Abstract
A [3H]-resiniferatoxin (RTX) binding assay utilizing rat spinal cord membranes was employed to identify novel vanilloids in a collection of natural products of fungal origin. Of the five active compounds found (scutigeral, acetyl-scutigeral, ovinal, neogrifolin, and methyl-neogrifolin), scutigeral (Ki=19 μM), isolated from the edible mushroom Albatrellus ovinus, was selected for further characterization.
Scutigeral induced a dose-dependent 45Ca uptake by rat dorsal root ganglion neurons with an EC50 of 1.6 μM, which was fully inhibited by the competitive vanilloid receptor antagonist capsazepine (IC50=5.2 μM).
[3H]-RTX binding isotherms were shifted by scutigeral (10–80 μM) in a competitive manner. The Schild plot of the data had a slope of 0.8 and gave an apparent Kd estimate for scutigeral of 32 μM.
Although in the above assays scutigeral mimicked capsaicin, it was not pungent on the human tongue up to a dose of 100 nmol per tongue, nor did it provoke protective wiping movements in the rat (up to 100 μM) upon intraocular instillation.
In accord with being non-pungent, scutigeral (5 μM) did not elicit a measurable inward current in isolated rat dorsal root ganglion neurons under voltage-clamp conditions. It did, however, reduce the proportion of neurons (from 61 to 15%) that responded to a subsequent capsaicin (1 μM) challenge. In these neurons, scutigeral both delayed (from 27 to 72 s) and diminished (from 5.0 to 1.9 nA) the maximal current evoked by capsaicin.
In conclusion, scutigeral and its congeners form a new chemical class of vanilloids, the triprenyl phenols. Scutigeral promises to be a novel chemical lead for the development of orally active, non-pungent vanilloids.
Keywords: triprenyl phenols, scutigeral, non-pungent vanilloids, resiniferatoxin, capsaicin, vanilloid receptors
Introduction
Natural products provide important new leads for the discovery of novel receptors as well as the manipulation of known ones. The very existence of vanilloid receptors was postulated based on the remarkable selectivity that primary sensory neurons show for capsaicin, the irritant principle in hot pepper (Jancsó, 1968; Szolcsányi & Jancsó-Gábor, 1975). Other `hot' spices, like piperine, the active ingredient in black pepper, and zingerone, isolated from ginger, also seem to act via vanilloid receptor activation (Szolcsányi, 1982; Liu & Simon, 1997a). Capsaicin, piperine, and zingerone are structurally similar. Resiniferatoxin (RTX), an ultrapotent vanilloid (Szallasi & Blumberg, 1989) isolated from the latex of the cactus-like perennial E. resinifera (Hergenhahn et al., 1975; Appendino & Szallasi, 1997), combines structural features of two classes of irritant compounds, capsaicinoids and phorbol esters. Although xenobiotics that activate vanilloid-sensitive neurons range from heavy metals to industrial irritants like toluene diisocyanate (Holzer, 1991; Maggi, 1991; Lundberg, 1996), the screening of compound libraries in vanilloid receptor assays was discouraged by the concept that the presence of a (homo)vanillyl moiety is a prerequisite for vanilloid-like activity.
Vanilloid receptors have long been considered to be non-selective cation channels with a preference for calcium (Marsh et al., 1987; Wood et al., 1988; Bevan & Szolcsányi, 1990). A vanilloid receptor (termed VR1) has been cloned recently (Caterina et al., 1997) which is a distant homolog of the TRP (transient release potential) family of store operated calcium channels (Clapham, 1996). In addition to vanilloids, the cloned VR1 appears to be activated by noxious heat and it was speculated that this is the reason why capsaicin is `hot' tasting (Caterina et al., 1997). Furthermore, VR1 is gated by low pH (protons) and thus can be viewed as a `molecular integrator of chemical and physical stimuli that elicit pain' (Tominaga et al., 1998).
The past years have yielded several important breakthroughs in our understanding of the pharmacology of vanilloid receptors. For instance, it turned out that pungent compounds structurally unrelated to capsaicinoids or resiniferanoids, as exemplified by isovelleral, isolated from the pungent mushroom Lactarius vellereus, can function as vanilloids, suggesting that the ligand recognition selectivity of vanilloid receptors is much broader than thought previously (Szallasi et al., 1996a). Furthermore, it was found that vanilloids evoke a number of currents in sensory neurons that differ both in onset and duration (Liu & Simon, 1996; 1997a; Liu et al., 1996; Petersen et al., 1996). The radiation inactivation size of vanilloid receptors, 290 kDA, implies the existence of a receptor oligomer (Szallasi & Blumberg, 1991). Thus, it is entirely feasible that the diversity of vanilloid-evoked currents reflects multisubunit receptors comprised of VR1 isoforms and/or related proteins.
Capsaicin and RTX induce different patterns of biological responses (Szallasi & Blumberg, 1990; 1996). Most important, RTX treatment results in a lasting desensitization with minimal initial pain response both in animal experiments (Szallasi & Blumberg, 1989; Craft et al., 1993) and clinical trials (Cruz et al., 1997). Another interesting example of this phenomenon is the pulmonary chemoreflex, which can repeatedly be evoked by capsaicin but shows rapid desentitization to RTX without any prior excitation (Szolcsányi et al., 1990).
In principle, there are two strategies to obtain vanilloids with unusual patterns of biological responses. The first approach is by systematic chemical modification of lead compounds. In fact, we have developed using our phorbol-based approach a ligand, phorbol 12-phenylacetate 13-acetate 20-homovanillate (PPAHV), which is devoid of hypothermia, a characteristic vanilloid effect (Appendino et al., 1996). Interestingly, PPAHV has at least two additional unexpected actions; first, it eliminates the positive cooperative behaviour of vanilloid receptors (Szallasi et al., 1996b), and second, it opens a unique conductance, not recognized by the competitive vanilloid receptor antagonist capsazepine (Liu et al., 1998). The second strategy is to assay collections of natural or synthetic compounds in order to identify new chemical leads. This is how we discovered previously that unsaturated dialdehydes and related terpenoids may function as vanilloids (Szallasi et al., 1996; 1998). In the present study, we report the discovery of triprenyl phenols, a fourth class of naturally occurring vanilloids.
Methods
Irritancy in the rat eye and on the human tongue
Irritancy by scutigeral was evaluated in the rat by the eye-wiping method of Jancsó et al. (1961), and on the human tongue as described by Karrer & Bartoshuk (1991). Briefly, increasing concentrations (up to 100 μM) of scutigeral made up in a solvent containing 10% EtOH and 10% Tween 80 in physiological saline were instilled into the eyes of female Sprague-Dawley rats weighing 200–250 g; the animals were placed into an observation chamber; and the number of protective eye-wiping movements was counted. To avoid any unnecessary discomfort to the animals, the experiment was started with a low (100 nM) concentration of scutigeral, the concentration was increased by 10 fold in the subsequent days, and the experiment was discontinued when rats did not respond to 100 μM scutigeral. To minimize the risk of compromising the experiment by desensitization, a full day was allowed to elapse between two applications. Furthermore, after the last scutigeral administration, rats were challenged with a 10 μM capsaicin solution, which evoked a similar number of wipings as in control animals.
Filter paper discs (1 cm in diameter) were impregnated with increasing amounts of scutigeral (up to 100 nmol) dissolved in acetone. The solvent was allowed to evaporate and then the discs were placed for 1 min on the tip of the tongue of volunteers (members of the Department of Organic Chemistry 2 at the University of Lund, Lund, Sweden).
The animal protocol was approved by the Animal Use Committee of the Karolinska Institute, Stockholm, Sweden. Since scutigeral was originally isolated from an edible mushroom, its testing on the tongue of informed volunteers did not require approval of an Ethics Committee.
Inhibition of [3H]-resiniferatoxin binding to rat spinal cord membranes
[3H]-Resiniferatoxin (RTX; 37 Ci mmol−1; Chemical Synthesis and Analysis Laboratory, NCI-FCRDC, Frederick, MD, U.S.A.) binding experiments utilizing rat spinal cord membranes were carried out as described previously (Szallasi et al., 1992; 1993). Briefly, female Sprague-Dawley rats weighing 200–250 g were sacrificed by decapitation under CO2 anaesthesia and the cervical segment of the spinal cord was collected into an ice-cold buffer (pH 7.4), which contained (in mM) KCl 5, NaCl 5.8, CaCl2 0.75, MgCl2 2, sucrose 320, and HEPES 10. Tissues were disrupted in the above buffer with the aid of a Polytron tissue homogenizer. Spinal cord homogenates were first centrifuged for 10 min at 1000×g (4°C), and then the resulting supernatant was further centriguted for 30 min at 35,000×g (4°C) to obtain a partially purified particulate preparation. Membranes resuspended in the above buffer were stored at −80°C until assayed.
Binding assay mixtures were set up on ice and contained 40 μg membrane protein, 0.25 mg ml−1 bovine serum albumin (Cohn fraction V, Sigma, St. Louis, MO, U.S.A.), [3H]-RTX, and non-radioactive ligands; the final volume was adjusted to 500 μl with the buffer described above. Non-specific binding was defined as that occurring in the presence of 1 μM non-radioactive RTX (LC Laboratories, Woburn, MA, U.S.A.). Binding was either analysed in the presence of a fixed concentration of [3H]-RTX (25 pM, the approximate Kd from the saturation binding experiments) and various concentrations of competing ligands or was determined using increasing (12–400 pM) concentrations of [3H]-RTX in the absence or presence of scutigeral (10, 20, 40 and 80 μM).
The binding reaction was initiated by transferring the assay mixtures into a 37°C water bath and was terminated following a 60 min incubation period by cooling the tubes on ice. Non-specific binding was reduced by adding 100 μg of bovine α1-acid glycoprotein (Sigma, St. Louis, MO, U.S.A.), a vanilloid-binding serum protein (Szallasi et al., 1992), to each tube. At 0°C, the dissociation of receptor-bound [3H]-RTX is unmeasurably slow (Szallasi & Blumberg, 1993); however, free [3H]-RTX is readily bound to α1-acid glycoprotein (Szallasi et al., 1992). Since free and non-specifically bound RTX are in equilibrium, α1-acid glycoprotein is able to remove most of the non-specifically bound RTX from the membrane lipid phase without compromizing specific binding by sequestering [3H]-RTX from the aqueous phase (Szallasi et al., 1992; 1993). Membrane-bound RTX was separated from the free as well as the α1-acid glycoprotein-bound RTX by pelleting the membranes in a Beckman 12 benchtop centrifuge (15 min, maximal velocity) and the radioactivity determined by scintillation counting.
Competition experiments were analysed by the curvilinear regression program LIGAND (Biosoft, Ferguson, MO, U.S.A.). Equilibrium binding parameters in saturation experiments were determined by fitting the allosteric Hill equation to the measured values as described before (Szallasi et al., 1993). RTX binding isotherms obtained in the presence of various concentrations of scutigeral were also analysed using the Schild equation (Arunlakshana & Schild, 1959).
45Ca2+ uptake by adult rat dorsal root ganglion neurons in culture
Dorsal root ganglia (DRGs) from all levels of the spinal column of Sprague-Dawley rats (females, weighing 200–250 g) were removed aseptically and collected into Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 4.5 mg ml−1 glucose, 0.5% heat-inactivated foetal bovine serum (Gibco BRL, Gaithersburg, MD, U.S.A.), 1 mM Na-pyruvate, 25 mM HEPES, and antibiotics as described previously (Wood et al., 1988; Acs et al., 1996). In order to obtain isolated neurons, DRGs were digested twice with 0.125% collagenase (Sigma, St. Louis, MO, U.S.A.) in DMEM for 90 min at 37°C and then triturated through a flame-polished Pasteur pipette. The myelin debris was removed by pelleting the cells through a cushion of DMEM containing 15% fatty acid free bovine serum albumin and the neurons were plated in MultiScreen-DV 96-well filtration plates (Millipore, Marlborough, MA, U.S.A.) at an approximate density of 5000 cells per well. In other experiments, isolated DRG neurons were enriched for small- to medium-size neurons using the procedure of Gilabert & McNaughton (1997). Briefly, after collagenase digestion the cell suspension was layered on the top of 5 ml of a 40% Ficoll (Sigma, St. Louis, MO, U.S.A.) solution and centrifuged at 100×g (15 min; 4°C). The upper band containing cells (approximately 3 ml) was collected from the interface between cell suspension and Ficoll. This fraction was diluted in DMEM, centrifuged (200×g; 5 min; 4°C) and the cells resuspended in DMEM.
To determine calcium uptake, DRG neurons were incubated for 30 min at 37°C with 1 μCi ml−1 45Ca2+ (23.55 mCi mg−1; DuPont-New England Nuclear, Boston, MA, U.S.A.) in the presence of 1.8 mM CaCl2; 0.25 mg ml−1 bovine serum albumin, and various concentrations of scutigeral (10 nM–100 μM). Maximal calcium uptake by scutigeral was compared to that evoked by capsaicin (30 nM–10 μM) and was also determined in the presence of the vanilloid receptor antagonist capsazepine (300 nM–30 μM; RBI, Natick, MA, U.S.A.). Cells were washed six times with ice-cold serum-free DMEM with the aid of a MultiScreen Vacuum Manifold (Millipore, Marlborough, MA, U.S.A.); filters were dried under a heat lamp; and the radioactivity determined by scintillation counting. For each scutigeral concentration, at least four wells were analysed and the resulting dose-response curve was fitted to the Hill equation using the computer program Fig. P (Biosoft, Ferguson, MO, U.S.A.). The goodness of fit was determined by the χ2 test.
Electrophysiological recordings
Electrophysiological measurements on adult rat DRG neurons were performed as described previously (Petersen et al., 1996). Briefly, DRGs were collected from all levels of the spinal column of adult Sprague-Dawley rats (males, weighing 180–220×g) killed by a sodium pentobarbital overdose and neurons were isolated from ganglia digested first with 0.25% (w v−1) collagenase type CLS II (273 U ml−1; Boehringer Mannheim, Mannheim, Germany) in DMEM for 90 min at 37°C and then for 11 min with a 25,000 U ml−1 trypsin solution. Dispersed cells were plated in Ham's F-12 medium supplemented with 10% heat-inactivated horse serum (Gibco BRL, Gaithersburg, MD, U.S.A.), 2 mM glutamine, 0.8% glucose, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin (all from Gibco BRL, Gaithersburg, MD, U.S.A.) and 100 ng ml−1 nerve growth factor 7S (Calbiochem-Novabiochem, Bad Soden, Germany) on glass-coverslips coated with 200 mg ml−1 poly-L-lysine (Sigma, St. Louis, MO, U.S.A.). Cells were maintained at 37°C in a humidified atmosphere of air, gassed with 3.5% CO2.
DRG neurons were voltage-clamped from 6–36 h after plating by the whole-cell patch-clamp method using an Axopatch 200 A amplifier (Axon Instruments, Foster City, CA, U.S.A.). A coverslip with the cells was placed in a recording chamber filled with 0.5 ml of a solution, which contained (in mM) NaCl 140, KCl 5, CaCl2 2, and MgCl2 1. The recording electrode was filled with a buffer (pH 7.3) containing (in mM) KCl 140, CaCl2 1, EGTA 11, HEPES 10, and Mg-ATP 2, and had a final resistance in the range of 2–6 ΩM. The clamp command signals were generated with the help of the pClamp software (Axon Instruments, Foster City, CA, U.S.A.). A total of 20 DRG neurons of small to medium size (545–1235 μm2 cross sectional area) were tested in the presence of various scutigeral solutions (100 nM–10 μM). Cells were then challenged with a 1 μM capsaicin solution. In all experiments, the application time for scutigeral or capsaicin was 60 s, with a 60 s washout period betweeen two challenges. The mean resting membrane potential was 40.1±5.3 mV (mean±s.e.mean).
Drugs and chemicals
Fungal triprenyl phenols were isolated as described (Dekermendjian et al., 1997). Briefly, fruit bodies of Albatrellus ovinus, collected in the vicinity of Lund, Sweden, were extracted with ethyl acetate. Initial fractionation was carried out on a PrepPak C18 Nova-Pak reverse phase column eluted with a linear gradient, in which the concentration of acetonitrile increased from 25 to 100% over a period of 60 min. Fractions were further purified on an analytical Nova-Pak reverse-phase column eluted with water:acetonitrile 27 : 73% (flow 1 ml min−1) to yield neogrifolin, ovinal, and scutigeral, respectively. Structures were confirmed by spectroscopy. Scutigeral was acetylated and neogrifolin was methylated as described elsewhere (Dekermendjian et al., 1997). Triprenyl phenol stock solutions were made up in DMSO, aliquoted, and stored at −80°C until assayed.
Results
Inhibition by triprenyl phenols of [3H]-RTX binding to rat spinal cord membranes
In competition experiments, the affinity of triprenyl phenols (structures shown in Figure 1) for specific RTX binding sites in rat spinal cord membranes (Figure 2) ranged from 5.5±1.3 μM (neogrifolin; mean±s.e.mean, n=5) to 60.7±6.0 μM (ovinal; mean±s.e.mean, n=3) (Table 1). In parallel experiments, capsaicin (see Figure 1 for structure) competed for specific RTX binding sites with a Ki of 4.8±0.7 μM (mean±s.e.mean, four determinations).
Figure 1.
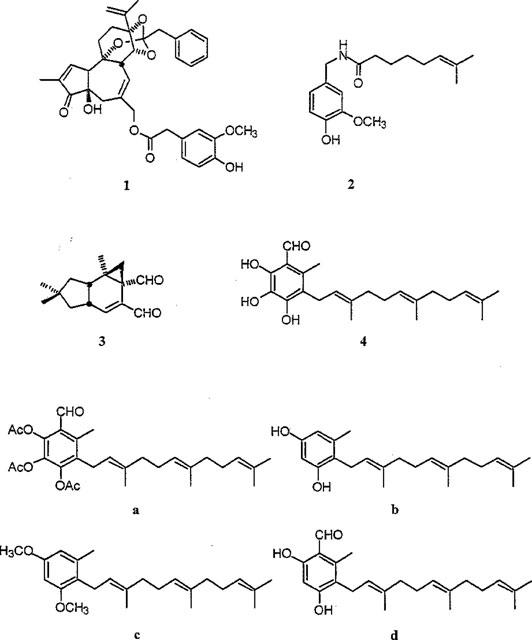
Vanilloid structures. (1), Resiniferatoxin; (2), capsaicin; (3), isovelleral and (4), scutigeral, representing the four known chemical classes of naturally occurring vanilloids. Other triprenyl phenols tested in this study: (a), acetyl-scutigeral; (b), neogrifolin; (c), methyl-neogrifolin and (d), ovinal.
Figure 2.
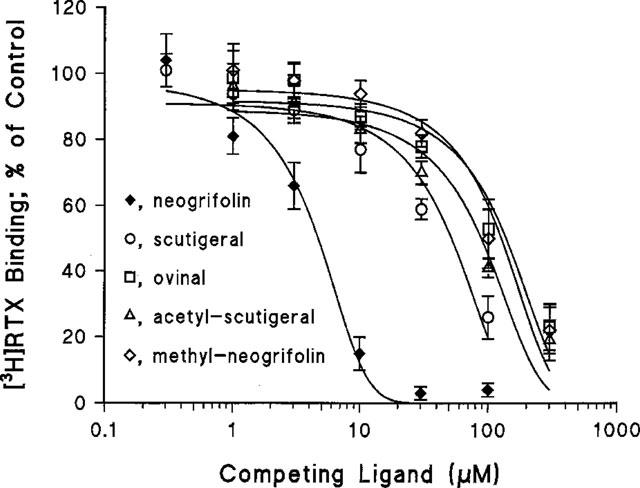
Inhibition of specific [3H]-resiniferatoxin (RTX) binding to rat spinal cord membranes by the triprenyl phenols neogrifolin, methyl-neogrifolin, scutigeral, acetyl-scutigeral, and ovinal. Data are from a single experiment performed using triplicate determinations. Curves were created by a computer fit of the Hill equation to the measured values. An additional 2–5 independent determinations gave similar results. See Table 1 for mean±s.e.mean affinities.
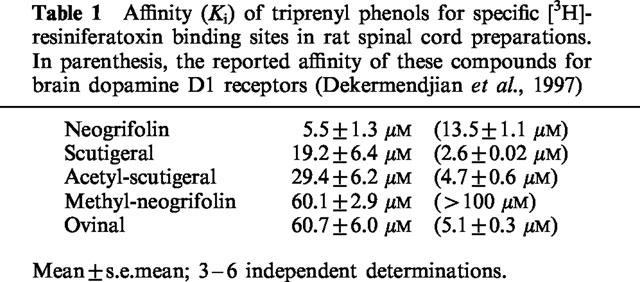
Table 1.
Affinity (Ki) of triprenyl phenols for specific [3H]-resiniferatoxin binding sites in rat spinal cord preparations. In parenthesis, the reported affinity of these compounds for brain dopamine D1 receptors (Dekermendjian et al., 1997)
In experiments in which the concentration of [3H]-RTX was varied, 10, 20, 40 and 80 μM scutigeral (structure shown in Figure 1) reduced the apparent affinity of specific binding sites for RTX from 28.8±3.5 to 39.0±2.1, 52.0±3.8, 65.9±9.3 and 81.3±7.5 pM (mean±s.e.mean, three determinations), respectively, without changing the Bmax values (71.6±4.5 fmol mg−1 protein in the absence of scutigeral; 70.1±8.3 fmol mg−1 protein in the presence of 10 μM scutigeral; 66.8±7.1 fmol mg−1 protein in the presence of 20 μM scutigeral; 69.2±6.9 fmol mg−1 protein in the presence of 40 μM scutigeral; and 69.4±11.2 fmol mg−1 protein in the presence of 80 μM scutigeral, respectively) (Figure 3). The Schild plot of the apparent binding affinities determined in the presence of various scutigeral concentrations had a slope of 0.8 and yielded an apparent Kd for scutigeral of 32.1 μM: this is in accord with a competitive binding mechanism. Moreover, the affinities for scutigeral determined in competition experiments (19.2 μM) and by the Schild plot (32.1 μM) are in good agreement.
Figure 3.
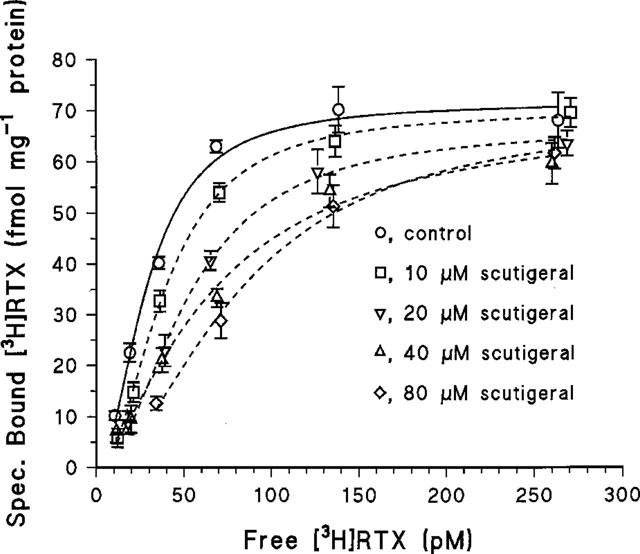
[3H]-resiniferatoxin (RTX) binding isotherms determined in the absence of scutigeral or in the presence of the following scutigeral concentrations; 10, 20, 40 and 80 μM. The Bmax values did not change significantly in the presence of scutigeral (see Results). As shown, values are from a single experiment performed using triplicates. The binding curves were determined by a fit of the allosteric Hill equation to the measured values. Two additional experiments yielded similar results; see Results for mean±s.e.mean values. The Schild plot of the mean apparent affinities had a slope of 0.8 and provided a Kd estimate for scutigeral of 32.1 μM.
Scutigeral-induced 45Ca2+ uptake by adult rat DRG neurons in culture
Scutigeral induced a dose-dependent, saturable 45Ca2+ uptake response by adult rat DRG neurons in culture with an EC50 of 1.6±0.3 μM (mean±s.e.mean, seven determinations; Figure 4A). The maximal 45Ca2+ uptake by scutigeral was similar to that evoked by capsaicin (Figure 4A), although further uptake was observed at high (>30 μM) concentrations of scutigeral (Figure 4B). The competitive vanilloid receptor antagonist capsazepine inhibited the 45Ca2+ influx response evoked by 10 μM scutigeral with an IC50 value of 5.2±2.1 μM (mean±s.e.mean, n=3) (Figure 5). Capsazepine (30 μM), however, did not prevent the additional 45Ca2+ uptake evoked by a high (100 μM) concentration of scutigeral.
Figure 4.

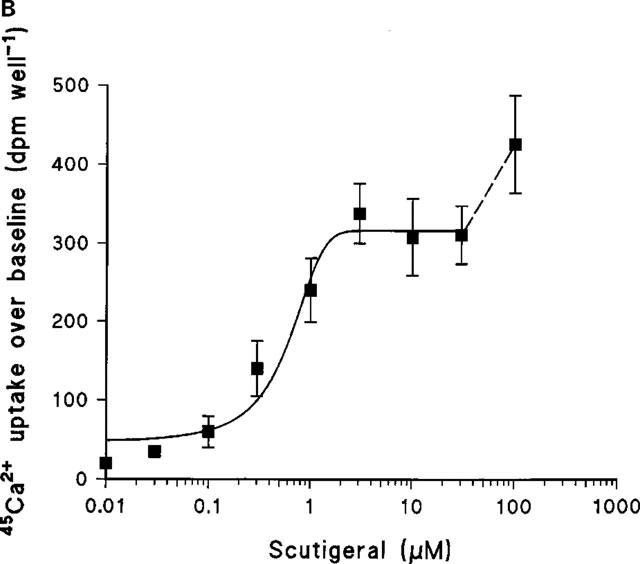
(A) Capsaicin- and scutigeral-induced 45Ca2+-uptake over baseline by rat dorsal root gangion neurons in culture. A representative experiment carried out in parallel; points are mean values of quintuple determinations; error bars indicate s.e.mean. For scutigeral, six additional experiments yielded similar results. (B) The scutigeral-induced 45Ca2+-influx is, in fact, bi-phasic. In addition to the first, saturable phase (which is mimicked by capsaicin and is inhibited by capsazepine), there is further uptake at high (>30 μM) scutigeral concentrations. Data (mean±s.e.mean) are from a single experiment.
Figure 5.
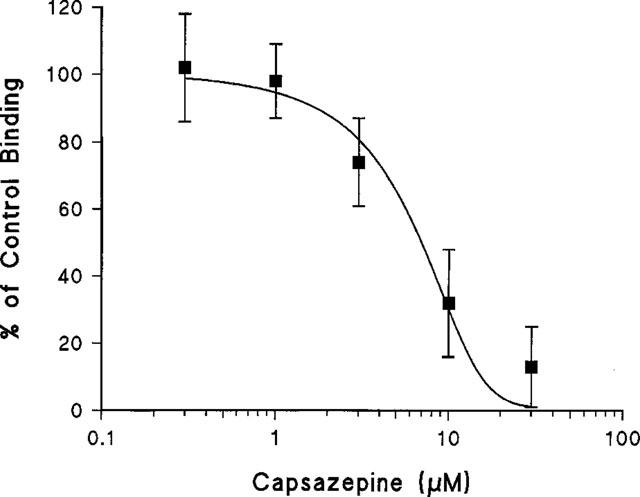
Inhibition by capsazepine of scutigeral (10 μM)-induced 45Ca2+-uptake over baseline by rat dorsal root ganglion neurons in culture. The curve was fitted using the Hill equation. As shown, the IC50 for capsazepine is 7.4 μM. Data are from a single experiment; mean±s.e.mean of quintuple determinations. Two additional experiments gave similar results.
Irritancy of scutigeral in the rat eye and on the human tongue
Scutigeral up to 100 nmol did not evoke a `hot' (or any other) sensation on the human tongue. Capsaicin and isovelleral, both at a dose of 2 nmol per tongue, were found to be pungent by the same experimenters. When instilled into the eye of rats, scutigeral, up to a concentration of 100 μM, provoked only few wiping movements with the forelegs (2±1 movements, mean±s.e.mean, five animals), which did not differ from the solvent-effect (1±1 wipings, mean±s.e.mean, five rats). The same rats, however, showed typical eye-wiping (16±5 wipings per rat, mean±s.e.mean; five rats) response upon subsequent challenge by 10 μM capsaicin.
Responses of voltage-clamped DRG neurons to scutigeral and capsaicin
A total of 20 DRG neurons of small to medium size (545–1235 μm2 in cross sectional area) were tested in the presence of scutigeral (100 nM–10 μM), but in no case did scutigeral elicit a detectable inward current. Nevertheless, 5 μM scutigeral did alter the responses of neurons to a subsequent capsaicin (1 μM) administration. Repeated applications of capsaicin to the same neuron led to strong tachyphylaxis, excluding the possibility of comparing responses to capsaicin before and after scutigeral challenge. To circumvent this problem, a comparison was done between populations of DRG neurons challenged with capsaicin (1 μM) only and tested with capsaicin (1 μM) following scutigeral (5 μM) treatment. These concentrations were chosen based on the 5 fold difference between capsaicin and scutigeral affinities in the RTX binding assay.
Previously, we found that 19 out of 31 DRG neurons (61%) displayed an inward current in response to capsaicin (Petersen et al., 1996). By contrast, only three out of the 20 neurons (15%) pretreated with 5 μM scutigeral responded to capsaicin in the present study. These findings indicate a significant reduction (chi2 test; P<0.025) in the proportion of DRG neurons that respond to capsaicin following scutigeral administration.
Figure 6A shows the lack of response in a DRG neuron to 1 μM scutigeral; the same neuron, however, did show an inward current in response to a subsequent application of 1 μM capsaicin. The maximal current in response to capsaicin occurred after 27 s in average and had a mean amplitude of 5.0±1.3 nA (mean±s.e.mean; three determinations). These values are similar to those determined without prior scutigeral application (not shown). Although an increase in the concentration of scutigeral to 5 μM did not result in any detectable inward currents, the parameters of a subsequent, capsaicin-evoked current changed substantially in two aspects (Figure 6B): first, the maximal current occurred later (72±12 s; mean±s.e.mean, n=3), and second, the current amplitude diminished from 5.0±1.3 to 1.9±0.7 nA (mean±s.e.mean, n=3).
Figure 6.
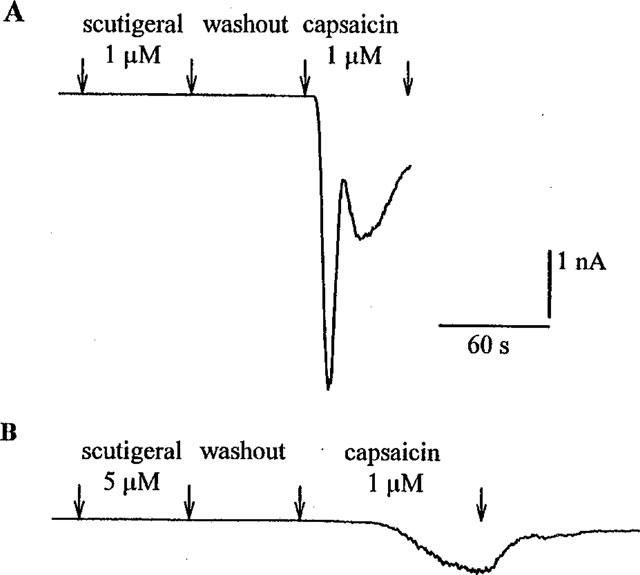
Responses of two rat dorsal root ganglion neurons held under voltage-clamp conditions to 1 μM capsaicin following scutigeral ((A) 1 μM; (B) 5 μM) administration. Note that the capsaicin-evoked current has two distinct components (a rapidly-activating current and a slowly-activating current) after pretreatment with the lower (1 μM) scutigeral concentration (upper panel). After administration of the higher (5 μM) scutigeral concentration, the capsaicin-evoked current becomes apparently monophasic (lower panel): the maximal current occurs later and is reduced from 4.4 to 0.8 nA. The resting membrane potentials were −60 mV (A) and −65 mV (B) and the cross-sectional areas were 809 μm2 (A) and 559 μm2 (B), respectively.
Discussion
Several compounds with affinity for vanilloid receptors have originally been isolated from natural sources. Prominent examples include capsaicin, the active ingredient in hot pepper, and resiniferatoxin (RTX), found in the irritant latex of E. resinifera, known as euphorbium. Both capsaicin and RTX are pungent and they share a (homo)vanillyl moiety essential for bioactivity (Jancsó, 1968; Szolcsányi, 1982; Szallasi & Blumberg, 1989). Unsaturated 1,4-dialdehydes and related terpenoids represent a third class of naturally occurring vanilloids (Szallasi et al., 1996; 1998). Although unsaturated dialdehydes are structurally dissimilar to capsaicin or RTX, they too are highly pungent (Kubo & Ganjian, 1981; Johnassohn & Sterner, 1997). As a matter of fact, such dialdehydes were long noted to have a `distinct, pepper-like taste' (Canonica et al., 1969) and gained culinary uses in exotic cuisines. The identification of pungent terpenoids as vanilloids (Szallasi et al., 1996 Szallasi et al., 1996) is of great importance in that it negates the concept of the (homo)vanillyl group being essential for vanilloid-like activity. In other words, this finding identifies the vanilloid receptor as a common target for a variety of structurally diverse compounds provoking a similar sensory modality rather than a receptor that recognizes a narrowly defined chemical structure.
Although the discovery that terpenoids are pungent by interacting at vanilloid receptors (Szallasi et al., 1996 Szallasi et al., 1996) clearly demonstrates the power of the human tongue to detect novel vanilloids, `tasting' plant extracts as a screening method is neither efficient nor safe. Therefore, in the present study, we have employed a [3H]-RTX binding assay utilizing rat spinal cord membranes to discover novel vanilloid structures and identified three fungal triprenyl phenols, neogrifolin, ovinal, and scutigeral, along with their semisynthetic derivatives methyl-neogrifolin and acetyl-scutigeral, as potential vanilloids. These compounds were originally isolated based on their affinity for dopamine D1 receptors, as determined by inhibition of specific [3H]-SCH 23390 binding to rat striatal membranes (Dekermendjian et al., 1997). In the dopamine D1 receptor assay, ovinal, scutigeral, and acetyl-scutigeral displayed similar potencies in the range of 2.6–5.1 μM, neogrifolin was somewhat less active with an IC50 of 13.5 μM, whereas methyl-neogrifolin was devoid of activity (Dekermendjian et al., 1997). For specific RTX binding sites, neogrifolin yielded the highest affinity (5.5 μM), whereas methyl-neogrifolin was similar in potency to ovinal, indicating that structure-activity relations are different for dopamine D1 and for vanilloid receptor binding.
Scutigeral evokes a 45Ca2+-uptake response, which is similar in magnitude to that evoked by capsaicin and is blocked by the competitive vanilloid receptor antagonist capsazepine; therefore, it is very likely that scutigeral opens the vanilloid receptor-operated cation channel. Under voltage-clamp conditions, however, no scutigeral-induced inward currents could be detected. We speculate that scutigeral gates the channel with prolonged kinetics compared to capsaicin or other pungent vanilloids. Thus, scutigeral, like olvanil, another non-pungent vanilloid (Sietsema et al., 1988; Brand et al., 1990), may cause a sustained increase in intracellular calcium concentrations (Liu & Simon, 1997b), which is not sufficient to generate action potentials but is able to block voltage-gated channels (Docherty et al., 1991; Liu & Simon, 1996). Interestingly, at high (>30 μM) concentrations scutigeral induces a further increase in 45Ca2+-uptake: this second phase is, however, not inhibited by capsazepine, thus it is probably not mediated by vanilloid receptors. It is also notable that scutigeral shows a 10- to 20 fold higher affinity in the calcium uptake assay (EC50=1.6 μM) compared to binding (19–32 μM determined by two different approaches). In this respect, scutigeral resembles capsaicin which evokes calcium uptake with a potency of 0.3 μM but inhibits RTX binding with a lower affinity of 4.9 μM (Ács et al., 1996). As yet, the reason for this discrepancy between vanilloid receptor binding and resulting calcium uptake is not known.
Under voltage-clamp conditions, capsaicin evokes two kinetically distinct currents, a rapidly- and a slowly-activating current, which may occur either separately or together in 61–73% of the primary sensory neurons tested (Liu & Simon, 1996; Liu et al., 1996; Petersen et al., 1996). The fast and slow components attain their maximal amplitudes after 5–12 s, and 35–45 s, respectively. Scutigeral (5 μM) significantly decreases (to 15%) the proportion of DRG neurons that respond to capsaicin. Furthermore, in those neurons that maintain their responsiveness to capsaicin, scutigeral delays the maximal current amplitude to 72 s. A possible interpretation of these findings is that scutigeral is able to prevent the capsaicin-evoked currents in some neurons and to abolish the first, rapidly-activating current in response to 1 μM capsaicin in other neurons, leaving the second, slowly-activating component both retarded and reduced. This model implies a differential sensitivity of the two components to scutigeral.
Desensitization of sensitive neuronal pathways by vanilloids is exploited to treat a variety of disease states ranging from urinary bladder hyperreflexia (Fowler et al., 1992; Barbanti et al., 1993) to diabetic neuropathy (Capsaicin Study Group, 1991). Pungency is a major limiting factor on the clinical use of capsaicin. As suggested by animal experimentation (Craft et al., 1993), RTX provides a more favourable ratio of desensitization to initial irritancy. In the treatment of urinary bladder hyperreflexia, topical RTX has been found to be clearly superior to capsaicin (Cruz et al., 1997; Lazzeri et al., 1997). RTX is not devoid of shortcomings, either. Most importantly, it is expensive to isolate from natural sources or to produce by complete synthesis (Wender et al., 1997). Regardless of the mechanism(s) that underlie the lack of irritancy, scutigeral is of interest because it represents the first member of a new structural class of non-pungent vanilloids (i.e. triprenyl phenols) and thus provides a new chemical lead for drug development.
Abbreviations
- DRGs
dorsal root ganglia
- DMEM
Dulbecco's Modified Eagle's Medium
- PPAHV
phorbol 12-phenylacetate 13-acetate 20-homovanillate
- RTX
resiniferatoxin
- TRP
transient release potential
- VR1
vanilloid receptor 1
References
- ÁCS G., LEE J., MARQUEZ V.E., BLUMBERG P.M. Distinct structure-activity relations for stimulation of 45Ca uptake and for high affinity binding in cultured rat dorsal root ganglion neurons and dorsal root ganglion membranes. Mol. Brain Res. 1996;35:173–182. doi: 10.1016/0169-328x(95)00204-6. [DOI] [PubMed] [Google Scholar]
- APPENDINO G., CRAVOTTO G., PALMISANO G., ANNUNZIATA R., SZALLASI A. Synthesis and evaluation of phorboid-20-homovanillates. Discovery of a class of ligands binding to the vanilloid receptor with different degrees of cooperativity. J. Med. Chem. 1996;39:3123–3131. doi: 10.1021/jm960063l. [DOI] [PubMed] [Google Scholar]
- APPENDINO G., SZALLASI A. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBANTI G., MAGGI C.A,. , BENEFORTI P., BAROLDI P., TURINI D. Relief of pain following intravesical capsaicin in patients with hypersensitive disorders of the lower urinary tract. J. Urol. 1993;71:686–694. doi: 10.1111/j.1464-410x.1993.tb16066.x. [DOI] [PubMed] [Google Scholar]
- BEVAN S., SZOLCSÁNYI J. Sensory neuron-specific actions of capsaicin; mechanisms and applications. Trends Pharmacol. Sci. 1990;11:330–335. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- BRAND L.M., SKARE K.L., LOOMANS M.E., RELLER H.H., SCHWEN R.J., LADE D.A., BOHNE R.L., MADDIN C.S., MOOREHEAD D.P., FANELLI R., CHIABRANDO C., CASTELLI M.G., TAI H.H. Antiinflammatory pharmacology and mechanism of the orally active capsaicin analogs, NE-19950 and NE-28345. Agents Actions. 1990;31:329–340. doi: 10.1007/BF01997628. [DOI] [PubMed] [Google Scholar]
- CANONICA L., CORBELLA P., GARIBOLDI P., JOMMI G., KREPINSKY J., FERRARI G., CASAGRANDE C. Sesquiterpenoids of Cinnamosma fragrans Baillon. Structure of cinnamolide, cinnamosmolide and cinnamodial. Tetrahedron. 1969;25:3895–3902. [Google Scholar]
- CAPSAICIN STUDY GROUP Treatment of painful diabetic neuropathy with topical capsaicin: a multi-centre, double-blind, vehicle-controlled study. Arch. Intern. Med. 1991;151:2225–2229. doi: 10.1001/archinte.151.11.2225. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMIGANA M., ROSEN T.A., LEVEIN J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E. TRP is cracked, but is CRAC TRP. Neuron. 1996;16:1069–1072. doi: 10.1016/s0896-6273(00)80132-4. [DOI] [PubMed] [Google Scholar]
- CRAFT R.M., CARLISI V.J., MATTIA A., HERMAN R.M., PORRECA F. Behavioral characterization of the excitatory and desensitizing effects of intravesical capsaicin and resiniferatoxin in the rat. Pain. 1993;55:205–215. doi: 10.1016/0304-3959(93)90149-J. [DOI] [PubMed] [Google Scholar]
- CRUZ F., GUIMARÃES M., SILVA C., REIS M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet. 1997;350:640–641. doi: 10.1016/S0140-6736(05)63330-2. [DOI] [PubMed] [Google Scholar]
- DEKERMENDJIAN K., SHAN R., NIELSEN M., STADLER M., STERNER O., WITT M.R. The affinity to the brain dopamine D1 receptor in vitro of triprenyl phenols isolated from the fruit bodies of Albatrellus ovinus. Eur. J. Med. Chem. 1997;32:351–356. [Google Scholar]
- DOCHERTY R.J., ROBERTSON B., BEVAN S. Capsaicin causes prolonged inhibition of voltage-activated calcium currents in adult dorsal root ganglion neurons in culture. Neurosci. 1991;40:513–521. doi: 10.1016/0306-4522(91)90137-d. [DOI] [PubMed] [Google Scholar]
- FOWLER C.A., JEWKES D., MCDONALD W.I., LYNN B., DE GROAT W.C. Intravesical capsaicin for neurogenic bladder dysfunction. Lancet. 1992;339:1239. doi: 10.1016/0140-6736(92)91186-c. [DOI] [PubMed] [Google Scholar]
- GILABERT R., MCNAUGHTON P. Enrichment of the fraction of nociceptive neurones in cultures of primary sensory neurones. J. Neurosci. Meth. 1997;71:191–198. doi: 10.1016/s0165-0270(96)00144-6. [DOI] [PubMed] [Google Scholar]
- HERGENHAHN M., ADOLF W., HECKER E. Resiniferatoxin and other esters of novel polyfunctional diterpenes from Euphorbia resinifera and unispina. Tetrahedron Lett. 1975;19:1595–1598. [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- JANCSÓ N.Desensitization with capsaicin and related acylamides as a tool for studying the function of pain receptors Pharmacology of Pain 196833, Oxford: Pergamon Press–55.eds. Lin, K., Armstrong, D. & Pardo, E.G.
- JANCSÓ N., JANCSÓ-GÁBOR A., TAKÁTS J. Pain and inflammation induced by nicotine, acetylcholine and structurally related compounds, and their prevention by desensitizing agents. Acta Physiol. Acad. Sci. Hung. 1961;19:113–131. [PubMed] [Google Scholar]
- JONASSOHN M., STERNER O. Terpenoid unsaturated 1,4-dialdehydes, occurrence and biological activities. Trends Org. Chem. 1997;6:23–43. [Google Scholar]
- KARRER T., BARTOSHUK L. Capsaicin desensitization and recovery on the human tongue. Physiol. Behav. 1991;49:757–764. doi: 10.1016/0031-9384(91)90315-f. [DOI] [PubMed] [Google Scholar]
- KUBO I., GANJIAN I. Insect antifeedant terpenes, hot-tasting to humans. Experientia. 1981;37:1063–1064. doi: 10.1007/BF02085009. [DOI] [PubMed] [Google Scholar]
- LAZZERI M., BENEFORTI P., TURINI D. Urodynamic effects of intravesical resiniferatoxin in humans: preliminary results in stable and unstable detrusor. J. Urol. 1997;158:2093–2096. doi: 10.1016/s0022-5347(01)68164-3. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Capsaicin-induced currents with distinct desensitization and Ca++ dependence in rat trigeminal ganglion cells. J. Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Similarities and differences in the currents activated by capsaicin, piperine and zingerone in rat trigeminal ganglion cells. J. Neurophysiol. 1997a;76:1858–1869. doi: 10.1152/jn.1996.76.3.1858. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. The responses of rat trigeminal ganglion neurons to capsaicin and two non pungent vanilloid receptor agonists: olvanil and glyceryl nonamide. J. Neurosci. 1997b;17:4101–4111. doi: 10.1523/JNEUROSCI.17-11-04101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L., SZALLASI A., SIMON S.A. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13-acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigeminal ganglion neurons. Neuroscience. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- LIU L., WANG Y., SIMON S.A. Capsaicin activated currents in rat dorsal root ganglion cells. Pain. 1996;64:191–195. doi: 10.1016/0304-3959(94)00097-2. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- MAGGI C.A. The pharmacology of the efferent function of sensory nerves. J. Auton. Pharmac. 1991;11:173–208. doi: 10.1111/j.1474-8673.1991.tb00317.x. [DOI] [PubMed] [Google Scholar]
- MARSH S.J., STANSFELD C.E., BROWN D.A., DAVEY R., MCCARTHY D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neurosci. 1987;23:275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- PETERSEN M., LAMOTTE R.H., KLUSCH A., KNIFFKI K.D. Multiple capsaicin-evoked currents in isolated rat sensory neurons. Neurosci. 1996;75:495–505. doi: 10.1016/0306-4522(96)00259-x. [DOI] [PubMed] [Google Scholar]
- SIETSEMA W.K., BERMAN E.F., FARMER R.W., MADDIN C.S. The antinociceptive effect and pharmacokinetics of olvanil following oral and subcutaneous dosing in the mouse. Life Sci. 1988;43:1385–1391. doi: 10.1016/0024-3205(88)90305-0. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., ÁCS G., CRAVOTTO G., BLUMBERG P.M., LUNDBERG J.M., APPENDINO G. A novel agonist, phorbol 12-phenylacetate 13-acetate 20-homovanillate, abolishes positive cooperativity of binding by the vanilloid receptor. Eur. J. Pharmacol. 1996b;299:221–228. doi: 10.1016/0014-2999(95)00864-0. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BÍRÓ T., MODARRES S., GARLASCHELLI L., PETERSEN M., KLUSCH A., VIDARI G., JONASSOHN M., DE ROSA S., STERNER O., BLUMBERG P.M., KRAUSE J.E. Dialdehyde sesquiterpenes and other terpenoids as vanilloids. Eur. J. Pharmacol. 1998;356:81–89. doi: 10.1016/s0014-2999(98)00514-7. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neurosci. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sci. 1990;47:1399–1408. doi: 10.1016/0024-3205(90)90518-v. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Molecular target size of the vanilloid (capsaicin) receptor in pig dorsal root ganglia. Life Sci. 1991;48:1863–1869. doi: 10.1016/0024-3205(91)90242-4. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. [3H]resiniferatoxin binding by the vanilloid receptor: species-related differences, effects of temperature and sulfhydryl reagents. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:84–91. doi: 10.1007/BF00168777. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., JONASSOHN M., ÁCS G., BÍRÓ T., ÁCS P., BLUMBERG P.M., STERNER O. The stimulation of capsaicin-sensitive neurones in a vanilloid receptor-mediated fashion by pungent terpenoids possessing an unsaturated 1,4-dialdehyde moiety. Br. J. Pharmacol. 1996a;119:283–290. doi: 10.1111/j.1476-5381.1996.tb15983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZALLASI A., LEWIN N.E., BLUMBERG P.M. Identification of α1-acid glycoprotein (orosomucoid) as a major vanilloid-binding protein in serum. J. Pharmacol. Exp. Ther. 1992;262:883–888. [PubMed] [Google Scholar]
- SZALLASI A., LEWIN N.E., BLUMBERG P.M. Vanilloid (capsaicin) receptors in the rat: positive cooperativity of resiniferatoxin binding and its modulation by reduction and oxidation. J. Pharmacol. Exp. Ther. 1993;266:678–683. [PubMed] [Google Scholar]
- SZOLCSÁNYI J.Capsaicin type pungent agents producing pyrexia Handbook of Experimental Pharmacology 1982Vol. 60Berlin Heidelberg: Springer-Verlag; 437–478.ed. Milton, A.S. [Google Scholar]
- SZOLCSÁNYI J., JANCSÓ-GÁBOR A. Sensory effects of capsaicin congeners. I. Relationship between chemical structure and pain-producing potency. Drug Res. 1975;25:1877–1881. [PubMed] [Google Scholar]
- SZOLCSÁNYI J., SZALLASI A., SZALLASI Z., JOÓ F., BLUMBERG P.M. Resiniferatoxin, an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 1990;255:923–928. [PubMed] [Google Scholar]
- TOMIGANA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- WENDER P.A., JESUDASON C.D., NAKAHIRA H., TAMURA N., TEBBE A.L., UENO Y. The first synthesis of a daphnane diterperne: the enantiocontrolled total synthesis of (+)-resiniferatoxin. J. Am. Chem. Soc. 1997;119:12976–12977. [Google Scholar]
- WOOD J.N., WINTER J., JAMES L.F., RANG H.P., YEATS J., BEVAN S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


