Abstract
8-epi prostaglandin (PG) F2α, a vasoconstrictor isoprostane, is synthesized under conditions of oxidative stress. This study was undertaken to investigate the vasoconstrictor effect of 8-epi PGF2α in the coronary circulation before and after a period of oxidative stress.
The effects of the isoprostane 8-epi PGF2α and the thromboxane mimetic U46619 were compared in the isolated rat heart perfused in the Langendorff mode at a constant pressure of 80 mmHg.
In normal hearts U46619 caused a dose-related reduction in coronary flow (ED50 4.7±2.2 nmol). In contrast, 8-epi PGF2α had no effect.
After reducing perfusion pressure to 20 mmHg for 30 min and reperfusing at 80 mmHg, the dose-response curve to U46619 was unaffected. In contrast, 8-epi PGF2α caused a dose-dependent drop in coronary flow (ED50 52.6±12.7 nmol), producing a similar maximal reduction to U46619.
Similarly, after perfusion with xanthine and xanthine oxidase for either 15 or 30 min there was little change in the response to U46619 in comparison to control hearts. In contrast, 8-epi PGF2α caused a reduction in coronary flow similar to that produced by U46619, the magnitude of the response being related to the length of xanthine/xanthine oxidase perfusion.
Responses to both U46619 and 8-epi PGF2α after xanthine/xanthine oxidase perfusion were blocked by the selective thromboxane receptor antagonist SQ29548 10−7 M.
These results show that oxidative stress in the isolated perfused rat heart reveals a potent vasoconstrictor effect of the isoprostane 8-epi PGF2α by an action on the thromboxane receptor.
The data also suggest that, since 8-epi PGF2α is a partial agonist at the thromboxane receptor, thromboxane receptor reserve is increased by oxidative stress.
Keywords: Isoprostane, thromboxane, prostaglandin, coronary circulation, oxidative stress, hypoxia
Introduction
Isoprostanes, like the prostaglandins, are metabolites of arachidonic acid. However, unlike the prostaglandins, they are principally derived by free radical attack on membrane phospholipids (Morrow et al., 1990) and are produced in vivo in conditions of oxidative stress (Delanty et al., 1996; 1997). They are formed in situ esterified to phospholipids and are thought to be released by the action of a phospholipase (Morrow et al., 1992). We have previously reported that one of the isoprostanes, 8-epi prostaglandin (PG) F2α, has a vasoconstrictor effect on isolated arterial rings (Kromer & Tippins, 1996; 1998). In porcine coronary artery 8-epi PGF2α has a partial agonist action on the thromboxane (TP) receptor (Kromer & Tippins, 1996). Increased 8-epi PGF2α production has been reported in ischaemic heart disease (Kromer et al., 1997), and urinary levels of 8-epi PGF2α increase in acute myocardial infarct patients after thrombolytic therapy or coronary artery bypass surgery (Delanty et al., 1997) and after percutaneous transluminal coronary angioplasty (Reilly et al., 1997).
The aim of this study was to investigate the vasoconstrictor properties of 8-epi PGF2α in comparison to the thromboxane mimetic U46619 in isolated perfused rat hearts and in hearts subjected to oxidative stress.
Methods
Male Sprague-Dawley rats weighing 200–250 g were anaesthetized with Hypnorm and Hypnovel (2 mg ml−1) in the ratio of 2 : 5 : 1 part of water at a dose of 2.7 ml kg−1 i.p. and 400 units sodium heparin given i.v. via the tail vein. The heart was removed and immediately placed into ice-cold Krebs (mM) NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24, Glucose 11 and sodium pyruvate 2.0. The ascending aorta was then cannulated rapidly and retrogradely perfused with Krebs solution (pH 7.4, bubbled with 95% O2-5% CO2, 37°C) at a constant pressure of 80 mmHg measured with an Elcomatic pressure transducer (EM750) attached to a side arm of the aortic perfusion cannula. Left ventricular pressure (LVP) was measured by a fluid-filled Clingfilm balloon inserted into the left ventricle (Curtis et al., 1986) and connected to a second pressure transducer (Bell and Howell Ltd. Type 4-422-0001). Left ventricular end diastolic pressure was set to approximately 15 mmHg (Curtis et al., 1986) which was optimal for maximal left ventricular developed pressure. Heart rate (HR) was derived from the left ventricular pressure trace by a cardiotachometer. The perfusion flow was continuously measured with a Gould blood flowmeter (SP2202) by positioning a flow probe in-line with the cannula. Data were recorded on a six channel recorder (Lectromed; MX-6). In xanthine/xanthine oxidase perfusion experiments hearts were electrically paced at 350 beats min−1.
After a 25 min stabilization period, responses to U46619 or to 8-epi PGF2α were recorded. Agonists were added as 10 μl bolus doses into the aortic perfusion cannula. The hearts were left for 10 min between doses to allow values to return to baseline or until flow and systolic LVP stabilized. In separate experiments, oxidative stress was created either by reduction of perfusion pressure to 20 mmHg for 30 min and then reperfusion at 80 mmHg, or by perfusion with Krebs containing 0.23 mM xanthine and 0.5 mU ml−1 xanthine oxidase for 15 or 30 min and then reperfusion with normal Krebs (Basu & Karmazyn, 1987). In both cases responses to U46619 or to 8-epi PGF2α were then repeated.
Drugs
8-epi PGF2α, U46619 (11, 9, epoxymethano PGH2) and the thromboxane antagonist SQ29548 ([(1S-[1,2b(5Z),3b,4]]-7-[3-[[-2-[(phenylamino) carbonyl hydrazino]methyl]-7-oxabicyclo [2.2.1]-hept-2-yl]-5-heptenoic acid] were purchased from Cayman Chemicals (Ann Arbor, MI, U.S.A.). All compounds were dissolved in ethanol and subsequently diluted to the required concentrations in Krebs solution. The highest ethanol concentration in the perfusate was approximately 0.2% which had no effect on the heart. Xanthine was dissolved in 5 M NaOH and diluted to the appropriate concentration in Krebs solution. The final concentration of NaOH in the perfusate was approximately 0.001 M which on its own had no effect on any measured parameter.
Statistical analysis
Results are expressed as mean±s.e.mean. ED50 values and Emax values were calculated using Prism software (GraphPad Inc., San Diego, CA, U.S.A.). Student's t-test was used to investigate differences in responses before and after oxidative stress. A P value <0.05 was taken to be the minimum level of statistical significance with n number of animals tested.
Results
Under resting conditions mean coronary flow at a constant pressure of 80 mmHg was 17.5±3.5 ml min−1 (n=6), systolic LVP 86±7 mmHg and HR 246±36 beats min−1. U46619 caused a dose-related reduction in flow (ED50 4.7±2.2 nmols, n=5, Figure 1). HR was unaffected by U46619 and systolic and developed LVP only decreased when there was a marked reduction in coronary flow (maximal decrease in systolic LVP of 26.5 mmHg after the administration of 32 nmols U46619). 8-epi PGF2α had no effect on any parameter measured in control rat hearts, even up to a dose of 256 nmols.
Figure 1.
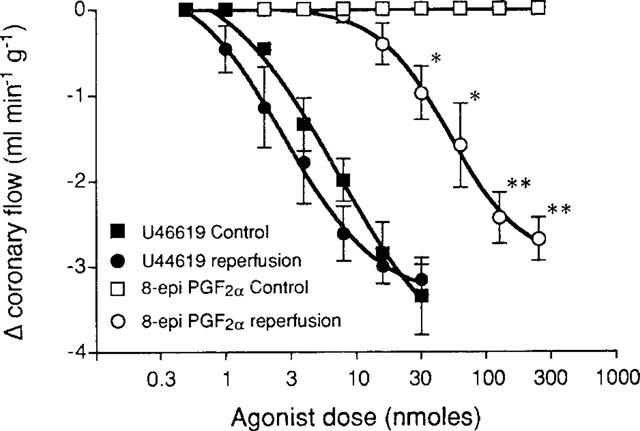
Effect of low flow on vasoconstrictor responses to U46619 and 8-epi PGF2α in the isolated rat heart. *P<0.05, **P<0.005, unpaired t-test, n=5.
Perfusion at 20 mmHg resulted in a drop in flow to between 2 and 5 ml min−1. After 30 min of 20 mmHg perfusion followed by reperfusion at 80 mmHg, flow increased briefly (29.3±6.4 ml min−1) and then reached a steady rate of 11.9±6.3 ml min−1. After reperfusion the dose-response curve to U46619 shifted slightly to the left, but this was not statistically significant (ED50 3.1±2.7 nmols; P>0.05, Student's t-test, Figure 1). However, 8-epi PGF2α caused a dose-dependent vasoconstriction after reperfusion with a threshold dose of 16 nmols and an ED50 of 52.6±12.7 nmols (Figure 1). 8-epi PGF2α had a similar effect to U46619 on other parameters measured, a large decrease in coronary flow associated with a decrease in systolic and developed LVP, but no effect on HR.
In hearts perfused with xanthine and xanthine oxidase, resting coronary flow was 11.8±1.2 ml min−1 (n=5) and systolic LVP was 112±19 mmHg. U46619 caused a dose-dependent decrease in flow with an ED50 of 0.8±0.1 nmol (n=5, Figure 2). After perfusion with xanthine and xanthine oxidase for either 15 or 30 min, the U46619 dose-response curves gave ED50 values of 0.8±0.1 nmol (n=4) and 0.6±0.1 nmol (n=5) for 15 and 30 min perfusions respectively (P>0.05; Student's t-test). As in the low flow experiments, 8-epi PGF2α had no effect on normal rat coronary vasculature even up to doses of 256 nmols. However, after xanthine and xanthine oxidase perfusion, 8-epi PGF2α caused a marked coronary constriction (Emax 2.1±0.2 ml min−1 g−1, ED50 26.7±4.8 nmols after 15 min and Emax 3.3±0.3 ml min−1 g−1, ED50 32.8±6.7 nmols after 30 min perfusion, Figure 2).
Figure 2.

Effect of xanthine/xanthine oxidase perfusion on vasoconstrictor responses to U46619 and 8-epi PGF2α in the isolated rat heart. *P<0.005 and **P<0.001 compared to control; #P<0.005 and ##P<0.001 compared to perfusion for 15 min, unpaired t-test, n=5.
After perfusion of the heart with xanthine and xanthine oxidase for 15 min, the reduction in coronary flow produced by submaximal doses of both U46619 (8 nmols) and 8-epi PGF2α (56 nmols) was virtually abolished by perfusion of the tissue with Krebs containing 10−7 M SQ29548, a TP receptor antagonist (4.7±0.9 ml min−1 g−1 control, 0.6±0.3 ml min−1 g−1 SQ29548 and 2.5±0.1 ml min−1 g−1 control, 0.1±0.1 ml min−1 g−1 SQ29548, for U46619 and 8-epi PGF2α respectively).
Discussion
This study shows that constriction of the rat coronary vasculature occurs in response to 8-epi PGF2α following oxidative stress induced either by low flow perfusion or by perfusion with xanthine and xanthine oxidase, with the isoprostane being completely devoid of constrictor activity before the tissue has been subjected to oxidative stress. The response to the thromboxane mimetic U46619 is unaffected by oxidative stress. Secondly, responses to both U46619 and 8-epi PGF2α after oxidative stress were blocked by the TP receptor antagonist SQ29548, suggesting that they were mediated by an action on the TP receptor. To our knowledge, no other study has reported a similar increase in reactivity to other mediators in response to oxidative stress.
It is widely, though not universally, accepted that the action of 8-epi PGF2α is mediated by an action on the TP receptor (Banerjee et al., 1992; Crankshaw, 1995; John & Valentin, 1997; Kang et al., 1993; Kromer & Tippins, 1996; Takahashi et al., 1992; Zhang et al., 1996). We have reported previously that 8-epi PGF2α acts as a partial agonist at the TP receptor (Kromer & Tippins, 1996). In the present study, the response to 8-epi PGF2α increased to 80±7% of the response to U46619 after ischaemia and to 50±5% and 80±7% of the response to U46619 after 15 or 30 min respectively of perfusion with xanthine and xanthine oxidase. This increase in intrinsic activity to the isoprostane may be explained therefore by an increase in TP receptor density (Kenakin, 1993) after oxidant stress. The response to a partial agonist is dependent upon receptor density and the stimulus response coupling. As we showed previously, when TP receptor reserve is low 8-epi PGF2α acts only as an antagonist, whereas a high efficacy agonist such as U46619 induces a maximum response (Kromer & Tippins, 1996). Conversely, when receptor reserve is high 8-epi PGF2α will produce a maximum response similar to that evoked by U46619 (Kromer & Tippins, 1996). The present data support the suggestion that TP receptor reserve primarily determines the degree of intrinsic activity exhibited by 8-epi PGF2α in a given preparation (John & Valentin, 1997).
It has been suggested that the platelet TP receptor exists in two states, a reduced (inactive) and an oxidized (active) state with the oxidized-active state being the predominant native form, comprising approximately two-thirds of the total number of receptors (Dorn, 1990). Hence in situations of tissue ischaemia, such as acute myocardial infarction, the active platelet TP receptor number is acutely increased (Dorn et al., 1990). A similar increase in functional or active TP receptors would explain the increased response to 8-epi PGF2α in the present study. Since U46619 is a full agonist in this tissue, any increase in functional TP receptor number would have little effect on responses to this compound.
The fact that 8-epi PGF2α is a more potent constrictor in conditions of oxidative injury suggests that the isoprostanes could play an important role in control of vessel tone during conditions of vascular injury such as myocardial ischaemia and angina. Clinical data suggest that thromboxane synthase inhibitors give symptomatic improvement in patients with unstable angina but are ineffective in every other group tested (Fiddler & Lumley, 1990). Thromboxane receptor antagonists on the other hand are effective in the treatment of peripheral vascular disease, in preventing restenosis after angioplasty, occlusion of coronary artery bypass grafts, and the deleterious effects of thromboxane in renal disease (Fiddler & Lumley, 1990). Synthesis of isoprostanes in such circumstances might explain the greater benefit derived from thromboxane receptor antagonists compared to thromboxane synthase inhibitors.
In conclusion, the Langendorff perfused rat heart is normally unresponsive to 8-epi PGF2α whereas U46619, the thromboxane mimetic, is a potent vasoconstrictor. After oxidant stress, 8-epi PGF2α evokes a vasoconstrictor response of similar amplitude, but lower potency compared to U46619. Responses to both agonists are inhibited by the TP receptor antagonist SQ29548, suggesting that they act upon the same receptor and that TP receptor reserve is increased by oxidant stress.
Acknowledgments
We thank the British Heart Foundation for funding.
Abbreviations
- 8-epi PGF2α
8-epi prostaglandin F2α
- HR
heart rate
- LVP
left ventricular pressure
- TP
thromboxane receptor
References
- BANERJEE M., KANG K.H., MORROW J.D., ROBERTS L.J., NEWMAN J.H. Effects of a novel prostaglandin, 8-epi PGF2α, in rabbit lung in situ. Am. J. Physiol. 1992;263:H660–H663. doi: 10.1152/ajpheart.1992.263.3.H660. [DOI] [PubMed] [Google Scholar]
- BASU D.K., KARMAZYN M. Injury to rat hearts produced by an exogenous free radical generating system. Study into the role of arachidonic acid and eiconsanoids. J. Pharmacol. Exp. Ther. 1987;242:673–685. [PubMed] [Google Scholar]
- CRANKSHAW D. Effects of the isoprostane, 8-epi prostaglandin F2α, on the contractility of the human myometrium in vitro. Eur. J. Pharmacol. 1995;285:151–158. doi: 10.1016/0014-2999(95)00398-5. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., MACLEOD B.A., TABRIZCHI R., WALKER M.J. An improved perfusion apparatus for small animal hearts. J. Pharm. Methods. 1986;15:87–94. doi: 10.1016/0160-5402(86)90008-2. [DOI] [PubMed] [Google Scholar]
- DELANTY N., REILLY M., PRATICO D., FITZGERALD D.J., LAWSON J.A., FITZGERALD G.A. 8-Epi PGF2α-specific analysis of an isoeicosanoid as an index of oxidant stress in vivo. Br. J. Clin. Pharmacol. 1996;42:15–19. doi: 10.1046/j.1365-2125.1996.03804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELANTY N., REILLY M.P., PRATICO D., LAWSON J.A., MCCARTHY J.F., WOOD A.E., OHNISHI S.T., FITZGERALD D.J., FITZGERALD G.A. 8-Epi PGF2α generation during coronary reperfusion–A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–2499. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- DORN G.W. Cyclic oxidation-reduction reactions regulate thromboxane A2/prostaglandin H2 receptor number and affinity in human platelet membranes. J. Biol. Chem. 1990;265:4240–4246. [PubMed] [Google Scholar]
- DORN G.W., LIEL N., TRASK J.L., MAIS D.E., ASSEY M.E., HALUSHKA P.V. Increased platelet thromboxane A2 prostaglandin H2 receptors in patients with acute myocardial infarction. Circulation. 1990;81:212–218. doi: 10.1161/01.cir.81.1.212. [DOI] [PubMed] [Google Scholar]
- FIDDLER G.I., LUMLEY P. Preliminary clinical studies with thromboxane synthase inhibitors and thromboxane receptor blockers. A review. Circulation. 1990;81:I69–I78. [PubMed] [Google Scholar]
- JOHN G.W., VALENTIN J.P. Analysis of the pulmonary hypertensive effects of the isoprostane derivative, 8-iso-PGF2α, in the rat. Br. J. Pharmacol. 1997;122:899–905. doi: 10.1038/sj.bjp.0701441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG K.H., MORROW J.D., ROBERTS L.J., NEWMAN J.H., BANERJEE M. Airway and vascular effects of 8-epi prostaglandin F2α in isolated perfused rat lung. J. Appl. Physiol. 1993;74:460–465. doi: 10.1152/jappl.1993.74.1.460. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Pharmacologic analysis of drug-receptor interaction. New York: Raven Press; 1993. [Google Scholar]
- KROMER B.M., KASKI J.C., MURDAY A., MADDEN B., TIPPINS J.R. Evidence of a role for 8-epi prostaglandin F2α in ischaemic heart disease. Br. J. Clin. Pharmacol. 1997;45:197P–198P. [Google Scholar]
- KROMER B.M., TIPPINS J.R. Coronary artery constriction by the isoprostane 8-epi prostaglandin F2α. Br. J. Pharmacol. 1996;119:1276–1280. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROMER B.M., TIPPINS J.R. The actions of 8-epi prostaglandin F2α on isolated rat aorta. J. Cardiovasc. Pharmacol. 1998;32:471–478. doi: 10.1097/00005344-199809000-00019. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., AWAD J.A., BOSS H.J., BLAIR I.A., ROBERTS L.J. Non-cyclooxygenase derived prostanoids (F2 isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J.D., HILL K.E., BURK R.F., NAMMOUR T.M., BADR K.F., ROBERTS L.J. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclogoxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REILLY M.P., DELANTY N., ROY L., ROKACH J., O'CALLAGHAN P., CREAN P., LAWSON J.A., FITZGERALD G.A. Increased formation of the isoprostanes IPF2α-I and 8-epi-prostaglandin F2α in acute coronary angioplasty. Evidence for oxidant stress during coronary reperfusion in humans. Circulation. 1997;96:3314–3320. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K., NAMMOUR T.M., FUKUNAGA M., EBERT J., MORROW J.D., ROBERTS L.J., HOOVER R.L., BADR K.F. Glomerular actions of a free radical generated novel prostaglandin, 8-epi prostaglandin F2α, in the rat. Evidence for interaction with thromboxane A2 receptors. J. Clin. Invest. 1992;90:136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG R.G., OGLETREE M.L., MORELAND S. Characterization of thromboxane A2/prostaglandin endoperoxide receptors in aorta. Eur. J. Pharmacol. 1996;317:91–96. doi: 10.1016/s0014-2999(96)00697-8. [DOI] [PubMed] [Google Scholar]


