Abstract
Possible nitric oxide (NO)-mediated effects on lipolysis were investigated in vivo in human subcutaneous adipose tissue using microdialysis, as well as in vitro on isolated fat cells of non-obese, healthy volunteers. NO donors were added to the ingoing dialysate solvents.
Changes in lipolysis and local blood flow were investigated by measuring glycerol levels and ethanol ratios, respectively, in the microdialysates.
It was shown that the NO synthase inhibitor, NG-monomethyl L-arginine (L-NMMA), but not the biologically inactive enantiomer NG-monomethyl D-arginine (D-NMMA), increased glycerol levels in the microdialysates without causing a change of local blood flow. In addition, L-NMMA increased glycerol levels in the microdialysate when local blood flow was stimulated with hydralazine.
Nitric oxide gas as well as the NO donor, nitroglycerine, reduced glycerol release from isolated adipocytes in vitro.
Expression of inducible nitric oxide synthase (iNOS) in human adipose tissue was shown by Western blot analysis. Biologically active NOS was demonstrated by measuring total enzymatic activity.
In conclusion, the data demonstrate that inhibition of NO release in subcutaneous adipose tissue results in an increased lipolysis in vivo. These effects, which were also observed in vitro, are independent of local blood flow changes. Furthermore, the demonstration of enzymatic NOS activity and the expression of inducible nitric oxide synthase (iNOS) in adipose tissue indicate that locally synthesized NO may play a role in the physiological control of lipolysis in human adipose tissue.
Keywords: Adipose tissue, nitric oxide synthase, glycerol, microdialysis, isolated fat cells
Introduction
Nitric oxide (NO) is formed in cells from L-arginine, which in the presence of oxygen, is converted to L-citrulline by NO synthases (NOS). Three different isoforms of NOS have been cloned and sequenced (reviewed in Michel & Feron, 1997). Two of the isoforms, neuronal NOS (nNOS) and endothelial NOS (eNOS), are constitutive, and produce small amounts of NOS following stimuli that raise intracellular Ca2+ concentrations. The third isoform, inducible NOS (iNOS), is expressed after induction by such agents as cytokines and bacterial lipopolysaccharide.
The action of NO is difficult to study since it reacts readily with oxygen, superoxide radicals or hydrogen peroxide to produce NO2, peroxynitrite and nitrate/nitrite, respectively (Henry et al., 1991). However, the effects of NO can be studied indirectly by adding NO donors, using scavengers, or by reduction of NO synthesis using NOS inhibitors such as the L-arginine analogue, NG-monomethyl L-arginine (L-NMMA).
NOS isoforms are expressed in several different cell types and NO is believed to be involved in the regulation of many events, including inflammation, vascular tone and metabolism (reviewed in Christopherson & Bredt, 1997; Michel & Feron, 1997). However, it is not known if NO also regulates lipid metabolism. Adipose tissue is the largest reservoir of lipids in the body. These are mobilized by lipolysis in fat cells. Lipolysis in adipose tissue is under hormonal regulation, the most well known being insulin and catecholamine effects on antilipolytic and lipolytic mechanisms. Recently, we suggested a dual cholinergic mechanism that also regulates lipolysis in vivo (Andersson & Arner, 1995). In theory, the latter regulatory system could utilize NO as a mediator, since NO plays a role in the parasympathetic modulation of myocardial contractility (Hare et al., 1998). Nitric oxide synthase has been demonstrated in white adipose tissue of the rat (Ribiere et al., 1996) indicating this tissue as a potential source of NO production. To date, no evidence for an involvement of NO in lipolysis has been presented. The major aim of this study was therefore to investigate whether NO has effects on lipolysis in vivo. We used microdialysis to investigate the effects of L-NMMA on local lipolysis in situ in human abdominal subcutaneous adipose tissue (Lafontan & Arner, 1996). We also studied total NOS activity and expression of NOS in this tissue, as well as the effect of NO on lipolysis in isolated fat cells in vitro.
Methods
Subjects
The experimental group comprised of 15 healthy, drug-free, non-obese subjects (four men and 11 women), none of whom were receiving medication. The ages ranged from 20–50 years (mean±s.e.mean=33±2.4 years) and body mass indices from 18–25 kg m−2 (mean±s.e.mean=22.2±0.66 kg m−2). The subjects were investigated on one or two occasions. For the in vitro experiments on isolated fat cells and tissue pieces, we used subcutaneous adipose tissue obtained from the abdominal area during elective surgery for non-malignant disorders. Subjects were fasted overnight and received only intravenous saline until the biopsy was taken at the start of the operation. Anaesthesia was induced with a short-acting barbiturate and then maintained with fentanyl in combination with a mixture of oxygen and nitrous oxide. Subjects were not selected on the basis of age, sex or body-weight.
The study was approved by the Ethical Committee at Karolinska Institutet. The subjects were given a detailed description of the study prior to their consent being obtained.
Microdialysis probes
Microdialysis probes were supplied by CMA Microdialysis, Stockholm, Sweden. A detailed description of the microdialysis method (Lafontan & Arner, 1996) and the microdialysis probes used (Tossman & Ungerstedt, 1986) have been presented elsewhere. Briefly, a dialysis tube (10×0.5 mm, 20,000 mol.wt. cut-off) was glued to the end of a double-lumen steel cannula and perfused with neutral dialysis solvent at low speed using precision pumps (CMA Microdialysis, Stockholm, Sweden). With these probes the perfusion solvent enters through the inner cannula, passes the dialysis membrane, and leaves through the outer cannula, from which it is collected.
Experimental design
The volunteers participating in the microdialysis experiments were investigated, in a supine position between the hours of 08.00–12.00, following an overnight fast, as described by Galitsky et al., (1993). Four to six probes were inserted into the abdominal adipose tissue surrounding the umbilicus, at a distance of at least 30 mm from each other. Throughout the experiment, including a pre-experimental period of 30 min, the inlet tubings of the probes were connected to microinjection pumps (CMA Microdialysis, Stockholm, Sweden) and probes continuously perfused with an ethanol-based (50 mmol l−1) Ringer-solution, composed as described by Arner et al. (1991). L-NMMA (1–10 mmol l−1) or D-NMMA (10 mmol l−1) was added to the ingoing solvent perfusate during periods of 1 h (60–120 min) or 2 h (60–180 min) of the 3 h experimental period. The effects of the vasodilator agent hydralazine (12.5 mg ml−1) alone were tested by adding the drug to the perfusate during a 1 h period (60–120 min). In the experiments investigating the effects of NOS inhibition during hydralazine clamping, the drug was included in the solvent perfusate throughout the entire experiment (0–180 min). In these experiments L-NMMA was added to the ingoing perfusate over 2 h (0–120 min).
Determination of glycerol levels and ethanol ratios in the microdialysate
In each experiment, fractions of the outgoing dialysate were collected for glycerol analysis using a bioluminometric method (Helmér et al., 1989) or ethanol analysis using an enzymatic method (Bernt & Gutman, 1974). Ethanol was also determined in the ingoing perfusate and the ratio of ethanol leaving versus that entering calculated. Changes in this ratio reflect changes in local blood flow of the adipose tissue (as discussed by Arner & Bülow, 1993). It has been demonstrated that 50 mmol l−1 ethanol does not alter lipolysis and has no effect on adipose tissue blood flow (Galitsky et al., 1993). This microdialysis technique also gives similar results to those obtained with local injection of xenon, when the two techniques to measure local blood flow in human subcutaneous adipose tissue are compared (Felländer et al., 1996). The coefficient of variations for baseline glycerol levels and ethanol ratios were 10% and 5%, respectively.
Determination of glycerol release from isolated human adipocytes
In additional experiments, lipolysis was investigated in vitro (as described by Lönnqvist et al., 1992). Briefly, isolated fat cells were prepared from a subcutaneous fat biopsy and incubated for 2 h at 37°C in diluted conditions (2%, v v−1) in an albumin-containing buffer (Lönnqvist et al., 1992). Aliquots of the medium were taken after 2 h for determination of glycerol as an index for lipolysis (Helmér et al., 1989). In one set of experiments, the isolated fat cells were exposed to nitric oxide gas for either 5 or 15 min. The tubes were then sealed and glycerol levels determined after a total experimental period of 2 h. Isoprenaline (10 μmol l−1) was added to the incubation medium in order to stimulate lipolysis. In another set of experiments, effects of the nitric oxide donor, nitroglycerine (1–10 mmol l−1) in the presence of dl-cysteine (10 mmol l−1) (Feelisch, 1991), were studied. Initial experiments showed that the rate of glycerol release was linear with incubation times of at least 2 h in both the absence and presence of NO gas or isoprenaline.
Measurement of total NOS activity
NOS activities were measured as described previously (Ribiere et al., 1996). Human adipose tissues were homogenized at 4°C using an Ultra-Turrax in a freshly prepared buffer containing Tris-HCl (10 mmol l−1) buffer (pH 7.4), EDTA (1.5 mmol l−1), dithiothreitol (0.5 mmol l−1) and protease inhibitors (10 μmol l−1 benzamidine and 100 μmol l−1 phenylmethyl-sulphonylfluoride). Homogenates were then centrifuged at 500×g for 10 min at 4°C, the supernatants collected and then centrifuged again at 100,000×g for 45 min at 4°C. The resulting supernatants (soluble fraction) were used for assays of NOS activity and Western blotting.
Nitric oxide synthase activities were quantified by following the conversion of [3H]-arginine into [3H]-citrulline, as described elsewhere (Slater et al., 1991). Control samples of soluble fractions (100 to 300 μg protein in 50 μl) were incubated at 37°C for 15 min in 50 μl of 50 mmol l−1 HEPES buffer (pH 7.4) containing L-valine 50 mmol l−1, NADPH 1 mmol l−1, CaCl2 1 mmol l−1, L-arginine 20 μmol l−1 and [3H]-arginine 97 nmol l−1 (specific activity: 68 Ci mmol−1, Amersham, France). Total NOS activity was determined as the difference between [3H]-citrulline produced by control samples and those containing 2 mmol l−1 EGTA plus 2 mmol l−1 L-NAME, an inhibitor of NOS. All reactions were stopped by addition of 1.5 ml of 1 : 1 (v v−1) H2O/Dowex 50W-X8 (Na+ form) resin. Four ml H2O was added to the resin incubate mixtures, which were left to settle for 10 min. Aliquots of the supernatants were removed, liquid scintillation cocktail added (emulsifier Safe-Packard) and samples counted for radioactivity. Enzyme activities were expressed as pmol [3H]-citrulline min−1 mg−1 protein. Protein concentrations were determined according to the method of Bradford (Bradford, 1976).
Western blot analysis
Western blotting was used to characterize iNOS in human adipose tissue. Soluble fractions (100 μg protein of adipose tissue) were subjected to SDS–PAGE (7.5% acrylamide) (Laemmli, 1970). After electrophoresis, proteins were transferred to nitro-cellulose membranes. Blots were blocked with 2.5% gelatine in Tris-saline buffer containing 0.05% Tween 20 and then incubated with polyclonal iNOS antibodies (Transduction Lab Lexington, KY, U.S.A.). Previous studies have shown that iNOS is the major NOS form expressed in rat fat cells (Ribiere et al., 1996). After washing, membranes were incubated for 1 h with goat anti-rabbit secondary antibody conjugated to peroxidase. Specific proteins were detected by enhanced chemiluminescence (ECL, Amersham, France).
Materials
The following agents were added to the microdialysate solvent as sterile solutions: NG-monomethyl L-arginine acetate (L-NMMA, Research Biochemicals International, Natick, MA, U.S.A.), NG-monomethyl D-arginine acetate (D-NMMA, Research Biochemicals International, Natick, MA, U.S.A.), hydralazine (Ciba-Geigy, Basel, Switzerland). In the experiments on isolated fat cells isoprenaline (ACO, Stockholm, Sweden), nitroglycerine (Research Biochemicals International, Natick, MA, U.S.A.), dl-cysteine (Sigma Chemical Co., St. Louis, MO, U.S.A.) and NO gas (AGA AB, Stockholm, Sweden) were added to the incubation medium. In the analysis of NOS activity NG-nitro-L-arginine methylester (L-NAME, Research Biochemicals International, Natick, MA, U.S.A.) and ethylene blycol-bis (β-aminoethyl-ether) N,N′ tetraacetic acid (EGTA) (Sigma Chemical Co., St. Louis, MO, U.S.A.) were used to obtain total inhibition of NOS activity. Other reagents were of highest purity available (Kebo, Stockholm, Sweden).
Statistical analyses
Values (mean±s.e.mean) for glycerol in the microdialysates are presented as a percentage of the mean glycerol levels during the first 30 min of microdialysis (pre-experiment baseline value). Due to the variability in absolute microdialysate levels between individuals, the statistical calculations were made on percentage changes using analysis of variance (ANOVA) followed by post-hoc Fisher's PLSD-tests. P<0.05 was regarded as significant. Comparisons were made to percentage changes obtained from intra-individual control microdialysis probes perfused with Ringer-ethanol solution alone. Data were also transformed to summary measurements in terms of peak effects. When two groups of values were compared, a Student's t-test was used.
Values for ethanol in the microdialysate are presented as percentage of the concentration in the perfusion solvent since these values have been demonstrated to mirror local blood flow (Arner & Bülow, 1993).
Results
To investigate if changes in local adipose tissue NO levels could influence lipolysis in vivo, changes in glycerol levels following NOS inhibition were studied with L-NMMA (Knowles & Moncada, 1994). Figure 1 (upper panel) shows the effects of 1 h L-NMMA exposure on microdialysate glycerol levels. A concentration-dependent increase in glycerol was observed during L-NMMA treatment (peak effects 129±4 and 142±7% for L-NMMA at 1 mmol l−1 and 10 mmol l−1, respectively, compared to baseline peak effect levels of 110±3%) which was followed by a return to baseline levels 1 h after termination of L-NMMA administration. However, L-NMMA had no effect on the ethanol ratio (an indirect measurement of local blood flow, Figure 1 lower panel). Figure 2 (upper panel), shows the effects of 10 mmol l−1 of L-NMMA during a 2 h perfusion period. In these experimental conditions, a pronounced and sustained increase in dialysate glycerol levels to approximately 150% of baseline (peak effect=159±10%) as compared with Ringer's solution (peak effect=117±5%) was observed. Again, no significant change in the ethanol ratios were found (Figure 2 lower panel). To test the specificity of L-NMMA, the effect of the biologically inactive enantiomer D-NMMA were compared. D-NMMA (10 mmol l−1) did not significantly change glycerol levels (peak effect=125±4%, compared to 117±5% for Ringer's solution) or ethanol ratios in the microdialysates during the 2 h perfusion period (Figure 2 upper and lower panels).
Figure 1.
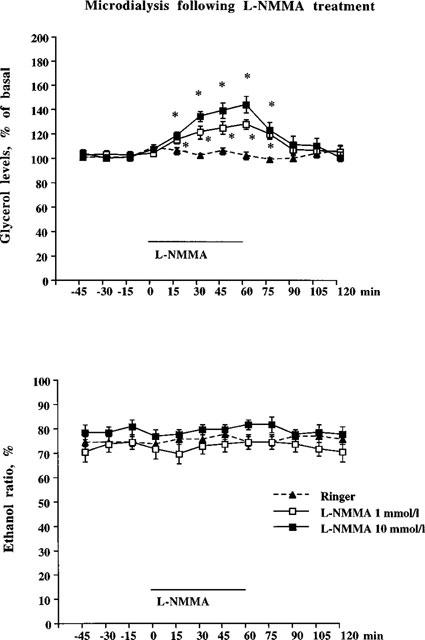
Subcutaneous adipose tissue microdialysate glycerol levels (top panel) and ethanol ratio (bottom panel) following L-NMMA treatment. Experimental protocol: 1 h of pre-treatment (probes perfused with Ringer-ethanol), 1 h of solvent or L-NMMA (1 or 10 mmol l−1), 1 h of post-treatment with solvent. L-NMMA versus solvent was statistically tested using ANOVA, followed by Fisher PLSD-test as post-hoc analysis. *Significant differences (P<0.05) from control group (Ringer alone). Microdialysate glycerol levels for both doses of L-NMMA were significantly increased compared to Ringer's solution and also differed from each other. No statistical significant differences were found comparing ethanol ratios. n=9–12 in each group. Mean glycerol levels (μmol l−1) during the pre-treatment period for the different groups were 29±2, 32±3 and 30±3, respectively.
Figure 2.
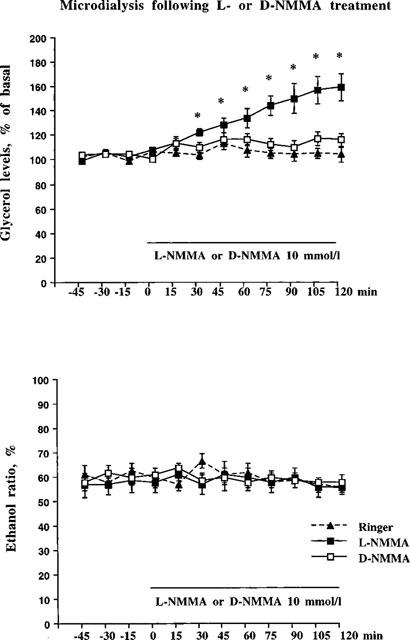
In the top panel subcutaneous adipose tissue microdialysate glycerol levels are shown following a 2 h L-NMMA or D-NMMA treatment. Experimental protocol: 1 h of pre-treatment (the probe were perfused with Ringer-ethanol), 2 h of solvent, L-NMMA (10 mmol l−1) or D-NMMA (10 mmol l−1). L-NMMA or D-NMMA versus solvent were statistically tested using ANOVA, followed by Fisher PLSD-test as post-hoc analysis. *P<0.05. n=8–10 in each group. Mean glycerol levels (μmol l−1) during the pre-treatment period for the different groups were 60±4 (Ringer), 45±4 (L-NMMA) and 47±6 (D-NMMA), respectively. In the lower panel subcutaneous adipose tissue microdialysate ethanol ratio are shown following a 2 h L-NMMA or D-NMMA treatment. No statistical differences were found on ethanol ratio (ANOVA, followed by Fisher PLSD-test as post-hoc). Number of probes=8–10 in each group.
To test if blood flow could influence the lipolytic action of NO, we investigated the effect of NOS inhibition by L-NMMA during hydralazine-stimulated blood flow. In other words, in conditions in which local blood flow was ‘clamped' by hydralazine prior to the addition of L-NMMA. Hydralazine (12.5 mg ml−1) treatment reduced the ethanol ratio by approximately 15% indicating that hydralazine itself slightly increases local blood flow (Figure 3). During hydralazine-clamping, treatment with L-NMMA resulted in a rapid increase in glycerol levels that reached a plateau level of 150–160% of hydralazine baseline levels by 30 min (Figure 4 upper panel, peak effect=177±10%). No change in the ethanol ratio caused by L-NMMA treatment was observed (Figure 4 lower panel).
Figure 3.
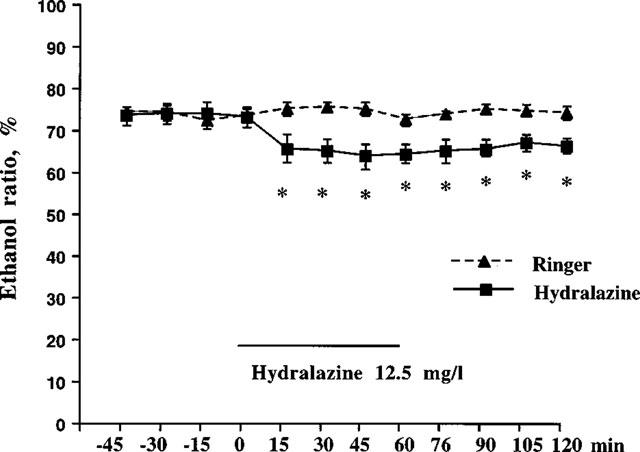
Subcutaneous adipose tissue microdialysate ethanol ratio following hydralazine treatment. Hydralazine (12.5 mg ml−1) was given during 1 h in accordance with the experimental protocol described in Figure 1. The microdialysate ethanol ratio for hydralazine was significantly decreased, compared to Ringer's solution. *P<0.05. n=5–6 in each group.
Figure 4.
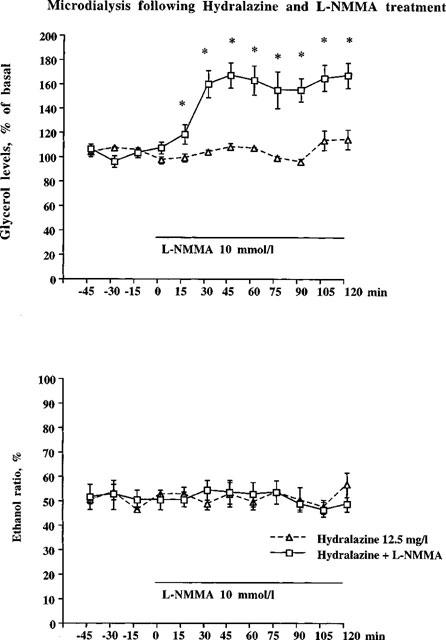
In the upper panel hydralazine (12.5 mg ml−1) was present during the whole experiment. L-NMMA (10 mmol l−1) was added to the microdialysate after 1 h. Microdialysate glycerol levels for hydralazine+L-NMMA were significantly increased as compared to hydralazine+Ringer (*P<0.05, ANOVA). n=5 in hydralazine+Ringer group, n=8 in hydralazine+L-NMMA group. Mean glycerol levels (μmol l−1) during the pre-treatment period for the different groups were 39±2 (Ringer) and 39±5 (L-NMMA), respectively. In the bottom panel hydralazine (12.5 mg ml−1) was present during the whole experiment. L-NMMA (10 mmol l−1) was added to the microdialysate after 1 h. No statistical differences were found on ethanol ratio (ANOVA, followed by Fisher PLSD-test as post-hoc). n=5 in hydralazine+Ringer group, n=8 in hydralazine+L-NMMA group.
In order to determine whether the effects of L-NMMA noted above resulted from NOS inhibition in adipose tissue, we investigated total NOS activity and iNOS protein in adipose tissue from three subjects. Enzyme activities in these subjects were 2.2, 0.86 and 0.52 pmol citrulline min−1 mg−1 protein. Western blot analysis of human adipocyte extracts with antibodies against iNOS (Ribiere et al., 1996) gave an immunoreactive band of about 130 kDa, which was clearly detected in both female and male adipose tissues. These data confirm that iNOS is expressed in human adipose tissue (Figure 5).
Figure 5.
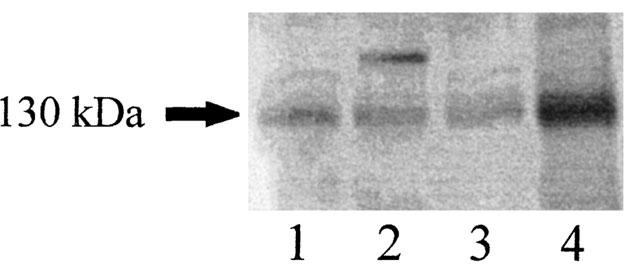
Identification of iNOS in the soluble fraction of human adipose tissue homogenates by Western blot analysis. Lines 1 and 2 (women), lines 3 and 4 (men).
Lipolysis can be determined in vitro by studying glycerol release in isolated adipocytes. The lipolysis rate can be increased by stimulation of fat cell β-adrenoceptors by isoprenaline. We also investigated if NO as a gas could alter lipolysis when administered to isolated fat cells. Gaseous exposure of fat cells to NO gas resulted in a significant and time-dependent reduction of glycerol release (Figure 6, upper panel).
Figure 6.
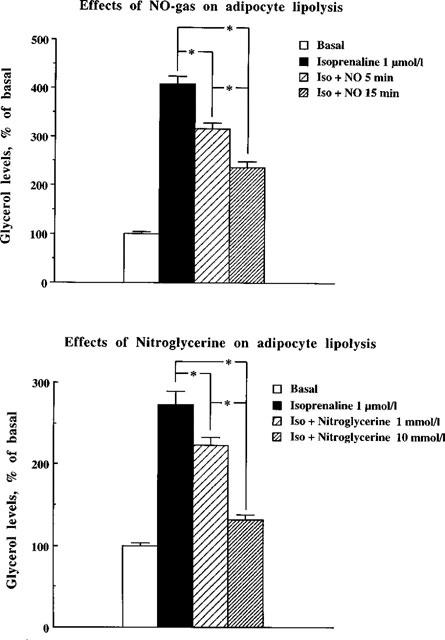
Effects of NO gas and nitro-glycerine on adipocyte lipolysis. Glycerol levels are shown as a percentage of basal (without isoprenaline) from isolated fat cells exposed to NO gas during 5 or 15 min on isoprenaline-induced lipolysis during 2 h (top panel). Means±s.e.mean are given as a percentage of basal glycerol levels, 100%=0.89±0.07 μmol g−1 triglycerides. n=6 experiments. In the bottom panel glycerol levels are shown from isolated adipocytes exposed to nitro-glycerine (1 or 10 mmol l−1) on isoprenaline-induced lipolysis during 2 h. Means±s.e.mean are given as a percentage of basal glycerol levels, 100%=0.45±0.06 μmol g−1 triglycerides. n=6 experiments. Dose versus time was statistically tested using ANOVA, followed by Fisher's PLSD-test. *P<0.05.
The effect of nitric oxide was also studied by adding the nitric oxide donor nitroglycerine in the presence of cysteine. In Figure 6 (lower panel), it is clear that nitroglycerine caused a concentration-dependent reduction of the isoprenaline-induced increase in glycerol levels.
In further experiments, it was noted that NO gas significantly inhibited glycerol release from adipocytes incubated in vitro in the absence of isoprenaline, representing basal lipolysis (control=100±4% and NO gas 60 min=18±4%).
Discussion
In this study, we investigated whether NO is involved in the regulation of lipolysis in human adipose tissue. It is suggested, that NO mediates an inhibitory action on lipolysis in human subcutaneous adipose tissue in vivo. The role of NO in human lipolysis is supported by the present demonstration of (i) an inhibitory role of NO on lipolysis in fat cells in vitro and (ii) demonstration of the expression and biological activity of NOS in human adipose tissue. The in vivo effect was demonstrated with a well established method, involving blocking NO synthesis with L-NMMA (Knowles & Moncada, 1994).
Glycerol levels, as determined by microdialysis were used as an index of lipolysis in adipose tissue, with increased dialysate glycerol levels being interpreted as an increased lipolysis and vice versa (Lafontan & Arner, 1996). We were also able to demonstrate increased adipose tissue-derived glycerol levels following inhibition of NOS by L-NMMA. This effect appeared to be concentration-dependent, sustained and specific, as indicated by the lack of effects of the inactive enantiomer D-NMMA. Moreover, effects of L-NMMA were abolished when the drug was removed from the dialysate.
In order to investigate whether NO has direct actions on adipocytes, isolated fat cells were exposed to both NO gas and to the NO-donor nitroglycerine. NO gas exposure of fat cells reduced glycerol release, as early as 5 min following gas exposure. Moreover, nitroglycerine treatment, in the presence of cysteine (Feelisch & Noack, 1987), gave a concentration-dependent reduction of isoprenaline-induced lipolysis. However, it must be noted, that the effects of NO gas requires cautious interpretation due to the instability of NO, which can be converted by oxygen into nitrite and nitrate (Feelisch, 1991). In the present experimental set-up, where it is impossible to achieve a complete absence of oxygen in order to still have viable fat cells, a significant conversion of NO into nitrite and nitrate is unavoidable.
What is the source of NO that inhibits lipolysis? Although this study was not primarily designed to answer this question, several possibilities can be discussed. NO produced by nNOS in nerves is thought to act as a neurotransmitter (see Bredt & Snyder, 1992). Although knowledge about adipose tissue innervation is limited, the existence of sympathetic catecholaminergic, probably noradrenergic, terminals has been documented in adipose tissue (Lindh & Hökfelt, 1990). The local noradrenergic neuronal network is considered to mediate, at least in part, lipolysis induced by physical exercise through stimulation of fat cell β-adrenoceptors (Arner, 1995; Arner et al., 1990; Wahrenberg et al., 1991). It has also been suggested that as many as 20% of postganglionic sympathetic neurones could be cholinergic (Lindh & Hökfelt, 1990). The existence of a cholinergic innervation of adipose tissue may relate to our previous demonstration of a dual cholinergic mechanism regulating lipolysis (Andersson & Arner, 1995). However, it remains to be determined whether human adipose tissue nerve terminals contain NO.
Another source of NO could be local adipose tissue production. In this respect, NOS activity was recently demonstrated in rat fat tissue. Most of this activity could be accounted for by iNOS, some by eNOS and none by nNOS (Ribiere et al., 1996). In the present study, we measured total NOS enzyme activity and iNOS protein. Data showed that human adipose tissue expresses iNOS indicating that the tissue per se is a potential source of NO. We did not try to measure selectively iNOS enzyme activity because recent data suggests that rat adipose tissue and several non-adipose human cells contain an inducible form of NOS, which is partly calcium-dependent (see Ribiere et al. (1996) for discussion and references).
One source of NO in adipose tissue could be the endothelial cells belonging to the blood vessels that irrigate adipose tissue. Since NO is a potent vasodilator, some of the effects of L-NMMA could be related to blood flow changes in adipose tissue. It has previously been shown that NO is released following activation of vessel wall cholinoceptors inducing vasodilatation (Furchgott & Zawadzki, 1980; Ignarro et al., 1987; Palmer et al., 1987). In the present study, we did not observe any changes in local blood flow following L-NMMA treatment, as determined using the ethanol escape technique (Galitsky et al., 1993; Hickner, 1995; Hickner et al., 1991). Local blood flow can also be measured by microspheres or xenon washout. On the other hand, there is no alternative to the ethanol technique for the present type of experiments where local blood flow must be measured at the place where the microdialysis probe is situated. However, to address further the issue of blood flow, we performed a hydralazine-clamping experiment. Hydralazine is known to markedly increase blood flow through an unknown NO-independent mechanism, that involves an as yet unidentified molecule in the vascular endothelium (Wei et al., 1997). Hydralazine itself increased local blood flow, as monitored by an approximately 15% reduction in ethanol ratio. During this hydralazine-induced stimulation of blood flow, perfusion of the microdialysis probe with L-NMMA still produced a marked increase in dialysate glycerol levels. In relative terms, L-NMMA was as effective in increasing adipose tissue glycerol levels in the absence and presence of hydralazine, i.e. approximately 60% increase above baseline. No changes in ethanol ratios were observed during the L-NMMA treatment, again indicating that the L-NMMA-induced changes in glycerol levels are independent of blood flow changes. These data taken together, strongly suggest that the action of L-NMMA on lipolysis is independent of NO effects on local blood flow.
In summary, this study provides evidence that NO is involved in the in vivo regulation of lipolysis in man. Nitric oxide synthase enzyme activity is present in human adipose tissue and may produce NO through iNOS. This NO is suggested to inhibit lipolysis both in vivo and in vitro. The demonstration of an interaction between NO and lipolysis represents a new principle for metabolic regulation with potential physiological and pathophysiological implications. In addition, adipose tissue derived NO could be a future target for pharmacological treatment of lipid disorders.
Acknowledgments
Skilful technical laboratory assistance of Mrs Katarina Hertel, Mrs Britt-Marie Leijonhufvud, Mrs Eva Sjölin, and Mrs Kerstin Wåhlen is greatly acknowledged. This study was supported by grants from the Swedish Medical Research Council, the Foundations of Söderberg, Lars Hierta, Belvén, and Novo Nordisk, as well as, the Swedish Diabetes Foundation, Swedish Heart and Lung Foundation, the Karolinska Institute Research Funds, and the Institute National de la Santé et de la Recherche Medicale (INSERM CJF 94-02).
Abbreviations
- D-NMMA
NG-monomethyl D-arginine
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- L-NMMA
NG-monomethyl L-arginine
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
References
- ANDERSSON K., ARNER P. Cholinoceptor-mediated effects on glycerol output from human adipose tissue using in situ microdialysis. Br. J. Pharmacol. 1995;115:1155–1162. doi: 10.1111/j.1476-5381.1995.tb15018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNER P. Impact of exercise on adipose tissue metabolism in humans. Int. J. Obes. 1995;19 suppl. 4:S18–S21. [PubMed] [Google Scholar]
- ARNER P., BÜLOW J. Assessment of adipose tissue metabolism in man: comparison of Fick and microdialysis techniques. Clin. Sci. 1993;85:247–256. doi: 10.1042/cs0850247. [DOI] [PubMed] [Google Scholar]
- ARNER P., KRIEGHOLM E., ENGFELDT P. In vivo interactions between beta1- and beta2-adrenoceptors regulate catecholamine tachyphylaxia in human adipose tissue. J. Pharmacol. Exp. Ther. 1991;259:317–322. [PubMed] [Google Scholar]
- ARNER P., KRIEGHOLM E., ENGFELDT P., BOLINDER J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J. Clin. Invest. 1990;53:1080–1090. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNT E., GUTMAN I.Determination of ethanol with alchohol dehydrogenase and NAD Methods of Enzymatic Analysis 1974Weinheim: Verlag Chemie GmbH; 1499–1505.ed. Bergmeyer, H.U. [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., SNYDER S.H. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- CHRISTOPHERSON K.S., BREDT D.S. Perspectives series: Nitric oxide and nitric oxide synthases. J. Clin. Invest. 1997;100:2424–2429. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEELISCH M. The biochemical pathways of nitric oxide formation from nitrovasodilatators: Appropriate choice of exogenous NO donors and aspects of preparation and handling of aqueous NO solutions. J. Cardiovasc. Pharmacol. 1991;17 Suppl. 3:S25–S33. [Google Scholar]
- FEELISCH M., NOACK E.A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur. J. Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- FELLÄNDER G., LINDE B., BOLINDER J. Evaluation of the microdialysis ethanol technique for monitoring of subcutaneous adipose tissue blood flow in humans. Int. J. Obes. 1996;20:220–226. [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smotth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GALITSKY J., LAFONTAN M., ARNER P. Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J. Clin. Invest. 1993;91:1997–2003. doi: 10.1172/JCI116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARE J.M., GIVERTZ M.M., CREAGER M.A., COLUCCI W.S. Increased sensitivity to nitric oxide synthase inhibition in patients with heart failure. Circulation. 1998;97:161–166. doi: 10.1161/01.cir.97.2.161. [DOI] [PubMed] [Google Scholar]
- HELMÉR J., ARNER P., LUNDIN A. Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal. Biochem. 1989;177:132–137. doi: 10.1016/0003-2697(89)90027-4. [DOI] [PubMed] [Google Scholar]
- HENRY Y., DUCROCQ C., DRAPIER C., SERVENT D., PELLAT C., GUISSANI A. Nitric oxide, a biological effector electron paramagnetic resonance detection of nitrosyl-iron-protein complexes in whole cells. Eur. Biophys. J. 1991;20:1–15. doi: 10.1007/BF00183275. [DOI] [PubMed] [Google Scholar]
- HICKNER R.C.Microdialysis in skeletal muscle Development and application of the microdialysis ethanol technique 1995Sweden: Karolinska Institutet; 1–67.Repro Print AB, Stockholm [Google Scholar]
- HICKNER R.C., ROSDAHL H., BORG I., UNGERSTEDT U., JORFELT L., HENRIKSSON J. Muscle blood flow during intermittent exercise: comparison of the microdialysis ethanol technique and 133Xe clearance. Clin. Sci. 1991;86:15–25. doi: 10.1042/cs0860015. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUGA G.M., WOOD K.S., BYRNS R.E., CHAUDHURI G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOWLES R.G., MONCADA S. Nitric oxide synthases in mammals. Biochem. J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LAFONTAN M., ARNER P. Application of in situ microdialysis to measure metabolic and vascular responses in adipose tissue. TIPS. 1996;17:309–313. [PubMed] [Google Scholar]
- LINDH B., HÖKFELT T.Structural and functional aspects of acetylcholine peptide coexistence in the autonomic nervous system Progress in Brain Research 1990London: Elsevier Science Publisher B.V; 175–191.ed. Aquilonius, S.M. & Gilberg, P.G. [DOI] [PubMed] [Google Scholar]
- LÖNNQVIST F., WAHRENBERG H., HELLSTRÖM L., REYNISDOTTIR S., ARNER P. Lipolytic catecholamine resistance due to decreased beta2-adrenoceptor expression in fat cells. J. Clin. Invest. 1992;90:2175–2186. doi: 10.1172/JCI116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL T., FERON O. Nitric oxide synthases: Which, how, and why. J. Clin. Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- RIBIERE C., JAUBERT A.M., GAUDIOT N., SABOURAULT D., MARCUS M.L., BOUCHER J.L., DENIS-HENRIOT D., GIUDICELLI Y. White adipose tissue nitric oxide synthase: A potential source for NO production. Biochem. Biophys. Res. Comm. 1996;222:706–712. doi: 10.1006/bbrc.1996.0824. [DOI] [PubMed] [Google Scholar]
- SLATER M., KNOWLES R.G., MONCADA S. Widespread tissue distribution, species distribution and changes in activity of Ca2+-dependent and Ca2+-independent nitric oxide synthases. FEBS Lett. 1991;1:145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- TOSSMAN U., UNGERSTEDT U. Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta. Physiol. Scand. 1986;128:9–14. doi: 10.1111/j.1748-1716.1986.tb07943.x. [DOI] [PubMed] [Google Scholar]
- WAHRENBERG H., BOLINDER J., ARNER P. Adrenergic regulation of lipolysis in human fat cells during exercise. Europ. J. Clin. Invest. 1991;85:1614–1621. doi: 10.1111/j.1365-2362.1991.tb01406.x. [DOI] [PubMed] [Google Scholar]
- WEI S., KASUYA Y., YANAGISAWA M., KIMURA S., MASAKI T., GOTO K. Studies on endothelium-dependent vasorelaxation by hydralazine in porcine coronary artery. Europ. J. Pharmacol. 1997;321:307–314. doi: 10.1016/s0014-2999(96)00972-7. [DOI] [PubMed] [Google Scholar]


