.
Keywords: Human atrial cells; K+ channels, anti-arrhythmic drugs; propafenone; whole-cell voltage-clamp method
Introduction
Wang et al. (1993b) reported that the transient outward current (Ito) and the sustained or ultra-rapid delayed rectifier K current (Isus or Ikur) contribute significantly to action potential duration in the human atrium. In fact, the activation and inactivation of Ito are both rapid, and thus it contributes to the phase 1 repolarization of the atrial action potential. On the other hand, Ikur or Isus activates very rapidly but inactivates slowly, and it contributes to the whole repolariz-ation phase of the atrial action potential.
The class Ic antiarrhythmic agents flecainide and propafenone are widely used for the conversion and treatment of atrial tachyarrhythmia (Harrison, 1985). According to Kosmala et al. (1994), Botto et al. (1994), Capucci et al. (1994) and Chimienti et al. (1996), these two drugs are equally effective at converting atrial fibrillation. Further, Stroobandt et al. (1997) found that low-dose propafenone was well tolerated and that it was effective in maintaining sinus rhythm in atrial fibrillation (af) patients for 6 months after pharmacological or electrical restoration of sinus rhythm. As mentioned above, these anti-arrhythmic drugs have been shown to be clinically useful in cases of atrial tachyarrhythmia; however, relatively few studies have evaluated the effects of these agents on membrane currents in the human atrium. Wang et al. (1995) have demonstrated that flecainide at clinically relevant concentrations inhibits Ito without affecting Isus in the human atrium, but the effects of propafenone on K currents in human atrial cells have not been properly evaluated. Although flecainide and propafenone both belong to the same class of drugs, we cannot take for granted that they have the same effect on voltage-gated K channels in the human atrium. The present study was designed to study the effects of propafenone on K currents in human atrial cells using the whole-cell patch-clamp method.
Methods
Preparation of single cells
The cell-isolation procedure for human atrial myocytes was derived from a technique described previously (Escande et al., 1987; Sakai et al., 1995; Kajimoto et al., 1997). Small segments of myocardium sampled from right atrial appendages were obtained at the time of open-heart surgery, in accordance with institutional guidelines governing research on human subjects. Each patient had a normal right atrial pressure and a normal sinus rhythm and was between 1 and 56 years of age. The clinical diagnosis was congenital heart disease in 35 patients and valvular disease in five patients. In brief, each segment of human myocardium was cut into pieces and incubated in warm Tyrode's solution for 10 min. The strips were then cut into even smaller pieces and left in nominally Ca2+-free Tyrode's solution for 17 min. They were then transferred to nominally Ca2+-free Tyrode's solution containing 0.8 mg ml−1 collagenase (Yakult, Tokyo, Japan) with 1 mg ml−1 protease (Type XX VII, Sigma Chemical Co, St. Louis, MO, U.S.A.) for 70–80 min at 37°C. The collagenase was then washed out by rinsing with high-K+, low Cl− solution (Isenberg & Klöckner, 1982) and the digested tissue was stored in the same solution.
Electrical recordings
The whole-cell voltage-clamp method used was one described previously (Hamill et al., 1981; Hagiwara et al., 1992a,1992b; Matsuda et al., 1996; Sakai et al., 1996; Kajimoto et al., 1997; Shoda et al., 1997). Patch pipettes were pulled from 1.25 mm borosilicate capillaries (D941-8.5-85 Clinitubes: Radiometer A/S, Copenhagen, Denmark). The resistance of each electrode, when filled with the pipette solution, was within the range 2–3 MΩ. The amplifier (TM-1000, ACT ME Laboratory, Tokyo, Japan) employed a 100 MΩ feedback resistor, and series resistance was partially compensated. The current-voltage (I-V) signals were stored on a video recorder (S-6000, Victor, Tokyo, Japan) via a PCM converter system (RP-880, NF Electronic Instruments, Tokyo, Japan) being used for computer analysis (PC 9801 RA, NEC, Tokyo, Japan). The current signals were fed from the video recorder to the computer via a 2.5 kHz, 8 pole Bessel-type low-pass filter. No leakage correction was applied. From a holding potential of −40 mV, ramp pulses of amplitude ±90 mV (0.72 V/sec) were applied to enable measurement of cell membrane capacitance (Hagiwara et al., 1992a). The value so obtained was 72.6±4.4 pF (n=33). In accordance with the previous reports (Wang et al., 1993b, 1995), we used a double-pulse protocol to separate Ito and Isus (see legend to Figure 2), however, the Isus derived by the second pulse in double-pulse protocol did not differ from that at the end of the first 200 ms pulse. Therefore, we measured Isus as the amplitude of the current at the end of the first or second pulse relative to the zero current level. We have measured the amplitude of Ito as the difference between the peak current and the sustained current at the end of the pulse to minimize the contamination of Isus. Experiments were performed at room temperature.
Figure 2.
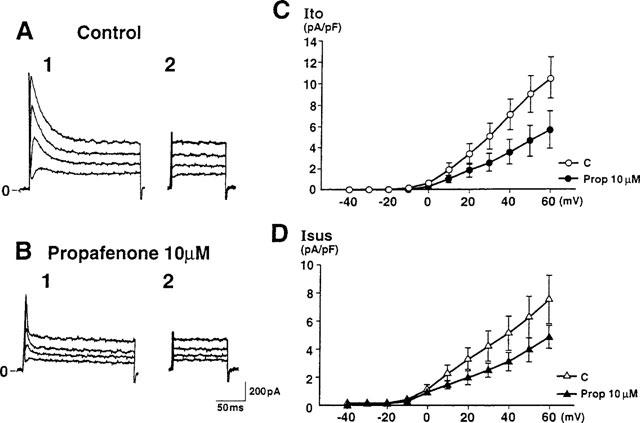
Effect of propafenone on Ito and Isus in a human atrial cell. Current traces were obtained in the absence (A) or presence (B) of 10 μM propafenone. Ito was elicited by 200 ms depolarizing pulses from a holding potential of −50 mV to 0, +20, +40 and +60 mV (A1 and B1). Isus was elicited by 100 ms depolarizing pulses from the same holding potential 10 ms after a 200 ms prepulse to +60 mV had been delivered to inactivate Ito (A2 and B2). (C and D) Current-voltage (I-V) relationships for Ito and Isus (n=8) before and after application of 10 μM propafenone showing that both Ito and Isus were reduced by propafenone at every membrane potential examined. Currents were normalized with respect to cell capacitance. Current densities of Ito and Isus were 10.39±1.94 pA/pF and 7.47±1.73 pA/pF, respectively, at +60 mV under control conditions, while the corresponding values after application of propafenone were 5.57±1.81 pA/pF and 4.83±0.87 pA/pF, respectively.
Solutions
The normal Tyrode's solution contained (in mM): NaCl 136.9, KCl 5.4, CaCl2 1.8, NaH2PO4 0.33, glucose 5 and HEPES 5 (pH adjusted to 7.4 with NaOH). The standard pipette solution contained (in mM): KOH 110, KCl 20, EGTA 10, MgCl2 2, K2-ATP 5, K2-creatine phosphate 5 and HEPES 5 (pH=7.4 with aspartic acid of approximately 60 mM). The standard external solution contained (in mM): N-methyl-D-glucamine (NMG) 150, KCl 5.4 and HEPES pH=7.4 with HCl) 5; CdCl2 0.2 and BaCl2 1 were added to eliminate contamination by Ca currents, inward rectifying K currents and delayed rectifying K currents (Wang et al., 1995).
Drugs
Propafenone HCl was kindly provided by Yamanouchi Pharmaceuticals (Tokyo, Japan) and dissolved in dimethyl sulphoxide (DMSO) as a 250 mM stock solution. The final concentration of DMSO did not exceed 0.1%; this concentration was found to have no effect on the control membrane currents.
Statistical Analysis
Paired and unpaired Student's t-tests were used to evaluate the statistical significance of differences between means. Values of P<0.05 were considered to indicate statistical significance. All statistical data are given as mean±s.e.mean.
Results
Effects of propafenone on Ito and Isus
To evaluate the effects of propafenone on the transient outward current (Ito) and the sustained delayed rectifier K current (Isus) in human atrial cells, we used an external solution containing NMG, Cd2+ and Ba2+ in order to block the Na current, Ca current and other K currents (IK1 and IK; Wang et al., 1995). Figure 1 illustrates the effects of propafenone on K currents in a human atrial cell. The current traces elicited by a 300 ms depolarization pulse from a holding potential of −50 mV to +60 mV exhibited Ito followed by a sustained outward current (A1). Ten μM propafenone inhibited both Ito and the sustained outward current (by 52.3 and 51.8%, respectively, in the cell illustrated) (A2). Inhibition of these currents by propafenone was completely reversed after a 6-min wash-out (A3).
Figure 1.
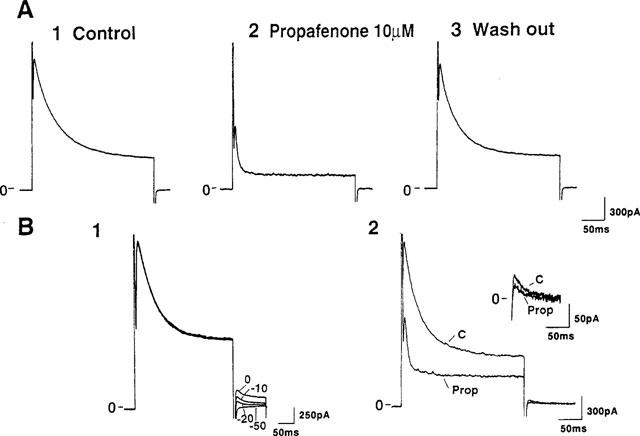
Effects of propafenone on K currents in a human atrial cell. (A) Current traces elicited by a 300 ms depolarization pulse from a holding potential of −50 mV to +60 mV exhibited Ito followed by a sustained outward current. Current traces were obtained under control conditions (A1), in the presence of 10 μM propafenone (A2) and after a 6-min wash-out (A3). (B) The tail current of the sustained outward current. Tail currents were obtained at −50, −20, −10 and 0 mV after 300 ms depolarizing pulses from a holding potential of −50 mV to +60 mV (B1). Ten μM propafenone inhibited both the sustained current and the tail current obtained at −20 mV (B2). Inset shows (at a greater gain) the change in the tail current determined in the same cell.
To determine whether the sustained outward current observed in Figure 1A is indeed the Isus previously observed in the human atrium, we measured the tail currents of this sustained outward current. Figure 1B1 shows the tail currents obtained at −50, −20, −10 and 0 mV. The amplitude of the tail current was largest at 0 mV and it became progressively smaller as greater and greater negative potentials were used. Ten μM propafenone inhibited both the sustained current and the tail current obtained at −20 mV (B2) and these currents were completely blocked by 1 mM 4-AP (results not illustrated). These results indicated that the sustained outward current observed in Figure 1 is indeed the Isus previously reported in human atrial myocytes (Wang et al., 1993b).
Figure 2 shows current traces obtained in the absence and presence of 10 μM propafenone (A and B). Current traces elicited by 200 ms depolarizing pulses from a holding potential of −50 mV to various depolarizing potentials (A1 and B1) exhibited Ito followed by Isus (A2 and B2). Since reactivation of Ito is known to occur at around 50–60 ms (Wang et al., 1993b), we used a double-pulse protocol (see legend to Figure 2) to separate Ito and Isus. Current-voltage relationships for Ito and Isus are shown in Figure 2C and D, respectively. Ito and Isus were both reduced by 10 μM propafenone at all membrane potentials examined suggesting that, whereas flecainide only blocks Ito (Wang et al., 1995), propafenone inhibits both Ito and Isus in human atrial cells.
Concentration-dependence of inhibition of Ito and Isus
Dose-response relationships for the inhibition of Ito and Isus by propafenone are illustrated in Figure 3. In the presence of propafenone, current amplitude was reduced and the inactivation of Ito was accelerated, both effects occurring in a dose-dependent manner. Propafenone blocked Ito and Isus with IC50 values of 4.9 μM and 8.6 μM, respectively.
Figure 3.
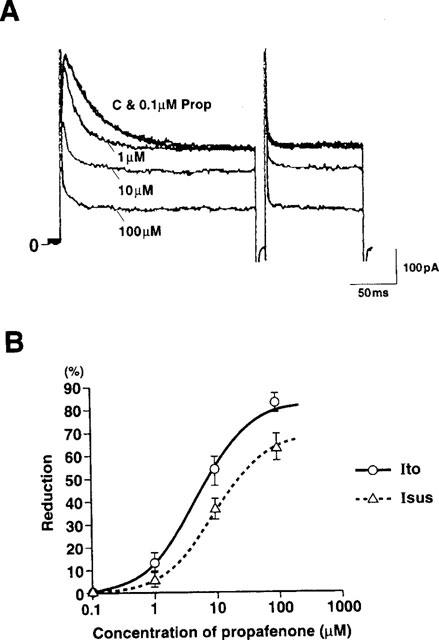
Concentration-dependence of inhibitory effect of propafenone on Ito and Isus. (A) Tracings showing the effects of 0.1, 1, 10 and 100 μM propafenone. Ito was elicited by 200 ms depolarizing pulses from a holding potential of −50 mV to +60 mV. Isus was elicited as in Figure 2. (B) Dose-response relationships for the effects of propafenone on Ito and Isus. Data points show the percentage reduction in Ito and Isus induced by propafenone. Ito and Isus were not affected by 0.1 μM propafenone. Mean reduction in Ito was by 14.2±3.8, 53.7±6.1 and 83.1±4.1% (n=14) of control and that in Isus was by 7.3±2.7, 38.0±4.4 and 63.8±5.7% (n=12) of control at 1, 10 and 100 μM propafenone, respectively. Lines were derived by a nonlinear least-squares fit of the values for the mean percentage reduction to the Michaelis equation: percentage reduction=Emax (propafenone)/[(propafenone)+IC50]×100. Ordinate shows percentage reduction in Ito and Isus. The values of Emax and IC50 obtained from these curves were 83.3% and 4.9 μM, respectively (for Ito) and 70.1% and 8.6 μM, respectively (for Isus).
Voltage-dependence of effects of propafenone on Ito and Isus
To examine the voltage-dependence of the effects of propafenone on these K currents, the relationships between the amplitude of the test potential and the reduction in Ito and Isus induced by 10 μM propafenone were analysed (Figure 4). For a given current, the reduction induced by 10 μM propafenone was much the same regardless of the test potential, indicating that propafenone blocked Ito and Isus in a voltage-independent fashion.
Figure 4.
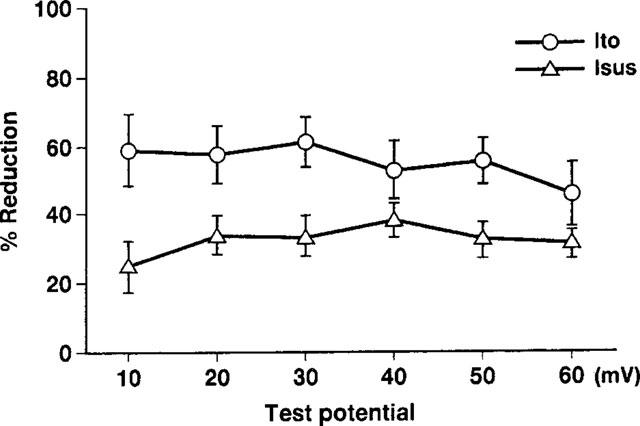
Dependence of drug effects of propafenone on test potential. Currents were elicited using the same experimental protocol as in Figure 2. The values for the mean percentage reductions in Ito and Isus induced by 10 μM propafenone are plotted against the corresponding test potential. No significant voltage-dependence was observed for the blocks induced by propafenone (n=4 for Ito, n=6 for Isus).
Use-dependence of effects of propafenone on Ito and Isus
The actions of anti-arrhythmic drugs are known to be state-dependent, resulting in effects that depend on both the voltage and frequency of depolarization. To determine the use-dependency of the effect of propafenone on Ito and Isus, a train of depolarizing pulses to +60 mV from a holding potential of −80 mV was delivered at 2 Hz once the cells had fully equilibrated following application of 10 μM propafenone. Under control conditions, Ito was not altered by repetitive pulsing at 2 Hz (Figure 5A1). Figure 5A2 shows representative current traces evoked by a 2 Hz train of constant amplitude stimuli (to +60 mV) in the presence of 10 μM propafenone, and in Figure 5B, the percentage inhibition of each current is plotted against pulse number. The currents did not alter in the course of a train of stimuli. Similarly, the currents did not alter in the course of a 1 Hz train of stimuli from −80 mV (holding potential) to +60 mV or +30 mV (results not illustrated). These results indicated that propafenone blocked Ito and Isus each in a use-independent manner, and it produced tonic block of these currents.
Figure 5.
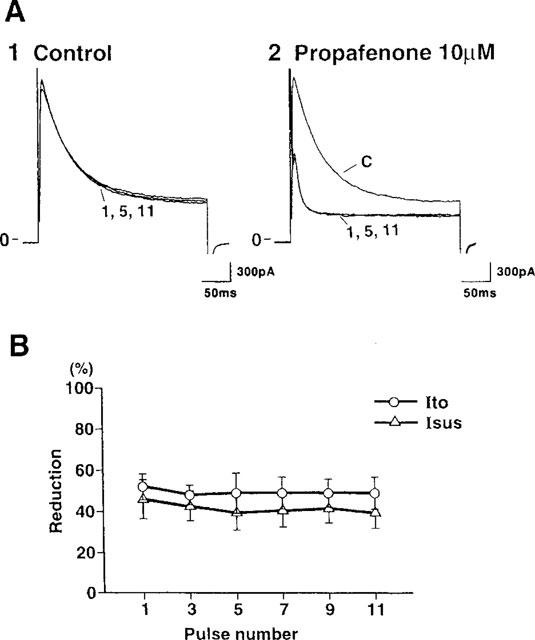
Use-dependence of effects of propafenone on Ito and Isus. A train of 300 ms depolarizing pulses was applied at 2 Hz from a holding potential of −80 mV to +60 mV under control conditions (A1) and 2 min after superfusion of the cell with 10 μM propafenone (A2). Current traces were obtained from the control, 1st, 5th and 11th pulses. (B) The mean percentage reduction in Ito and Isus induced by 10 μM propafenone when a train of 2 Hz stimuli was used (n=3) is plotted against the appropriate pulse number. No significant interaction was observed between pulse number and drug effect.
Changes in the kinetic properties of Ito induced by propafenone
To evaluate whether propafenone affects the kinetic properties of Ito, we also analysed the time course of inactivation and the steady-state inactivation curve in both the absence and presence of 10 μM propafenone (Figures 6 and 7). After exposure to 10 μM propafenone, the inactivation of Ito was clearly accelerated. Under control conditions, the inactivation of Ito was well fitted by a single exponential relation with a time constant of 28.3±2.2 ms at +60 mV (τ(C), n=10). After addition of 10 μM propafenone, τ (Prop) was 6.7±0.6 ms (n=10). In fact, the time course of inactivation was accelerated by propafenone in a dose-dependent manner, the time constants being 23.1±2.8 (n=6) and 6.6±0.8 ms (n=5), respectively, with 1 and 100 μM propafenone.
Figure 6.
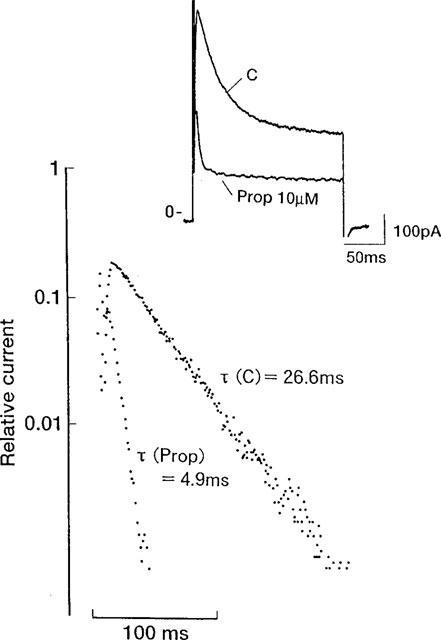
Change induced by propafenone in the inactivation time course of Ito. The graph shows semilogarhithmic plots of Ito. The inactivation of Ito was well fitted by a single exponential relation with a time constant of 26.6 ms (τ (C)) under control conditions. After the addition of 10 μM propafenone, τ (Prop) was 4.9 ms. Inset shows current traces before and after addition of 10 μM propafenone. Basic experimental protocol was as in Figure 3.
Figure 7.
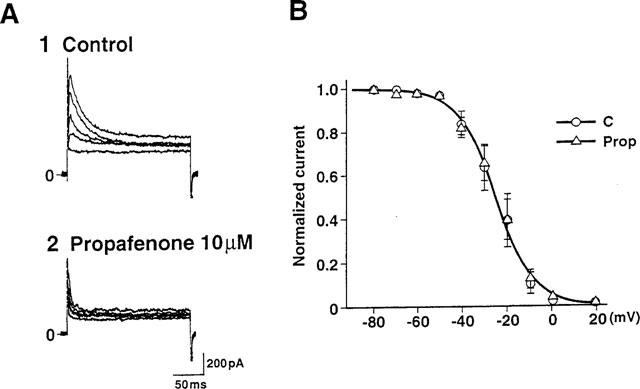
Effects of propafenone on the voltage-dependence of the steady-state inactivation curve for Ito. Steady-state inactivation curves were obtained in both the absence (n=14) and presence of 10 μM propafenone (n=9). (A) Superimposed current tracings elicited by depolarizing pulses to +60 mV from various holding potentials between −80 mV and +20 mV before (A1) and after (A2) application of 10 μM propafenone. (B) Steady-state inactivation curves for Ito before and after addition of 10 μM propafenone.
Next, we examined the effect of propafenone on the steady-state inactivation curve for Ito (Figure 7). The membrane potential was held at various levels between −80 mV and +20 mV and depolarizing test pulses to +60 mV were applied. The peak amplitudes of Ito were normalized, plotted against the membrane potential, and fitted to a Boltzmann equation as follows:
where y∞ is the inactivation parameter, Vm is the membrane potential, V0.5 is the potential required to give a half value, and s is the slope factor. The slope factor under control conditions was 8.2 mV and V0.5 was −25.3 mV. The corresponding values were 8.5 mV and −24.9 mV, respectively, in the presence of 10 μM propafenone. These results indicated that propafenone did not modify the voltage-dependence of the steady-state inactivation of Ito.
The voltage-dependent activation of Ito was also analysed by examining the tail currents obtained on repolarization to −20 mV after a 5 ms pulse to test potentials between −40 and +80 mV. These data showed that the slope factor under control conditions was 8.6 mV and that V0.5 was 13.5 mV. The corresponding values were 9.1 mV and 13.9 mV, respectively, in the presence of 10 μM propafenone (results not illustrated), suggesting that propafenone did not alter the voltage-dependence of the steady-state activation of Ito.
Changes in the kinetic properties of Isus induced by propafenone
We also examined the effect of propafenone on the activation kinetics for Isus (Figure 8). The steady-state activation curve of Isus was constructed from the tail current measurements at −20 mV (see legend to Figure 8). These data were well described by a single Boltzmann equation. The slope factor under control conditions was 6.1 mV and V0.5 was 4.8 mV. The corresponding values were 6.1 mV and 3.1 mV, respectively, in the presence of 10 μM propafenone, indicating that propafenone did not modify the voltage-dependence of the steady-state activation of Isus.
Figure 8.
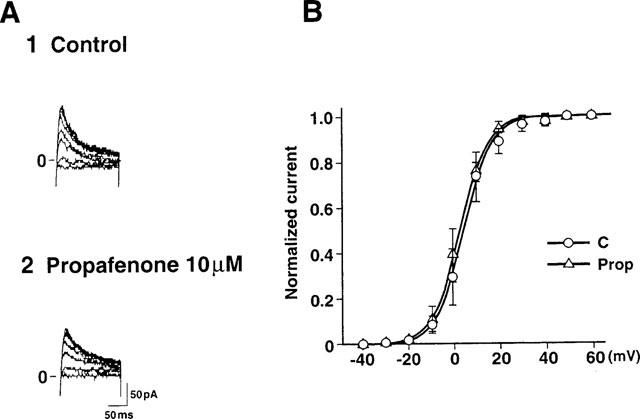
Effects of propafenone on the voltage-dependence of the steady-state activation curve for Isus. (A) Tail currents were obtained at −20 mV after a 300 ms depolarization pulse to between 0 and +60 mV from a holding potential of −50 mV before (A1) and after (A2) application of 10 μM propafenone. (B) Steady-state activation curves for Isus before (n=6) and after addition of 10 μM propafenone (n=6).
Time-dependence of inhibition of Ito by propafenone
An acceleration of the inactivation of Ito is known to result from a rapid blocking of open channels (Dukes et al., 1990). To determine whether this occurred with propafenone, we plotted the degree of propafenone-induced Ito blockade against the time elapsed after the onset of the depolarizing clamp pulse (Figure 9). In the presence of propafenone, the initial current at depolarizing potentials did not differ from that obtained under control conditions. However, as shown in Figure 9, the current gradually declined with time after depolarization, indicating that propafenone blocked Ito in an open state. The time constants obtained for the onset of the block were averaged for three experiments giving a value of 3.4±0.4 ms at 10 μM propafenone. This value was of the same order as that obtained with 5 μM flecainide (6.3±1.2 ms), an agent known to reduce Ito by producing a rapid open-channel block in the human atrium (Wang et al., 1995). In addition, these values were of the same order as the inactivation time constant (6.7±0.6 ms) obtained with 10 μM propafenone (Figure 6), supporting the conclusion that the acceleration of the inactivation of Ito induced by propafenone was due to an open-channel block.
Figure 9.
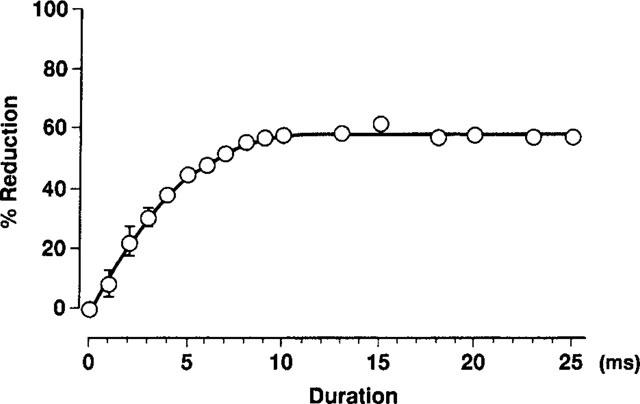
Time-dependence of inhibition of Ito by propafenone. Propafenone-induced Ito blockade is plotted against the time elapsed after the onset of the depolarizing pulse. The time constant for the onset of the block was averaged for three experiments: the value so obtained was 3.4±0.4 ms at 10 μM propafenone. The curve was derived by a least-squares fit to the equation: B=Bmax [1-exp (−kt)], where B is the current block (percentage reduction in Ito), Bmax is the maximum reduction produced by 10 μM propafenone (found to be 58%), k is the rate constant and t is pulse duration.
Effects of propafenone on the reactivation of Ito
The recovery of Ito from inactivation in the presence of drug reflects the dissociation rate constant of the drug from the Ito channel. A rapid dissociation of propafenone from its binding site was suggested by the observed absence of a use-dependent block of Ito (Figure 5). To elucidate the time-dependence of the cessation of the propafenone block that occurred on returning to the holding potential, we measured the reactivation of Ito both in the absence and presence of propafenone. Figure 10A shows current traces obtained by applying 200 ms (P1) and 100 ms (P2) depolarizing pulses from a holding potential of −80 mV to +60 mV when the P1-P2 interval was varied between 10 and 300 ms. The curves derived from such data revealed no significant difference in the reactivation of Ito whether 10 μM propafenone was or was not present (35.3±6.8 ms and 26.4±5.6 ms, n=6, C & Prop, Figure 8B). Similarly, the reactivation of Ito was not significantly altered by propafenone at a holding potential of −50 mV (54.6±8.4 ms and 68.4±16.7 ms, n=7, C & Prop). This result, suggesting that propafenone dissociates rapidly from the Ito channel, is consistent with the lack of use-dependency reported above.
Figure 10.
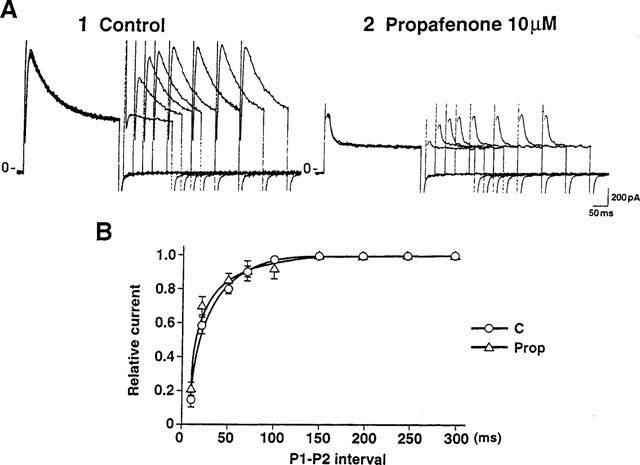
Effects of propafenone on reactivation of Ito. (A) Current traces before (1) and after (2) application of 10 μM propafenone obtained using 200 ms (P1) and 100 ms (P2) depolarizing pulses to +60 mV from a holding potential of −80 mV. The P1-P2 interval was varied between 10 and 300 ms. (B) The normalized currents (P2/P1) obtained in the absence or presence of 10 μM propafenone were plotted as a function of the P1-P2 interval (n=6). This process is well described by a single exponential with a time constant of 35.3±6.8 ms (control) or 26.4±5.6 ms (Prop). No significant change in the reactivation of Ito was seen following the application of 10 μM propafenone.
Discussion
As mentioned in Introduction, Ito and Isus both make contributions to the repolarization phase of the action potential, and hence to action potential duration, in human atrial cells. It is known that a low dose of 4-aminopyridine (4-AP) blocks Isus selectively in human atrial cells. In fact, Wang et al. (1993b) reported that 50 μM 4-AP prolongs action potential duration (APD90) by approximately 66% in human atrial myocytes. This implies that Isus plays an important role in the repolarization phase of the atrial action potential. In addition, the recovery from inactivation of human Ito (time constant 35.3±6.8 ms, Figure 10) is much faster than that previously observed in the rabbit atrium (time constant 650.6±159.0 ms; Fermini et al., 1992). Thus, the contribution made by Ito to the action potential may be important in the human atrium even in atrial tachyarrhythmias.
The efficacy of anti-arrhythmic drugs in the treatment of atrial fibrillation (af) is related to their ability to prolong atrial refractoriness (Rensma et al., 1988; Kirchhof et al., 1991; Wang et al., 1992, 1993a), generally by prolonging the duration of the atrial action potential (Wang et al., 1990). In actual fact, inhibiting K channels in atrial cells may be an important way of treating supraventricular arrhythmia. However, the effects of anti-arrhythmic drugs on K channels have not yet been thoroughly evaluated in the human atrium. It was reported by Wang et al. (1995) that flecainide blocks Ito without affecting Isus in human atrial cells, and propafenone, another class Ic anti-arrhythmic agent, is known to be effective at converting atrial fibrillation (Capucci, 1994; Weiner, 1994) and maintaining a normal sinus rhythm (Chimienti et al., 1996). However, the effects of propafenone on K currents in human atrial cells have not previously been evaluated. For this reason, we decided to investigate the effects of propafenone on K channels in human atrial cells in the present experiments.
Mechanism underlying the block of Ito by propafenone in human atrial myocytes
Propafenone inhibited Ito with an IC50 value of 4.9 μM (Figure 3) and its effect on Ito showed no significant voltage- or use-dependence (Figures 4 and 5). Propafenone accelerated the inactivation time constant for Ito in a dose-dependent manner (Figures 3 and 6) and, while it did not affect the initial current at depolarizing potentials, it did produce a block that increased as a function of time after depolarization (with a time constant of 3.4±0.4 ms) (Figure 9). These results indicated that channel opening was required for the blocking action of propafenone on Ito. A shift of the steady-state inactivation curve was not observed in the present experiment, suggesting a lack of drug effect on the inactivation gating of Ito (Figure 7). The reactivation time constant of Ito was not modulated by propafenone, indicating that the dissociation of the drug from the Ito channel might be rapid, a conclusion consistent with the lack of a use-dependent effect of this drug on Ito (Figure 10).
In other mammalian cardiac species, propafenone is known to inhibit Ito channels. For example, it has been reported that propafenone inhibits Ito in the rabbit atrium and rat ventricle (Duan et al., 1992; Slawsky & Castle, 1993) with IC50 values of 5.9 μM and 3.3 μM, respectively. In the present experiment, propafenone inhibited Ito with an IC50 of 4.9 μM, a value similar to that obtained in these other species. The following two pieces of evidence obtained in rabbit atrial and rat ventricular myocytes (Duan et al., 1992; Slawsky & Castle, 1993) suggest that propafenone inhibits Ito through a preferential interaction with the open state of the channel. First, there appears to be no inhibition of Ito at the onset of an activating depolarization; however, during continued depolarization, inhibition develops in an exponential manner at a rate and magnitude that are both concentration-dependent. Second, although there is an increase in the rate constant of apparent Ito inactivation in cells exposed to the drug, there is a minimal shift in the voltage-dependence of the steady-state inactivation. These results, together with our present data, strongly suggest that propafenone inhibits the Ito channel through a preferential interaction with the open state in mammalian cardiac cells.
Blocking mechanisms underlying the state-dependent Ito block: comparison between propafenone and other drugs
Quinidine blocks Ito in human atrial cells (Wang et al., 1995) in a manner similar to that observed with propafenone in the present experiment: viz. quinidine accelerates the inactivation time course, produces no inhibition at the onset of depolarization and does not affect the steady-state inactivation curve. These results indicate that quinidine, too, preferentially blocks Ito in an open state. However, the profiles of the blocks produced by these two drugs differ in certain important respects. First, quinidine blocks Ito in a voltage-dependent manner, suggesting that the block would be enhanced at more positive potentials. Second, the time constant of the onset of the block during continued depolarization is 14.5±4.2 ms at 16°C and it is 5.5±1.1 ms even at 36°C, values slower than that obtained here with propafenone (3.4±0.4 ms at room temperature). Third, quinidine slows the reactivation of Ito, the time constant increasing from a control value of 20 ms to 41 ms. This result indicates a slow removal of quinidine from the channel on returning to the holding potential, a characteristic that would result in the appearance of a use-dependent block. To judge from these results, although propafenone and quinidine both block Ito in an open state, the time course of the development of the inhibition and the dissociation from the Ito channel were both more rapid for propafenone than for quinidine.
An analysis of its effect on Ito in human atrial cells shows that flecainide exhibits a kinetic profile similar to that of propafenone. Flecainide shows no use-dependency and produces no obvious change in the reactivation time constant (Wang et al., 1995). These results indicate that flecainide dissociates rapidly from the Ito channel, as suggested here for propafenone. However, flecainide shifts the steady-state inactivation curve in the hyperpolarizing direction, suggesting that it inhibits Ito through a preferential interaction with the inactivated state of the channel (Wang et al., 1995). Thus, we conclude that although propafenone and flecainide belong to the same class of antiarrhythmic agents (class Ic), they inhibit Ito by acting on different gating states of the channel. The former drug interacts preferentially with the open state, while the latter interacts with the inactivated state of the Ito channel.
Effect of propafenone on Isus
Wang et al. (1993b) reported that Isus is highly K+ selective in human atrial myocytes. In the present experiment, the reversal potential of the sustained outward current was more positive (Figure 1B1) than the theoretical equilibrium potential for K+ (estimated EK=−86 mV). One possibility of this discrepancy is that the amplitude of the tail current was too small to measure and the inward capacitive current overlapped this component, therefore, we could not detect an outward tail current at −50 mV. Another possibility is that this current was not carried only by the K+ ion. However, 1 mM of 4-AP completely blocked both the sustained current and the tail current (result not illustrated). These results indicated that the sustained outward current observed in the present experiment is indeed the Isus previously reported in human atrial myocytes (Wang et al., 1993b).
Propafenone blocked Isus with an IC50 value of 8.6 μM, and it blocked Isus in a voltage- and use-independent manner (Figures 3, 4, and 5). Quinidine has been reported to block Isus with an IC50 of around 5 μM in human atrial cells (Wang et al., 1995) but it blocks Isus in a voltage-dependent manner, suggesting an open-channel block. In the present experiment, propafenone blocked Isus in a voltage-independent manner, implying that the blocking profiles of the two drugs may be different. A quinidine-induced change in the tail current of a cloned human cardiac potassium channel (HK2), which is believed to be the equivalent of the native Isus, was clearly demonstrated by Snyders et al. (1993). Quinidine blocks HK2 in a voltage-dependent manner and induces the tail ‘crossover' phenomenon in which a rising phase appears in the tail current and there is a retardation of the tail current's decline (Snyders et al., 1991; Delpo'n et al., 1997). Since blocked channels do not conduct, a conversion to open channels would result initially in a rising phase in the tail current; subsequently, the tail current would display a slower decline if some of the open channels became blocked again, rather than closing irreversibly. These results indicate that quinidine preferentially blocks the Isus channel in the open state. In our preliminary experiments on human atrial cells, propafenone slowed the time course of the onset of the Isus tail current relative to control (Figure 1B2, inset). The retardation of Isus tail current was much the same regardless of the changes in depolarizing potentials or pulse durations, indicating that this phenomenon was not due to depletion nor accumulation of K+ ions. The retardation of Isus tail current also occurred when quinidine was applied to the HK2 channel, suggesting that propafenone blocked Isus in the open state. The mechanism by which propafenone blocks Isus needs to be evaluated by analysing the onset of its blocking action and the change it induces in the time course of the tail current. In the present experiments, we were not able to analyse the onset of the block of Isus produced by propafenone because it was very difficult to isolate Isus from Ito. For this reason, future studies should be performed using a cloned K channel.
Anti-arrhythmic effect of propafenone
Propafenone is clinically effective for the treatment of atrial fibrillation (af). In fact, with this drug the reported conversion rate for paroxysmal or chronic af ranges from 29 to 87% (Kosmala et al., 1994; Weiner et al., 1994; Aliot & Denjoy, 1996; Stroobandt et al., 1997), the average being 66.2%. Maintenance of a normal sinus rhythm was achieved in 30 to 75% of cases (Antmann et al., 1988; Porterfield and Porterfield 1988; Pritchett et al., 1991; Reimold et al., 1993; Cobbe & Rae, 1995; Chimienti et al., 1996; Crijns et al., 1996; Stroobandt et al., 1997), the average being 52.9%. This suggests that propafenone should be an effective anti-arrhythmic agent for use against atrial tachyarrhythmias.
Recently, the ionic changes occurring during atrial fibrillation were described by Yue et al. (1997) in the canine atrium. Rapid atrial pacing decreases the action potential duration (APD) and its adaptation to changes in rate. The APD changes are accompanied by decreases in Ca current and Ito, without alterations in the other ionic currents flowing during the action potential plateau. The authors suggested that in the canine model these effects would produce changes in atrial refractoriness associated with an enhanced ability to maintain af. If this is also true for patients with atrial fibrillation, both the Ito and the Ca current would be already suppressed in such patients. Thus, inhibition of other outward currents, such as Isus, should be effective in prolonging the atrial APD. At the time of the conversion to sinus rhythm from atrial fibrillation, the plasma level of propafenone was 1.26±0.71 mg l−1 (around 3.3 μM) following its intravenous administration (Suttorp et al., 1990). If we take into account the 70 to 90% plasma protein binding of propafenone (Gillis and Kates, 1986), the estimated free concentration of this drug would have been within the range 0.33 to 1 μM. Therefore, the present experiments demonstrate that propafenone blocks both Ito and Isus at clinically relevant concentrations. This therefore suggests that propafenone may be effective in improving atrial refractoriness by inhibiting Isus in patients with atrial tachyarrhythmias.
Acknowledgments
We thank Dr Kazuhiro Seo, Dr Masatsugu Terada and Dr Mitsuru Aoki, Department of Cardiac Surgery, The Heart Institute of Japan for providing human atrial specimens. This work was supported by a research grant from Ministry of Education, Science and Culture of Japan.
Abbreviations
- Isus or Ikur
sustained or ultra-rapid delayed rectifier K current
- Ito
transient outward K current
References
- ALIOT E., DENJOY I. Comparison of the safety and efficacy of flecainide versus propafenone in hospital out-patients with symptomatic paroxysmal atrial fibrillation/flutter. The flecainide af French study group. Am. J. Cardiol. 1996;77:66A–71A. doi: 10.1016/s0002-9149(97)89120-5. [DOI] [PubMed] [Google Scholar]
- ANTMANN E.M., BEAMER A.D., CANTILLON C., MCGOWAN N., GOLDMAN L., FRIEDMAN P.L. Long-term propafenone therapy for suppression of refractory symptomatic atrial fibrillation and atrial flutter. J. Am. Coll. Cardiol. 1988;12:1005–1011. doi: 10.1016/0735-1097(88)90468-8. [DOI] [PubMed] [Google Scholar]
- BOTTO G.L., BONINI W., BROFFONI T., CAPPELLETTI G., FALCONE C., LOMBARDI R., PAULESU A., PEDRAGLIO E., FERRARI G. Regular ventricular rhythms before conversion of recent onset atrial fibrillation to sinus rhythm. Pacing & Clin. Electrophysiol. 1994;17:2114–2117. doi: 10.1111/j.1540-8159.1994.tb03810.x. [DOI] [PubMed] [Google Scholar]
- CAPUCCI A., BORIANI G., BOTTO G.L., LENZI T., RUBINO I., FALCONE C., TRISOLINO G., DELLA CASA S., BINETTI N., CAVAZZA M., SANGUINETTI M., MAGNANI B. Conversion of recent-onset atrial fibrillation by a single oral loading dose of propafenone or flecainide. Am. J. Cardiol. 1994;74:503–505. doi: 10.1016/0002-9149(94)90915-6. [DOI] [PubMed] [Google Scholar]
- CHIMIENTI M., CULLEN M.T., CASADEI G. Safety of long-term flecainide and propafenone in the management of patients with symptomatic paroxysmal atrial fibrillation: report from the flecainide and propafenone Italian study investigators. Am. J. Cardiol. 1996;77:60A–75A. doi: 10.1016/s0002-9149(97)89119-9. [DOI] [PubMed] [Google Scholar]
- COBBE S.M., RAE A.P., UK Propafenone PSVT Study Group A randomized, placebo-controlled trial of propafenone in the prophylaxis of paroxysmal supraventricular tachycardia and paroxysmal atrial fibril. Circulation. 1995;92:2550–2557. [PubMed] [Google Scholar]
- CRIJNS H.J.G.M., GROSSELINK A.T.M., LIE K.I. Propafenone versus disopyramide for maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation: a randomized, double-blind study. Cardiovasc. Drugs and Ther. 1996;10:145–152. doi: 10.1007/BF00823592. [DOI] [PubMed] [Google Scholar]
- DELPO'N E., VALENZUELA C., GAY P., FRANQUEZA L., SNYDERS D.J., TAMARGO J. Block of human cardiac Kv 1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels. Cardiovasc. Research. 1997;35:341–350. doi: 10.1016/s0008-6363(97)00121-1. [DOI] [PubMed] [Google Scholar]
- DUAN D., FERMINI B., NATTEL S. Potassium channel blocking properties of propafenone in rabbit atrial myocytes. J. Pharmacol. Exp. Ther. 1992;264:1113–1123. [PubMed] [Google Scholar]
- DUKES I.D., CLEEMANN L., MORAD M. Tedisamil blocks the transient and delayed rectifier K currents in mammalian cardiac and glial cells. J. Pharmacol. Exp. Ther. 1990;254:560–569. [PubMed] [Google Scholar]
- ESCANDE D., COULOMBE A., FAIVRE J.-F., DEROUBAIX E., CORABOEUF E. Two types of transient outward currents in adult human atrial cells. Am. J. Physiol. 1987;252:H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- FERMINI B., WANG Z., DUAN D., NATTEL S. Differences in rate dependence of transient outward current in rabbit and human atrium. Am. J. Physiol. 1992;263:H1747–H1754. doi: 10.1152/ajpheart.1992.263.6.H1747. [DOI] [PubMed] [Google Scholar]
- GILLIS A.M., KATES R.E. Influence of protein binding on the myocardial uptake and pharmacodynamics of propafenone. J. Cardiovasc. Pharmacol. 1986;8:1163–1167. doi: 10.1097/00005344-198611000-00011. [DOI] [PubMed] [Google Scholar]
- HAGIWARA N., IRISAWA H., KASANUKI H., HOSODA S. Background current in the sino-atrial node cells of the rabbit heart. J. Physiol. 1992a;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA N., MATSUDA N., SHODA M., IRISAWA H. Stretch-activated anion currents of rabbit cardiac myocytes. J. Physiol. 1992b;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers. Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARRISON D.C. Antiarrhythmic drug classification: new science and practical applications. Am. J. Cardiol. 1985;56:185–187. doi: 10.1016/0002-9149(85)90591-0. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., KLÖCKNER U. Calcium tolerant ventricular myocytes prepared by preincubation in a ‘KB-medium'. Pflügers. Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- KAJIMOTO K., HAGIWARA N., KASANUKI H., HOSODA S. Contribution of phosphodiesterase isozymes to the regulation of the L-type calcium current in human cardiac myocytes. Br. J. Pharmacol. 1997;121:1549–1556. doi: 10.1038/sj.bjp.0701297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRCHHOF C., WIJFFELS M., BRUGADA J., PLANELLAS J., ALLESSIE M. Mode of action of a new class Ic drug (ORG 7797) against atrial fibrillation in conscious dogs. J. Cardiovasc. Pharmacol. 1991;17:116–124. doi: 10.1097/00005344-199101000-00017. [DOI] [PubMed] [Google Scholar]
- KOSMALA W., KUCHARSKI W., HACZYNSKI J., KOBUSIAK-PROKOPOWICZ M. Propafenone, flecainide, procainamide in the treatment of a fresh attack of atrial fibrillation. Wiadomosci Lekarskie. 1994;47 (21–24):801–807. [PubMed] [Google Scholar]
- MATSUDA N., HAGIWARA N., SHODA M., KASANUKI H., HOSODA S. Enhancement of the L-type Ca2+ current by mechanical stimulation in single rabbit cardiac myocytes. Circ. Res. 1996;78:650–659. doi: 10.1161/01.res.78.4.650. [DOI] [PubMed] [Google Scholar]
- PORTERFIELD J.G., PORTERFIELD L.M. Therapeutic efficacy and safety of oral propafenone for atrial fibrillation. Am. J. Cardiol. 1988;63:114–116. doi: 10.1016/0002-9149(89)91091-6. [DOI] [PubMed] [Google Scholar]
- PRITCHETT E.L.C., MCCARTHY E.A., WILKINSON W.E. Propafenone treatment of symptomatic paroxysmal supraventricular arrhythmias. Ann. Int. Med. 1991;114:539–543. doi: 10.7326/0003-4819-114-7-539. [DOI] [PubMed] [Google Scholar]
- REIMOLD S.C., CANTILLON C.O., FRIEDMAN P.L., ANTMAN E.M. Propafenone versus sotalol for suppression of recurrent symptomatic atrial fibrillation. Am. J. Cardiol. 1993;71:558–563. doi: 10.1016/0002-9149(93)90511-a. [DOI] [PubMed] [Google Scholar]
- RENSMA P.L., ALLESSIE M.A., LAMMERS W.J.E.P., BONKE F.I.M., SCALIJ M.J. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ. Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- SAKAI R., HAGIWARA N., KASANUKI H., HOSODA S. Chloride conductance in human atrial cells. J. Mol. Cell. Cardiol. 1995;27:2403–2408. doi: 10.1016/s0022-2828(95)92199-0. [DOI] [PubMed] [Google Scholar]
- SAKAI R., HAGIWARA N., MATSUDA N., KASANUKI H., HOSODA S. Sodium-potassium pump current in rabbit sino-atrial node cells. J. Physiol (Lond). 1996;490:51–62. doi: 10.1113/jphysiol.1996.sp021126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHODA M., HAGIWARA N., KASANUKI H., HOSODA S. ATP-activated cationic current in rabbit sino-atrial node cells. J. Mol. Cell. Cardiol. 1997;29:689–695. doi: 10.1006/jmcc.1996.0311. [DOI] [PubMed] [Google Scholar]
- SLAWSKY M.T., CASTLE N.A. K+ channel blocking actions of flecainide compared with those of propafenone and quinidine in adult rat ventricular myocytes. J. Pharmacol. Exp. Ther. 1993;269:66–74. [PubMed] [Google Scholar]
- SNYDERS D.J., KNOTH K.M., ROBERDS S.L., TAMKUN M.M. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac channel. Molecular Pharmacology. 1991;41:322–330. [PubMed] [Google Scholar]
- SNYDERS D.J., TAMKUN M.M., BENNETT P.B. A rapidly activating slowly inactivating potassium channel cloned from human heart. J. Gen. Physiol. 1993;101:513–543. doi: 10.1085/jgp.101.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROOBANDT R., STIELS B., HOEBRECHTSA R. Propafenone for conversion and prophylaxis of atrial fibrillation. Propafenone atrial fibrillation trial investigators. Am. J. Cardiol. 1997;79:418–423. doi: 10.1016/s0002-9149(96)00779-5. [DOI] [PubMed] [Google Scholar]
- SUTTORP M.J., KINGMA J.H., JESSURUN E.R., LIE-A-HUEN L., VAN HEMEL N.M., LIE K.I. The value of class Ic antiarrhythmic drugs for acute conversion of paroxysmal atrial fibrillation or flutter to sinus rhythm. J. Am. Coll. Cardiol. 1990;16:1722–1727. doi: 10.1016/0735-1097(90)90326-k. [DOI] [PubMed] [Google Scholar]
- WANG J., BOURNE G.W., WANG Z., VILLEMAIRE C., TALAJIC M., NATTEL S. Comparative mechanisms of antiarrhythmic drug action in experimental atrial fibrillation. Circulation. 1993a;88:1030–1044. doi: 10.1161/01.cir.88.3.1030. [DOI] [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Sustained depolarization-induced outward current in human atrial myocytes. Circ. Res. 1993b;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J. Pharmacol. Exp. Ther. 1995;272:184–196. [PubMed] [Google Scholar]
- WANG Z., PAGE P., NATTEL S. Mechanism of flecainide's antiarrhythmic action in experimental atrial fibrillation. Circ. Res. 1992;71:271–287. doi: 10.1161/01.res.71.2.271. [DOI] [PubMed] [Google Scholar]
- WANG Z., PELLETIER L.C., TALAJIC M., NATTEL S. Effects of flecainide and quinidine on human atrial action potentials. Circulation. 1990;82:274–283. doi: 10.1161/01.cir.82.1.274. [DOI] [PubMed] [Google Scholar]
- WEINER P., GANAM R., GANEM R., ZIDAN F., RABNER M. Clinical course of recent-onset atrial fibrillation treated with oral propafenone. Chest. 1994;105:1013–1016. doi: 10.1378/chest.105.4.1013. [DOI] [PubMed] [Google Scholar]
- YUE L., FENG J., GASPO R., LI G.-R., WANG Z., NATTEL S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]


