Abstract
The effects of treating groups of rats with four different anabolic androgenic steroids (AAS) (testosterone, nandrolone, methandrostenolone, and oxymetholone) on 5-hydroxytryptamine (5-HT) and dopamine (DA) neurones in different brain regions were examined. The AAS was injected six times with 1 week's interval and the rats were sacrificed 2 days after the final injection. 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA), DA and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were measured. The effect on DA and 5-HT synthesis rate was analysed as the accumulation of 3,4-dihydroxyphenyl-alanine (DOPA) and 5-hydroxytryptophan (5-HTP), respectively, after inhibition of the amino acid decarboxylase with NSD-1015 (3-hydroxy-benzylhydrazine dihydrochloride). Additionally, the monoamine oxidase (MAO) activity was analysed in the hypothalamus.
The DOPAC+HVA/DA ratio was increased in the striatum in all treatment groups. However, the synthesis rate of DA was significantly increased only in the methandrostenolone treated group.
The 5-HIAA/5-HT ratio was increased in all treatment groups in the hippocampus, in the frontal cortex in the methandrostenolone-treated animals and in the hypothalamus in the testosterone- and oxymetholone-treated rats, while the 5-HT synthesis rate was not affected by the AAS-treatments.
The MAO-A activity was increased in the oxymetholone-treated rats while the other treatment groups were unaffected. The MAO-B activity was not changed.
The results indicate that relatively high doses of AAS increase dopaminergic and 5-hydroxytryptaminergic metabolism in male rat brain, probably due to enhanced turnover in these monaminergic systems.
Keywords: Rat brain, dopamine, 5-hydroxytryptamine, anabolic androgenic steroids, frontal cortex, striatum, hippocampus, hypothalamus
Introduction
The term anabolic androgenic steroids (AAS) comprises testosterone and structurally related synthetic compounds (Hoberman & Yesalis, 1995). AAS have been medically used for treatment of various somatic and psychiatric conditions (Hoberman & Yesalis, 1995). However, complications and the development of more efficacious drugs have reduced the medical use of anabolic-androgenic steroids (Bahrke et al., 1990). Today AAS are mainly used illicitly both by athletes and non-athletes (Williamson & Young, 1992). AAS are commonly used in cycles of 6–12 weeks in doses up to 100 times the recommended therapeutic dose (Williamson & Young, 1992). A wide spectrum of psychiatric side-effects have been described following the abuse of AAS. In the early career of abuse, positive effects on mood, e.g. euphoria, increased energy and self-esteem, have commonly been described (Corrigan, 1996; Lukas, 1996; Su et al., 1993). These symptoms may occasionally take hypomanic or manic proportions (Su et al., 1993; Pope & Katz, 1994). After prolonged abuse of AAS, and possibly also escalated dose regimens, labile mood with quick swings and lack of impulse control can occur (Corrigan, 1996). At this stage the behavioural changes following AAS abuse may result in acts of violence, including homicide (Choi & Pope, 1994; Corrigan, 1996; Schulte et al., 1993; Thiblin et al., 1997; Yates et al., 1992). Depressive symptoms are frequently appearing in connection to discontinuation of AAS use (Corrigan, 1996; Pope & Katz, 1994).
The reported behavioural changes following AAS abuse resemble the impulsive, aggressive and depressive symptoms that have been associated with a dysregulation of central 5-hydroxytryptaminergic activity in man (Coccaro, 1992; Linnoila & Virkkunen, 1992; Virkkunen et al., 1995). This has led to speculations that at least some of the AAS-mediated behavioural changes could be mediated through changes in the 5-hydroxytryptaminergic activity in the CNS (Galligani et al., 1996; Thiblin et al., 1997). Increased aggressive behaviour in animals as a result of AAS administration is well established (Bonson et al., 1994; Albert et al., 1992; Lumia et al., 1994). 5-HT agonists, selective 5-hydroxytryptamine reuptake inhibitors as well as other agents increasing the 5-HT transmission have been reported to reduce aggressive behaviour in several animal species (Fuller, 1996; Muehlenkamp et al., 1995; Olivier et al., 1995). Bonson et al. (1994) have shown that the 5-hydroxytryptamine system may play an important role in the control of AAS-induced aggressive dominance in rats. These findings, together with other experimental studies concerning effects of steroids on various neurotransmitters, in which 5-hydroxytryptaminergic markers are usually the ones most influenced by steroids (Biegon, 1990), indicate that effects on central 5-hydroxytryptaminergic activity may be an important factor in AAS-related alterations in aggressive behaviour.
The positive effects on mood (Corrigan, 1996; Lukas, 1996), together with observations of a possible addictive potential of AAS (Brower, 1992), have inspired research concerning the effects of AAS on the mesocorticolimbic reward system. Johansson et al. (1997) have shown that treatment of rats with nandrolone increased brain levels of β-endorphins in the ventral tegmental area (VTA). VTA projects to the nucleus accumbens in the striatum, where several central stimulants, e.g. amphetamine and cocaine, act by enhancing dopaminergic activity. A connection between AAS and central dopaminergic activity has been reported in animal studies (Vermes et al., 1979). In man, treatment with nandrolone has resulted in significant increases in serum HVA concentrations (Hannan et al., 1991).
The aim of the present study was to investigate the effects of four different AAS, on the dopaminergic- and 5-hydroxytryptaminergic neurones in rat brain.
Methods
Animals
Male Sprague-Dawley rats (B&K International AB, Sollentuna, Sweden), weighing 240–260 g, were housed five per cage under controlled temperature (21°C) and humidity with a 12 h light-dark cycle (lights on 0600 h). Food and water were freely available. The compounds were injected subcutaneously (s.c.) in a volume of 1.0 ml kg−1 body weight. The animal experiments were approved by the local Animal Ethical Committee.
Animal treatment
Experiment I: (5-HT and DA metabolism and MAO-activity)
Forty rats were divided in five groups with eight animals group−1 receiving one of the following treatments: (1) Peanut oil (control), (2) Testosterone propionate 5 mg kg−1, (3) Nandrolone propionate 5 mg kg−1, (4) Methandrostenolone 5 mg kg−1, or (5) Oxymetholone 5 mg kg−1. The animals received one injection per week for 6 weeks. The weight of the animals were monitored before each injection. The animals were sacrificed with a guillotine 48 h following the last treatment. Brains were rapidly removed and the brain regions (hippocampus, striatum, frontal cortex and hypothalamus) were dissected and immediately frozen on dry ice. The samples were stored at −70°C until assayed.
Experiment II: (5-HTP and DOPA accumulation)
Forty rats were divided in five groups with eight animals group−1 and treated as described above. Forty-eight hours following the last treatment the rats were injected with NSD 1015, 100 mg kg−1 s.c. (in water solution with pH brought to about five with sodium hydroxide) and the animals were killed with a guillotine 30 min after the NSD 1015 injections. Brains were rapidly removed and the brain regions (hippocampus, striatum, frontal cortex and hypothalamus) were dissected and immediately frozen on dry ice. The samples were stored at −70°C until assayed.
Determination of biogenic monoamines and metabolites in brain tissue
The following endogenous compounds in various brain regions were determined by use of high performance liquid chromatography (HPLC) with electrochemical detection according to the method of Magnussion et al. (1980) with some modifications (Larrson et al., 1990): 5-HT, 5-HIAA, 5-HTP, DA, DOPAC, HVA, and DOPA. The mobile phase was 0.1 M phosphate buffer (pH 2.5) : methanol : acetonitrile −89 : 9 : 2 v v−1, containing 1 mM octylsulphate. The frozen samples were weighed and homogenized in perchloric acid 0.1 M, containing sodium bisulphite 2.5 mM, ethylendiamine tetra-acetic acid (EDTA) 1 mM and isoprenaline as internal standard. The supernatants were injected directly onto a Supelcosil LC-18-DB (3 μm) column, connected to a detector (ESA Coulochem 5100A), set to 0.05/0.30 V.
Tissue preparation for MAO assay
One half of each hypothalamus was used for the MAO assays. The tissues were homogenized in 40 volumes (v w−1 of a sucrose solution 0.32 M. Aliquots of the homogenates were immediately used for the MAO activity assay using 5-HT synaptosomes (nerve terminals) and the rest was frozen at −20°C for the assay of total MAO-A and MAO-B activities and protein determination.
MAO assay
MAO activity was determined according to a modified method of Wurtman & Axelrod (1963) using substrates for the A-form ([14C]-5-HT) and B-form ([14C]-phenethylamine, PEA) of MAO. The incubation medium mixture consisted of 25 μl of the homogenate, 950 μl of 0.11 M sodium phosphate buffer, pH 7.4. Following 10 min preincubation at 37°C, 25 μl of the radioactive substrate, either [14C]-5-HT (50 μM final concentration) or [14C]-PEA (0.9 μM final concentration) was added. The incubation was continued for 5 min in a water bath at 37°C. The reaction was stopped by addition of 1 ml of 1 M HCl. Blanks were obtained by addition of HCl before [14C]-5-HT. The acid metabolite was extracted into 6 ml ethyl acetate by vigorous shaking in a multi-tube vortex mixer (Model 2601, Scientific Manufactoring Industries). After centrifugation, 4 ml ethyl acetate was transferred to a counting vial containing 10 ml liquid scintillation cocktail (Ultima Gold, Packard). The radioactivity was measured in a liquid scintillation counter (LS6000TA, Beckman). MAO activity was expressed as pmol product mg protein−1 5 min−1.
MAO activity in 5-HT synaptosomes
The deamination of [14C]-5-HT by the homogenate in sucrose containing intact synaptosomes was determined as described previously by Ask et al. (1983). After a 10-min preincubation of 50 μl of the homogenate in 925 μl of Krebs-Henseleit's buffer, pH 7.4, containing glucose 5.6 mM, ascorbic acid 1.1 mM and disodium edetate 0.13 mM, the incubation was continued for a further 10 min at 37°C with [14C]-5-HT (0.1 μM) in the absence and presence of 0.12 μM citalopram. The deaminated product, [14C]-5-HIAA, was extracted into ethyl acetate as described above. The total deamination was obtained in the absence of citalopram and the deamination in the 5-HT synaptosomes was calculated from the difference of the deamination in the absence and the presence of citalopram. MAO activity was expressed as pmol 5-HIAA mg protein−1 5 min−1.
Protein determination
The protein concentration in hypothalamus homogenates was analysed according to a slightly modified method of Lowry et al. (1951).
Compounds
Testosterone propionate (4-androsten-17b-ol-3-one 17-propionate), nandrolone propionate (19-nortestosterone 17-propionate), methandrostenolone (1-dehydro-17a-methyltestosterone) and oxymetholone (17b-hydroxy-2-hydroxymethylene-17-methyl-5a-androstan-3-one) were all purchased from Sigma, St. Louis, MO, U.S.A. They were all dissolved in peanut oil at a concentration of 5 mg ml−1, containing 10% ethanol. 5-Hydroxytryptamine [14C]-creatinine sulphate (55 mCi mmol−1) and 2-phenyl[1-14C]ethylamine hydrochloride (50 mCi mmol−1) were bought from Amersham International plc, Buckinghamshire, U.K. NSD 1015 was purchased from Sigma. Other compounds used were of highest purity available.
Statistics
All statistical analyses were calculated in the computer program Statistica® Version 5. Analysis of variance was tested with ANOVA followed by Tukey HSD post-hoc test, with a significance level of P<0.05.
Results
Dopamine and its metabolites
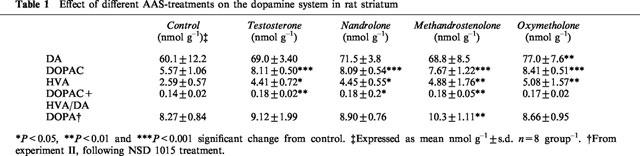
The concentration of DA was increased in the striatum in the AAS-treated rats as compared to the controls (Table 1). This increase met significance (P<0.01) in the oxymetholone-treated rats. DOPAC and HVA concentrations were significantly increased in the striatum in all treatment groups (DOPAC: P<0.001; HVA: testosterone and nandrolone P<0.05, methandrostenolone and oxymetholone P<0.01) (Table 1). The DOPAC+HVA/DA-ratio was significantly increased in striatum in the following groups: testosterone and methandrostenolone P<0.01 and nandrolone: P<0.05 (Table 1). No changes were seen in the DA, DOPAC or HVA concentrations in the frontal cortex (data not shown).
Table 1.
Effect of different AAS-treatments on the dopamine system in rat striatum
DA synthesis rate
There was a significant increase in the DA-synthesis rate measured as DOPA accumulation in the striatum in the methandrostenolone-treated rats (P<0.01) compared to the control group. No significant changes in the other treatment groups were seen (Table 1).
5-Hydroxytryptamine and its metabolites
There was a significant (P<0.01) increase in 5-HT concentrations in the hippocampus in the oxymethenolone-treated animals compared with the controls. No changes in the other regions were seen (Table 2).
Table 2.
Effect of different AAS-treatments on 5-HT, 5-HIAA and 5-HTP concentrations in the rat brain
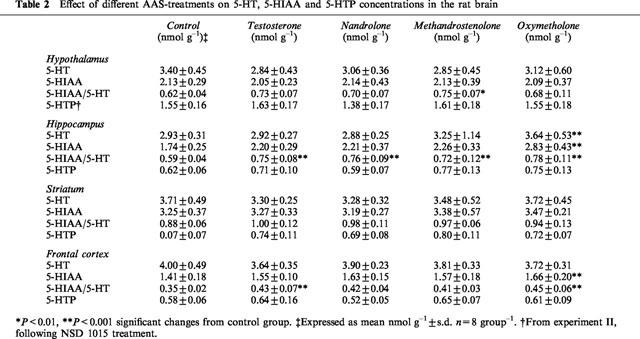
In the hippocampus and frontal cortex there was a general pattern of increased levels of 5-HIAA in the AAS-treated rats. Significance was reached in the oxymethenolone-treated animals in both brain areas (P<0.001 and 0.01, respectively) and nearly reached significance in the hippocampus in the nandrolone and testosterone-treated groups. In the other brain regions examined there were no changes in the 5-HIAA concentrations (Table 2).
A significant increase in the 5-HIAA/5-HT ratio was found in the hippocampus for all AAS-treated groups (P<0.01) as compared with controls (Table 2). A similar pattern of general increase in the 5-HIAA/5-HT ratio for AAS-treated animals were seen in the other brain areas examined, but significance was only reached for the methandrostenolone group in the hypothalamus (P<0.05) and for testosterone (P<0.01) and oxymetholone (P<0.01) in the frontal cortex (Table 2). There were no significant changes in the striatum.
5-HT synthesis rate
There was a tendency for increased hippocampal 5-HTP accumulation in the methandrostenolone-treated animals, which, however, did not reach statistical significance (Table 2). No changes in the 5-HTP accumulation were seen in the other brain regions following either of the AAS treatments.
MAO-A and MAO-B
MAO-A and MAO-B activity in the hypothalamic homogenates were analysed with [14C]-5-HT and [14C]-PEA, respectively, as substrates. There was a significant (P<0.01) increase of MAO-A activity in the oxymetholone-treated group, while the other treatment groups were unaffected (Table 3). There were no changes in MAO-B activity between the groups (Table 3).
Table 3.
MAO-A and MAO-B activities in hypothalamic homogenates in AAS-treated rats
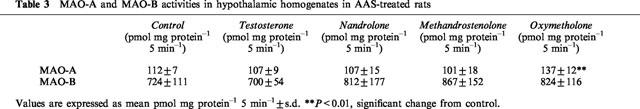
MAO activity within 5-HT synaptosomes
The MAO activity within 5-HT synaptosomes in the hypothalamic homogenates were analysed with a low concentration of [14C]-5-HT that is actively accumulated in these synaptosomes. The difference in the deamination of 5-HT to 5-HIAA in the absence or presence of the selective 5-HT reuptake inhibitor citalopram, is supposed to be a measure of the MAO activity in the 5-HT synaptosomes.
There were no changes in total MAO activity or extra-5-hydroxytryptaminergic MAO activity between the treatment groups (Table 4). Although there was a tendency for decreased intra-5-hydroxytryptaminergic MAO activity in the oxymetholone- and methandrostenolone treated animals these changes did not reach statistical significance (Table 4).
Table 4.
MAO activity in hypothalamic 5-HT synaptosomes from AAS-treated rats

Discussion
The doses given in our study (5 mg kg−1 week−1) were calculated to roughly correspond to those used by body builders who self-administer AAS, i.e. 1–10 mg kg−1 week−1 (Mats Garle, Doping Laboratory, Huddinge Hospital, personal communication). Doses at these levels are regarded as supra-physiological in man but have been described as both physiological (Mendelson & McEwen, 1990) and supra-physiological in rats (Knoth et al., 1993). Because it has been shown that androgens interact with other steroid receptors (Janne, 1990) we chose a relatively moderate dose-regimen believing that such a strategy might minimize the risk of ‘spill over' effects on other steroid receptors such as glucocorticoid and progesterone receptors.
In the present study an increased DA-metabolism (measured as the ratio between DOPAC/DA) was seen in the striatum in the four AAS-treated animal groups. However, when measuring the effect of these treatments on DOPA accumulation following NSD-1015 treatment, a significant increase was seen only in the methandrostenolone group. Also the 5-hydroxytryptaminergic system was affected by AAS treatment. Thus, it was found that 5-HT metabolism (expressed as 5-HIAA/5-HT ratio) was significantly increased in the hippocampus. However, 5-HT synthesis rate (measured as accumulation of 5-HTP after inhibition of aromatic amino acid decarboxylase activity), was not significantly enhanced. The possibility that the increased levels of 5-HIAA and DOPAC+HVA are caused by an increase in MAO-activity is contradicted by the MAO-analysis that showed no significant change in the total activity of MAO and the intra-5-hydroxytryptamine MAO activity measured in vitro. The results could be interpreted as a primary effect of AAS on neuronal activity, reflected by the increased DOPAC+HVA/DA and 5-HIAA/5-HT ratios. This should result in an increased demand of transmitter leading to increased transmitter synthesis. However, a significant increase in DA synthesis in striatum was only seen in the oxymetholone group and no significant increase in 5-HT synthesis was observed in any of the treatment regimes or brain regions analysed. It is possible that the increase in the synthesis rate is too low to be detected with the method used. Furthermore, it has recently been reported that NSD 1015 in itself can increase 5-HTP accumulation in the rat brain (Mück-Seler & Dicsik, 1995). This increase may blunt the enhancement of 5-HTP (and possibly also DOPA) accumulation evoked by other means, e.g. AAS treatment.
In addition to the increases in DA and 5-HT, exclusively found in the oxymetholone treated rats, there was a significant increase in the levels of MAO-A in this group of animals that was not observed in the other groups. The principal substrates for MAO-A are noradrenaline and 5-hydroxytryptamine, thus, the increase in MAO-A activity (which was assessed in the hypothalamus) found in the oxymetholone-treated animals may reflect a compensatory increase in enzymatic activity due to marked increase in 5-hydroxytryptaminergic and noradrenergic activity.
The increase in 5-hydroxytryptaminergic metabolism found in the present study is in contrast with the results of earlier studies where treatment with testosterone propionate 30 mg kg−1 day−1 during a 14 day period resulted in decreased levels of 5-HT and 5-HIAA in the hippocampus (Bonson & Winter, 1992) and where 45 days of weekly treatment with testosterone propionate (10 mg kg−1) resulted in decreased levels of 5-HT in the diencephalon (Martinez-Conde et al., 1985). One explanation for the conflicting results might be that the decreased levels of 5-HT and/or 5-HIAA found in the earlier studies reflect compensatory down regulatory mechanisms of a stressed 5-hydroxytryptaminergic system and that the doses used in the present study were too low to trigger these mechanisms.
In earlier studies reported by Mitchell & Stewart (1989) decreased concentrations of both DA and DOPAC were found in the nucleus accumbens following castration of male rats. This decrease could be prevented by treatment of the rats with testosterone or estradiol. It has been shown that DA and DOPAC concentrations in the caudate-putamen do not vary with sexual behaviours (Ungerstedt, 1974), behaviours that are influenced by AAS and that are related to changes in mesolimbic DA alterations (Mitchell & Stewart, 1989). Thus, it seems likely that the increased DA metabolism in the striatum found in the present study mainly reflects a stimulatory influence on the mesolimbic DA system rather than the nigro-striatal DA system. Stimulation of the mesolimbic DA system is known to be associated with reinforcement of behaviour (Koob, 1992). Taken together, these data indicate that an increase in the DA neuronal activity might account for some of the positive effects, e.g., euphoria, increased self-esteem and confidence that frequently appear as early effects following AAS administration in man (Corrigan, 1996; Su et al., 1993). In this context it is worth noting that AAS have been ascribed a sensitizing action on the brain reward system similar to that of various psychoactive substances, e.g. d-amphetamine (Clark et al., 1996).
In conclusion, in the present study it was found that treatment with various AAS leads to increased DA and 5-HT metabolism in brain regions regulating affective, emotional, and motivational behaviour. These effects are likely to reflect increased neuronal activity rather than enhanced enzymatic activity since no major changes in the synthesis rates of DA and 5-HT or MAO-enzymatic activity could be detected. The increase in the DA and 5-HT metabolism may account for some of the observed central stimulatory properties that have been reported following AAS abuse.
Abbreviations
- AAS
anabolic androgenic steroid
- DA
dopamine
- DOPAC
3,4-dihydroxypheylacetic acid
- 5-HIAA
5-hydroxy nidole acetic acid
- HPLC
high performance liquid chromatography
- 5-HT
5-hydroxytryptamine
- 5-HTP
5-hydroxytryptophan
- HVA
homovanillic acid
- NSD 1015
3-hydroxybenzylhydrazine dihydrochloride
- PEA
phenylethylamine
- VTA
ventral tegmental area
References
- ALBERT D., JONIK R., WALLSH M. Hormone-dependent aggression in male and female rats: Experimental, hormonal and neural foundations. Neurosci. Biobehav. Rev. 1992;16:177–192. doi: 10.1016/s0149-7634(05)80179-4. [DOI] [PubMed] [Google Scholar]
- ASK A., FAGERVALL I., ROSS S. Selective inhibition of monoamine oxidase in monoaminergic neurons in the rat brain. Naunyn-Schmiedebergs Arch. Pharmacol. 1983;324:79–87. doi: 10.1007/BF00497011. [DOI] [PubMed] [Google Scholar]
- BAHRKE M., YESALIS C., WRIGHT J. Psychological and behavioural effects of endogenous testosterone levels and anabolic-androgenic steroids among males. Sports Medicine. 1990;10:303–337. doi: 10.2165/00007256-199010050-00003. [DOI] [PubMed] [Google Scholar]
- BIEGON A. Effects of steroid hormones on the serotonergic system. Ann. N.Y. Acad. Sci. 1990;200:427–432. doi: 10.1111/j.1749-6632.1990.tb16899.x. [DOI] [PubMed] [Google Scholar]
- BONSON K., WINTER J. Reversal of testosterone-induced dominance by the serotonergic agonist quipazine. Pharmacol. Biochem. Behav. 1992;42:809–813. doi: 10.1016/0091-3057(92)90034-d. [DOI] [PubMed] [Google Scholar]
- BONSON K.R., JOHNSON R.G., FIORELLA D., RABIN R.A., WINTER J.C. Serotonergic control of androgen-induced dominance. Pharmacol. Biochem. Behav. 1994;49:313–322. doi: 10.1016/0091-3057(94)90427-8. [DOI] [PubMed] [Google Scholar]
- BROWER K. Addictive potential of anabolic steroids. Psychiatric Annals. 1992;22:31–34. [Google Scholar]
- CHOI P., POPE H. Violence towards women and illicit androgenic-anabolic steroid use. Annals of Clinical Psychiatry. 1994;6:21–25. doi: 10.3109/10401239409148835. [DOI] [PubMed] [Google Scholar]
- CLARK A., LINDENFELD R., GIBBONS C. Anabolic-androgenic steroids and brain reward. Pharmacol. Biochem. Behav. 1996;53:741–745. doi: 10.1016/0091-3057(95)02082-9. [DOI] [PubMed] [Google Scholar]
- COCCARO E.F. Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behaviour relationship. Int. Clin. Psychopharmacol. 1992;7:3–12. doi: 10.1097/00004850-199200710-00001. [DOI] [PubMed] [Google Scholar]
- CORRIGAN B. Anabolic steroids and the mind. Med. J. Aust. 1996;165:222–226. doi: 10.5694/j.1326-5377.1996.tb124932.x. [DOI] [PubMed] [Google Scholar]
- FULLER R. Fluoxetine effects on serotonin function and aggressive behaviour. Ann. N.Y. Acad. Sci. 1996;794:90–97. doi: 10.1111/j.1749-6632.1996.tb32512.x. [DOI] [PubMed] [Google Scholar]
- GALLIGANI N., RENCK A., HANSEN S. Personality profile of men using anabolic androgenic steroids. Hormones and Behavior. 1996;30:170–175. doi: 10.1006/hbeh.1996.0021. [DOI] [PubMed] [Google Scholar]
- HANNAN C., FRIEDL K., ZOLD A., KETTLER T., PLYMATE S. Psychological and serum homovanillic acid changes in men administered androgenic steroids. Psychoneuroendocrinology. 1991;16:4:335–343. doi: 10.1016/0306-4530(91)90019-p. [DOI] [PubMed] [Google Scholar]
- HOBERMAN J.M., YESALIS C.E. The history of synthetic testosterone. Scientific American. 1995;272:76–81. doi: 10.1038/scientificamerican0295-76. [DOI] [PubMed] [Google Scholar]
- JANNE O.Androgen interaction through multiple steroid receptors 1990Rockville: National Institute on Drug Abuse; Erinoff & Linn (ed.) [PubMed] [Google Scholar]
- JOHANSSON P., RAY A., ZHOU Q., HUANG W., KARLSSON K., NYBERG F. Anabolic androgenic steroids increases b-endorphin levels in the ventral tegmental area in the male rat brain. Neurosci. Res. 1997;27:185–189. doi: 10.1016/s0168-0102(96)01141-8. [DOI] [PubMed] [Google Scholar]
- KNOTH R., PARK R., EGGENBRECHT K., CLEGG A. Effects of chronic exposure to testosterone on spatial learning in the rat. Soc. Neurosci. Abstr. 1993;19:367. [Google Scholar]
- KOOB G. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- LARSSON L., RENYI L., ROSS S., SVENSSON B., ÄNGEBY-MÖLLER L.K. Different effects on the responses of functional pre- and postsynaptic 5-HT1A receptors by repeated treatment of rats with the 5-HT1A receptor agonist 8-OH-DPAT. Neuropharmacol. 1990;29:85–91. doi: 10.1016/0028-3908(90)90047-u. [DOI] [PubMed] [Google Scholar]
- LINNOILA M., VIRKKUNEN M. Biologic correlates of suicidal risk and aggressive behavioral traits. J. Clin. Psychopharmacol. 1992;12:19. [PubMed] [Google Scholar]
- LOWRY O., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- LUKAS S.E. CNS effects and abuse liability of anabolic-androgenic steroids. Ann. Rev. Pharmacol. Toxicol. 1996;36:333–357. doi: 10.1146/annurev.pa.36.040196.002001. [DOI] [PubMed] [Google Scholar]
- LUMIA A., THORNER K., MCGINNIS M. Effects of chronically high doses of the anabolic androgenic steroid, testosterone, on intermale aggression and sexual behavior in male rats. Physiol. Behav. 1994;55:331–335. doi: 10.1016/0031-9384(94)90142-2. [DOI] [PubMed] [Google Scholar]
- MAGNUSSON O., NILSSON L., WESTERLUND D. Simultaneous determination of dopamine, DOPAC, and homovanillic acid. Direct injection of supernatants from brain tissue homogenates in a liquid chromatography-electrochemical detection system. J. Chromatogr. 1980;221:237–247. [PubMed] [Google Scholar]
- MARTINEZ-CONDE E., LERET E., DIAZ M.L., SILVIAA The influence of testosterone in the brain of the male rat on levels of serotonin (5-HT) and hydroxyindole-acetic acis (5-HIAA) Comp. Biochem. Physiol. 1985;80:411–414. doi: 10.1016/0742-8413(85)90077-5. [DOI] [PubMed] [Google Scholar]
- MENDELSON S., MCEWEN B. Chronic testosterone propionate treatment decrease the concentration of (3H)quipazine binding at 5-HT3 receptors in the amygdala of the castrated male rat. Brain Res. 1990;528:339–343. doi: 10.1016/0006-8993(90)91679-b. [DOI] [PubMed] [Google Scholar]
- MITCHELL J., STEWART J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in male rat. Brain Res. 1989;491:116–127. doi: 10.1016/0006-8993(89)90093-0. [DOI] [PubMed] [Google Scholar]
- MÜCK-SELER D., DICSIK M. The acute effects of reserpine and NSD-1015 on the brain serotonin synthesis rate measured by an autoradiographic method. Neuropsychopharmacology. 1995;12:251–262. doi: 10.1016/0893-133X(94)00084-D. [DOI] [PubMed] [Google Scholar]
- MUEHLENKAMP F., LUCION A., VOGEL W. Effects of selective serotonergic agonists on aggressive behavior in rats. Pharmacol. Biochem. Behav. 1995;50:671–674. doi: 10.1016/0091-3057(95)00351-7. [DOI] [PubMed] [Google Scholar]
- OLIVIER B., MOS J., VAN OORSCHOT R., HEN R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28:80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- POPE H., KATZ D. Psychiatric and medical effects of anabolic-androgenic steroid use. Arch. Gen. Psychiatry. 1994;51:375–381. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- SCHULTE H., HALL M., BOYER M. Domestic violence associated with anabolic steroid abuse. Am. J. Psychiatry. 1993;150:348. doi: 10.1176/ajp.150.2.348a. [DOI] [PubMed] [Google Scholar]
- SU T., PAGLIARO M., SCHMIDT P., PICKAR D., WOLKOWITZ O., RUBINOW D. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;131:2760–2764. [PubMed] [Google Scholar]
- THIBLIN I., KRISTIANSSON M., RAJS J. Anabolic androgenic steroids and behavioural patterns among violent offenders. J. Forensic Psychiatry. 1997;8:299–310. [Google Scholar]
- UNGERSTEDT U.Functional dynamics of central monoamine pathways The Neurosciences: Third Study Program 1974695–704.Worden F (ed)
- VERMES I., VÁRSZEGI M., TÓTH É., TELEGDY G. Action of androgenic steroids on brain neurotransmitters in rats. Neuroendocrinology. 1979;28:386–393. doi: 10.1159/000122887. [DOI] [PubMed] [Google Scholar]
- VIRKKUNEN M., GOLDMAN D., NIELSEN D.A., LINNOILA M. Low brain serotonin metabolism rate (low CSF 5-HIAA) and impulsive violence. J. Psychiatry and Neuroscience. 1995;20:271–275. [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., YOUNG A. Psychiatric effects of androgenic and anabolic-androgenic steroid abuse in men: a brief review of the literature. J. Psychopharmacol. 1992;6:1:20–26. doi: 10.1177/026988119200600107. [DOI] [PubMed] [Google Scholar]
- WURTMAN R., AXELROD J. A sensitive and specific assay for the estimation of monoamine oxidase. Biochem. Pharmacol. 1963;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- YATES W., PERRY P., MURRAY S. Aggression and hostility in anabolic steroid users. Biol. Psychiatry. 1992;31:1232–1234. doi: 10.1016/0006-3223(92)90344-y. [DOI] [PubMed] [Google Scholar]


