Abstract
The effects of physiological substrates of multidrug resistance-associated proteins (MRPs) on cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel currents were examined using patch clamp recording from CFTR-transfected mammalian cell lines.
Two MRP substrates, taurolithocholate-3-sulphate (TLCS) and β-estradiol 17-(β-D-glucuronide) (E217βG) caused a voltage-dependent block of macroscopic CFTR Cl− currents when applied to the intracellular face of excised membrane patches, with mean apparent dissociation constants (KDs) of 96±10 and 563±103 μM (at 0 mV) respectively. The unconjugated bile salts taurocholate and cholate were also effective CFTR channel blockers under these conditions, with KDs of 453±44 and 3760±710 μM (at 0 mV) respectively.
Reducing the extracellular Cl− concentration from 154 to 20 mM decreased the KD for block intracellular TLCS to 54±1 μM, and also significantly reduced the voltage dependence of block, by suggesting that TLCS blocks Cl− permeation through CFTR by binding within the channel pore.
Intracellular TLCS reduced the apparent amplitude of CFTR single channel currents, suggesting that the duration of block is very fast compared to the gating of the channel.
The apparent affinity of block by TLCs is comparable to that of other well-known CFTR channel blockers, suggesting that MRP substrates may comprise a novel class of probes of the CFTR channel pore.
These results also suggest that the related proteins CFTR and MRP may share a structurally similar anion binding site at the cytoplasmic face of the membrane.
Keywords: CFTR, chloride channel, multidrug resistance-associated protein, ABC protein, channel block, sulphonylurea, cystic fibrosis
Introduction
Cystic fibrosis is caused by mutations in a single gene, that encoding the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is a member of the ATP-binding cassette (ABC) family of membrane proteins, most members of which are thought to form ATP-dependent pumps of a wide range of substances (Higgins, 1995). In contrast, CFTR is a phosphorylation- and ATP-dependent ion channel which mediates the passive electrodiffusion of Cl− ions (Gadsby et al., 1995; Hanrahan et al., 1995). However, recent evidence has suggested some degree of functional similarity between CFTR and the closely related ABC proteins, multidrug resistance-associated protein (MRP) and canalicular multispecific organic anion transporter (cMOAT; also known as MRP2), both of which function as ATP-dependent pumps of a broad range of large intracellular organic anions (see below). We have shown that the CFTR Cl− channel is permeable to a number of large organic anions from the cytoplasmic side of the membrane (Linsdell & Hanrahan, 1998a), including the MRP- and cMOAT-substrate oxidized glutathione (GSSG; Linsdell & Hanrahan, 1998b). Such large anions are also able to block Cl− permeation through CFTR when present in the intracellular solution (Linsdell & Hanrahan, 1996a; 1998b), suggesting that large anions and Cl− ions pass through the same pore in CFTR, but that large anions have a longer residency time within the pore and therefore slow the overall rate of permeation.
MRP and cMOAT show similar yet broad substrate specificities, both transporting a diverse range of glutathione, glucuronide, and sulphate conjugates (Oude Elferink et al., 1995; Keppler et al., 1997). Physiological substrates for these transporters may include cysteinyl leukotrienes such as leukotriene C4 (LTC4; Leier et al., 1994; Oude Elferink et al., 1995), conjugated bile salts such as taurolithocholate-3-sulphate (TLCS; Oude Elferink et al., 1995; Jedlitschky et al., 1996) and conjugated steroids such as β-estradiol 17-(β-D-glucuronide) (E217βG; Loe et al., 1996; Keppler et al., 1997).
We have investigated the effects of two physiological MRP/cMOAT substrates on CFTR Cl− channel activity. The high affinity MRP/cMOAT substrates TLCS and E217βG both caused a voltage-dependent block of macroscopic CFTR Cl− currents when applied to the cytoplasmic face of the membrane. The unconjugated bile salts taurocholate and cholate were also effective voltage-dependent blockers. Intracellular TLCS caused a voltage-dependent reduction in unitary CFTR Cl− current, suggesting a blocking mechanism with kinetics much faster than those of channel opening and closing. These results identify MRP/cMOAT substrates as a novel class of probes of the CFTR Cl− channel, and suggest some structural similarity between CFTR and other related ABC proteins at the cytoplasmic face of the membrane.
Methods
CFTR macroscopic and single channel current recordings were both made using the excised, inside-out configuration of the patch clamp technique, as described previously (Linsdell & Hanrahan, 1996b; 1998a; Linsdell et al., 1997). Baby hamster kidney (BHK) cells were used for macroscopic recordings and Chinese hamster ovary (CHO) cells for single channel recordings (Linsdell & Hanrahan, 1998a). Briefly, channels were activated by exposure of the cytoplasmic face of the patch to 40–180 nM protein kinase A catalytic subunit (PKA; prepared in the laboratory of Dr M. P. Walsh, University of Calgary, Alberta, Canada, as described previously; Tabcharani et al., 1991) plus 1 mM MgATP (Sigma, St Louis, MO, U.S.A.). Macroscopic current-voltage (I-V) relationships were constructed using depolarizing ramp protocols and have had the background (leak) current recorded before the addition of PKA subtracted digitally, as described previously (Linsdell & Hanrahan, 1996b; 1998a). Current traces were filtered at 100 Hz (for macroscopic currents, I) or 50 Hz (for single channel currents, i) using an 8-pole Bessel filter, and digitized at 250 Hz. Macroscopic currents were analysed using pCLAMP6 computer software (Axon Instruments, Foster City, CA, U.S.A.), while single channel currents were analysed using custom-written pCLAMP-compatible DRSCAN software (Hanrahan et al., 1998).
All recording solutions contained (mM) NaCl 150, MgCl2 2, TES (pH 7.4) 10 except where the effects of reducing extracellular Cl− concentration were studied (Figure 4), when the pipette solution contained (mM) NaCl 20, Na·gluconate 134, Mg(OH)2 2, TES (pH 7.4) 10. Channel blockers (all obtained from Sigma, except glibenclamide (glyburide; Calbiochem, La Jolla, CA, U.S.A.), 4,4′-dinitrostilbene-2,2′-disulphonic acid (DNDS; Pfaltz & Bauer, Waterbury, CT, U.S.A.) and diphenylamine-2-carboxylate (DPC; N-phenylanthranilic acid; Fluka Chemika, Buchs, Switzerland)) were added to the intracellular solution from concentrated stocks made up in recording solution and stored frozen until just before the experiment.
Figure 4.
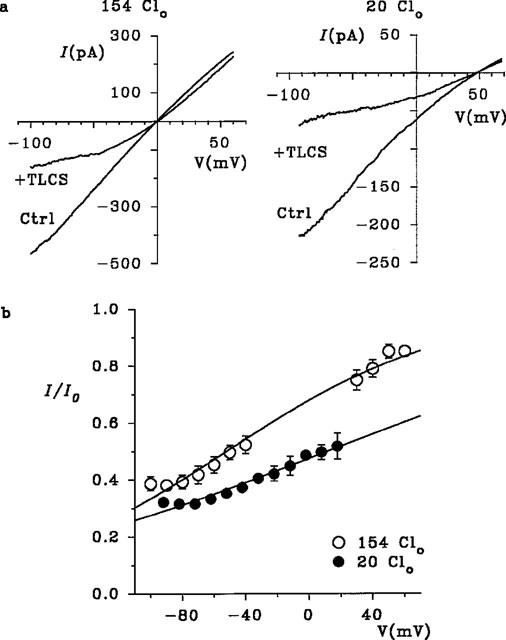
Dependence of block by intracellular TLCS on extracellular Cl− concentration. (a) Effects of 60 μM TLCS on macroscopic DFTR currents recorded with 154 mM (left) or 20 mM (right) Cl− in the pipette (extracellular) solution (Clo). (b) Mean fraction of control current remaining (I/I0) following addition of 60 μM TLCS as a function of voltage. The data have been fitted by equation 1, giving a KD(0) of 109 μM and a −z′ of 0.36 with 154 mM Cl−, and a KD(0) of 54 μM and a −z′ of 0.22 with 20 mM Cl−.
The effects of CFTR channel blockers were analysed according to the Woodhull (1973) model of voltage dependent block:
where I is the amplitude of the current remaining in the presence of blocker, I0 is the control, unblocked current amplitude, [B] is the blocker concentration, and KD(V) is the voltage dependent dissociation constant, the voltage dependence of which is given by:
where KD(0) is the dissociation constant at 0 mV, z′ is the apparent valence of the blocking ion, that is the real valence (−2 for TLCS and DNDS, −1 for all other blockers studied here) multiplied by δ, the fraction of the membrane electric field experienced by the blocking ion, V is the membrane potential, and F, R and T have their normal thermodynamic meanings.
Experiments were carried out at room temperature, 21–23°C. Mean values are presented as mean±s.e.mean. For graphical presentation of mean values, error bars represent±s.e.mean, where this is larger than the size of the symbol.
Results
The effects of the MRP/cMOAT substrates TLCS and E217βG on macroscopic CFTR Cl− currents were examined in inside-out membrane patches excised from BHK cells stably expressing CFTR (Linsdell & Hanrahan, 1996b; see Methods). As shown in Figure 1, the addition of TLCS (200 μM) or E217βG (200 μM) to the intracellular solution caused a marked reduction in CFTR Cl− current. In both cases the block was clearly voltage-dependent, with the reduction in current amplitude being strongest at negative voltages, as would be expected for block of the channel pore within the transmembrane electric field by a negatively charged molecule acting from the inside. Block occurred immediately on application of either TLCS or E217βG, and in both cases block was readily reversible on washing (not shown). The unconjugated bile salts taurocholate and cholate were also effective voltage dependent blockers of CFTR Cl− current when added to the intracellular solution (Figure 1). Although these bile salts are thought not to be transported by MRP or cMOAT in the unconjugated form, they may inhibit MRP- or cMOAT-mediated transport of other substances (Holló et al., 1996; Loe et al., 1996; Saxena & Henderson, 1996). The blocking effects of all of these substances was analysed using the Woodhull (1973) model (see Methods); results are summarized in Table 1. The chemical structures of these substances are given in Figure 2.
Figure 1.
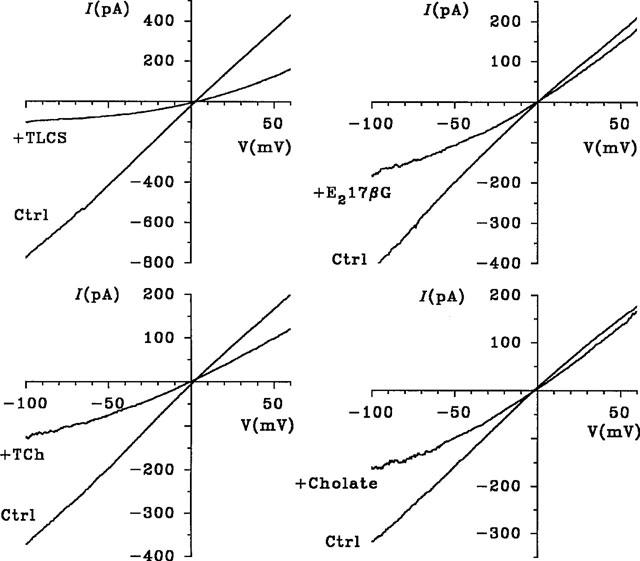
Voltage-dependent block of macroscopic CFTR Cl− currents by MRP/cMOAT substrates. Each panel shows an example macroscopic I-V relationship recorded before (ctrl.) and immediately after addition of TLCS (200 μM), E217βG (200 μM), taurocholate (TCh; 500 μM) or cholate (1 mM) to the intracellular solution. Experiments with different blockers were carried out on different patches. CFTR currents were activated by addition of PKA in the presence of MgATP, and reached steady-state levels 2–5 min after addition of PKA. Patches were held at 0 mV and depolarizing voltage ramps applied at a rate of 100 mV s−1. Current-voltage relationships were leak subtracted as described in Methods.
Table 1.
Blockers of macroscopic CFTR Cl− current in inside-out BHK cell patches
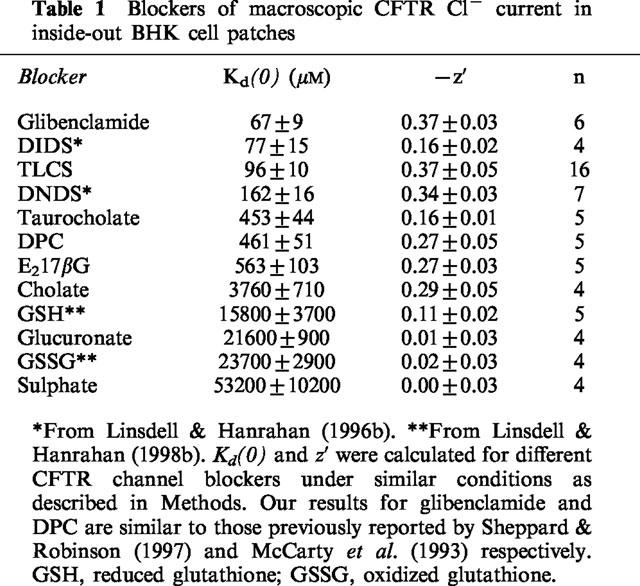
Figure 2.
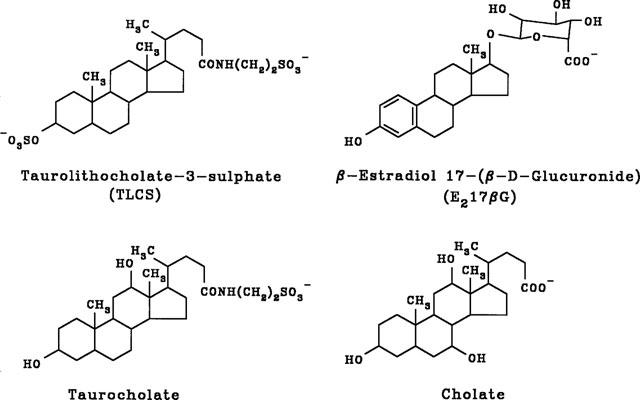
Chemical structures of the blockers used in Figure 1.
Of those substances tested in Figure 1, TLCS was the highest affinity blocker of CFTR Cl− current (Table 1). The concentration dependence of block of macroscopic CFTR current by intracellular TLCS is described in Figure 3a. At each concentration studied, the mean fraction of control current remaining following addition of TLCS (I/I0) was calculated from 3–4 patches at −100, −50 and +50 mV. At each potential, the data have been fitted by equation 1 (see Methods), suggesting that a single TLCS molecule blocks the channel with a KD of 25.4 μM at −100 mV, 45.3 μM at −50 mV, and 186.8 μM at +50 mV. Similar analyses at other potentials allowed examination of the voltage dependence of block (Figure 3b). The fitted straight line gives a KD of 93.2 μM at 0 mV, and its slope an apparent valency, z′, of −0.35 for TLCS, according to equation 2 (see Methods).
Figure 3.
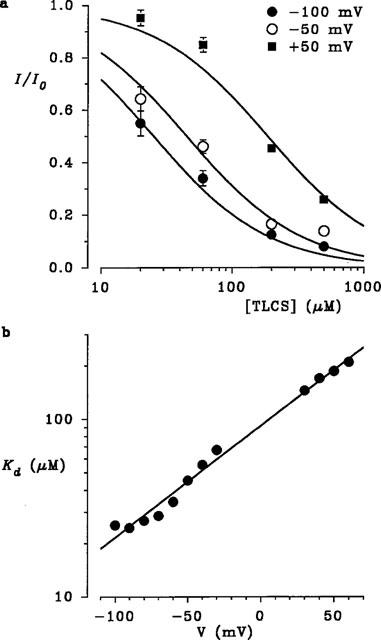
Concentration and voltage dependence of block by TLCS. (a) Mean fraction of control current remaining (I/I0) following addition of different concentrations of TLCS, at different membrane potentials. n=3–4 patches. The data at each voltage have been fitted by equation 1, as described in the text. (b) Dissociation constant, KD, for TLCS block as a function of membrane potential. KD was calculated from equation 1 as described in the text. The fitted straight line indicates a KD of 93.2 μM at 0 mV at and apparent valency (z′) of −0.35 for TLCS.
The voltage dependence of block by MRP substrates suggests that these substances block CFTR Cl− currents by binding within the pore. Consistent with this hypothesis, block by intracellular TLCS was enhanced by reducing the extracellular Cl− concentration (Figure 4). With 154 mM Cl− in the pipette solution, 60 μM TLCS blocked macroscopic currents with a mean KD(0) of 109±16 μM and a −z′ of 0.36±0.02 (n=6); note that these values are similar to those obtained from the concentration dependence of block shown in Figure 3. Reducing external Cl− concentration to 20 mM (by replacement with gluconate) significantly reduced both the KD0) (to 54±1 μM, n=5) and also the −z′ (to 0.22±0.01, n=5) (P<0.01 in both cases, Student's two-tailed t-test).
The effect of intracellular TLCS on CFTR single channel currents was examined in inside-out membrane patches excised from CHO cells stably expressing CFTR (Tabcharani et al., 1991; see Methods). Addition of 50 μM TLCS to the intracellular solution caused a voltage-dependent reduction in single channel current amplitude (Figure 5), suggesting that TLCS blocks the channel by a relatively fast mechanism, with individual blocking and unblocking events occurring too rapidly to resolve using patch clamp recording at the time resolution used. Intracellular TLCS did not appear to affect channel gating or open probability; indeed, the reduction in unitary amplitude was sufficient to account for the reduction in macroscopic current amplitude due to TLCS. Inclusion of 50 μM TLCS in the pipette solution has no discernible effect on CFTR single channel current amplitude or gating (not shown), suggesting that TLCS blocks the channel preferentially from its cytoplasmic end. A number of other anions have previously been shown to block CFTR only from the cytoplasmic side of the membrane (e.g. gluconate (Linsdell & Hanrahan, 1996a), disulphonic stilbenes (Linsdell & Hanrahan, 1996b), glibenclamide (Sheppard & Robinson, 1997)).
Figure 5.
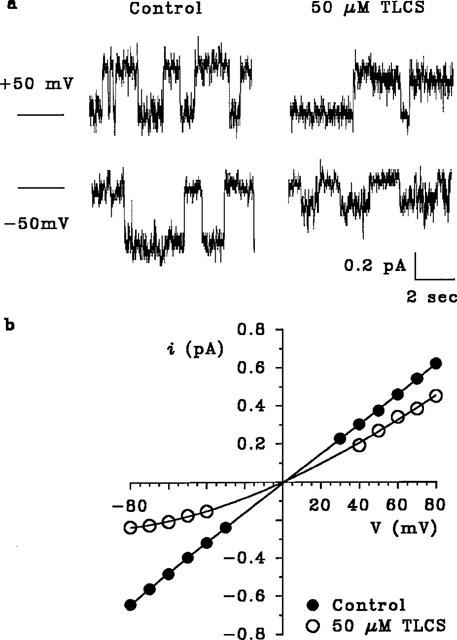
Block of unitary CFTR Cl− currents by TLCS. (a) Single CFTR channel currents recorded at +50 mV (top) or −50 mV (bottom), before (control) and after addition of 50 μM TLCs to the intracellular solution. In each case the closed state of the channel is indicated by the line to the left. TLCS causes a voltage-dependent reduction in apparent single channel current amplitude, and effect which is illustrated more clearly by the mean single channel i-V relationships in (b). Mean of data from 3–18 patches.
The apparent affinity with which TLCS and E217βG block CFTR Cl− currents are much higher than those of unconjugated glutathione (Linsdell & Hanrahan, 1998b; Table 1), glucuronide, or sulphate (data not shown; Table 1), and are comparable to those previously described for other classes of CFTR channel blockers, such as the sulphonylureas glibenclamide and tolbutamide (Sheppard & Welsh, 1992; Schultz et al., 1996; Venglarik et al., 1996; Sheppard & Robinson, 1997), the arylaminobenzoates diphenylamine-2-carboxylate (DPC) and flufenamic acid (McCarty et al., 1993), and the disulphonic stilbenes 4,4′-dinitrostilbene-2,2′-disulphonic acid (DNDS) and 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS; Linsdell & Hanrahan, 1996b). The effects of intracellular glibenclamide, DNDS and DPC on macroscopic CFTR Cl− currents in excised BHK cell patches are shown in Figure 6 for comparison. The affinity and voltage dependence of these blockers measured under the same conditions as in Figure 1 are summarized in Table 1. This table does not represent an exhaustive list of known CFTR channel blockers, but rather selected examples of different classes of blockers. For example, other arylaminobenzoates may block cardiac CFTR with a higher affinity than any blocker listed in Table 1 (Walsh & Wang, 1998). The chemical structures of glibenclamide, DNDS and DPC, as shown in Figure 6, indicate that they are unrelated to the novel CFTR channel blockers identified in the present study. However, MRP substrates and unconjugated bile sales are structurally related to oestrogens which have previously been reported to bind to and inhibit CFTR (Singh et al., 1994; 1995).
Figure 6.
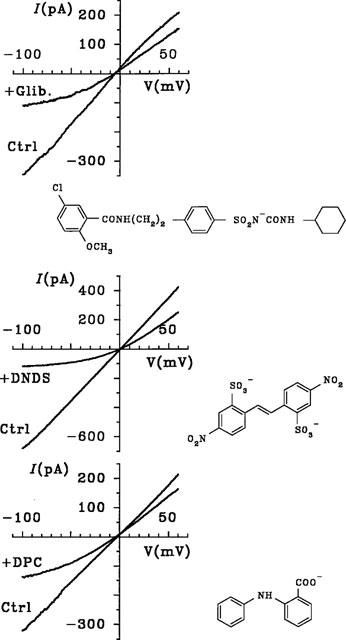
Voltage-dependent block of macroscopic CFTR Cl− currents by different classes of CFTR channel blockers. As in Figure 1, example macroscopic I-V relationships were recorded before (ctrl) and immediately after addition of glibenclamide (Glib; 60 μM), DNDS (200 μM) or DPC (200 μM) to the intracellular solution. The structures of these chemically diverse blockers are also shown.
Discussion
Based on its structural similarity to other ABC proteins, CFTR was originally proposed to function as a pump (Hyde et al., 1990). However, subsequent experimental evidence proved that the primary (although perhaps not exclusive) function of CFTR is that of a Cl− channel (see Gadsby et al., 1995; Hanrahan et al., 1995). Nevertheless, recent evidence has suggested that CFTR may share some functional similarities with other ABC proteins which do function as pumps, in particular its close structural relatives MRP and cMOAT (Lallemand et al., 1997; Linsdell & Hanrahan, 1998a, 1998b). Our present results indicate that TLCS and E217βG , both of which are high affinity substrates for both MRP- and cMOAT-mediated active transport, also act as blockers of the CFTR Cl− channel. Whether these substances are actually permeant in CFTR, as is the case for lower affinity anionic blockers such as gluconate and glutamate (Linsdell & Hanrahan, 1998a) and glutathione (Linsdell & Hanrahan, 1996b) is not addressed in the present study. Nevertheless, our results suggest some similarity between CFTR and other ABC proteins in terms of anion binding.
MPR and cMOAT transport a broad range of glutathione, glucuronide and sulphate conjugates (Oude Elferink et al., 1995; Keppler et al., 1997). We find that both the sulphate TLCS and the glucuronide E217βG cause voltage dependent block of Cl− permeation in CFTR when applied to the cytoplasmic face of excised patches (Figure 1). In both cases the affinity of the blocking reaction (at 0 mV) was at least an order of magnitude higher than that associated with block by unconjugated glutathione, glucuronate or sulphate (Table 1).
The unconjugated bile salts taurocholate and cholate were also effective voltage dependent blockers of CFTR. Although these bile salts are thought not to be substrates for MRP- or cMOAT-mediated transport, they do inhibit transport of other substrates and thus probably interact with an anion binding site on the cytoplasmic face of these proteins (Holló et al., 1996; Loe et al., 1996; Saxena & Henderson, 1996). Unconjugated bile salts are transported by the hepatocanalicular protein known as canalicular bile acid transporter (cBAT; Müller & Jansen, 1997) or bile salt export pump (BSEP; Gerloff et al., 1998). The gene encoding this protein was recently cloned from rat liver and named spgp (sister of P-glycoprotein; Gerloff et al., 1998). Although the spgp protein shows closer homology to P-glycoprotein than to MRP or cMOAT, a yeast bile salt transporter (bile acid transporter, bat1p; Ortiz et al., 1997) is homologous to both MRP and cMOAT. Thus a broad range of organic anions may bind to a homologous anion binding site on all these ABC proteins (CFTR, MRP, cMOAT, cBAT/BSEP), which contributes either to a cytoplasmic substrate binding site (in MRP, cMOAT and cBAT/BSEP) or to the Cl− channel pore in CFTR. Similarly, substrates of P-glycoprotein inhibit an outwardly rectifying Cl− channel in multidrug-resistant CHO cells overexpressing P-glycoprotein (Bear, 1994).
The affinity of CFTR channel block by MRP substrates, in particular TLCS, is comparable to that of other well known classes of CFTR channel blockers, namely sulphonylureas, disulphonic stilbenes, and arylaminobenzoates (Table 1), although block by DPC (McCarty et al., 1993) and glibenclamide (Schultz et al., 1996; Sheppard & Robinson, 1997) appear kinetically slower than that by TLCS (Figure 5), with individual blocking events being resolved at the single channel level. MRP substrates may therefore comprise a novel class of probes with which to investigate the molecular architecture of the CFTR channel pore. The dependence of block by TLCS on voltage (Figures 1, 3 and 5) and on extracellular Cl− concentration (Figure 4) are both consistent with binding within the channel pore. Previously, it has been shown that DPC interacts with CFTR residues serine 341 (located in the sixth transmembrane region, TM6) and threonine 1134 (in TM12) (McDonough et al., 1994), and that DNDS interacts with TM6 residue arginine 347 (Linsdell & Hanrahan, 1996b), which suggests that these residues may contribute to intrapore anion binding sites. An interesting difference between the MRP substrates studied here and other CFTR channel blockers studied previously is that all of the blockers identified in the present study occur naturally within the body, raising the possibility that an endogenous cytoplasmic blocker may regulate CFTR conductance in vivo (Tabcharani et al., 1991; Linsdell & Hanrahan, 1996a).
Block of the CFTR Cl− channel is potentially of clinical as well as biophysical interest. Selective, high affinity CFTR channel blockers may be useful in the treatment of diarrhoea (Sheppard & Welsh, 1992). Furthermore, by analogy with other ion channels, it has been suggested that CFTR channel blockers might serve as lead compounds for the development of CFTR channel openers (Sheppard & Welsh, 1992; Schultz et al., 1996), of great potential therapeutic relevance to cystic fibrosis. Anionic MRP/cMOAT substrates represent a novel class of CFTR channel blockers which might guide the development of clinically useful modulators of CFTR activity.
Acknowledgments
We thank Jie Liao for maintaining the cell cultures. This work was supported by the Canadian Cystic Fibrosis Foundation (CCFF), Medical Research Council (MRC) of Canada, and National Institute of Diabetes and Digestive and Kidney Diseases. P.L. was supported by postdoctoral fellowships from CCFF and MRC. J.W.H. is an MRC scientist.
Abbreviations
- ABC
ATP-binding cassette
- BHK
baby hamster kidney
- BSEP
bile salt export pump
- cBAT
canalicular bile acid transporter
- CFTR
cystic fibrosis transmembrane conductance regulator
- CHO
Chinese hamster ovary
- cMOAT
canalicular multispecific organic anion transporter
- DIDS
4,4′-diisothiocyanostilbene-2,2′-disulphonic acid
- DNDs
4,4′-dinitrostilbene-2,2′-disulphonic acid
- DPC
diphenylamine-2-carboxylate
- E217βG
β-estradiol 17-(β-D-glucuronide)
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- MRP
multidrug resistance-associated protein
- PKA
protein kinase A catalytic subunit
- TLCS
taurolithocholate-3-sulphate
- TM
transmembrane region
- z′
apparent valence
References
- BEAR C.E. Drugs transported by P-glycoprotein inhibit a 40pS outwardly rectifying chloride channel. Biochem. Biophys. Res. Commun. 1994;200:513–521. doi: 10.1006/bbrc.1994.1478. [DOI] [PubMed] [Google Scholar]
- GADSBY C. C., NAGEL G., HWANG T.-C. The CFTR chloride channel of mammalian heart. Annu Rev. Physiol. 1995;57:387–416. doi: 10.1146/annurev.ph.57.030195.002131. [DOI] [PubMed] [Google Scholar]
- GERLOFF T., STIEGER B., HAGENBUCH B., MADON J., LANDMANN L., ROTH J., HOFMANN A.F., MEIER P.J. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- HANRAHAN J.W., KONE Z., MATTHEWS C.J., LUO J., JIA Y., LINSDELL P. Patch clamp studies of the CFTR chloride channel. Meths. Enzymol. 1998;293:169–194. doi: 10.1016/s0076-6879(98)93014-2. [DOI] [PubMed] [Google Scholar]
- HANRAHAN J.W., TABCHARANI J.A., BECQ F., MATHEWS C.J., AUGUSTINAS O., JENSEN T.J., CHANG X.-B., RIORDAN J.R.Function and dysfunction of the CFTR chloride channel Ion Channels and Genetic Diseases 1995New York: Rockefeller University Press; 125–137.eds. Dawson, D.C. & Frizzell, R.A. [PubMed] [Google Scholar]
- HIGGINS C.F. The ABC of channel regulation. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- HOLLÓ Z., HOMOLYKA L., HEGEDÜS T., SARKADI B. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Letts. 1996;383:99–104. doi: 10.1016/0014-5793(96)00237-2. [DOI] [PubMed] [Google Scholar]
- HYDE S.C., EMSLEY P., HARTSHORN M.J., MIMMACK M.M., GILEADI U., PEARCE S.R., GALLAGHER M.P., GILL D.R., HUBBARD R.E., HIGGINS C.F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- JEDLITSCHKY G., LEIER I., BUCHHOLZ U., BARNOUIN K., KURZ G., KEPPLER D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988–994. [PubMed] [Google Scholar]
- KEPPLER D., LEIER I., JEDLITSCHKY G. Transport of glutathione conjugates and glucuronides by the multidrug resistance proteins MRP1 and MRP2. Biol. Chem. 1997;378:787–791. [PubMed] [Google Scholar]
- LALLEMAND J.Y., STOVEN V., ANNEREAU J.P., BOUCHER J., BLANQUET S., BARTHE J., LENOIR G. Induction by antitumoral drugs of proteins that functionally complement CFTR: a novel therapy for cystic fibrosis. Lancet. 1997;350:711–712. doi: 10.1016/s0140-6736(05)63510-6. [DOI] [PubMed] [Google Scholar]
- LEIER I., JEDLITSCHKY G., BUCHHOLZ U., COLE S.P.C., DEELEY R.G., KEPPLER D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994;269:27807–27810. [PubMed] [Google Scholar]
- LINSDELL P., HANRAHAN J.W. Flickery block of single CFTR chloride channels by intracellular anions and osmolytes. Am. J. Physiol. 1996a;271:C628–C634. doi: 10.1152/ajpcell.1996.271.2.C628. [DOI] [PubMed] [Google Scholar]
- LINSDELL P., HANRAHAN J.W. Disulphonic stilbene block of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a mammalian cell line and its regulation by a critical pore residue. J. Physiol. 1996b;496:687–693. doi: 10.1113/jphysiol.1996.sp021719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSDELL P., HANRAHAN J.W. Adenosine triphosphate-dependent asymmetry of anion permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. J. Gen. Physiol. 1998a;11:601–614. doi: 10.1085/jgp.111.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSDELL P., HANRAHAN J.W. Glutathione permeability of CFTR. Am. J. Physiol. 1998b;275:C323–C326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- LINSDELL P., TABCHARANI J.A., HANRAHAN J.W. Multi-ion mechanism for ion permeation and block in the cystic fibrosis transmembrane conductance regulator chloride channel. J. Gen. Physiol. 1997;110:365–377. doi: 10.1085/jgp.110.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE D.W., ALMQUIST K.C., COLE S.P.C., DEELEY R.G. ATP-dependent 17β-estradiol 17-(β-D-glucuronide) transport by multidrug resistance protein (MRP). Inhibition by cholestatic steroids. J. Biol. Chem. 1996;271:9683–9689. doi: 10.1074/jbc.271.16.9683. [DOI] [PubMed] [Google Scholar]
- MCCARTY N.A., MCDONOUGH S., COHEN B.N., RIORDAN J.R., DAVIDSON N., LESTER H.A. Voltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl− channel by two closely related arylaminobenzoates. J. Gen. Physiol. 1993;102:1–23. doi: 10.1085/jgp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONOUGH S., DAVIDSON N., LESTER H.A., MCCARTY N.A. Novel pore-lining residues in CFTR that govern permeation and open-channel block. Neuron. 1994;13:623–634. doi: 10.1016/0896-6273(94)90030-2. [DOI] [PubMed] [Google Scholar]
- MÜLLER M., JANSEN P.L.M. Molecular aspects of hepatobiliary transport. Am. J. Physiol. 1997;272:G1285–G1303. doi: 10.1152/ajpgi.1997.272.6.G1285. [DOI] [PubMed] [Google Scholar]
- ORTIZ D.F., STPIERRE M.V., ABDULMESSIH A., ARIAS I.M. A yeast ATP-binding cassette-type protein mediating ATP-dependent bile acid transport. J. Biol. Chem. 1997;272:15358–15365. doi: 10.1074/jbc.272.24.15358. [DOI] [PubMed] [Google Scholar]
- OUDE ELFERINK R.P.J., MEIJER D.K.F., KUIPERS F., JANSEN P.L.M., GROEN A.K., GROOTHUIS G.M.M. Hepatobiliary secretion of organic compounds; molecular mechanisms of membrane transport. Biochim. Biophys. Acta. 1995;1241:215–268. doi: 10.1016/0304-4157(95)00006-d. [DOI] [PubMed] [Google Scholar]
- SAXENA M., HENDERSON G.B. MOAT4, a novel multispecific organic-anion transporter for glucuronides and mercapturates in mouse L1210 cells and human erythrocytes. Biochem. J. 1996;320:273–281. doi: 10.1042/bj3200273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ B.D., DEROOS A.D.G., VENGLARIK C.J., SINGH A.K., FRIZZELL R.A., BRIDGES R.J. Glibenclamide blockade of CFTR chloride channels. Am. J. Physiol. 1996;271:L192–L200. doi: 10.1152/ajplung.1996.271.2.L192. [DOI] [PubMed] [Google Scholar]
- SHEPPARD D.N., ROBINSON K.A. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a murine cell line. J. Physiol. 1997;503:333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPPARD D.N., WELSH M.J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J. Gen. Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH A.K., BRADBURY N.A., SCHULTZ B.D., PRICE E., FRIZZELL R.A., BRIDGES R.J.Photoaffinity labeling of CFTR by radiolabeled estrogens: evidence for direct binding of estrogens to CFTR Pediatr. Pulmonol. 199512197(abstract) [Google Scholar]
- SINGH A.K., SCHULTZ B.D., VENGLARIK C.J., FRIZZELL R.A., BRIDGES R.J.Estrogen inhibition of CFTR-mediated Cl− secretion Pediatr. Pulmonol. 199410186(abstract) [Google Scholar]
- TABCHARANI J.A., CHANG X.-B., RIORDAN J.R., HANRAHAN J.W. Phosphorylation-regulated Cl− channel in CHO cells by stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- VENGLARIK C.J., SCHULTZ B.D., DEROOS A.D.G., SINGH A.K., BRIDGES R.J. Tolbutamide causes open channel blockade of cystic fibrosis transmembrane conductance regulator Cl− channels. Biophys. J. 1996;70:2696–2703. doi: 10.1016/S0006-3495(96)79839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSH K.B., WANG C. Arylaminobenzoate block of the cardiac cyclic AMP-dependent chloride channel. Mol. Pharmacol. 1998;53:539–546. doi: 10.1124/mol.53.3.539. [DOI] [PubMed] [Google Scholar]
- WOODHULL A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


