Abstract
Migraine headache pain is thought to result from an abnormal distention of intracranial, extracerebral blood vessels and the consequent activation of the trigeminal nervous system. Migraine is also often accompanied by extracranial sensory disturbances from facial tissues. These experiments investigate whether meningeal dilation produces central sensitization of neurones that receive convergent input from the face.
Single unit extracellular activity was recorded from the trigeminal nucleus caudalis of anaesthetized rats in response to either noxious stimulation of the dura mater, innocuous stimulation of the vibrissae or to a transient dilation of the meningeal vascular bed.
Rat α-CGRP (calcitonin gene-related peptide; 1 μg kg−1, i.v.) caused a dilation of the middle meningeal artery and facilitated vibrissal responses by 36±7%.
The 5-HT1B/1D agonist, L-741,604 (3 mg kg−1, i.v.), inhibited responses to noxious stimulation of the dura mater (16±7% of control) and, in a separate group of animals, blocked the CGRP-evoked facilitation of vibrissal responses.
L-741,604 (3 mg kg−1, i.v.) also inhibited responses to innocuous stimulation of the vibrissa (14±10% of control) with neurones that received convergent input from the face and from the dura mater, but not with cells that received input only from the face (70±12% of control).
These data show that dilation of meningeal blood vessels causes a sensitization of central trigeminal neurones and a facilitation of facial sensory processing which was blocked by activation of pre-synaptic 5-HT1B/1D receptors.
Sustained dural blood vessel dilation during migraine may cause a sensitization of trigeminal neurones. This may underlie some of the symptoms of migraine, such as the headache pain and the extracranial allodynia. Inhibition of this central sensitization may therefore offer a novel strategy for the development of acute and/or prophylactic anti-migraine therapies.
Keywords: 5HT1B/1D receptors, trigeminal nucleus caudalis, dura mater, migraine, central sensitization, electrophysiology
Introduction
Migraine headache is believed to involve a painful distention of cerebral and dural blood vessels. Mechanical, thermal or electrical stimulation of these structures produces sensations of intense, burning pain which is often referred to somatic loci, such as the orbit or forehead (Ray & Woolf, 1940; Coffey & Rhoton, 1993). In some cases, migraine headache can be accompanied by extracranial pain, usually of the neck and jaw, and an increased sensitivity to innocuous sensory stimuli, such as a facial hypersensitivity and a marked scalp tenderness (Dalessio & Silberstein, 1993).
Preclinical studies show that mechanical and electrical stimulation of the dura mater can evoke single unit, electrophysiological responses in the caudal trigeminal nucleus (Davis & Dostrovsky, 1986; Strassman et al., 1986; Kaube et al., 1992) and that there is a convergence of cranial and extracranial inputs onto the same neurones (Davis & Dostrovsky, 1986; Strassman et al., 1986). The extracranial sensory disturbances associated with migraine suggest that there is a convergence of nociceptive input from intracranial blood vessels with non-nociceptive input from extracranial tissues so that innocuous stimuli are perceived as painful. Indeed, clinical evidence for this effect has been reported by Thomsen et al. (1996) who found that decreased nociceptive thresholds in myofascial tissues accompanied nitroglycerin induced headache.
This may be the result of central sensitization, a phenomenon in which neurones of the spinal dorsal horn become hyper-excitable following an intense depolarizing stimulus such as might occur during peripheral inflammation (Woolf, 1983). When this sensitization occurs responses to noxious and innocuous stimuli become enhanced and there is poor discrimination of stimulus intensity or modality; this can last from minutes to hours. The observation that extracranial hyperalgesia and allodynia (of the face and scalp) often accompany migraine headache pain suggests that a central sensitization occurs in the trigeminal dorsal horn when neurones receive a prolonged noxious input from distended intracranial blood vessels.
Current theories propose that local vasodilation of intracranial extracerebral blood vessels, such as those supplying the dura mater, and a consequent stimulation of surrounding trigeminal perivascular afferent neurones are important mechanisms underlying migraine headache pain (Moskowitz, 1992). In addition to relaying nociceptive information, meningeal perivascular nociceptive afferents have also been suggested to release sensory neuropeptides (CGRP and substance P) which cause vasodilation and inflammation of pain-producing intracranial blood vessels. This integration between trigeminal sensory neurones and cranial blood vessels has been termed the ‘trigemino-vascular system'.
The ‘triptans' are a series of 5-HT1B/1D receptor agonists based upon the novel anti-migraine compound sumatriptan (Humphrey & Feniuk, 1991). Preclinical and clinical pharmacological studies suggest that the triptans have potentially three modes of action in the trigemino-vascular system to mediate their anti-migraine effects. First, the triptans are thought to have a vascular mechanism of action at 5-HT1B receptors to constrict painfully distended intracranial blood vessels by a direct action on the vascular smooth muscle (Humphrey & Feniuk, 1991; Friberg et al., 1991). Secondly, the triptans may also have a neurogenic mechanism of action by inhibiting the release of vasoactive neuropeptides (e.g. CGRP and/or substance P) from trigeminal perivascular afferent nerves, also by an action at 5-HT1B/1D receptors (Moskowitz, 1992; Williamson et al., 1997b). Thirdly, brain penetrant 5-HT1B/1D agonists may have an additional central mechanism of action that reduces nociceptive neurotransmission within the trigeminal sensory nuclei (Goadsby & Hoskin, 1996; Goadsby & Knight, 1997; Cumberbatch et al., 1997b, 1997c), possibly by inhibiting neuropeptide and/or glutamate release from the central terminals of trigeminal afferent fibres (Cumberbatch et al., 1997a).
Whilst several studies have now shown the central anti-nociceptive actions of brain penetrant triptans (Goadsby & Hoskin, 1996; Goadsby & Knight 1997; Cumberbatch et al., 1997b,1997c) the aims of the current study were to extend these findings by combining the putative vascular and neuronal components involved in migraine headache pain pathogenesis. Previous studies have shown that CGRP caused a potent dilation of the middle meningeal artery in the rat (Williamson et al., 1997a). We have used novel methodology combining electrophysiology and intravital microscopy to simultaneously monitor the changes in dural blood vessel diameter and trigeminal neuronal activity in anaesthetized rats following systemic CGRP. The primary goals of this study were to investigate if dilation of the middle meningeal artery could influence trigeminal neuronal activity and generate a central sensitization and if these nociceptive inputs from the dura mater could be blocked by a 5-HT1B/1D agonist. Secondary objectives were to ascertain the role of 5HT1B/1D receptors in the processing of non-nociceptive information as a comparison with nociceptive neurotransmission from the dura mater. These objectives were addressed using four experimental protocols: (1) to test the effects of meningeal vasodilation on trigeminal neuronal activity and on convergent responses to innocuous stimulation of the face; (2) to test the effects of a 5-HT1B/1D agonist on responses of trigeminal neurones to noxious stimulation of the dura mater; (3) to test the effects of a 5-HT1B/1D agonist on any vasodilation induced changes in sensory processing of these neurones; and (4) to test the direct effects of a 5-HT1B/1D receptor agonist on responses of trigeminal neurones to innocuous stimulation of the face. The 5-HT1B/1D agonist, L-741,604 (Sternfeld et al., 1996), was used in these studies, since it has been shown to have high affinity at rat 5-HT1B/1D receptors and is brain penetrant (unpublished observations); see Sternfeld et al. (1996) for receptor affinities compared to clinically effective ‘triptan' 5HT1B/1D receptor agonists. Some of these data have been published previously in abstract form (Cumberbatch et al., 1997a,1997d; Williamson et al., 1998).
Methods
Animal preparation
All experiments were conducted and terminated under general anaesthesia in accordance with a project licence issued by the U.K. Home Office under the Animals (Scientific Procedures) Act 1986. Male Sprague-Dawley rats (320–375 g; n=30) were anaesthetized with halothane (1–2% in O2, n=14) or with sodium pentobarbitone (80 mg kg−1, i.v., n=16). The trachea was cannulated to provide artificial ventilation and to monitor end tidal CO2. Either the carotid artery and jugular veins (14 rats) or the femoral artery and veins (16 rats) were cannulated to monitor blood pressure and to administer drugs and/or anaesthetics. The brainstem was exposed by retracting the overlying muscle and removing the atlanto-occipital membrane. The skull was thinned using a saline cooled drill to form a transparent layer of intact bone over the middle meningeal artery (MMA). A tungsten bipolar stimulating electrode was positioned on the thinned surface of the skull adjacent to the MMA using a micromanipulator. All exposed surfaces were covered with mineral oil to prevent desiccation. Anaesthesia was maintained with sodium pentobarbitone (constant i.v. infusion of 20–30 mg kg−1 h−1) and neuromuscular blockade was initiated and maintained with pancuronium bromide (1 mg kg−1 h−1). General anaesthesia was monitored throughout and was ensured by the absence of any cardiovascular and pupillary responses to noxious stimuli. Body temperature was maintained between 36–37.5°C using a feedback controlled homeothermic blanket system. Animals were killed with an overdose of pentobarbitone at the end of the experiment.
Dural blood vessel measurements
This methodology has been described in detail previously (Williamson et al., 1997a). In brief, an intravital microscope was positioned over a closed thin cranial window to allow visualization and tracking of the middle meningeal artery. Arterial diameter was continuously monitored using a video dimension analyzer and plotted on computer for subsequent analysis.
Single unit extracellular recordings from the trigeminal nucleus caudalis
Extracellular action potentials were recorded from single caudal trigeminal neurones using a glass microelectrode filled with 0.5 M NaCl (4–5 MΩ). The signal was amplified (1 k) and filtered (500 Hz–5 kHz band-width) and action potentials were displayed on oscilloscopes to enable accurate isolation of a single unit from adjacent cell activity. Cells were selected for study that responded to vibrissal stimulation and that could be classified as low threshold mechanoreceptive (LTMR) in terms of their facial receptive field, irrespective of nociceptive input from the dura mater. Receptive field characteristics were determined using brief (0.5–1 s) applications of a directable air-jet to deflect vibrissae and a blunt probe (2 mm tip diameter for up to 5 s) to apply maintained pressure to the skin of the lower face and vibrissal pad. Neurones were considered to have LTMR receptive fields if they responded to innocuous stimulation (i.e. air-jet or light stroking) of the vibrissae and/or surrounding face and if they had rapidly adapting responses to maintained pressure (from a blunt probe). Neurones that responded with sustained firing to application of constant noxious pressure or failed to respond to vibrissal stimulation were rejected. After determining the facial receptive field an electrical stimulus (1–3 mA; 100–200 μs) was applied to the dura mater, through the closed cranial window, to determine if the cell received convergent input. Action potentials were counted, using a window discriminator, in response to either noxious electrical stimulation of the dura mater (trains of 20×1–3 mA, 100–200thinsp;μs stimuli applied at 1 Hz) or to innocuous stimulation of the vibrissa (trains of 10×100 ms air-jet pulses at 1 Hz). Stimuli were applied in repeated cycles until responses were stable. The interval between stimuli was either 1 min for air-jet or 3 min for electrical stimuli.
Experimental protocols
Protocol 1: To determine the effects of CGRP-evoked dural vasodilation on trigeminal neuronal activity
This protocol examined the effects of CGRP-evoked meningeal vasodilation on the responses of convergent trigeminal neurones to vibrissal stimulation. Air-jet stimuli were cycled until the responses were stable, then CGRP was administered i.v. at 1 μg kg−1 to evoke a meningeal vasodilation. Neuronal activity was monitored constantly and any changes were calculated as a percentage of the mean of the three control responses immediately before CGRP administration. For each cell the effects of CGRP were calculated from the three responses that coincided with the recovery phase of the vasodilation.
Protocol 2: To determine the effects of 5-HT1B/1D receptor activation on dural nociceptive neurotransmission
The second protocol examined the effects of the 5HT1B/1D agonist, L-741,604, on responses to noxious stimulation of the dura mater. Regular cycles of noxious electrical stimuli (trains of 20 stimuli at 1–3 mA and 100–200 μs) were applied to the dura mater every 3 min until responses were stable. L-741,604 was administered in a cumulative dose regimen of 0.3, 1 and 3 mg kg−1 (i.v.) and the drug effects were calculated as a percentage of the mean of three pre- L-741,604 control responses.
Protocol 3: To determine the effects of 5-HT1B/1D receptor activation on the central sensitization following CGRP-evoked dural vasodilation
In separate experiments, L-741,604 (3 mg kg−1, i.v.) was administered as a pre-treatment immediately after surgery and prior to locating a neurone. CGRP (1 μg kg−1) was administered to evoke a dilation of the middle meningeal artery and the effects on vibrissal responses were compared with those of the control group described in Protocol 1.
Protocol 4: To determine the effects of 5-HT1B/1D receptor activation on responses to innocuous stimulation of the face
The fourth protocol examined the effects of the 5-HT1B/1D agonist, L-741,604 on the responses evoked by innocuous mechanical stimulation of the vibrissae. Stimuli were cycled until responses were stable, then L-741,604 was administered i.v. in a cumulative dose regimen of 0.3, 1 and 3 mg kg−1. Two populations of neurones were studied, one group of cells that received convergent inputs from the dura mater and the face and another group that only received inputs from the face. Drug effects were calculated as the percentage inhibition relative to the mean of the three pre-drug control responses.
Statistics
All data are expressed as means±s.e.mean. Statistical tests were performed using either a 2-way ANOVA with repeated measures or a t-test; P⩽0.05 was considered to be significant.
Drugs
L-741,604 was used as a selective 5-HT1B/1D agonist (N,N-Dimethyl - 2 - [ 5- (1,2,4-Triazol-4-yl)-1H-indol-3-yl]ethylamine; Sternfeld et al., 1996). Rat-αCGRP (calcitonin gene-related peptide; Cambridge Research Biochemicals) was aliquoted (1 μg ml−1) and frozen at −70°C and defrosted immediately before use. All drugs were dissolved in sterile isotonic saline and dosed in a volume of 1 ml kg−1. Doses of L-741,604 are expressed as base weight.
Results
Cell data
Extracellular single unit electrophysiological recordings were made from 44 cells in the trigeminal dorsal horn of 30 rats. Cells were located 1–2 mm caudal to obex and at a depth of 800±45 μm (range of 240–1800 μm) from the pial surface. Cells within this range have been histologically verified, in a representative series of experiments, to be located in the trigeminal nucleus caudalis (unpublished observations). Of these cells, 37 responded to electrical stimulation (1–3 mA, 200 μs pulse width) of the dura mater with latencies of 2–14 ms (mean of 3.8±0.5 ms) and also had convergent inputs from the face. Seven cells had input exclusively from the face. Cutaneous receptive fields were mainly in the lower facial region and all cells were classified as LTMR and responded to innocuous stimulation of the vibrissa.
Protocol 1: Effects of CGRP-induced vasodilation on neuronal responses
Intravenous CGRP (1 μg kg−1) caused a robust vasodilation of the middle meningeal artery in 16 rats. The magnitude of this dilation was 190±7% of the pre-CGRP resting control and the mean duration was 5.5±0.3 min. A representative example can be seen in Figure 1A and the mean data from the ten rats used for the control group are shown in Figure 1B. CGRP also caused a transient decrease in mean arterial blood pressure from 113±4 to 87±3 mmHg. This hypotension was shorter than the vasodilation (3.7±0.2 min).
Figure 1.
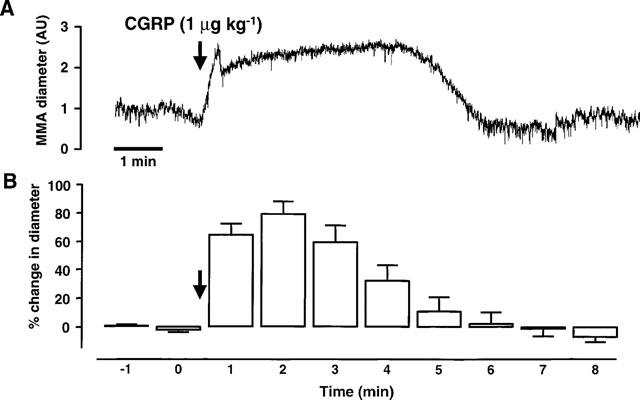
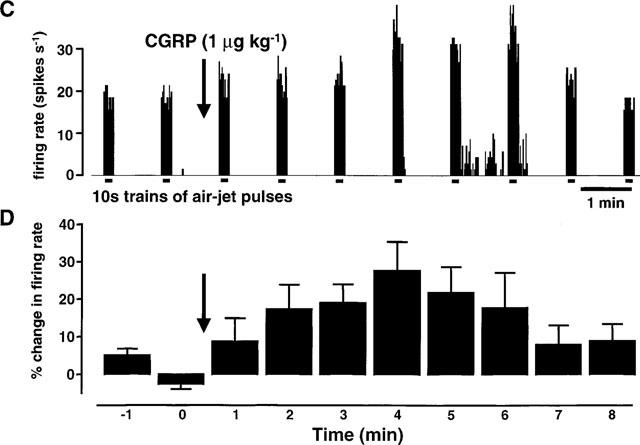
This figure shows the effects of rat-αCGRP (1 μg kg−1; i.v.) on the middle meningeal artery (MMA) diameter and on neuronal activity from the trigeminal dorsal horn. The top trace (A) shows an original experimental record of the MMA diameter in arbitrary units (AU). The mean responses from 12 CGRP applications in ten rats (B) are displayed as the percentage change in MMA diameter relative to the pre-CGRP control period. The effects of CGRP-evoked vasodilation on trigeminal neuronal activity are shown in the third trace (C). In this panel extracellular, single unit action potentials are displayed as the firing rate (spikes s−1) response to innocuous stimulation of the vibrissae using an air-jet. The mean data (D) from 12 cells are shown as the percentage change in neuronal activity relative to the mean of three pre-CGRP stable controls. (A) and (C) are time-matched data from the same experiment and (B) and (D) are mean data from simultaneous recordings of MMA diameter and neuronal activity following application of CGRP.
Innocuous stimulation of the vibrissa evoked reproducible and stable firing activity with cells that received convergent inputs from the face and from the dura mater. Figure 1C shows the ratemeter recording from a convergent neurone that responded to innocuous stimulation of the vibrissa. CGRP (1 μg kg−1, i.v.) evoked a dilation of the middle meningeal artery that facilitated responses to vibrissal stimulation, reaching a maximum of 170% of control 4 min after the dose. This value was calculated from the mean of the three responses at the end phase of the dilation, min 4, 5 and 6 (Figure 1C). The facilitation was sustained for 3 min beyond the duration of the dilation before returning to control values. This time course of response was typical throughout the experiments.
A total of 13 durally convergent cells were tested using this protocol. Vibrissal responses were stable and varied by only±5% from the mean baseline prior to CGRP. Following administration of CGRP responses to vibrissal stimulation were facilitated by >5% with nine cells (mean 136±7% of control), there were no changes with three cells (mean 100±2% of control) and with one cell vibrissal responses were reduced to 31% of control. Because of the atypical response, the cell that responded with reduced firing after CGRP was not included in the mean analysis. Figure 1D shows the mean data from the 12 cells with which there was either facilitation or no change in the responses to vibrissal stimulation. As with the representative cell in Figure 1C the period of maximum facilitation corresponds with the end of the vasodilation, 4.8±0.3 min after the CGRP injection.
The vasodilation did not evoke any significant neuronal activity per se. Only two cells displayed spontaneous activity prior to CGRP and only three cells had background activity after the dilation; this activity was 0.2–2.4 spikes s−1 (n=3).
The effects of CGRP-evoked dural vasodilation was also tested on four cells that received input from the vibrissa but did not respond to electrical stimulation of the dura mater. CGRP-evoked vasodilation (248±16% of control) caused no change in neuronal activity (100±5% of control) with these cells.
Protocol 2: Effects of L-741,604 on responses to dural stimulation
With neurones that received convergent input from the face and from the dura mater, responses to noxious electrical stimulation of the dura mater were dose dependently inhibited by L-741,604 at 0.3, 1 and 3 mg kg−1 (n=6; Figure 2). Figure 2A shows example responses from a single neurone and Figure 2B shows the mean data. Approximately 30 min (28±2 min) after the 3 mg kg−1 dose the responses were maximally inhibited by 84±7%.
Figure 2.
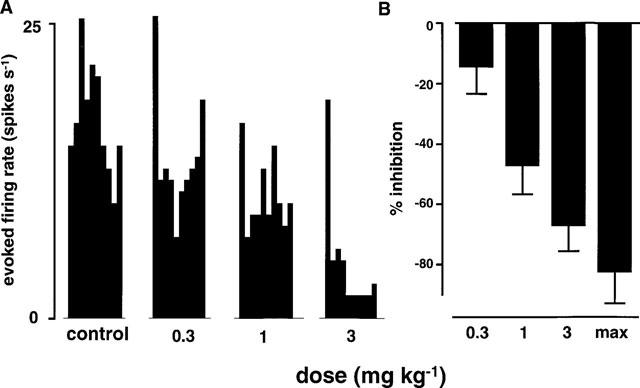
This figure shows the effects of the 5HT1B/1D agonist, L-741,604 (0.3, 1 and 3 mg kg−1), on the trigeminal responses to noxious stimulation of the dura mater. Effects of L-741,604 on responses of a single trigeminal neurone are shown in the left panel as post-stimulus histograms (A) of electrically evoked action potentials (spikes s−1). The mean data (n=6) are shown in the left panel (B) and are expressed as the percentage inhibition relative to the mean of three pre-L-741,604 control responses.
Protocol 3: Effects of L-741,604 on the central sensitization following CGRP-evoked dural vasodilation
A separate group of five animals were given the 5-HT1B/1D agonist L-741,604 (3 mg kg−1, i.v.) immediately after surgery. Thirteen cells were studied, all of which received convergent input from the dura mater and the vibrissa. Figure 3 shows the effects of CGRP on the diameter of the MMA and on the neuronal responses evoked by innocuous stimulation of the vibrissa. CGRP dilatated the MMA by the same amount as in the control group (see Figure 1). However, the dilation had no effect on the vibrissal responses with the group pre-treated with L-741,604. There was a highly significant difference in the effects of CGRP-evoked vasodilation on neuronal activity when the L-741,604 group was compared with the control group (P<0.0005; compare Figures 1D and 3B).
Figure 3.
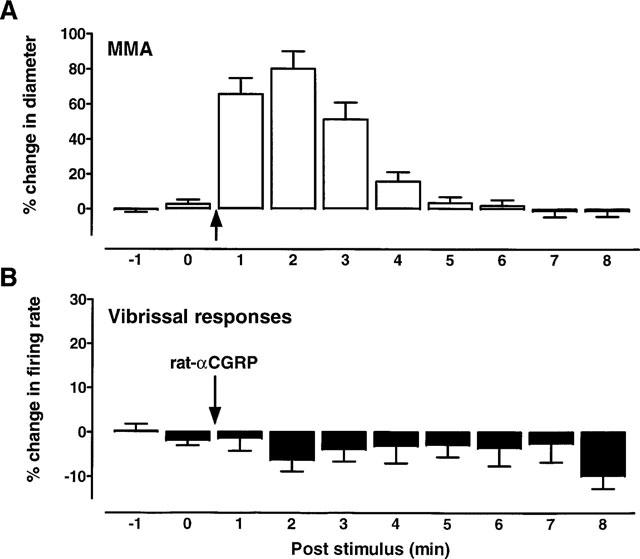
The effects of CGRP on the middle meningeal artery (MMA) and on trigeminal neuronal activity were tested in animals pre-treated with the 5HT1B/1D agonist, L-741,604 (n=5 rats; 3 mg kg−1; i.v.). (A) shows the effects of CGRP (1 μg kg−1 i.v.; arrow) on the diameter of the MMA (n=13) and (B) shows the effects of this dilation on trigeminal neuronal responses to vibrissal stimulation (n=13 cells). Data are expressed as the percentage inhibition relative to the stable pre-CGRP control period.
Protocol 4: Effects of the 5-HT1B/1D agonist L-741,604 on vibrissal responses
Single unit responses to regular cycles of innocuous vibrissal stimulation were tested with L-741,604. Figure 4A shows a typical trace from a single neurone that received input from both the dura mater and the vibrissa. Repeated innocuous stimulation of the vibrissa evoked regular, reproducible responses that were dose dependently inhibited by L-741,604 at 0.3, 1 and 3 mg kg−1, i.v. In contrast, Figure 4B shows an experimental record from a neurone that received input from the face only. With this cell, responses to vibrissal stimulation were unaffected by L-741,604 at the same doses. In both cell types these response profiles were consistent in that non-nociceptive responses were inhibited significantly (P<0.05) more with cells that received convergent input from the dura and from the vibrissa (n=5 cells from five rats; Figure 4C), than those cells that did not receive dural input (n=3 cells from three rats; Figure 4D).
Figure 4.
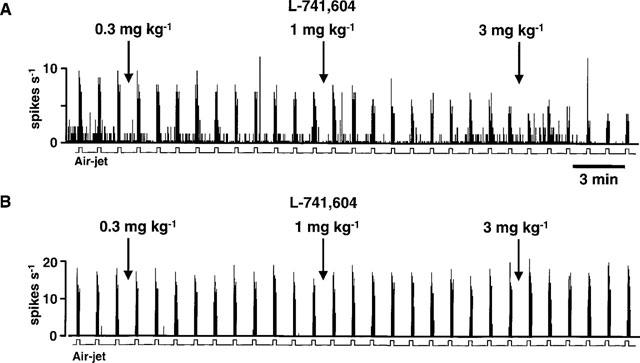
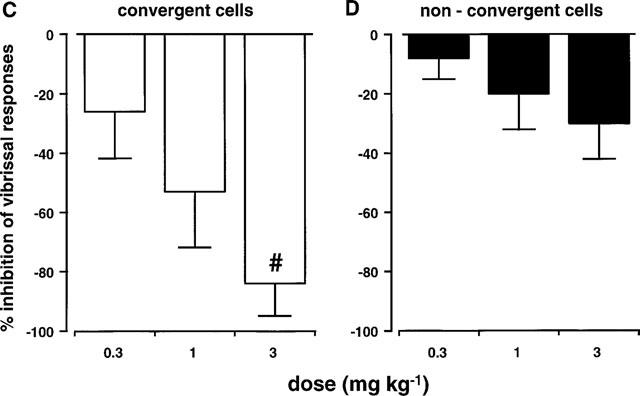
(A) and (B) show the effects of the 5HT1B/1D agonist, L-741,604 (0.3, 1 and 3 mg kg−1; i.v.), on responses to stimulation of the vibrissae from a cell that also received convergent nociceptive input from the dura mater (A) and from a cell that received facial inputs only (B). The mean data for the effects of L-741,604 on responses to vibrissal stimulation can be seen in (C) and (D). All convergent cells (C; n=5) received inputs from the face and from the dura mater. Non-convergent cells (D; n=3) received no dural inputs. These mean data are expressed as a percentage of the mean of three pre-L-741,604 stable control responses. There was a significant difference (#P<0.05) between the two groups at 3 mg kg−1.
Discussion
Migraine is characterized by a throbbing headache, probably due to distention of cranial blood vessels, and by an extracranial allodynia which is thought to result from convergence of nociceptive and non-nociceptive inputs within the trigeminal nucleus caudalis (Thomsen et al., 1996). The current experiments support this hypothesis since they have demonstrated that dilation of the meningeal vascular bed, such as may occur during migraine headache, results in a sensitization of the trigeminal nucleus caudalis and a facilitation of convergent sensory responses. Moreover, we have also shown that 5-HT1B/1D receptor activation can block transmission of inputs from the dura mater and the development of the central sensitization. These data may provide further understanding of the trigeminal processing that underlies migraine headache and the central mechanism of action of the brain penetrant 5-HT1B/1D agonist anti-migraine compounds.
Effects of meningeal vasodilation on trigeminal neuronal activity
The present studies show that dilation of meningeal blood vessels can cause a central sensitization of trigeminal neurones and thereby facilitate non-nociceptive central sensory transmission mediated by these cells. These experiments are in agreement with those of Burstein et al. (1998) which show that chemical stimulation of the dura mater with an ‘inflammatory soup' resulted in decreased mechanical and thermal thresholds and increased facial cutaneous receptive fields, again consistent with a central sensitization. Under the conditions of the present experiment vasodilation of dural blood vessels by the potent vasoactive neuropeptide CGRP did not evoke neuronal firing in cells within the trigeminal nucleus caudalis per se. However, these studies utilized extracellular recording techniques and we cannot discount the possibility that intracellular recordings may have shown sub-threshold changes in neuronal activity. Indeed, sub-threshold inputs from cutaneous regions have been proposed to be involved in receptive field expansion of neurones in the spinal dorsal horn (Woolf & King, 1987). It is interesting to note that under the conditions of the current experiments it was difficult to find cells that did not receive convergent inputs from the face and from the dura mater. This is reflected in the relatively low number of non-convergent cells used in this study.
An important observation is the time lag between the vasodilation and the sensitization (see Figure 1A); neurones became maximally sensitized after the peak increase in vessel diameter and remained in a hyper-responsive state for a period that exceeded the vasodilation. This progressive build up of the sensitization may reflect a gradual increase in sub-threshold inputs from the dural perivascular afferents that innervate the distended blood vessels. A similar event occurs during the wind-up of spinal dorsal horn neurones. Following a train of noxious electrical stimuli, neuronal responses become progressively enhanced after each sequential stimulus and remain facilitated for a few minutes after cessation of the conditioning stimulus (Mendell, 1966). Sensitization of central trigeminal neurones during a migraine attack could be much more pronounced and maintained due to a more prolonged vasodilation than has been observed in the present studies. This sensitization may represent a critical process in the pathogenesis of migraine and may underlie the symptoms of extracranial allodynia and hyperalgaesia associated with migraine headache pain.
Effects of the 5-HT1B/1D agonist, L-741,604
Noxious electrical stimulation of the dura mater evoked trigeminal neuronal responses that were potently inhibited by L-741,604. This is consistent with previous studies using brain penetrant anti-migraine 5-HT1B/1D agonists (Goadsby & Hoskin, 1996; Goadsby & Knight, 1997; Cumberbatch et al., 1997a, 1997b, 1997c). Although the 5-HT1B/1D agonists are known to be vasoconstrictors, the inhibition of trigeminal responses by L-741,604 is unlikely to be a consequence of a direct constriction of dural blood vessels and removal of a tonic excitatory drive since, under the conditions of these experiments, L-741,604 did not affect the resting diameter of the middle meningeal artery when administered at 3 mg kg−1 i.v. (data not shown). Both rizatriptan (Williamson et al., 1997c) and sumatriptan (Williamson et al., 1997b) also had no effect on resting meningeal blood vessel diameter in rats. Since the 5HT1B/1D agonist, sumatriptan, has been shown to have no effect on trigeminal ganglion neurones (O'Shaughnessy et al., 1993), the actions of L-741,604 in the current experiments were unlikely to have been on peripheral neurones and were presumably within the trigeminal nucleus caudalis.
The results from the current study are consistent with an activation of pre-synaptic 5HT1B/1D receptors on dural nociceptive afferents and an inhibition of neurotransmitter release in the trigeminal nucleus caudalis. Responses to innocuous stimulation of the vibrissa were inhibited by L-741,604, but only with cells that received convergent input from the dura mater. This suggests that there was a maintained sub-threshold input from the meninges which produced a tonic facilitation of facial responses and that this meningeal tone was reduced by activation of 5-HT1B/1D receptors. In addition, L-741,604 also blocked the facilitation of vibrissal responses by CGRP-evoked dilation of the meningeal blood vessels. These experiments suggest that 5-HT1B/1D receptors are located pre-synaptically on meningeal nociceptive primary afferents, but not on non-nociceptive extracranial fibres, since L-741,604 had no effect on non-convergent neurones. A post synaptic site of action is unlikely because anatomical studies show that 5HT1B/1D receptors are located in trigeminal ganglia and on primary afferent neurones in the nucleus caudalis, but not on cell bodies (Longmore et al., 1997). These observations are also consistent with pre-clinical studies which show that 5-HT1B/1D receptor activation inhibited dural, but not facial cutaneous, neurogenic extravasation (Buzzi et al., 1995; Shepheard et al., 1995).
The current experiments did not include an assessment of cells that responded to noxious facial stimulation, but previous experiments have shown that another 5-HT1B/1D agonist, naratriptan, inhibited trigeminal mechanical nociceptive responses (Cumberbatch et al., 1997b). Interestingly, naratriptan had no effect on spinal responses to similar intensities of noxious mechanical stimuli. This is consistent with the clinical observations that the anti-migraine triptans are not generally analgaesic (Dao et al., 1995; Roberts-Thomson et al., 1996) and supports the notion that 5-HT1B/1D receptors may be specifically involved in the modulation of dural nociceptive processing.
These data show that dilation of meningeal blood vessels evokes a sensitization of central trigeminal neurones that may underlie the symptoms of extracranial pain and allodynia that often accompany migraine headache. 5-HT1B/1D receptors may specifically modulate neurotransmission of dural afferent fibres to prevent the development of the central sensitization and thus may offer a new target for acute and prophylactic treatment of migraine and other vascular headaches.
Acknowledgments
The authors would like to thank F. Sternfeld and the Department of Medicinal Chemistry, Terlings Park, for synthesizing L-741,604.
Abbreviations
- LTMR
low threshold mechanoreceptive
- MMA
middle meningeal artery
References
- BURSTEIN R., YAMAMURA H., MALICK A., STRASSMAN A.M. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–82. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- BUZZI M.G., BONAMINI M., MOSKOWITZ M.A. Neurogenic model of migraine. Cephalalgia. 1995;15:277–280. doi: 10.1046/j.1468-2982.1995.1504277.x. [DOI] [PubMed] [Google Scholar]
- COFFEY R.J., RHOTON A.L.Pain-sensitive cranial structures Woolf's Headache 1993Oxford University Press; 19–41.ed. Dalessio, D.J. & Silberstein, S.D. [Google Scholar]
- CUMBERBATCH M.J., HEFTI F.F., HILL R.G., HARGREAVES R.J. Drug-induced modulation of dural nociceptive neurotransmission. Proc. Soc. Neurosci. 1997a;27:601.17. [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. Differential effects of naratriptan on spinal vs. trigeminal nociceptive responses. Cephalalgia. 1997b;17:381. doi: 10.1046/j.1468-2982.1998.1810659.x. [DOI] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. Rizatriptan has central antinociceptive effects against durally evoked responses. Eur. J. Pharmacol. 1997c;328:37–40. doi: 10.1016/s0014-2999(97)83024-5. [DOI] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., MASON G. S., HILL R.G., HARGREAVES R.J. Trigeminal neurones that receive non-nociceptive vibrissal input in the anaesthetized rat: effects of convergent nociceptive input from the dura mater and of a 5-HT1B/1D agonist. J. Physiol. 1997d;504P:103P–104P. [Google Scholar]
- DALESSIO D.J., SILBERSTEIN S.D.Diagnosis and classification of headache Wolffs Headache 1993Oxford University Press; 3–18.eds. Dalessio, D.J. & Silberstein, S.D. [Google Scholar]
- DAO T.T., LUND J.P., REMILLARD G., LAVIGNE G.J. Is myofascial pain of the temporal muscles relieved by oral sumatriptan? A cross-over pilot study. Pain. 1995;62:241–244. doi: 10.1016/0304-3959(95)00025-N. [DOI] [PubMed] [Google Scholar]
- DAVIS K.D., DOSTROVSKY J.O. Activation of trigeminal brain-stem nociceptive neurons by dural artery stimulation. Pain. 1986;25:395–401. doi: 10.1016/0304-3959(86)90244-7. [DOI] [PubMed] [Google Scholar]
- FRIBERG L., OLESEN J., IVERSEN H.K., SPERLING B. Migraine pain associated with middle cerebral artery dilation: reversal by sumatriptan. Lancet. 1991;338:13–17. doi: 10.1016/0140-6736(91)90005-a. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., HOSKIN K.L. Inhibition of trigeminal neurones by intravenous administration of the serotonin (5-HT)1B/D receptor agonist zolmitriptan (311C90): are brain stem sites therapeutic target in migraine. Pain. 1996;67:355–359. doi: 10.1016/0304-3959(96)03118-1. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., KNIGHT Y.E. Inhibition of trigeminal neurones after intravenous administration of naratriptan through an action at 5-hydroxy-tryptamine (5-HT1B/1D) receptors. Br. J. Pharmacol. 1997;122:918–922. doi: 10.1038/sj.bjp.0701456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY P.P., FENIUK W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol. Sci. 1991;12:444–446. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- KAUBE H., HOSKIN K.L., GOADSBY P.J. Activation of the trigeminovascular system by mechanical distention of the superior sagittal sinus in the cat. Cephalalgia. 1992;12:133–136. doi: 10.1046/j.1468-2982.1992.1203133.x. [DOI] [PubMed] [Google Scholar]
- LONGMORE J., SHAW D., SMITH D., HOPKINS R., MCALLISTER G., PICKARD J.D., SIRINATHSINGHJI D.J., BUTLER A.J., HILL R.G. Differential distribution of 5-HT1D- and 5-HT1B-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia. 1997;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- MENDELL L.M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp. Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol. Sci. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- O'SHAUGHNESSY C., CONNOR H.E., FENIUK W. Extracellular recordings of membrane potentials from guinea-pig isolated trigeminal ganglion: lack of effect of sumatriptan. Cephalalgia. 1993;13:175–179. doi: 10.1046/j.1468-2982.1993.1303175.x. [DOI] [PubMed] [Google Scholar]
- RAY B.S., WOLFF H.G. Experimental studies on headache. Archives of Surgery. 1940;41:813–855. [Google Scholar]
- ROBERTS-THOMSON I., ARGYRIDES J., PANNALL P., FREWIN D. Sumatriptan and episodic pain syndromes other than migraine. Pain. 1996;67:226–227. doi: 10.1016/0304-3959(96)03144-2. [DOI] [PubMed] [Google Scholar]
- SHEPHEARD S.L., WILLIAMSON D.J., BEER M.S., HILL R.G., HARGREAVES R.J. Differential effects of 5-HT1B/1D receptor agonists on neurogenic dural plasma extravasation and vasodilation in anaesthetized rats. Neuropharmacology. 1997;36:525–533. doi: 10.1016/s0028-3908(97)00057-9. [DOI] [PubMed] [Google Scholar]
- SHEPHEARD S.L., WILLIAMSON D.J., WILLIAMS J., HILL R.G., HARGREAVES R.J. Comparison of the effects of sumatriptan and the NK1 antagonist CP-99,994 on plasma extravasation in dura mater and c-fos mRNA expression in trigeminal nucleus caudalis of rats. Neuropharmacol. 1995;34:255–261. doi: 10.1016/0028-3908(94)00153-j. [DOI] [PubMed] [Google Scholar]
- STERNFELD F., BAKER R., BROUGHTON H.B., GUIBLIN A.R., JELLEY R.A., MATASSA V.G., REEVE A.J., BEER M.S., STANTON J.A., HARGREAVES R.J., SHEPHEARD S.L., LONGMORE J., RAZZAQUE Z., GRAHAM M.I., SOHAL B., STREET L.J. The chemical evolution of N,N-Dimethyl-2-[5-(1,2,4-triazol-4-yl)-1H-indol-3-yl]ethylamine (L-741,604) and analogues: potent and selective agonists for 5-HT1D receptors. Bioorgan. & Med. Chem. Lett. 1996;6:1825–1830. [Google Scholar]
- STRASSMAN A., MASON P., MOSKOWITZ M., MACIEWICZ R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986;379:242–250. doi: 10.1016/0006-8993(86)90777-8. [DOI] [PubMed] [Google Scholar]
- THOMSEN L.L., BRENNUM J., IVERSEN H.K., OLESEN J. Effect of a nitric oxide donor (glyceryl trinitrate) on nociceptive thresholds in man. Cephalalgia. 1996;16:169–174. doi: 10.1046/j.1468-2982.1996.1603169.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., CUMBERBATCH M.J., SHEPHEARD S.L., HARGREAVES R.J. Sensitization of caudal trigeminal neurones by dural artery dilation. Neurology. 1998;50:A227. [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural vessel diameter in the anaesthetized rat. Cephalalgia. 1997a;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat-intravital microscope studies. Cephalalgia. 1997b;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., HILL R.G., HARGREAVES R.J. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur. J. Pharmacol. 1997c;328:61–64. doi: 10.1016/s0014-2999(97)83028-2. [DOI] [PubMed] [Google Scholar]
- WOOLF C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- WOOLF C.J., KING A.E. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. J. Neurophysiol. 1987;58:460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]


