Abstract
The thermogenic activity of the serotonin and noradrenaline reuptake inhibitor sibutramine (BTS 54524; Reductil) was investigated by measuring oxygen consumption (VO2) in rats treated with sibutramine or its two pharmacologically-active metabolites.
Sibutramine caused a dose-dependent rise in VO2, with a dose of 10 mg kg−1 of sibutramine or its metabolites producing increases of up to 30% that were sustained for at least 6 h, and accompanied by significant increases (0.5–1.0°C) in body temperature.
Based on the accumulation in vivo of radiolabelled 2-deoxy-[3H]-glucose, sibutramine had little or no effect on glucose utilization in most tissues, but caused an 18 fold increase in brown adipose tissue (BAT).
Combined high, non-selective doses (20 mg kg−1) of the β-adrenoceptor antagonists, atenolol and ICI 118551, inhibited completely the VO2 response to sibutramine, but the response was unaffected by low, β1-adrenoceptor-selective (atenolol) or β2-adrenoceptor-selective (ICI 118551) doses (1 mg kg−1).
The ganglionic blocking agent, chlorisondamine (15 mg kg−1), inhibited completely the VO2 response to the metabolites of sibutramine, but had no effect on the thermogenic response to the β3-adrenoceptor-selective agonist BRL 35135.
Similar thermogenic responses were produced by simultaneous injection of nisoxetine and fluoxetine at doses (30 mg kg−1) that had no effect on VO2 when injected individually.
It is concluded that stimulation of thermogenesis by sibutramine requires central reuptake inhibition of both serotonin and noradrenaline, resulting in increased efferent sympathetic activation of BAT thermogenesis via β3-adrenoceptor, and that this contributes to the compound's activity as an anti-obesity agent.
Keywords: Sibutramine, serotonin, noradrenaline, thermogenesis, oxygen consumption, body temperature, glucose utilization, β3-adrenoceptor, brown adipose tissue
Introduction
Sibutramine hydrochloride monohydrate (N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine hydrochloride monohydrate; BTS 54 524; Reductil) is a novel drug that has been registered recently in the U.S.A., Mexico and Brazil for the treatment of obesity. Sibutramine is a 5-hydroxytryptamine (5-HT) and noradrenaline (NA) reuptake inhibitor (Buckett et al., 1988; Luscombe et al., 1989) and is, therefore, a member of a new class of drug called serotonin-noradrenaline reuptake inhibitors, or SNRIs. The compound is a tertiary amine, and when administered to animals and humans it is rapidly demethylated to form the secondary amine, Metabolite 1, and then the primary amine, Metabolite 2. Studies in vitro using rat brain tissue show that sibutramine is a weak inhibitor of NA and 5-HT reuptake, whereas Metabolites 1 and 2 are approximately equipotent as the selective NA reuptake inhibitor desipramine and as the selective 5-HT reuptake inhibitor fluoxetine (Cheetham et al., 1993; 1996).
Sibutramine has been shown to cause weight loss in humans (Weintraub et al., 1991; Ryan et al., 1995; Lean, 1997) and rats (Fantino et al., 1995; Stricker-Krongrad et al., 1995). Studies in rats have shown that sibutramine reduces food intake by enhancing satiety (Halford et al., 1995), and more recently this effect has been shown to be attenuated by antagonists of 5-HT2A/2C and β1-adrenergic receptors and completely inhibited by the α1-adrenoceptor antagonist, prazosin (Jackson et al., 1997a). This same group (Jackson et al., 1997b) went on to show that not only other SNRIs markedly reduce food intake in rats, but that this effect could be mimicked by combined administration of a selective 5-HT reuptake inhibitor (fluoxetine) and a selective NA reuptake inhibitor (nisoxetine) at doses which neither drug alone had an effect on food intake.
The ability of sibutramine to cause weight loss in animals (and humans) was initially ascribed to its effects on food intake, but in a study of obese Zucker (fa/fa) rats treated chronically with subutramine (Connoley et al., 1995) it was found that after an initial, transient effect on food intake, average intake over the 2-week study period was not significantly lower than the control intake. Nevertheless, there was a consistent, sustained effect on body weight (i.e. decreased body fat) that suggested that sibutramine must have affected energy balance by increasing metabolic rate–i.e. it had thermogenic properties. A dual effect on energy intake and expenditure was not altogether surprising since it is well known that experimental manipulations affecting food intake usually have opposite effects on thermogenesis. For example, inducing hyperphagia with central injections of neuropeptide-Y or lesioning the ventromedial hypothalamus inhibits thermogenesis. Conversely, inhibiting food intake by lesioning the lateral hypothalamus, or by raising levels of corticotrophin-releasing factor, 5-HT or several cytokines, results in a stimulation of thermogenesis (see Rothwell et al., 1989; Dryden et al., 1994; Rothwell, 1997 for reviews). Given this reciprocal relationship between food intake and thermogenesis and the results from the Zucker rat experiment, the present study was undertaken to investigate the thermogenic properties of sibutramine and its pharmacologically-active metabolites using measurements of oxygen consumption, body temperature and tissue glucose utilization. Further studies showed that the thermogenic effects could be blocked by β-adrenoceptor antagonists and a ganglionic blocking agent, and that similar thermogenic effects could be produced by simultaneous injection of nisoxetine (noradrenaline reuptake inhibitor) and fluoxetine (serotonin reuptake inhibitor). Some of the results have been published previously in abstract form (Connoley et al., 1995; 1996; Liu et al., 1996).
Methods
Animals
A preliminary dose-ranging study (data not shown) showed that responses to sibutramine were similar in both sexes, and because females were more readily available, it was decided to use female rats for all subsequent studies. Adult (180–220 g) female Wistar were obtained from the colony maintained at St George's Hospital Medical School, and housed in pairs in a room maintained at 21±1°C with a 12 h light cycle (lights on 0700 h). The animals were maintained on a conventional pelleted stock diet, and were transferred in the normal, fed state to the laboratory for metabolic measurements approximately 1 h before starting measurements. Apart from the glucose utilization experiment (see below), each animal received every treatment in the protocol, with 1 week between treatments, which meant that the animals were usually kept for at least 1 month.
Measurement of oxygen consumption
Oxygen consumption (VO2) was determined in closed-circuit respirometers maintained at the thermoneutral temperature for rats (29°C). The system is an improved version of that originally described by Stock (1975) that allows for eight rats to be individually studied at one time. VO2 is recorded on a computer every 5 min and expressed as ml O2 per kg metabolic body size per min (i.e. ml kg−0.75 min−1). Each animal had its VO2 measured for 1–2 h before (baseline) and 2–4 h after treatment with drugs (precise times are given in the description of each experiment). Thirty minute averages of VO2 were used to plot the time-course of responses, and the increase in VO2 for each rat was determined by comparing the mean of the last 30 min of baseline VO2 with the mean value obtained for the post-treatment period. All animals were accustomed to the respirometers and procedures by measuring VO2 for 2 h on at least one (usually two) occasions before testing the effects of the test compounds.
Measurement of body temperature
Colonic temperature (Tc) was measured using a rectal thermocouple probe inserted approximately 6 cm and held until a steady, peak temperature had been recorded. The rats were allowed to sit unrestrained on the bench during this procedure and had been pretrained (see above). Measurements were made before injecting the drugs, and again at the end of the test VO2 measurement.
Measurement of tissue glucose utilization
The method used for measuring tissue glucose utilization (GU) was that employed previously to investigate the effect of adrenoceptor agonists on muscle and adipose tissue metabolism in anaesthetized rats (Liu & Stock, 1995). Briefly, the rats were anaesthetized (1.2 g kg−1 urethane, i.p.), and the right jugular vein cannulated. Colonic temperature was recorded every 15 min and 60 min later 30 μCi of 2-deoxy-[3H]-glucose (2DG; 20 Ci mmol−1) were injected via the jugular cannula. Serial blood samples (50 μl) were taken through the same cannula at 1, 3, 5, 10, 20, 40 and 60 min after the 2DG injection. Samples were immediately deproteinized, centrifuged and the supernatant used for the determination of blood glucose with a glucose oxidase kit (Boehringer, Germany) and plasma radioactivity (Beckman Ready Value scintillation cocktail and a Beckman LS6000 counter) and rats were killed 60 min after the administration of the 2DG, and the following tissues were dissected, freeze-clamped and stored in liquid N2 prior to extraction and determination of radioactive 2DG-6-phosphate: gastrocnemius, soleus, tibialis anterior, extensor digitorum longus, adductor longus, diaphragm, heart, brain, periovarian white adipose tissue and interscapular brown adipose tissue (BAT). Tissue GU was calculated by dividing the radioactivity (d.p.m.) of 2DG-6-phosphate in the tissues by the calculated 60-min integral of the ratio of blood 2DG/blood glucose (d.p.m. ng−1), and the results were expressed as ng glucose min−1 mg−1 wet weight of tissue.
Drugs
Sibutramine hydrochloride monohydrate (BTS 54 524; Knoll Pharmaceuticals) was administered orally (gavage) by dissolving in sterile water at a concentration designed to provide the appropriate doses in 1 ml kg−1 body weight. In the GU experiment, sibutramine was administered by intraperitoneal injection after dissolving in sterile saline. Other drugs were dissolved in sterile saline and given by intraperitoneal or subcutaneous injection (see individual experiments). The other drugs used were: sibutramine Metabolite 1 (BTS 54354; N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-methylamine hydrochloride) and Metabolite 2 (BTS 54505; 1[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine hydrochloride), both supplied by Knoll Pharmaceuticals; the β-adrenoceptor antagonists atenolol (β1-adrenoceptor antagonist; Zeneca Pharmaceuticals) and ICI 118551 (β2-adrenoceptor antagonist; 1 -[2,3 -dihydro- (7-methyl - 1H-inden -4- yl)oxy]- 3 - [(1- methylethyl) amino ] - 2 - butanolhydrochloride; Zeneca Pharmaceuticals); the β3-adrenoceptor agonist BRL 35135 (R*,R*-(±)-methyl - 4 - [2 - [2-hydroxy-2-(3-chlorophenyl)ethyl-amino]-propyl]-phenoxyacetate hydrobromide; SmithKline Beecham) the long-acting ganglionic blocking agent chlorisondamine (Ciba-Geigy); the noradrenaline reuptake inhibitor nisoxetine (Research Biochemicals International) and the 5-HT reuptake inhibitor fluoxetine (Lilly). Radiolabelled 2-deoxy-[3H]-glucose was purchased from Amersham International.
Statistics
The results have been presented as means±s.e.mean and significant differences assessed using Student's t-test for paired or unpaired data, as appropriate. Analysis of variance, followed by the Dunnett test was used for multiple comparisons of differences from control values. All probabilities quoted are two-tailed, with P<0.05 being taken as the level for significance.
Details of the design of the seven experiments carried out are described below with results.
Results
Thermogenic effect of different doses of sibutramine
This experiment used 16 rats, with each receiving four different doses (0.3, 1, 3 and 10 mg kg−1 and vehicle) in a balanced design with 1 week between tests. VO2 was measured for 2 h before (baseline) and 2 h after (test) oral dosing with sibutramine. The results (Table 1) show that, compared to vehicle, a significantly greater VO2 was seen only at the 3 and 10 mg kg−1 doses, and only the 10 mg kg−1 dose produced a significant rise in colonic temperature. Nevertheless, test VO2 exhibited a very strong (r2=0.999) dose-dependency (Figure 1). Over the test period (120 min), the 10 mg kg−1 dose produced a 23% increase in VO2 above baseline (Table 1), but as the plot of VO2 against time shows (Figure 2), VO2 was approximately 30% above baseline and still rising at 120 min post-treatment.
Table 1.
Effect of sibutramine on oxygen consumption (VO2) and colonic temperature (Tc)

Figure 1.
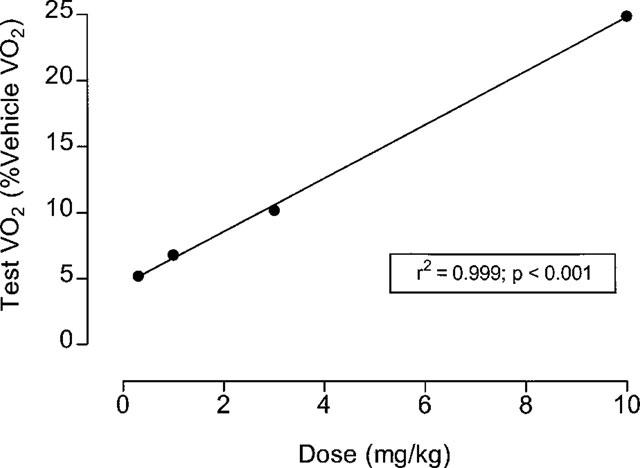
Sibutramine dose: VO2 response. Plot of Test VO2 (% vehicle test VO2) versus different doses (0.03–10 mg kg−1) of sibutramine calculated using data taken from Table 1.
Figure 2.
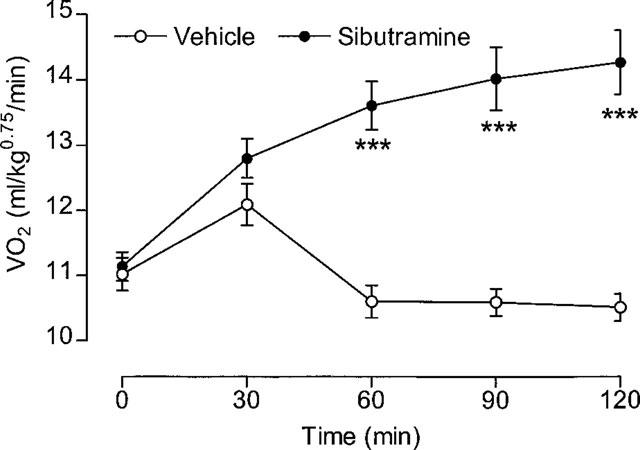
Effect of 10 mg kg−1 sibutramine on VO2. Animals were dosed (p.o.) At 0 min. Mean values±s.e.mean (n=16). ***P<0.001 vs vehicle (paired t-test).
Thermogenic effect of sibutramine over 2–6 h
The VO2 response to 10 mg kg−1 sibutramine shown in Figure 2 suggested that VO2 had yet to reach an established plateau by the end of 2 h. Extending the post-treatment VO2 measurements much beyond 4 h means changing the absorbers for carbon dioxide and water in the respirometers (thus disturbing the animals), or starting the measurements later, after dosing the animals. In the next experiment, therefore, vehicle or sibutramine (10 mg kg−1, p.o.) was given 1 h before starting VO2 measurements over the next 5 h (i.e. up to 6 h post-treatment). Thus, there was no baseline VO2, and in addition the results for the first 30 min settling-down period were discarded. The time plot of results (Figure 3) shows the mean 30 min VO2 for the second 30 min period (i.e. ending 2 h post-treatment) and every 30 min thereafter up to 6 h post-treatment. It can be seen that VO2 reached a steady plateau level between 2–3 h post-treatment, and remained elevated for up to 5 h, with the response just beginning to decline by the end of the 6 h measurement period. At this point, colonic temperature was more than 1°C higher in the sibutra- mine-treated rats (vehicle=37.2±0.16°C, sibutramine= 38.3±0.1°C, P<0.001).
Figure 3.
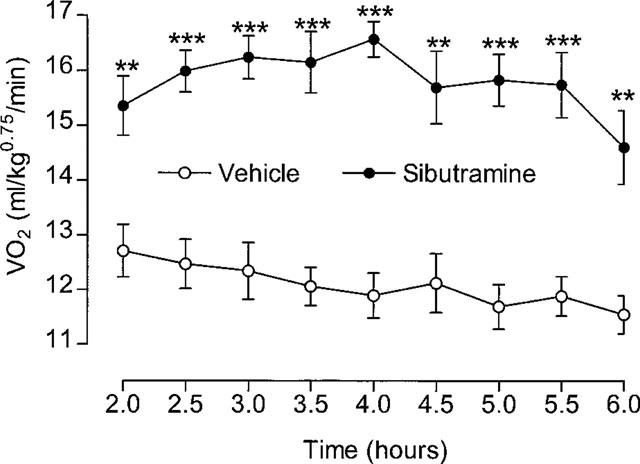
Effect of sibutramine on VO2 over 2–6 h. Animals were dosed 1 h before starting VO2 measurements; VO2 at 2 h=mean of previous 30 min. Mean values±s.e.mean (n=8). **P<0.01, ***P<0.001 vs vehicle (paired t-test).
Effect of sibutramine on tissue GU
Two groups (n=8) of rats were used to measure GU following treatment with sibutramine or vehicle. Immediately after preparing the anaesthetized animals for the measurement of GU they were injected with sibutramine (10 mg kg−1, i.p.) or vehicle and left for 60 min before starting the determination of GU with the injection of 2-deoxy-[3H]-glucose. Two rats, (one sibutramine, one vehicle) were studied simultaneously, with a gap of 30 min at the start to allow for the initial rapid blood sampling and the dissection and freeze-clamping of tissues at the end of the experiment. Due to a blocked cannula on the last experimental day, only seven data sets were obtained for rats treated with sibutramine.
It was not possible to monitor VO2 response to sibutramine in these experiments, but the colonic temperature recordings show (Figure 4) that there was a steady decline in the temperature of control rats from a relatively high 38°C down to 36°C during the first 60 min; thereafter it remained steady at ∼36°C. In the sibutramine-treated rats, however, the initial decline was reversed after 60 min, and colonic temperature had returned to 38°C before the end of the experiment. As a result, the colonic temperature of these rats was 2°C higher than that of the control rats (vehicle=35.9±0.5°C, sibutramine= 38.0±0.2°C, P<0.01; n=8). The GU results (Table 2) show that sibutramine had no significant effect on GU in the various tissues studied, apart from gastrocnemius muscle, diaphragm and interscapular brown adipose tissue (BAT). The increases in gastrocnemius (29%) and diaphragm (77%) were relatively modest compared to the 18 fold increase seen in BAT.
Figure 4.
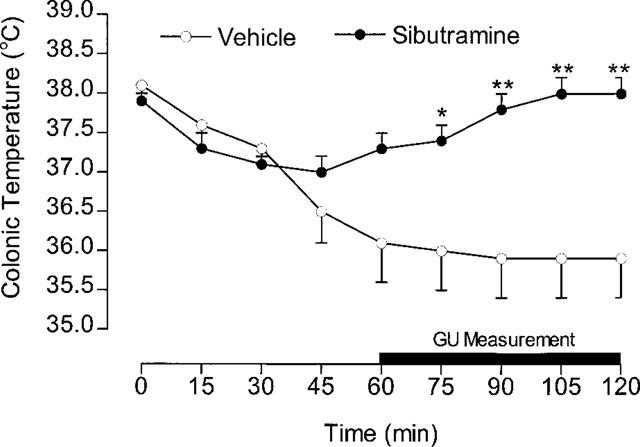
Colonic temperature during GU measurement. Animals received sibutramine (10 mg kg−1, i.p.) at 0 min, and the GU measurement started by injecting [3H]-2DG at 60 min. Mean values±s.e.mean (n=8 control; n=7 sibutramine). *P<0.05, **P<0.01 vs control (unpaired t-test).
Table 2.
Effect of sibutramine on tissue glucose utilization
Effect of β-adrenoceptor antagonists on the thermogenic response to sibutramine
This experiment involved 16 rats, with each rat receiving each treatment in a balanced design with 1 week between treatments. VO2 was measured for 1.5 h before, and for 4 h after dosing with sibutramine (10 mg kg−1, p.o.). In addition to sibutramine, each rat received on separate occasions subcutaneous injections of vehicle, atenolol (1 mg kg−1), ICI 118551 (1 mg kg−1) or 20 mg kg−1 (s.c.) of both atenolol and ICI 118551. The subcutaneous injections of these β-adrenoceptor antagonists were given approximately 5 min before the oral dose of sibutramine.
Figure 5 shows the mean baseline (0 min) and 30 min VO2 values for the 4 h following treatment with subutramine and the β-adrenoceptor antagonists. The most noticeable feature is the almost complete absence of a thermogenic response to sibutramine when the animals were treated with the combined high doses of atenolol and ICI 118551. There was no effect of the individual low doses of either β-adrenoceptor antagonist on the thermogenic response to sibutramine, except that VO2 tends to be a little lower than sibutramine alone towards the end of the 4 h period. There was no significant difference in the VO2 over the 4 h post-treatment period between sibutramine (15.44±0.45 ml kg−0.75 min−1) and the sibutramine plus the low-dose of the two β-adrenoceptor antagonists (atenolol=14.76±0.55 ml kg−0.75 min−1; ICI 118551=14.68±0.46 ml kg−0.75 min−1, n=8), but the combined high-dose of the two antagonists resulted in a significantly lower VO2 (13.14±0.52 ml kg−0.75 min−1; P<0.01, Dunnett test). As a result, the thermogenic response (increase above baseline) to sibutramine was reduced from 21 to 4% (P<0.01; Dunnett test) by the high-dose atenolol and ICI 118551 combination.
Figure 5.
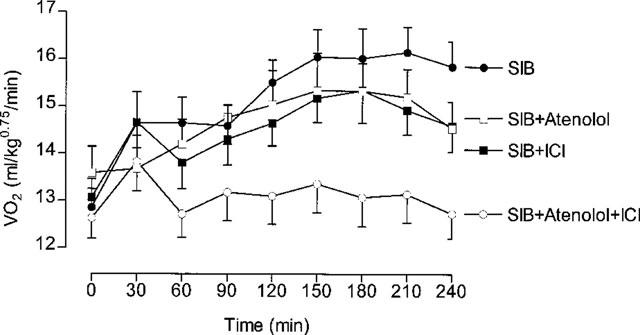
Effect of β-adrenoceptor antagonists on the VO2 response to sibutramine (10 mg −1). The dose of atenolol and ICI 118551 (ICI) was 1 mg kg−1 when given alone, but 20 mg kg−1 of each when injected in combination. Mean values±s.e.mean (n=16).
Thermogenic activity of Metabolite 1 and Metabolite 2
Sixteen rats were used in this experiment, with eight rats being treated with vehicle and Metabolite 1 (M1), and the other eight rats being treated with vehicle and Metabolite 2 (M2); rats received both vehicle and the appropriate metabolite at 1 week intervals in a balanced design. VO2 was measured for 1.5 h before, and 4 h after dosing with the metabolites (10 mg kg−1, i.p.).
The time-course of the VO2 response to both metabolites and their respective vehicle control response is shown in Figure 6, which indicates that the magnitude and pattern of response was almost identical for the two metabolites. This is confirmed by inspection of the VO2 results shown in Table 3, in which it can be seen that both metabolites produced a 17% increase in VO2 over the 4 h post-treatment period as well as significant increases in body temperature.
Figure 6.
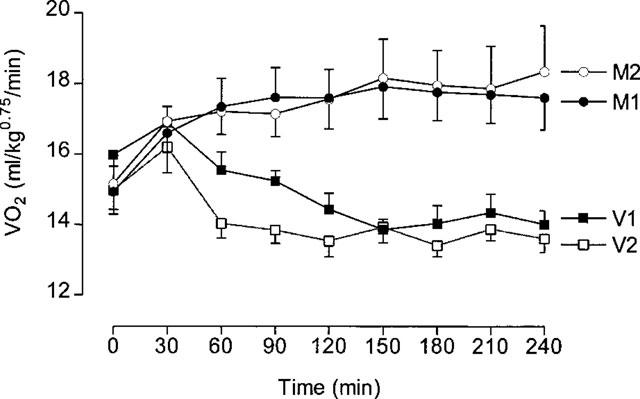
VO2 responses to Metabolite 1 and Metabolite 2. Animals were dosed at 0 min with 10 mg kg−1 (i.p.). Metabolite 1 (M1) or saline (V1), and Metabolite 2 (M2) or saline (V2). Mean values±s.e.mean (n=8).
Table 3.
Thermogenic responses to Metabolites 1 and 2
Effect of ganglionic blockade on the thermogenic response to Metabolites 1 and 2
This experiment was carried out using two different groups of 16 rats to test responses to Metabolites 1 and 2. Each group was dosed with the test metabolite (10 mg kg−1, i.p.) on two occasions, but on one occasion they were pre-treated (5 min earlier) with chlorisondamine (15 mg kg−1, i.p.) in a balanced design with 1 week between treatments. VO2 was measured for 1.5 h before and 4 h after treatment. Subsequently, eight rats previously used to test Metabolite 1 were tested in the same way, but with each rat being injected with the selective β3-adrenoceptor agonist BRL 35135 (10 μg kg−1, i.p.) instead of a sibutramine metabolite.
Figure 7 shows that chlorisondamine completely blocked the VO2 response to both metabolites throughout the 4 h test period, but had no effect on the response to BRL 35135. The extent of the block is also evident from the results shown in Table 4, where the thermogenic response to both metabolites (M1=14%; M2=29%) was reduced to zero by pre-treatment with chlorisondamine, as was the rise in body temperature. However, the marked (24%) thermogenic response to BRL 35135 was completely unaffected by chlorisondamine; if anything, the response was slightly enhanced (29%; not significant) after chlorisondamine. Colonic temperature was not affected by BRL 35135, probably because the thermogenic effect of BRL 35135 had disappeared by the end of the test period (see Figure 7).
Figure 7.
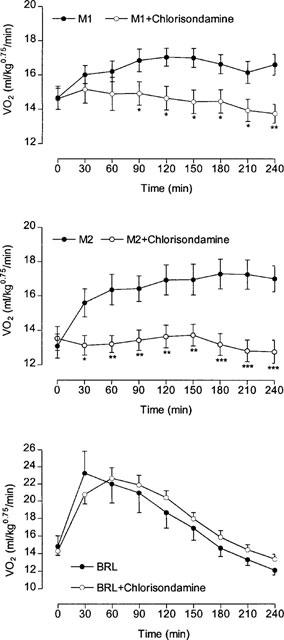
Effect of ganglionic blockade with chlorisondamine on the VO2 response to Metabolite 1 (M1; upper panel), Metabolite 2 (M2; middle panel) and BRL 35135 (BRL; lower panel). Animals were dosed with chlorisondamine (15 mg kg−1, i.p.) or saline prior to being injected at 0 min with 10 mg kg−1 of the appropriate drug. Mean values±s.e.mean (n=16; n=8 for BRL 35135). *P<0.05, **P<0.01, ***P<0.001 vs M1 or M2.
Table 4.
Effect of chlorisondamine on the thermogenic response to Metabolites 1 and 2 and BRL 351356
Individual and combined effects of nisoxetine and fluoxetine on thermogenesis
In this experiment 24 rats were divided into four groups (n=6), with the groups receiving either vehicle, nisoxetine (30 mg kg−1, i.p.), fluoxetine (30 mg kg−1, i.p.) or a combination of the two (both at 30 mg kg−1). VO2 was measured for 3 h after treatment.
Figure 8 shows that VO2 remained more or less at baseline levels when the rats were treated with vehicle, nisoxetine or fluoxetine, but combined treatment with the two drugs resulted in a rapid rise in VO2 that peaked at about 1 h after treatment. Thereafter, VO2 declined slightly, but remained well above the basal level for the remaining 2 h. This increase in VO2 was accompanied by a significant rise in colonic temperature (vehicle=−0.10±0.08°C; nisoxetine+fluoxetine=1.04± 0.46°C; P<0.05, Dunnett test); neither drug alone had an effect on body temperature (nisoxetine=−0.45±0.32°C; fluoxetine 0.35±0.22°C). The thermogenic responses to the four treatments, averaged over the 3 h test period, are shown in Figure 9. The 27% increase after combined injections of nisoxetine and fluoxetine compares with the 28% increase seen with Metabolite 2 over the first 3 h post-treatment (data extracted from those summarized in Table 4).
Figure 8.
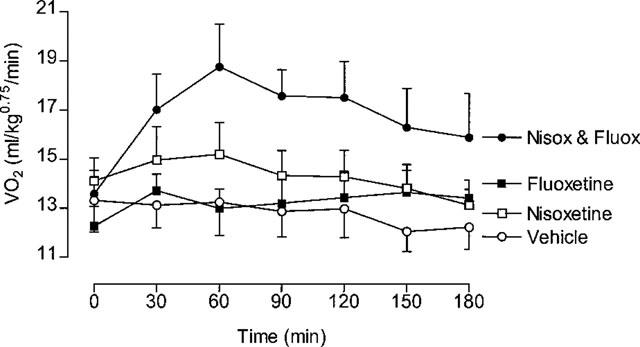
Individual and combined effects of nisoxetine and fluoxetine. Rats received either nisoxetine (30 mg kg−1, i.p.), fluoxetine (30 mg kg−1, i.p.) or a combination of the two (both at 30 mg kg−1). Mean values±s.e.mean (n=6).
Figure 9.
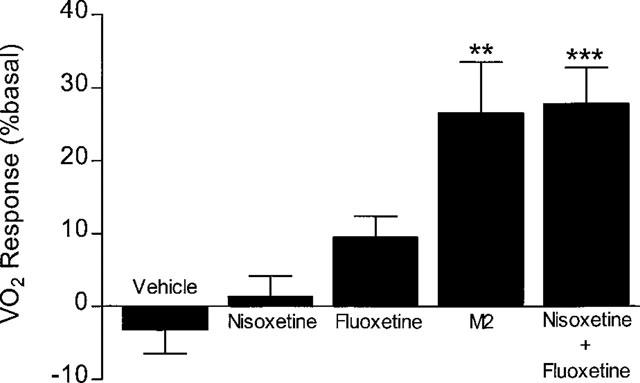
Effects of nixoxetine, fluoxetine and Metabolite 2 (M2) on thermogenesis. Mean test VO2 (0–180 min) from Figure 8 expressed as % basal VO2, and compared with response to Metabolite 2 (data over 0–180 min taken from Figure 7). Mean values±s.e.mean (n=6; n=16 for Metabolite 2). **P<0.01, ***P<0.001 vs respective vehicle (paired t-test).
Discussion
The results from these experiments demonstrate unequivocally the thermogenic properties of sibutramine and its two metabolites, with the 10 mg kg−1 doses of all three compounds producing significant, sustained 20–30% increases in VO2. These increases in VO2 were invariably associated with increases of 0.5–1.0°C in colonic temperature. However, it has to be remembered that these measurements were carried out at the thermoneutral ambient temperature for rats (29°C), and it is unlikely that body temperature would increase as much, if at all, at normal room temperature (21°C). Likewise, the VO2 would be expected to be slightly attenuated at an ambient temperature below thermoneutrality, since part of the thermogenic response to sibutramine would substitute for any existing cold-induced (non-shivering) thermogenesis, and would not contribute to the rise in VO2.
The dose-dependent effects of sibutramine on thermogenesis were clearly evident in the first experiment (see Figure 1), and the dose chosen for subsequent studies (10 mg kg−1) is also the one that causes more than a 50% decrease in nocturnal food intake and was used in previous studies investigating the mechanisms responsible for its hypophagic effects (Jackson et al., 1997a,1997b). These hypophagic effects appear within 2 h of treatment, and may be sustained for up to 8 h (Jackson et al., 1997b), which is consistent with a biological half-life of sibutramine's metabolite (M2) of approximately 5 h (unpublished data from Knoll Pharmaceuticals). The slow onset and sustained effect on food intake is almost identical to that observed here for the VO2 response to sibutramine (see Figures 2 and 3), and suggests that activation of thermogenesis and inhibition of food intake involves the same or very similar mechanisms. However, even supposing activation by sibutramine of the same central pathways, it is self-evident that enhanced satiety and increased thermogenesis must involve different efferent pathways and effectors.
Identifying and quantifying the contribution of different organs and/or tissues to any form of thermogenesis (cold-, diet- or drug-induced) is exceptionally difficult since it requires simultaneous measurements of tissue blood flow (e.g. from trapping of injected microspheres) and oxygen extraction (i.e. arteriovenous difference in O2 content) of individual organs or tissues. Even then, the nature of the measurements means that only a transient `snapshot' measurement of oxygen uptake is obtained. However, the measurement of tissue glucose utilization using the accumulation of radiolabelled 2DG-phosphate (Issad et al., 1987) provides an alternative method for estimating simultaneously the metabolic activity of all tissues (apart from liver, where the 2DG-phosphate can be hydrolysed). Moreover, by allowing the 2DG-phosphate to accumulate for up to an hour after injecting 2DG, an integrated estimate of metabolic activity, relatively unaffected by rapid transients, is obtained. This technique was particularly relevant to the present study using a compound like sibutramine with a slow onset of activity.
The recordings of rectal temperature during the measurement of glucose utilization (Figure 4) illustrate this slow onset, and show how the 60 min GU measurement covered the period when the thermogenic effect of sibutramine was causing body temperature to rise rapidly. Apart from a very small contribution from some muscles (gastrocnemius and diaphragm), BAT clearly accounts for practically all of the metabolic response to sibutramine. It should be noted that, although fatty acids provide the main fuel for BAT thermogenesis, there is a high glucose requirement to maintain the Krebs cycle and avoid cellular ketosis, and explains why GU is a reliable measure of BAT thermogenesis. The increase in BAT was quite remarkable, and in Figure 10 it has been compared with results obtained previously (Liu & Stock, 1995) using the same technique for measuring GU response to the selective β3-adrenoceptor agonist, BRL 35135. The 18 fold increase produced by sibutramine contrasts with the 12 fold increase produced by an equivalent thermogenic dose of BRL 35135. It is not known why sibutramine should produce a greater increase in GU than BRL 35135, although if sibutramine exerts its effects via increased sympathetic activity, this will activate all adrenoceptor subtypes (α and β), whereas the activity of BRL 35135 will be restricted mainly to β3-adrenoceptor.
Figure 10.
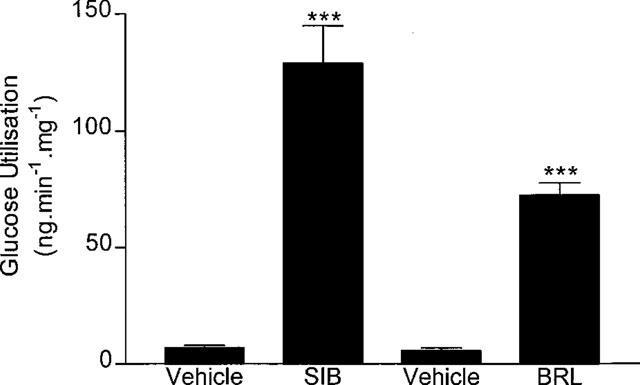
Comparison of the effects of sibutramine and BRL 35135 on BAT glucose utilization. Calculations based on data for sibutramine (SIB) in Table 2, and from Liu & Stock (1995) for BRL 35135 (BRL).
The GU experiment indicated that sibutramine, like BRL 35135, was a highly effective agonist of BAT thermogenesis, and prompted an experiment to determine if the effects of sibutramine on VO2 were mediated by β3-adrenoceptor. BAT thermogenesis is mainly due to sympathetic activation of β3-adrenoceptor, and explains the potent thermogenic activity of selective β3-adrenoceptor agonists such as BRL 35135 (see Stock, 1993). One of the key identifying pharmacological characteristics of the β3-adrenoceptor is its weak afinity for conventional β-adrenoceptor antagonists. The low pA2 of conventional selective and non-selective antagonists for the β3-adrenoceptor means it is possible to use doses in vivo of drugs such as atenolol, ICI 118551, propranolol and nadolol that selectively inhibit β1-adrenoceptor and β2-adrenoceptor responses while leaving β3-adrenoceptor responses intact (e.g. Carlisle & Stock, 1992; Liu et al., 1995). Previous studies with atenolol and ICI 118551 (Carlisle & Stock, 1992) have shown that the low doses used here (1 mg kg−1) block β1-adrenoceptor and β2-adrenoceptor responses, respectively, without affecting thermogenic responses to non-selective thermogenic β-adrenoceptor agonists (e.g. isoprenaline). However, high (20 mg kg−1) doses alone will block thermogenic responses to both non-selective and β3-adrenoceptor-selective thermogenic agonists. The complete inhibition of the thermogenic response to sibutramine by the combined high dose of atenolol and ICI 118551 (see Figure 5) clearly shows that the thermogenic response involves a β-adrenoceptor. On the other hand, the failure of the low β1-adrenoceptor-selective and β2-adrenoceptor-selective doses of atenolol and ICI 118551 to affect the sibutramine responses indicates that these are most likely to be mediated by the β3-adrenoceptor, and would be consistent with the potent effects of sibutramine on GU in BAT.
Given the known in vitro pharmacology of sibutramine and its metabolites (Buckett et al., 1988; Luscombe et al., 1989; Cheetham et al., 1993; 1996), and the fact that these compounds lack β3-adrenoceptor affinity (Stock, 1997), it seems extremely unlikely that the effects of sibutramine on thermogenesis and BAT GU could be due to direct peripheral activation of β3-adrenoceptor. Instead, it is far more likely that the metabolic effects result from increased sympathetic activity due to the central effects of sibutramine, possibly the same central pathways that cause the changes in feeding behaviour (Jackson et al., 1997a,1997b). However, before using a ganglionic blocking agent to test for central activation of sympathetic activity, it was decided to switch to using the metabolites (M1 and M2) of sibutramine since it was thought that these should produce faster and more reproducible responses than dosing with their precursor. Moreover, it was anticipated that future studies would at some stage require central (i.c.v.) injections, and for this it would be essential to use the metabolites and to have established previously the magnitude and time course of responses to parenteral administration. Surprisingly, however, both the magnitude and the time course of the response to M2 was the same as for M1, and very similar to the sibutramine responses; this can be seen by comparing Figure 6 (M1 and M2) with Figure 2 (sibutramine). These similarities suggest that the in vivo conversion of sibutramine to M1, and the conversion of that to M2 cannot account for the slow onset of the thermogenic response to sibutramine. The fact that it takes 60–90 min to see the peak effect of any of these compounds might suggest that penetration of the blood-brain barrier may be the limiting factor, but a more likely explanation is that it is the slow rise in synaptic concentrations of 5-HT and noradrenaline following inhibition of monoamine reuptake - i.e. synaptic concentrations will depend, mainly, on the level of neuronal activity (Gundlah et al., 1997). This explanation would be consistent with the observation that it takes about 2 h for 5-HT levels to peak following reverse dialysis of fluoxetine and nisoxetine into the hypothalamus (Gundlah et al., 1997).
Slow penetration of the blood-brain barrier and/or build-up of synaptic monoamine levels would be consistent with the suggestion (above) that the changes in peripheral metabolism were due to central stimulation by sibutramine or its metabolites of efferent sympathetic activity. This was confirmed in the experiments where pretreatment with the ganglionic blocking agent, chlorisondamine, caused a complete inhibition of the VO2 and colonic temperature responses to M1 and M2. The observation that the thermogenic response to the β3-adrenoceptor agonist BRL 35135 was not affected at all by pretreatment with chlorisondamine indicates that the drug had not affected the animals' thermogenic capacity or ability to respond to an agonist acting directly on tissue thermogenesis. Although chlorisondamine blocks both parasympathetic and sympathetic ganglia, given the evidence for sibutramine exerting its thermogenic effect via a β-adrenoceptor (specifically β3-adrenoceptor), it is assumed that the inhibition of thermogenesis by chlorisondamine was due to blockade of sympathetic ganglionic transmission. It is worth noting that, based on tissue GU, this increase in sympathetic activity appears to be restricted mainly to BAT, with no obvious effect on other sympathetically-innervated tissues such as WAT or heart. The absence of a thermogenic response to M1 and M2 in the presence of chlorisondamine indicates that any inhibition of peripheral NA reuptake is, by itself, incapable of stimulating thermogenesis. However, this does not rule out the possibility that under normal conditions the sympathetic activation of thermogenesis could be potentiated by inhibition of NA reuptake at sympathetic nerve terminals in, for example, BAT.
Jackson et al. (1997b) have shown that the effects of sibutramine on food intake could be mimicked by combined treatment with a selective NA reuptake inhibitor (nisoxetine) and a selective 5-HT reuptake inhibitor (fluoxetine) at doses that individually had no effect on food intake. Exactly the same interaction between nisoxetine and fluoxetine was seen in the present study as regards thermogenesis. The thermogenic response to combined treatment was the result of a synergistic interaction, and not an additive effect, since neither drug had any thermogenic activity on its own. The similarity between these metabolic responses and those obtained by Jackson et al. (1997b) when observing feeding behaviour is worth emphasizing since these workers also showed that these quite high doses (30 mg kg−1) of nisoxetine and fluoxetine had no effect individually on food intake, but in combination had effects comparable to those seen with 10 mg kg−1 sibutramine. Likewise, 10 mg kg−1 of M2 in this study produced a thermogenic response that was identical to that produced by combined treatment with 30 mg kg−1 each of nisoxetine and fluoxetine (see Figure 9).
In conclusion, this series of experiments demonstrates that simultaneous inhibition of 5-HT and NA reuptake produces a marked, up to 30%, increase in thermogenesis (VO2) and body temperature in the rat. These increases are due to central stimulation of efferent sympathetic nerves that activate thermogenesis via β3-adrenoceptor. The onset of the metabolic effects is slow (peaking at 60–90 min), but the thermogenesis is sustained for at least 6 h after treatment. These thermogenic effects could contribute, along with inhibition of food intake, to the anti-obesity effects of chronic treatment with sib-utramine. Moreover, thermogenesis alone must account for the negative energy balance seen in genetically obese rats (Connoley et al., 1995) and mice (Day & Bailey, 1998) in which sibutramine caused weight loss without affecting food intake. Associated effects on BAT and muscle GU might also explain the amelioration in insulin resistance seen in obese-diabetic ob/ob mice (Day & Bailey, 1998). Finally, it has been shown recently that humans also exhibit a thermogenic response to a single, acute dose of sibutramine (Hansen et al., 1998).
Abbreviations
- BAT
brown adipose tissue
- 2DG
2-deoxyglucose
- GU
glucose utilization
- 5HT
5-hydroxytryptamine
- M1
Metabolite 1
- M2
Metabolite 2
- NA
noradrenaline
- SNRI
serotonin and noradrenaline reuptake inhibitor
- VO2
oxygen consumption
References
- BUCKETT W.R., THOMAS P.C., LUSCOMBE G.P. The pharmacology of sibutramine hydrochloride (BTS 54 524), a new antidepressant which induces rapid noradrenergic down-regulation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1988;12:575–584. doi: 10.1016/0278-5846(88)90003-6. [DOI] [PubMed] [Google Scholar]
- CARLISLE H.J., STOCK M.J. Potentiation of thermoregulatory responses to isoproterenol by β-adrenergic antagonists. Am. J. Physiol. 1992;263:R915–R923. doi: 10.1152/ajpregu.1992.263.4.R915. [DOI] [PubMed] [Google Scholar]
- CHEETHAM S.C., VIGGERS J.A., BUTLER S.A., PROW M.R., HEAL D.J. [3H]Nisoxetine - A radioligand for noradrenaline reuptake sites: correlation with inhibition of [3H]noradrenaline uptake and effect of DSP-4 lesioning and antidepressant treatments. Neuropharmacol. 1996;35:63–70. doi: 10.1016/0028-3908(95)00134-4. [DOI] [PubMed] [Google Scholar]
- CHEETHAM S.C., VIGGERS J.A., SLATER N.A., HEAL D.J., BUCKETT W.R. [3H]Paroxetine binding in rat frontal cortex strongly correlates with [3H]-5-HT uptake: effect of administration of various antidepressant treatments. Neuropharmacol. 1993;32:737–743. doi: 10.1016/0028-3908(93)90181-2. [DOI] [PubMed] [Google Scholar]
- CONNOLEY I.P., FROST I., HEAL D.J., STOCK M.J. Role of β-adrenoceptors in mediating the thermogenic effects of sibutramine. Br. J. Pharmacol. 1996;117:170P. doi: 10.1038/sj.bjp.0702446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOLEY I.P., HEAL D.J., STOCK M.J. A study in rats of the effects of sibutramine on food intake and thermogenesis. Br. J. Pharmacol. 1995;114:388P. [Google Scholar]
- DAY C., BAILEY C.J. Effect of the antiobesity agent sibutramine in obese-diabetic ob/ob mice. Int. J. Obesity. 1998;22:619–623. doi: 10.1038/sj.ijo.0800636. [DOI] [PubMed] [Google Scholar]
- DRYDEN S., FRANKISH H., WANG Q., WILLIAMS G. Neuropeptide Y and energy balance. Eur. J. Clin. Invest. 1994;24:1–16. doi: 10.1111/j.1365-2362.1994.tb01089.x. [DOI] [PubMed] [Google Scholar]
- FANTINO M., MARTEL P., SOUQUET A.M., WIETESKA L., BRONDEL L., COURCIER S. Decrease of food intake and weight loss induced by sibutramine in the rat. Obesity Res. 1995;3 Suppl 4:628S. [Google Scholar]
- GUNDLAH C., MARTIN K.F., HEAL D.J., AUERBACH S.B. In vivo criteria to differentiate monoamine reuptake inhibitors from releasing agents: sibutramine is a reuptake inhibitor. J. Pharmacol. Exp. Therap. 1997;283:581–591. [PubMed] [Google Scholar]
- HALFORD J.C.G., HEAL D.J., BLUNDELL J.E. Effects in the rat of sibutramine on food intake and the behavioural satiety sequence. Br. J. Pharmacol. 1995;114:387P. [Google Scholar]
- HANSEN D.L., TOUBRO S., STOCK M.J., MACDONALD I.A., ASTRUP A. Thermogenic effects of sibutramine in humans. Am. J. Clin. Nutr. 1998;68:1180–1186. doi: 10.1093/ajcn/68.6.1180. [DOI] [PubMed] [Google Scholar]
- ISSAD T., PÉNICAUD L., FERRÉ P., KANDÉ J., BAUDON M.A., GIRARD J. Effects of fasting on tissue glucose utilization in conscious resting rats. Biochem. J. 1987;246:241–244. doi: 10.1042/bj2460241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON H.C., BEARHAM M.C., HUTCHINS L.J., MAZURKIEWICZ S.E., NEEDHAM A.M., HEAL D.J. Investigation of the mechanisms underlying the hypophagic effects of the 5-HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. Br. J. Pharmacol. 1997a;121:1613–1618. doi: 10.1038/sj.bjp.0701311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON H.C., NEEDHAM A.M., HUTCHINS L.J., MAZURKIEWICZ S.E., HEAL D.J. Comparison of the effects of sibutramine and other monoamine reuptake inhibitors on food intake in the rat. Br. J. Pharmacol. 1997b;121:1758–1762. doi: 10.1038/sj.bjp.0701312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAN M.E.J. Sibutramine - a review of clinical efficacy. Int. J. Obesity. 1997;21 Suppl 1:S30–S36. [PubMed] [Google Scholar]
- LIU Y.-L., KASHANI S.M.Z., HEAL D.J., STOCK M.J. Effect of sibutramine on tissue glucose utilisation in the rat. Br. J. Pharmacol. 1996;117:324P. [Google Scholar]
- LIU Y.-L., STOCK M.J. Acute effects of the β3-adrenoceptor agonist, BRL 35135, on tissue glucose utilisation. Br. J. Pharmacol. 1995;114:888–894. doi: 10.1111/j.1476-5381.1995.tb13287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.-L., TOUBRO S., ASTRUP A., STOCK M.J. Contribution of β3-adrenoceptor activation to ephedrine-induced thermogenesis in humans. Int. J. Obesity. 1995;19:678–685. [PubMed] [Google Scholar]
- LUSCOMBE G.P., HOPCROFT R.H., THOMAS P.C., BUCKETT W.R. The contribution of metabolites to the rapid and potent down-regulation of rat cortical β-adrenoceptors by the putative antidepressant sibutramine hydrochloride. Neuropharmacol. 1989;28:129–134. doi: 10.1016/0028-3908(89)90048-8. [DOI] [PubMed] [Google Scholar]
- ROTHWELL N.J. Neuroimmune interactions: the role of cytokines. Br. J. Pharmacol. 1997;121:841–847. doi: 10.1038/sj.bjp.0701248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHWELL N.J., LEFEUVRE R.A., STOCK M.J.Central control of thermogenesis Obesity in Europe 88 1989London: Libbey; 241–245.(eds). Bjorntorp, P. & Rossner, S. [Google Scholar]
- RYAN D.H., KAISER P., BRAY G.A. Sibutramine: a novel new agent for obesity treatment. Obesity Res. 1995;3:553S–559S. doi: 10.1002/j.1550-8528.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]
- STOCK M.J. An automatic, closed-circuit oxygen consumption apparatus for small animals. J. Appl. Physiol. 1975;39:849–850. doi: 10.1152/jappl.1975.39.5.849. [DOI] [PubMed] [Google Scholar]
- STOCK M.J. Experimental studies with selective β-adrenergic agonists and antagonists in animals. Int. J. Obesity. 1993;17 Suppl 3:S69–S72. [PubMed] [Google Scholar]
- STOCK M.J. Sibutramine: a review of the pharmacology of a novel anti-obesity agent. Int. J. Obesity. 1997;21 Suppl 1:S25–S29. [PubMed] [Google Scholar]
- STRICKER-KRONGRAD A., SOUQUET A.M., BURLET C. Effects of sibutramine on feeding behaviour in obese and lean Zucker rats. Int. J. Obesity. 1995;19 Suppl 2:p399. [Google Scholar]
- WEINTRAUB M., RUBIO A., GOLIK A., BYRNE L., SCHEINBAUM M.L. Sibutramine in weight control; a dose-ranging efficacy study. Clin. Pharmacol. Ther. 1991;50:330–337. doi: 10.1038/clpt.1991.144. [DOI] [PubMed] [Google Scholar]


