Abstract
The actions of opioid receptor agonists on the calcium channel currents (IBa) of acutely dissociated periaqueductal grey (PAG) neurons from C57B16/J mice and mutant mice lacking the first exon of the μ-opioid receptor (MOR-1) were examined using whole cell patch clamp techniques. These effects were compared with the GABAB-receptor agonist baclofen.
The endogenous opioid agonist methionine-enkephalin (met-enkephalin, pEC50 6.8, maximum inhibition 40%), the putative endogenous μ-opioid agonist endomorphin-1 (pEC50 6.2, maximum inhibition 35%) and the μ-opioid selective agonist DAMGO (Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin, pEC50 6.9, maximum inhibition 40%) inhibited IBa in 70% of mouse PAG neurons. The inhibition of IBa by each agonist was completely prevented by the μ-receptor antagonist CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2). The δ-opioid receptor agonists DPDPE ([D-Pen2,5]-enkephalin, 1 μM) and deltorphin II (1 μM), and the κ-opioid receptor agonist U-69593 (1–10 μM), did not affect IBa in any cell tested.
The GABAB agonist baclofen inhibited IBa in all neurons (pEC50 5.9, maximum inhibition 42%).
In neurons from the MOR-1 deficient mice, the μ-opioid agonists met-enkephalin, DAMGO and endomorphin-1 did not inhibit IBa, whilst baclofen inhibited IBa in a manner indistinguishable from wild type mice.
A maximally effective concentration of endomorphin-1 (30 μM) partially (19%), but significantly (P<0.005), occluded the inhibition of IBa normally elicited by a maximally effective concentration of met-enkephalin (10 μM).
This study indicates that μ-opioid receptors, but not δ- or κ-opioid receptors, modulate somatic calcium channel currents in mouse PAG neurons. The putative endogenous μ-agonist, endomorphin-1, was a partial agonist in mouse PAG neurons.
Keywords: Endomorphin-1, μ-opioid receptor, periaqueductal grey, calcium channels, partial agonist, gene knockout, baclofen
Introduction
The midbrain periaqueductal grey (PAG) plays a pivotal role in the integration of an animal's response to threat, stress and pain (reviewed by Bandler & Shipley, 1994). The PAG is an important central site of action for the analgesic actions of opioid drugs (summarized by Yaksh & Rudy, 1978), and there is also considerable evidence that the PAG is critically involved in an animal's responses to withdrawal of opioid drugs (reviewed in Christie et al., 1997). In rat PAG, μ-opioid receptor activation modulates the activity of single neurons in many ways. μ-Opioids inhibit both glutamatergic (Vaughan & Christie, 1997) and GABAergic (Vaughan et al., 1997) synaptic inputs to all PAG cells. In a sub-population of PAG cells, μ-opioids directly increase an inwardly rectifying potassium conductance (Chieng & Christie, 1994) and inhibit voltage dependent calcium channels (Kim et al., 1997; Connor & Christie, 1998). Nothing is known about the cellular actions of opioids in mouse PAG.
All of the potential endogenous opioid agonists identified to date, with the exception of the opioid receptor-like protein (ORL1) agonist nociceptin, interact with native μ-receptors (Lord et al., 1977; Chavkin et al., 1982). Similarly, both methionine enkephalin (met-enkephalin) and leucine enkephalin, β-endorphin and dynorphin stimulate heterotrimeric guanine nucleotide-binding (G) proteins via the cloned human μ-receptor transfected into C6 glioma cells (Alt et al., 1998). An additional two putative endogenous μ-receptor agonists, endomorphin-1 and endomorphin-2, have been identified in bovine brain (Zadina et al., 1997). Endomorphin-1 and -2 were notable in displaying affinities for rat μ-receptors at least 1000 fold higher than their affinities for guinea-pig κ- and rat δ-receptors, a selectivity which has subsequently been confirmed for murine opioid receptors (Goldberg et al., 1998). The selectivity for the μ-receptor led to the suggestion that the endomorphins may be the ‘natural ligands for this receptor' (Zadina et al., 1997). Subsequently, evidence from biochemical studies measuring μ-receptor mediated G-protein activation has suggested that both endomorphin-1 and -2 are partial agonists at cloned μ-opioid receptors (MOR-1) (Alt et al., 1998; Hosohata et al., 1998), as well as μ-receptors in brain and spinal cord membranes (Harrison et al., 1998; Narita et al., 1998; Sim et al., 1998). In this study we have examined the regulation of voltage dependent calcium channel currents in acutely dissociated mouse PAG neurons by a variety of opioid agonists, including met-enkephalin, endomorphin-1 and Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin (DAMGO). The actions of these agonists on neurons from both wild type C57B16/J mice and mutant mice lacking the first exon of MOR-1 were compared.
Methods
Wild type and knockout mice homozygous for a deletion of exon 1 of MOR-1 were used as sources of PAG neurons. Production and detailed initial phenotypic analysis of the μ-opioid receptor knockout mice Schuller et al. (1999). Briefly, the targeting vector used to produce the gene-targeted mice lacking exon 1 was constructed in two steps. First, a 6.5 kb HindIII/XhoI fragment corresponding to part of intron 1 of MOR-1 was cloned into the HindIII and XhoI restriction sites, located between the pMC1neo sequence and the HSV-TK gene of the plasmid knockout vector. Then a 2.5 kb NotI/BamHI fragment, located 5′ of exon 1 of MOR-1, was cloned into the NotI and BamHI sites 3′ of the Neo gene. Successful targeting events were detected by Southern blot, which was also used to identify embryonic stem cells in which one allele of MOR-1 had been replaced by the mutant allele during homologous recombination. Heterozygous mice were produced following germ-line transmission of microinjected embryonic stem cells, and homozygous MOR-1 knockout mice derived from heterozygote/heterozygote mating were viable and fertile. Knockout mice used in the present study were littermates derived from matings of homozygous MOR-1 knockout mice. Wild type mice were of the parental C57B16/J strain.
Tissue dissociation
Mice of either sex (post natal days 35–103) were anaesthetized with halothane and then killed by cervical dislocation. Horizontal or coronal midbrain slices (between 270–300 μm thick) containing the periaqueductal grey were cut with a vibratome in ice cold physiological saline of composition (mM): NaCl 126, KCl 2.5, MgCl2 1.2, CaCl2 2.4, NaH2PO4 1.2, NaHCO3, 24 and glucose 11; gassed with 95% O2/5% CO2 and stored for 30 min at 35°C. Cells were dissociated as previously described (Connor & Christie, 1998), using procedures based on those outlined by Ingram et al. (1997). Briefly, slices were transferred to a dissociation buffer of composition (mM): Na2SO4 82, K2SO4 30, HEPES 10, MgCl2 5, glucose 10, containing 20 units ml−1 papain, pH 7.3 and incubated for 2 min at 35°C. The slices were then placed in fresh dissociation buffer containing 1 mg ml−1 bovine serum albumin (BSA) and 1 mg ml−1 trypsin inhibitor and the periaqueductal grey region was subdissected from each slice with a fine tungsten wire. Cells were dissociated from the slices by gentle trituration, plated onto plastic culture dishes and kept at room temperature in dissociation buffer.
Electrophysiology
Recordings of currents through Ca2+ channels were made using standard whole cell patch clamp techniques (Hamill et al., 1981) at room temperature (22–24°C), as previously described (Connor & Christie, 1998). Immediately prior to recording, dishes of cells were superfused with a buffer of composition (mM): NaCl 140, KCl 2.5, CaCl2 2.5, MgCl2 1.5, HEPES 10, glucose 10, pH 7.3 in order to wash off the dissociation buffer. For calcium channel current (IBa) recordings, cells were perfused in solution containing (mM) tetraethylammonium chloride 140, BaCl2 4, CsCl 2.5, HEPES 10, glucose 10, pH 7.3. Recordings were made with fire polished borosilicate pipettes of resistance between 2–4 MΩ when filled with intracellular solution of the following composition (mM): CsCl 110, MgATP 5, Na2GTP 0.2, EGTA 10, CaCl2 2 and HEPES 10, pH 7.3. The peak IBa in each cell was determined by stepping the membrane potential from a holding potential of −90 mV to potentials between −60 and +60 mV, usually for 30 ms, in 10 mV increments. Following this procedure the peak current was evoked every 30 s, and monitored for at least a further 2 min before drugs were applied. The inhibition by drugs was quantified by measuring the current amplitude isochronically with the peak of the control IBa. Cells in which the IBa declined in the absence of drug treatment were discarded. Whole cell capacitance and series resistance were compensated manually by nulling the capacitive transient evoked by a 20 mV pulse from −90 mV. The series resistance had an average value of 5 MΩ; series resistance compensation of at least 80% was used in all experiments. An approximate value of whole cell capacitance was read from the amplifier capacitance compensation circuit (Axopatch 1D, Axon Instruments, Foster City CA, U.S.A.). Leak current was subtracted on line using a P/8 protocol, typically the leak conductance was of the order of 100 pS. Evoked IBa were filtered at 2 kHz, sampled at 5–10 kHz and recorded on hard disk for later analysis. Data was collected and analysed off line with the PCLAMP suite of programs (Axon Instruments). Cells were exposed to drugs via a series of flow pipes positioned above the cells. Drug reservoirs were made from silanized glass syringes and 0.05% bovine serum albumin was included in IBa recording buffers to prevent adherence of peptides to the tubing. All data are expressed as mean±s.e.mean, unless otherwise indicated.
Drugs and chemicals
Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2) was synthesized and purified by Chiron Mimotopes (Clayton, Victoria, Australia). Buffer salts were from BDH Australia or Sigma Australia. Papain was from Worthington Biochemical Corporation (Freehold, NJ, U.S.A.). DAMGO (Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin), DPDPE ([D-Pen2,5]-enkephalin), BSA and trypsin inhibitor (Type II-O) were from Sigma Australia. Met-enkephalin was from Auspep (Melbourne, Australia). Baclofen, deltorphin II and U-69593 ((+) - (5α,7α,8β) - N - methyl - N-[7 -(1- pyrrolidinyl)-1-oxaspiro [4.5]dec-8-yl]benzeneacetamide) were from Research Biochemicals International (Natick, MA, U.S.A.). Morphine hydrochloride was from Glaxo U.K. CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) was kindly donated by the National Institutes on Drug Abuse, U.S.A. In parallel experiments, the U-69593 used in this study inhibited the electrically stimulated contractions of the guinea-pig longitudinal muscle/myenteric plexus; the DPDPE and deltorphin II inhibited the electrically stimulated twitch of the rat vas deferens (data not shown).
Results
When PAG neurons were stepped from a holding potential of −90 mV to potentials between −60 mV and +60 mV the inward currents in most cells began to activate at about −40 mV and were invariably greatest at membrane potentials between −10 and +10 mV. The inward currents in PAG neurons were sensitive to Cd2+, a non-selective blocker of high voltage-activated calcium channels. At a concentration of 30 μM, Cd2+ blocked 95±0.5% (n=7) of the inward current.
The opioid receptor agonists met-enkephalin, DAMGO, endomorphin-1 and morphine inhibited IBa in 107 of 149 (72%) of PAG neurons tested. The inhibition of IBa by these agonists was rapid and completely reversible (Figure 1). The inhibition of IBa by submaximally effective concentrations of met-enkephalin (1 μM), DAMGO (3 μM) and endomorphin-1 (10 μM) was completely prevented by treatment of opioid-sensitive neurons with the μ-opioid receptor selective antagonist CTAP (1 μM, 2 min, Figure 2). Neither the selective κ-opioid receptor agonist U-69593 (1–10 μM, n=15), nor the selective δ-opioid receptor agonists DPDPE (1 μM, n=8) and deltorphin II (1 μM, n=7), inhibited IBa in any PAG neuron tested.
Figure 1.

Modulation of PAG calcium channel currents by μ-opioids and baclofen. IBa was elicited by repetitively stepping the membrane potential from −90 mV to 0 mV. (a) (i) A time plot of the peak amplitude of IBa illustrating the effects of application of the μ agonist DAMGO and GABAB agonist baclofen. (ii) Selected traces from the same experiment, showing the inhibition of IBa by each agonist. (b), Concentration-response relationships for met-enkephalin (EC50 140 nM), DAMGO (EC50 130 nM) and endomorphin-1 (EC50 800 nM) in PAG neurons. Each point represents at least five cells tested.
Figure 2.
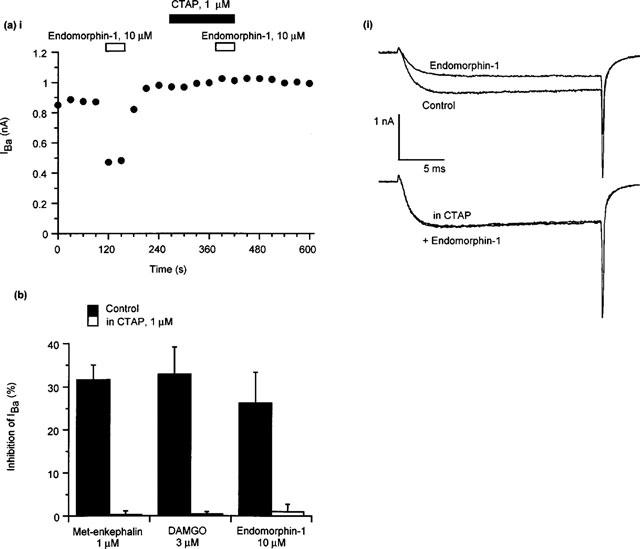
The μ-opioid receptor antagonist CTAP blocks the effects of met-enkephalin, DAMGO and endomorphin-1. The example traces were elicited by repetitively stepping the membrane potential of a PAG neuron from −90 mV to 0 mV. (a) The effects of endomorphin-1 in μ-opioid responsive cell are blocked by the selective μ-opioid receptor antagonist CTAP. (i) A time plot of the peak amplitude of IBa illustrating the effects of application of endomorphin-1 and subsequent co-application of CTAP and endomorphin-1. (ii) Selected traces from the same experiment. (b) In the presence of CTAP (1 μM) met-enkephalin (1 μM, P<0.0001 vs control, unpaired t-test, n=5 for each), DAMGO (3 μM, n=5, P<0.001 vs control, n=7) and endomorphin-1 (10 μM, P<0.01 vs control, n=5 for each) do not inhibit IBa to any significant extent.
A concentration-response relationship for μ-opioid agonist inhibition of PAG IBa was determined by application of one or more concentrations of agonist to cells stepped repetitively from −90 mV to the membrane potential that evoked the largest IBa in each neuron (either −10 or 0 mV) (Figure 1b). Desensitization of the inhibition of IBa by any of the agonists was not observed during brief applications of all but the highest concentrations tested. A logistic function was fitted to the concentration response relationship for each agonist inhibition of IBa, and a pEC50 and slope factor determined. Met-enkephalin inhibited IBa with a pEC50 of 6.8±0.1 and slope factor of 0.9±0.1, the maximum inhibition of IBa was about 40%. DAMGO inhibited IBa with a pEC50 of 6.9±0.1, a slope factor of 1.1±0.3, to a maximum of about 40%. Endomorphin-1 inhibited IBa with a pEC50 of 6.2±0.2, a slope factor of 0.5±0.1, to a maximum of about 35%. Morphine (10 μM) inhibited IBa by 25±4% (n=5). The GABAB agonist baclofen inhibited IBa in all PAG neurons tested (45/45), with a pEC50 of 5.9±0.2, a slope factor of 0.9±0.3, to a maximum of about 42%.
The maximal inhibition of IBa by endomorphin-1 appeared to be less than that by met-enkephalin or DAMGO. To determine if endomorphin-1 was acting as a partial agonist at μ-receptors the occlusion of the effects of a high concentration of met-enkephalin by a maximally effective concentration of endomorphin-1 was examined (Figure 3). In these experiments cells were exposed to endomorphin-1 (30 μM) and then switched to buffer containing endomorphin-1 (30 μM) and met-enkephalin (10 μM). The same cells were also exposed to met-enkephalin (10 μM) alone. The two treatments were separated by 5 min and the order of treatment was alternated between cells. A concentration of 30 μM endomorphin-1 was chosen because the inhibition of IBa by this concentration of agonist did not appear to desensitize significantly in the first 60 s of agonist exposure, while the inhibition of IBa by 100 μM endomorphin-1 did (data not shown). Met-enkephalin (10 μM) applied alone inhibited IBa by 38±2%; when met-enkephalin (10 μM) was applied to the same cells in the presence of endomorphin-1 (30 μM) the inhibition of IBa was only 31±4% (P<0.004, paired t-test, n=9) (Figure 4). Met-enkephalin (10 μM) applied in the presence of endomorphin-1 (30 μM) did not inhibit IBa more than endomorphin-1 alone (29±4%; P>0.1, paired t-test).
Figure 3.

Endomorphin-1 is a partial agonist in PAG neurons. (i) A time plot of the peak amplitude of IBa illustrating the effects of application of endomorphin-1, endomorphin-1 with met-enkephalin and subsequently met-enkephalin alone. IBa was elicited by repetitively stepping the membrane potential of the neuron from −90 mV to 0 mV. Note that the abscissa has been truncated so the effects of the agonists can be more easily compared. Endomorphin-1 occluded the effects of met-enkephalin by about 20% in nine cells tested. (ii) Selected traces from the same experiment, showing the IBa in each condition.
In neurons from mice containing a deletion of the first exon of the μ-opioid receptor (MOR-1), neither DAMGO (3 μM, n=9), met-enkephalin (3 μM, n=10) nor endomorphin (10 μM, n=9) inhibited IBa in any cell. There was also no inhibition of IBa by either U-69593 (1–10 μM, n=12), DPDPE (1–10 μM, n=3) or deltorphin II (1 μM, n=7). The inhibition of IBa by baclofen (10 μM) was not different between neurons from wild type and μ-receptor knock out mice. The inhibition of IBa by baclofen was 41±2% (n=10) in wild type mice and 36±4%, (n=12) in exon 1 deletion mice). There was no apparent difference in the amount of IBa between wild type or μ-receptor knock out mice. The peak IBa density was 253±21 pA pF−1 in μ-receptor knock out mice (n=29) and 271±17 pA pF−1 in parallel experiments in wild type animals (n=31).
Discussion
In mouse PAG neurons, μ-opioid receptor activation inhibited IBa in most neurons, while the GABAB receptor agonist, baclofen, inhibited IBa in all neurons. Neither δ- nor κ-opioid agonists affected IBa in any mouse PAG neuron examined. The results of the present study are broadly consistent with previous studies showing that μ-opioids inhibit IBa in a sub-population of acutely dissociated rat PAG neurons (Kim et al., 1997; Connor & Christie, 1998), whilst δ- and κ-opioid agonists are ineffective (Connor & Christie, 1998). Notably, μ-opioids inhibited IBa in a far greater proportion of mouse PAG neurons than rat PAG neurons (72% vs 38%). Autoradiographic studies have identified both κ- and δ-opioid receptor messenger RNA localized to the mouse PAG (DePaoli et al., 1994; Jenab et al., 1995; Kitchen et al., 1997) and a moderate to high density of [3H]-U-69593 binding has been reported in the PAG of C57BL/6 mice (Jamensky & Gianoulakis, 1997). The biochemical consequences of κ-opioid receptor activation in the PAG are poorly characterized but a number of studies have demonstrated δ-opioid receptor stimulation of G-protein activity in mouse PAG (see Garzon et al., 1997). The lack of effect of either δ- or κ-receptor agonists on IBa in mouse PAG is intriguing. A similar conundrum exists in the rat PAG where several studies have also failed to find any effects of δ- and κ-opioids on the membrane currents of PAG neurons or on synaptic transmission within PAG slices (Chieng & Christie, 1994; Vaughan & Christie, 1997), despite the presence of δ- and κ-opioid receptor messenger RNA and radioligand binding (Kalyuzhny et al., 1996; Mansour et al., 1996). It should be noted that the present study utilized Ba2+ as a charge carrier and strong intracellular Ca2+ buffering in order to minimize current rundown mediated by Ca2+-dependent processes. It is likely that any Ca2+-dependent mechanisms of IBa modulation were suppressed under these conditions and thus the possibility that δ- and κ-opioid receptors may act via such pathways in PAG neurones cannot be excluded.
The type(s) of calcium channel present in mouse PAG neurons is not known at present, however rat PAG neurons possess predominantly N-type and P/Q-type currents (approximately 40% of each), with lesser amounts of L-type and resistant current (Connor & Christie, 1998). We did not attempt to determine which types of calcium channel current were being modulated by μ-opioids in mouse PAG, however previous studies have shown that μ-opioids predominantly inhibit the N-type and to a lesser extent P/Q-type calcium channels in central neurons (e.g. Rhim & Miller, 1994; Kim et al., 1997; Soldo & Moises, 1997). In our previous study in rat PAG neurons, nociceptin, the endogenous ligand for the opioid-receptor like protein (ORL1), strongly inhibited both N-type and P/Q-type currents, while having little effect on the residual L- and R-type currents (Connor & Christie, 1998).
In mouse PAG neurons, the putative endogenous μ-opioid receptor selective agonist endomorphin-1 inhibited IBa as a partial agonist, with a relatively low potency. The partial agonist characteristics of endomorphin-1 have previously been noted by a number of investigators utilizing assays that measure G-protein interaction with both native (Harrison et al., 1998; Narita et al., 1998; Sim et al., 1998) and cloned (Alt et al., 1998; Hosohata et al., 1998) μ-opioid receptors. In those experiments endomorphin-1 and -2 consistently stimulate G-protein activity to levels between 40 and 70% of the maximal amounts stimulated by agonists such as DAMGO. In several studies endomorphin-1 was also shown to occlude the maximal stimulatory effects of DAMGO, confirming its partial agonist activity (Alt et al., 1998; Sim et al., 1998). The efficacy of endomorphin-1 in stimulating G-protein activity appears to be similar to that of morphine (Alt et al., 1998; Hosohata et al., 1998; Narita et al., 1998; Sim et al., 1998).
The actions of endomorphin-1 and -2 on single cells are poorly characterized. In NG108-15 neuroblastoma X glioma cells transfected with the rat μ-opioid receptor, both endomorphin-1 and -2 inhibited the voltage-dependent calcium currents with EC50s of about 10 nM (Mima et al., 1997; Higashida et al., 1998), which is much more potent than observed in mouse PAG cells. The high potency in the latter studies presumably results at least partly from the overexpression of μ-receptors in the NG108-15 cells.
Endomorphin-1 and -2 are potent analgesics when injected i.c.v. in mice (Zadina et al., 1997; Goldberg et al., 1998). This activity presumably arises at least in part from actions within the PAG, which is thought to be a major site of action for opioid mediated analgesia (Yaksh & Rudy, 1978). It is not known what part the inhibition of calcium channels may have in mediating opioid effects in mouse PAG but clearly μ-opioid agonists, including endomorphins, have the potential to directly modulate the somatic currents of most neurons in the mouse PAG.
Acknowledgments
This study was supported by The University of Sydney Medical Foundation. M.C. is the recipient of a Rolf Edgar Lake Fellowship from the Faculty of Medicine, University of Sydney. A.S. and J.E.P. supported by NIH Grant DA-09040. The donation of CTAP by the National Institutes on Drug Abuse is gratefully acknowledged.
Abbreviations
- BSA
bovine serum albumin
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- DAMGO
Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin
- >DPDPE
[D-Pen2,5]-enkephalin
- G-protein
heterotrimeric guanine nucleotide-binding protein
- IBa
calcium channel current
- met-enkephalin
methionine enkephalin
- MOR-1
μ-opioid receptor clone
- ORL1
opioid receptor-like protein
- PAG
periaqueductal grey
- U-69593
(+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide
References
- ALT A., MANSOUR A., AKIL H., MEDZIHRASKY F., TRAYNOR J.R., WOODS J.H. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J. Pharmacol. Exp. Ther. 1998;286:282–288. [PubMed] [Google Scholar]
- BANDLER R., SHIPLEY M. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression. TiNS. 1994;17:379–388. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- CHAVKIN C.I., JAMES I.F., GOLDSTEIN A. Dynorphin is a specific endogenous ligand of the κ opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- CHIENG B., CHRISTIE M.J. Hyperpolarization by opioids acting on μ-receptors of a subpopulation of rat periaqueductal gray neurones in vitro. Br. J. Pharmacol. 1994;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIE M.J., WILLIAMS J.T., OSBORNE P.B., BELLCHAMBERS C. Where is the locus in opioid withdrawal. TiPS. 1997;18:134–140. doi: 10.1016/s0165-6147(97)01045-6. [DOI] [PubMed] [Google Scholar]
- CONNOR M., CHRISTIE M.J. Modulation of the calcium channel currents of acutely dissociated rat periaqueductal gray neurones. J. Physiol. 1998;509:47–58. doi: 10.1111/j.1469-7793.1998.047bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PAOLI A.M., HURLEY K.M., YASADA K., REISINE T., BELL G. Distribution of κ opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Mol. Cell. Neurosci. 1994;5:327–335. doi: 10.1006/mcne.1994.1039. [DOI] [PubMed] [Google Scholar]
- GARZON J., MARTINEZ-PENA Y., SANCHEZ-BLAZQUEZ P. Gx/z is regulated by μ but not δ opioid receptors in the stimulation of low Km GTPase activity in mouse periaqueductal grey matter. Eur. J. Neurosci. 1997;9:1194–1200. doi: 10.1111/j.1460-9568.1997.tb01474.x. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I.E., ROSSI G.E., LETCHWORTH S.R., MATHIS J.P., RYAN-MORO J., LEVENTHAL L., SU W., EMMEL D., BOLAN E.A., PASTERNAK G.W. Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain. J. Pharmacol. Exp. Ther. 1998;286:1007–1013. [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high resolution current recording from cells and cell free membrane patches. Pflugers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARRISON L.M., KASTIN A.J., ZADINA J.E. Differential effects of endomorphin-1, endomorphin-2, and Tyr-W-MIF-1 on activation of G-proteins in SH-SY5Y human neuroblastoma membranes. Peptides. 1998;19:749–753. doi: 10.1016/s0196-9781(98)00022-9. [DOI] [PubMed] [Google Scholar]
- HIGASHIDA H., HOSHI N., KNIJNIK R., ZADINA J.E., KASTIN A.J. Endomorphins inhibit high-threshold Ca2+ channel currents in rodent NG108-15 cells overexpressing μ-opioid receptors. J. Physiol. 1998;507:71–75. doi: 10.1111/j.1469-7793.1998.071bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSOHATA K., BURKEY T.H., ALFARO-LOPEZ J., VARGA E., HRUBY V.J., ROESKE W.R., YAMAMURA H.I. Endomorphin-1 and endomorphin-2 are partial agonists at the human μ-opioid receptor. Eur. J. Pharmacol. 1998;346:111–114. doi: 10.1016/s0014-2999(98)00117-4. [DOI] [PubMed] [Google Scholar]
- INGRAM S., WILDING T.J., MCCLESKEY E.W., WILLIAMS J.T. Efficacy and kinetics of opioid action on acutely dissociated neurons. Mol. Pharmacol. 1997;52:136–143. doi: 10.1124/mol.52.1.136. [DOI] [PubMed] [Google Scholar]
- JAMENSKY N.T., GIANOULAKIS C. Content of dynorphins and κ-opioid receptors in distinct brain regions of C57BL/6 and DBA/2 mice. Alcohol. Clin. Exp. Res. 1997;21:1455–1464. [PubMed] [Google Scholar]
- JENAB S., KEST B., FRANKLIN S.O., INTURRISI C.E. Quantitative distribution of the delta opioid receptor mRNA in the mouse and rat CNS. Life Sci. 1995;56:2343–2355. doi: 10.1016/0024-3205(95)00228-x. [DOI] [PubMed] [Google Scholar]
- KALYUZHNY A.E., ARVIDSSON U., WU W., WESSENDORF M.W. μ-Opioid and δ-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract tracing. J. Neurosci. 1996;16:6490–6503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM C.J., RHEE J.-S., AKAIKE N. Modulation of high-voltage activated Ca2+ channels in the rat periaqueductal gray neurons by μ-type opioid agonist. J. Neurophysiol. 1997;77:1418–1424. doi: 10.1152/jn.1997.77.3.1418. [DOI] [PubMed] [Google Scholar]
- KITCHEN I., SLOWE S.J., MATTHES H.W.D., KIEFFER B. Quantitative autoradiographic mapping of μ-, δ- and κ-opioid receptors in knockout mice lacking the μ-opioid receptor gene. Brain Res. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- LORD J.A.H., WATERFIELD A.A., HUGHES J., KOSTERLITZ H.W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- MANSOUR A., BURKE S., PAVLIC R.J., AKIL H., WATSON S.J. Immunohistochemical localization of the cloned κ1 receptor in the rat CNS and pituitary. Neurosci. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- MIMA H., MORIKAWA H., FUKUDA K., KATO S., SHODA T., MORI K. Ca2+ channel inhibition by endomorphins via the cloned μ-opioid receptor expressed in NG108-15 cells. Eur. J. Pharmacol. 1997;340:R1–R2. [PubMed] [Google Scholar]
- NARITA M., MIZOGUCHI H., OJI G.S., TSENG E.L., SUGANUMA C., NAGASE H., TSENG L.F. Characterization of endomorphin-1 and -2 on [35S]GTPγS binding in the mouse spinal cord. Eur. J. Pharmacol. 1998;351:383–387. doi: 10.1016/s0014-2999(98)00395-1. [DOI] [PubMed] [Google Scholar]
- RHIM H., MILLER R.J. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J. Neurosci. 1994;14:7608–7615. doi: 10.1523/JNEUROSCI.14-12-07608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULLER A.G.P., KING M., ZHANG J., BOLAN E., PAN Y.-X., MORGAN D.J., CHANG A., CZICK M.E., UNTERWALD E., PASTERNAK G.W., PINTAR J.E. Retention of heroin and morphine-6-glucuronide analgesia in a new line of MOR-1 knockout mice insensitive to morphine. Nature Neurosci. 1999;2:1–8. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- SIM L.J., LIU Q., CHILDERS S.R., SELLEY D.E. Endomorphin-stimulated [35S]GTPγS binding in rat brain: evidence for partial agonist activity at μ-opioid receptors. J. Neurochem. 1998;70:1567–1576. doi: 10.1046/j.1471-4159.1998.70041567.x. [DOI] [PubMed] [Google Scholar]
- SOLDO B.L., MOISES H.C. μ-Opioid receptor activation decreases N-type Ca2+ current in magnocellular neurons of the rat basal forebrain. Brain Res. 1997;758:118–126. doi: 10.1016/s0006-8993(97)00206-0. [DOI] [PubMed] [Google Scholar]
- VAUGHAN C.W., CHRISTIE M.J. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J. Physiol. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN C.W., INGRAM S.L., CONNOR M.A., CHRISTIE M.J. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., RUDY T.A. Narcotic analgetics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4:299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- ZADINA J.E., HACKLER L., GE L.-J., KASTIN A.J. A potent and selective endogenous agonist for the μ-opioid receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]


