Abstract
The effects of long-term treatment with trandolapril, an angiotensin I-converting enzyme inhibitor, on exercise capacity of rats with chronic heart failure (CHF) following coronary artery ligation were examined. CHF was developed by 8 weeks after the coronary artery ligation.
The running time of rats with CHF in the treadmill test was shortened to approximately 65% of that of sham-operated rats (16.3±1.2 vs 25.1±1.6 min, n=7; P<0.05). ATP, creatine phosphate (CP), and lactate contents of the gracilis muscle of rats with CHF were similar to those of sham-operated rats before running. After running, ATP and CP were decreased and lactate was increased in both rats with CHF and sham-operated rats. There were no significant differences in the levels of energy metabolites between rats with CHF and sham-operated rats. The rates of decrease in ATP and CP and rate of increase in lactate in the gracilis muscle of rats with CHF during exercise were greater than those of sham operated rats (2.5, 2.0 and 1.5 fold high, respectively), suggesting wastage of energy during exercise in the animals with CHF.
Myofibrillar Ca2+-stimulated ATPase (Ca-ATPase) activity of skeletal muscle of rats with CHF was increased over that of the sham-operated control (62.03±1.88 vs 52.34±1.19 μmol Pi mg−1 protein h−1 n=7; P<0.05). The compositions of myosin heavy chain (MHC) isoforms of gracilis muscle were altered by CHF; decreases in MHC types I and IIb and an increase in MHC type IIa were found (P<0.05).
Rats with CHF were treated with 1 mg kg−1 day−1 trandolapril from the 2nd to 8th week after surgery. Treatment with trandolapril prolonged the running time, reversed the rates of decrease in ATP and CP and the rate of increase in lactate, and restored the Ca-ATPase activity (51.11±0.56 μmol Pi mg−1 protein h−1, n=7; P<0.05) and composition ratio of MHC isoforms in the gracilis muscle.
The results suggest that long-term trandolapril treatment of rats with CHF may restore their ability to utilize energy without wastage and thus improve exercise capacity.
Keywords: Angiotensin I-converting enzyme inhibitor; calcium-stimulated ATPase; exercise capacity; high-energy phos-phate; lactate; myosin heavy chain; treadmill; running time, trandolapril
Introduction
Chronic heart failure (CHF) is characterized by low cardiac output and increased peripheral resistance, and its development is closely related to alterations in cardiac muscle contractility. Numerous studies have examined the pathophysiology of CHF with respect to mechanical abnormalities in cardiac muscle function. In contrast, relatively little information is available concerning the pathophysiology of skeletal muscles in animals with CHF. Several studies have shown biochemical and structural abnormalities in skeletal muscles of patients and animals with CHF. Several studies have shown biochemical and structural abnormalities in skeletal muscles of patients and animals with CHF, including reduced activity of enzymes involved in oxidative phosphorylation (Sullivan et al., 1990; Drexler et al., 1992) and ultrastructural changes in myosin heavy chain isozymes (Mancini et al., 1989; Sullivan et al., 1990; Broqvist et al., 1992a, 1992b; Sabbah et al., 1993). Although these pathophysiological alterations are related to exercise intolerance, a major symptom of CHF in patients and animals (Coats, 1994; 1996), the correlation of biochemical and structural parameters with exercise capacity of patients and animals with CHF has not been fully established.
Angiotensin I-converting enzyme (ACE) inhibitors are one of the most potent therapeutic agents that have the ability to prolong life expectancy in patients and animals with heart failure (Pfeffer et al., 1987; SOLVD Investigators, 1991; SAVE Investigators, 1992; TRACE study group, 1995). Although treatment of patients with CHF and ACE inhibitors improves cardiac function, the effects of ACE inhibitors on skeletal muscle function of patients and animals with CHF remains unclear. Treatment of dogs with microsphere embolism-induced moderate heart failure with enarapril prevented a progressive decline in the proportion of type I skeletal muscle fibres (Sabbah et al., 1993), whereas therapy of patients with severe congestive heart failure by enalapril improved neither energy metabolism nor electrolytes of muscles (Broqvist et al., 1992a, 1992b). It is also unknown whether or not the effects of enalapril are generalized actions for ACE inhibitors. The present study was thus undertaken to determine if the ACE inhibitor trandolapril improves exercise capacity of rats with CHF following coronary artery ligation.
Methods
Animals
Male Wistar rats (SLC, Hamamatsu, Japan) weighing 220–250 g were used in the present study. The rats were fed standard rat chow and tap water ad libitum, and housed in polyethylene cages with 12-h light and 12-h dark cycle according to Guide for the Care and Use of Laboratory Animals as promulgated by the National Research Council. The protocol of this study was approved by the University Committee of Animal Use and Welfare.
Coronary artery ligation
Before starting the study, we selected rats that could run on a treadmill. Animals were subjected to treadmill exercise by the methods described below. The rats that could run on the treadmill for more than 20 min (approximately 50% of the acclimatized animals) were used for coronary artery ligation or sham-operation.
Myocardial infarction was induced in 200 rats by occluding the left coronary artery according to the method described previously (Sanbe et al., 1993). In brief, the rats were anaesthetized with 40 mg kg−1 intraperitoneal injection of sodium pentobarbitone and artificially ventilated with air. The heart was exteriorized, its coronary artery was ligated approximately 2 mm from its origin with a 5-0 suture, and then the heart was repositioned in the chest. The mortality of the coronary artery-ligated rats was approximately 40% within 1 week after the operation. Sham-operated rats were treated similarly except that no suture was tied around the coronary artery.
Experimental protocol and treatment with trandolapril
In the first set of experiments, haemodynamic parameters were determined 8 weeks after the operation. Then, the hearts were isolated and sampled for measurement of infarct size. In the second series of experiments, hearts isolated from rats with CHF and sham-operated rats 8 weeks after the operation were used for determination of pre-exercise levels of energy metabolites, myofibrillar Ca2+-ATPase activity, and isoforms of myosin heavy chain. In the third series of experiments, a treadmill test for the operated animals was performed 8 weeks after surgery. Then, energy metabolites after exercise were determined.
Rats were treated with an oral administration of either 1 or 3 mg kg−1 of trandolapril once daily, from the 2nd to the 8th week after coronary artery ligation or sham operation. Trandolapril suspended in 0.3 ml of 0.25% sodium carboxymethyl cellulose was administered into the stomach through a probe. The drug-treated rats were used for determination of haemodynamics and biochemical variables according to the same protocol as described above.
Measurement of haemodynamics
Haemodynamic parameters of rats with CHF and sham-operated rats treated with trandolapril or untreated were measured as described previously (Sanbe et al., 1993). The animals were anaesthetized with a gas mixture of nitrous oxide and oxygen (3 : 1) containing 2.5% halothane. The anaesthesia was continued with a gas mixture of nitrous oxide and oxygen containing 0.5% halothane at a flow rate of 1.2 L min−1 through a mask loosely placed on the nose. Body temperature of the animals was kept at 36.5±0.5°C by an electronic panel heater. A microchip pressure transducer (SPC320, Miller Instrument, Houston, TX, U.S.A.) was introduced into the left ventricular cavity through the right carotid artery to measure left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and left ventricular positive and negative dP/dt (±dP/dt) by means of a carrier amplifier (AP-621G, Nihon Kohden, Tokyo, Japan) and differentiator (ED-601G, Nihon Kohden), respectively. The mean arterial blood pressure (MAP) was measured by means of a pressure transducer (TP-200T, Nihon Kohden) connected to a cannula placed in the right femoral artery.
Treadmill exercise study
Treadmill exercise was performed according to the method of Davies et al. (1984) with some modifications. Acclimatized rats or coronary artery-ligated and sham-operated rats (with or without trandolapril treatment) were subjected to the treadmill test (KN-73, Natsume, Tokyo, Japan). The treadmill was equipped with a continuous belt at a slope of 5° and operated at a constant running speed of 25 m min−1. The end point for each test was decided by the inability of the rat to run any more on the treadmill belt. After a certain period of running, the animals experienced difficulty in continuing the running to match the pace of the treadmill. This resulted in a landing on the electric shock grid at the rear of the continuous belt. When given the electric shock, the animals again started running. The end point for every test was marked by a rat's inability to return to the treadmill belt from the shock grid, despite additional manual encouragement. The running time from the start to the end point of each rat was recorded.
At the end of exercise, the animals were immediately anaesthetized with diethylether, and then the right gracilis muscle was quickly excised. It took about 45 s from induction of anaesthesia to fixation of the isolated muscle. Due to animal welfare regulations, the direct fixation of skeletal muscle of awake animals with liquid nitrogen was not carried out in the present study. In another set of experiments, coronary artery-ligated and sham-operated rats that did not perform treadmill exercise were anaesthetized with diethylether. The gracilis muscles were quickly excised and frozen with liquid nitrogen. The frozen muscles were kept in liquid nitrogen until they were used for the biochemical assay.
Measurements of energy metabolites in muscle
High-energy phosphates and energy metabolites in the gracilis muscle of rats with CHF and sham-operated rats with and without exercise were extracted with a solution containing 0.3 N perchloric acid and 0.25 mM EDTA as described previously (Tanonaka et al., 1991). The resultant supernatant fluid was sampled for determination of tissue ATP, CP, lactate and pyruvate contents. Tissue ATP content was determined by an HPLC method. The perchloric acid extracts were applied to an HPLC (L-6000 series, Hitachi, Tokyo, Japan) equipped with an ion-exchange column (Asahipak ES-502N, Asahi Kasei, Nobeoka, Japan) and eluted with 250 mM KH2PO4 containing 15% CH3CN (pH 6.5) at the rate of 1 mL min−1 (Sanbe et al., 1995a). CP was converted to ATP by the enzymatic method of Lowry & Passoneau (1972) and determined as ATP content as described above. Lactate and pyruvate contents were determined by the method of Gutmann & Wahlfeld (1974). Furthermore, the consumption rate of ATP and CP during running was estimated by dividing the difference in the mean values of energy metabolites before and after running by running time. Production rate of lactate was estimated in a similar manner. The baseline values for ATP, CP and lactate determined in the present study were comparable to those of other investigators (Bernocchi et al., 1996).
Isolation of myofibrils and measurement of calcium ATPase activity
In another set of experiments, myofibrils were prepared from gracilis muscles according to the method of Solaro et al. (1971) with a minor modification. All procedures for isolation of myofibrils were carried out at 4°C. The frozen gracilis muscle was thawed and rinsed with a solution of the following composition (mM): sucrose 250, KCl 100, imidazole 20, and EDTA 5 (pH 6.8). The tissue was then homogenized in 10 vol. of the above solution with a Polytron homogenizer (PT-10, Kinematica, Littau, Switzerland). The homogenate was centrifuged at 1000×g for 10 min at 4°C. The resulting pellet was suspended in 10 vol of 175 mM KCl containing 0.5% Triton X-100 (pH 6.8), and then the suspension was centrifuged under the same conditions. The resulting myofibrillar pellet was washed twice in the same manner. After these washings, the purified myofibril pellet was subjected to two washings in the KCl/imidazole buffer containing 150 mM KCl and 20 mM imidazole (pH 7.0). The resulting pellet, i.e., the myofibrillar fraction, was suspended in the KCl/imidazole buffer. Protein concentrations of the samples were determined by the method of Lowry et al. (1951). There were no significant differences in the protein yield among the groups studied.
Separation of myosin heavy chain isoforms by SDS–PAGE
The isolated myofibrils were separated into myosin heavy chain (MHC) isoforms of the skeletal muscle by use of a 4% SDS–PAGE gel. Myofibrillar proteins (approximately 6 μg) were mixed with 15 μL of a buffer containing 100 mM Tris/HCl, 5% glycerol, 4% SDS, 5% 2-mercaptoethanol, and 0.05% bromphenol blue (pH 6.8). The mixture was boiled for 2 min in 100°C and then loaded into the sample wells. The gels were run in an electrophoresis apparatus (Rapidas AE-6220, Atto, Tokyo, Japan) at room temperature. The loaded mixture was subjected to electrophoresis at a constant current of 10 mA/slab gel until the dye front ran off the gel. Then, the gels were stained for 2 h with a staining solution containing 0.1% Coomassie blue R-250, 30% 2-propanol, and 10% acetic acid. After staining, the gels were washed with a mixture of 20% methanol and 10% acetic acid.
Measurement of calcium-activated ATPase (Ca-ATPase) in myofibrils
Myofibrillar Ca-ATPase activity was determined according to the method of Solaro et al. (1971). Briefly, a 0.1 mL myofibrillar sample (approximately 0.3 mg protein) was mixed with the reaction buffer (pH 7.0) of the following composition (mM): KCl 50, imidazole 20, MgCl2 2, sodium azide 10, and CaCl2 0.1 (total ATPase activity). The mixture was incubated for 2 min at 37°C after addition of 2 mM ATP/Tris, pH 7.0, and the reaction was thereafter terminated by the addition of 12% trichloroacetic acid. The denatured protein was separated by centrifugation at 3000×g for 15 min at 4°C. Inorganic phosphate liberated by hydrolysis of ATP by ATPases (total ATPase activity) was determined by the method of Tausky & Shorr (1953). Basal ATPase activity of myofibrils was also determined by incubation in the presence of 16 mM ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA) insted of CaCl2. The myofibrillar Ca-ATPase activity was estimated by subtracting basal ATPase activity from total ATPase activity.
Infarct size
After measurement of haemodynamic parameters, the myocardium was isolated for determination of skeletal muscle metabolites. Myocardial infarct sizes were determined according to the method of Sanbe et al. (1993). The myocardium was isolated and its vascular space was washed with 320 mM sucrose −20 mM Tris (pH 7.4) and sliced into six to seven 1-mm thick slices from the base. The slices were incubated with 1% trichlorophenyltetrazolium chloride (TTC) in saline for 10 min, after which the unstained areas were determined according to the methods described previously (Sanbe et al., 1993).
Statistical analysis
The values were expressed as the means±s.e.mean. Statistical significance was estimated by two-way ANOVA followed by Scheffe's multiple comparison. Differences with a probability of 5% or less were considered to be significant (P<0.05).
Results
Changes in haemodynamic parameters
In the first set of experiments, changes in haemodynamic parameters of sham-operated rats and rats with CHF and the effects of long-term treatment with 1 or 3 mg kg−1 day−1 trandolapril on the haemodynamics were determined (Table 1). The values of MAP, LVSP, LVEDP, +dP/dt and −dP/dt of the non-operated rats were 125±3 mmHg, 142±4 mmHg, 6.2±0.9 mmHg, 8850±347 mmHg s−1 and 7950±400 mmHg s−1, respectively (n=8). Similar values were recorded in sham-operated rats. A significant decrease in MAP was observed in rats with CHF 8 weeks after the coronary artery ligation. LVSP and ±dP/dt of rats with CHF were also lower than those of sham-operated rats, whereas LVEDP was significantly increased.
Table 1.
Changes in haemodynamic parameters of the sham-operated rats and the rats with CHF at the 8th week after coronary artery ligation
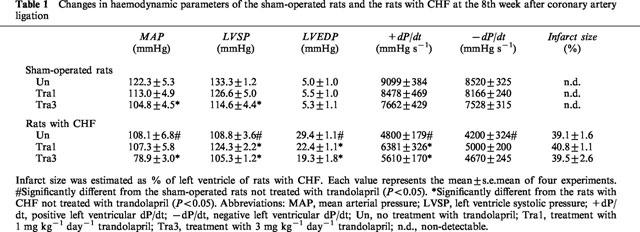
In the rats with CHF, the increase in LVEDP was attenuated and +dP/dt was partially restored by treatment with 1 mg kg−1 day−1 trandolapril, whereas MAP and LVSP were similar to those of drug-untreated rats with CHF. In contrast, MAP and LVSP of rats with CHF treated with 3 mg kg−1 day−1 trandolapril were lower than those of rats with CHF not treated with the drug, whereas the increase in LVEDP of rats with CHF was attenuated by treatment with the drug. Treatment of sham-operated rats with 1 mg kg−1 day−1 trandolapril did not affect these parameters, whereas treatment with 3 mg kg−1 day−1 trandolapril significantly decreased MAP and LVSP and tended to decrease ±dP/dt.
The infarct size of rats with CHF represented approximately 40% of the left ventricle. There were no significant differences in the infarct size of rats with CHF irrespective of treatment with or without trandolapril. No infarct areas were seen in sham-operated rats with or without trandolapril-treatment.
Exercise capacity
Figure 1 shows the running times of untreated and 1 and 3 mg kg−1 day−1 trandolapril-treated rats with CHF and those of sham-operated rats. Sham-operated rats ran for approximately 26 min on the treadmill. Sham-operated rats treated with 1 mg kg−1 day−1 trandolapril ran for a similar period, whereas those treated with 3 mg kg−1 ran for approximately 22 min. A significant reduction in running time was seen in the rats with CHF. The rats with CHF treated with 1 mg kg−1 day−1 trandolapril ran for approximately 25 min, whereas the running time was not prolonged by treatment with 3 mg kg−1 day−1 trandolapril.
Figure 1.
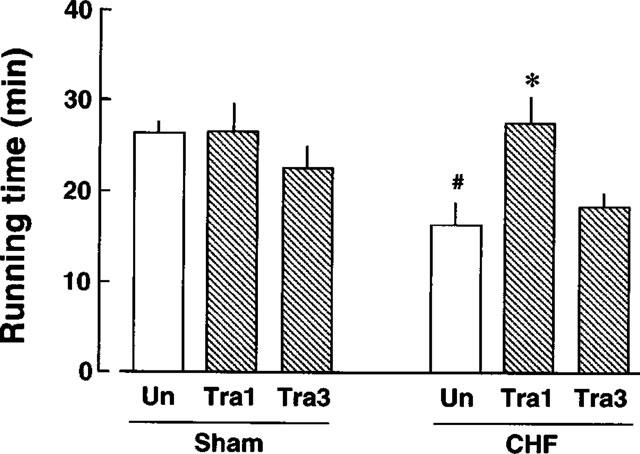
Running time of sham-operated rats and rats with CHF in the treadmill test. Each value represents the mean±s.e.mean of seven experiments. #Significantly different from sham-operated rats not treated with trandolapril (P<0.05). *Significantly different from rats with CHF not treated with trandolapril (P<0.05). Abbreviations: ‘Un', sham-operated rats or rats with CHF not treated with trandolapril; ‘Tra1', sham-operated rats or rats with CHF treated with 1 mg kg−1 day−1 trandolapril. ‘Tra3', sham-operated rats or rats with CHF treated with 3 mg kg−1 day−1 trandolapril.
Energy metabolite content
ATP, CP, lactate, and pyruvate contents of the gracilis muscle before exercise were measured in rats with CHF and in sham-operated rats treated or not with 1 or 3 mg kg−1 day−1 trandolapril (Table 2). ATP, CP, lactate and pyruvate contents of the gracilis muscle of non-operated, non-exercised rats were 5.89±0.27, 12.30±0.58, 6.06±0.24 and 0.30±0.04 μmoles g−1 frozen tissue (n=7), respectively. There were no differences in these energy metabolites between the rat with CHF and the sham-operated rat regardless of treatment status.
Table 2.
Contents of skeletal muscular energy metabolites of the sham-operated rats and the rats with CHF before and after running at the 8th week after coronary artery ligation
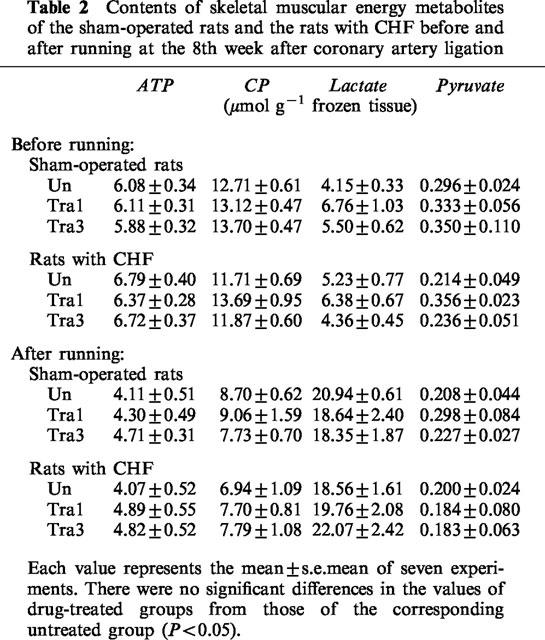
In another set of experiments, the energy metabolite contents of rats with CHF and sham-operated rats treated or not with trandolapril were measured after exercise (Table 2). After exercise, the ATP, CP, lactate and pyruvate contents of the non-operated rats were 4.54±0.38, 7.37±0.60, 18.73±1.38 and 0.23±0.03 μmoles g−1 frozen tissue (n=7), respectively. Exercise on the treadmill resulted in decreases in ATP and CP contents and an increase in lactate content as compared with those of the corresponding non-exercised rats. There was no significant difference in pyruvate content regardless of the presence or absence of exercise. These energy metabolite levels in the gracilis muscle of rats with CHF were similar to those of sham-operated rats. Treatment with 1 or 3 mg kg−1 day−1 trandolapril did not affect these levels in rats with CHF after exercise.
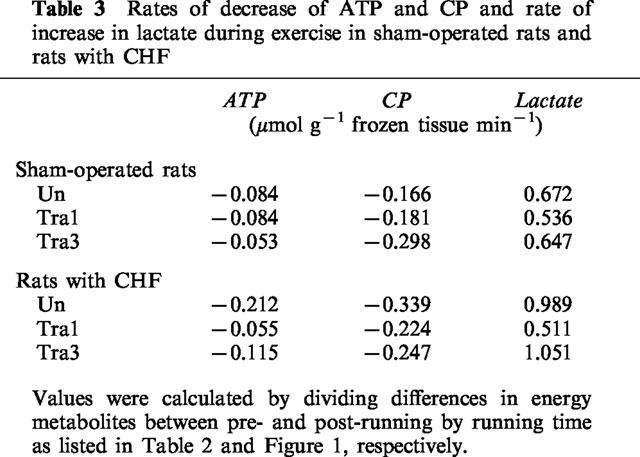
To further investigate skeletal muscle energy metabolism, we calculated the rate of decrease of muscular ATP and CP and the rate of increase of lactate during exercise (Table 3). These parameters of sham-operated rats treated with 1 or 3 mg kg−1 day−1 trandolapril were similar to those of sham-operated rats not treated with trandolapril. The rate of decrease of ATP and CP of rats with CHF were approximately 2.5 and 2.0 fold higher, respectively, than that of the sham-operated rats. The rate of increase of lactate content of rats with CHF was 1.5 fold higher than that of the sham-operated rats. Treatment with 1, but not 3 mg kg−1 day−1, trandolapril attenuated the changes in these parameters.
Table 3.
Rates of decrease of ATP and CP and rate of increase in lactate during exercise in sham-operated rats and rats with CHF
Ca-ATPase activity
Myofibrillar Ca-ATPase activity was determined (Figure 2). The Ca-ATPase activity of control rats was 49.7±1.1 μmoles Pi mg protein−1 h−1 (n=7). In rats with CHF not treated with the drug, the Ca-ATPase activity was significantly increased. Treatment of rats with CHF with 1 or 3 mg kg−1 day−1 trandolapril significantly attenuated the increase in the Ca-ATPase activity. In sham-operated rats, there were no changes in the Ca-ATPase activities regardless of treatment status.
Figure 2.
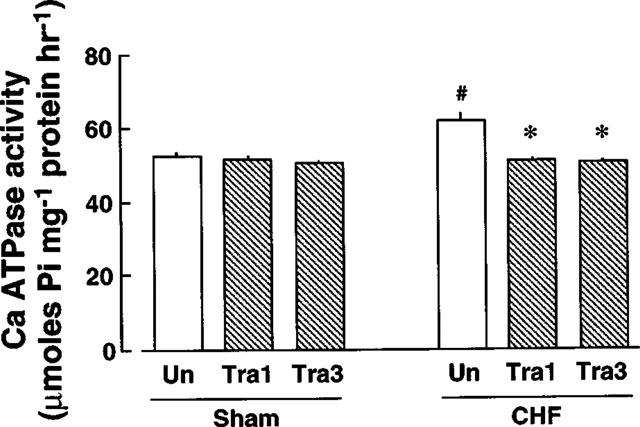
Myofibrillar Ca-ATPase activity of gracilis muscles of sham-operated rats and rats with CHF. Each value represents the mean±s.e.mean of seven experiments. #Significantly different from sham-operated rats not treated with trandolapril (P<0.05). *Significantly different from rats with CHF not treated with trandolapril (P<0.05). The abbreviations in the Figure are the same as those in Figure 1.
Isoforms of myosin heavy chain
Changes in the composition of myosin heavy chain isoforms were examined by SDS–PAGE (Figure 3) and semi-quantified estimation of these results by an image analyzer is shown in Figure 4. The proportions of MHC types I, IIa and IIb of gracilis muscles in non-operated rats were 9.5±1.4, 81.3±2.2 and 9.3±1.1%, respectively, n=7. In rats with CHF, MHC types I and IIb were significantly decreased, whereas type IIa tended to be increased, compared with the sham values. The proportions of these isoforms in rats with CHF treated with 1 or 3 mg kg−1 day−1 trandolapril were similar to those of the untreated, sham-operated rat. Treatment with 1 or 3 mg kg−1 day−1 trandolapril did not affect the composition of MHC isoforms of sham-operated rats.
Figure 3.
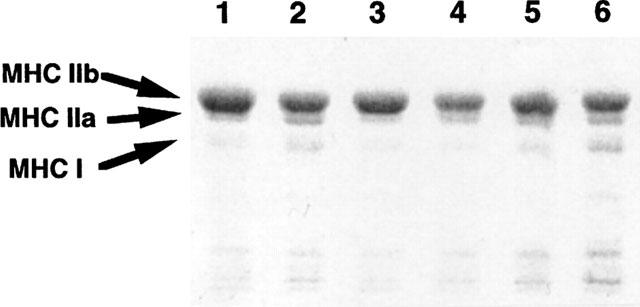
Representative SDS–PAGE of myosin heavy chain isoforms of gracilis muscles of rats with CHF and sham-operated rats treated or not treated with 1 mg kg−1 day−1 trandolapril or 3 mg kg−1 day−1 trandolapril. The gel was stained with Coomassie blue. Lane 1, rats with CHF; lane 2 sham-operated rats; lane 3, rats with CHF treated with 1 mg kg−1 day−1 trandolapril; lane 4, sham-operated rats treated with 1 mg kg−1 day−1 trandolapril; lane 5, rats with CHF treated with 3 mg kg−1 day−1 trandolapril; lane 6, sham-operated rats treated with 3 mg kg−1 day−1 trandolapril. MHC I, IIa and IIb represent myosin heavy chain isoform types I, IIa and IIb, respectively.
Figure 4.
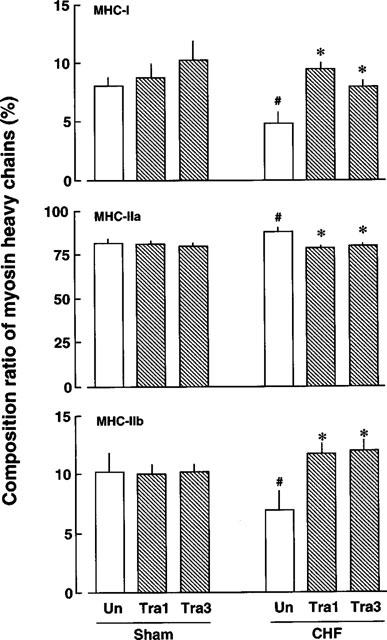
Composition ratios of myosin heavy chain isoforms in gracilis muscles of sham-operated rats and rats with CHF. Each value represents the mean±s.e.mean of seven experiments. #Significantly different from sham-operated rats not treated with trandolapril. *Significantly different from rats with CHF not treated with trandolapril. The abbreviations in the Figure are the same as those in Figure 1.
Discussion
In the present study, the rats with coronary artery ligation showed significant decreases in left ventricular systolic pressure (LVSP), LV ±dP/dt and MAP and a marked increase in left ventricular end-diastolic pressure (LVEDP), whose results are similar to those in previous studies (Sanbe et al., 1993; 1995a). This model also showed decreases in cardiac output and stroke volume indices 8 weeks after coronary artery ligation (Sanbe et al., 1993). Thus, we conclude that CHF with low cardiac output may be developed by 8 weeks after coronary artery ligation.
The most interesting finding in this study was the appreciable shortening of the exercise period of rats with CHF. As for alterations in metabolic parameters, there were no significant differences in energy metabolite contents, such as ATP, CP, lactate and pyruvate, of gracilis muscles before and after exercise between the rat with CHF and the corresponding sham-operated rat. These results suggest that energy production of the skeletal muscle of rats with CHF may be sufficiently executed in the resting as well as exercise state. ATP and CP contents in soleus and extensor digitorum muscles of rats were reduced 4 weeks after treatment with monocrotaline, an inducer of right ventricular dysfunction (Bernocchi et al., 1996). The inconsistency of the changes in muscular ATP and CP contents between our study and the one just cited may be due to differences in experimental models and periods examined. Furthermore, we detected increases in the consumption rates of ATP and CP and production rate of lactate in rats with CHF. This suggests that not only the consumption of ATP but also the production of lactate appeared to be enhanced in rats with CHF. Similarly, Mancini et al. (1989) detected in a 31P-NMR study that the decline in CP and the rise in inorganic phosphate levels of skeletal muscle during exercise were more rapid in patients with CHF than in control subjects. These observations suggest that skeletal muscle of patients and animals with CHF may fatigue more easily than that of control subjects.
We also determined myofibrillar Ca-ATPase activity, one of the most important factors in the energy consumption process of skeletal muscles. A higher Ca-ATPase activity was detected in rats with CHF, suggesting wastage of energy during exercise in the skeletal muscle of animals with CHF. This is consistent with the shortening of the running time of rats with CHF in the present study.
Seven sarcomeric MHC genes were found in the rat when analysis was made with an MHC complementary DNA probe (Mahdavi et al., 1986). In the adult rat skeletal muscle, three isoforms, that is, MHC types I, IIa and IIb, are expressed. MHC type I is a slow-twitch oxidative form encoded by the cardiac β-MHC gene (Lompre et al., 1984). This type effectively utilizes ATP of skeletal muscles, whereas it slowly generates muscular contractile force (Izumo et al., 1986; Mahdavi et al., 1986). MHC type IIa is a fast-twitch oxidative isoform, and type IIb is a fast-twitch glycolytic isoform (Izumo et al., 1986; Mahdavi et al., 1986). Thus, it is generally recognized that MHC type II isoforms can generate fast muscular contraction, and utilize ATP less effectively than MHC type I. Sabbah et al. (1993) demonstrated that the percentage of the triceps muscle type I fibres was reduced and that of type II fibres was increased in dogs with microsphere embolism-induced heart failure. In accord with this finding, we observed a decrease in the proportions of MHC types I and IIb and an increase in the proportion of MHC type IIa in rats with CHF. Since there were no significant differences in the yield of the myofibrillar fraction from the gracilis muscle among the experimental groups, the alteration of MHC isoform composition appears to reflect changes in the absolute amount of MHC isoform in the skeletal muscle. Our findings of the decreased levels of MHC type I and the increased levels of MHC type IIa in rats with CHF suggest a greater consumption rate of high-energy phosphates in the animals. This mechanism may, in part, account for the shortening of the running time of the rats with CHF.
We employed two doses of trandolapril in the present study to determine the appropriate dose for ACE inhibitor therapy of CHF. Treatment of rats with CHF with 1 mg kg−1 day−1 trandolapril from the 2nd to 8th week resulted in a restoration of the running time and biochemical and molecular alterations in myofibrillar Ca-ATPase, and muscular isoforms. The improvement was associated with attenuation in the increases in the rates of ATP consumption and lactate production. Treatment with 3 mg kg−1 day−1 trandolapril did not improve the running time, but did improve the biochemical and molecular abnormality of rats with CHF. Energy metabolite contents of rats with CHF or sham-operated rats before and after exercise were similar irrespective of the dose of trandolapril employed. Thus, the difference in the effects between the two dosages appeared to be focused on the running time. The results suggest that ACE inhibitor therapy definitely improves the biochemical and molecular abnormality of CHF, whereas another or additional factors may be necessary for restoration of the ability to exercise in animals with CHF.
The MAP of rats with CHF was decreased to 89% of that of the sham-operated rats. Long-term treatment with 3 mg kg−1 day−1 trandolapril further reduced this value to approximately 65% of the value for the sham-operated rats. Treatment of sham-operated rats with 3 mg kg−1 day−1 trandolapril also decreased the MAP and tended to shorten the running time. These results suggest that the lack of benefits in running time following treatment with 3 mg kg−1 day−1 trandolapril, despite improvement of Ca-ATPase activity and MHC composition, may be attributed to drug-induced hypotension. Despite no evidence in experimental animals, a clinical study showed that hypotension aggravates systemic circulatory function of patients with CHF, leading to limited perfusion to exercising skeletal muscles (Mabee et al., 1994). Thus, it is likely that restoration of exercise tolerance of animals with CHF requires at least two factors, improvement of systemic circulation and improvement of biochemical and molecular alterations in myofibrils. Treatment with 3 mg kg−1 day−1 trandolapril, in contrast to 1 mg kg−1 day−1 trandolapril, may have improved the latter abnormality of the skeletal muscle. The ineffectiveness of captopril on aerobic exercise capacity of patients after acute myocardial infarction was also attributed to a drug-induced hypotension (Ray et al., 1993).
Several investigators demonstrated therapeutic effects of long-term ACE inhibitor treatment on humoral factors, including attenuation of the increase in plasma levels of catecholamine (Kleber & Doering, 1991), natriuretic peptide (ANP), and brain natriuretic peptide (BNP) (Crozier et al., 1989; Kawahara et al., 1989; Yoshimura et al., 1994). Sanbe et al. (1995b) also showed that long-term treatment with trandolapril attenuated an increase in ACE activity of heart and plasma in this model. In a preliminary study, we found that the plasma level of humoral factors such as norepinephrine increased in rats with CHF (about 2 fold increase) and that long-term treatment with 3 mg kg−1 day−1 trandolapril could reverse the changes in the plasma norepinephrine level (unpublished data). If this improvement is elicited through ACE inhibition, circulatory angiotensin II may play an important role in the development of CHF-induced changes in skeletal muscle function and metabolism in animals with CHF. Although we did not measure ACE activity of the skeletal muscle in rats with CHF, an increase in plasma ACE activity may result in a rise of plasma concentration of circulatory angiotensin II (Sanbe et al., 1995b), which might lead to biochemical and molecular alterations in Ca-ATPase activity and composition of MHC isoforms.
Simonimi et al. (1996a, 1996b) showed that β-MHC mRNA expression and MHC type I level in soleus muscle decreased in rats with CHF. Despite the difference in preparations studied, the latter results on MHC levels agree with our findings. They also measured the mRNA of skeletal mitochondrial enzymes such as cytochrome c oxidase (COX III) and succinate dehydrogenase, whose expression levels decreased in the rats with CHF (Simonimi et al., 1996b). However, it is unlikely that the reduced expression level of COX III contributes to exercise intolerance, since the skeletal muscular activity of COX III was not correlated with its mRNA expression (Simonimi et al., 1996b). Nevertheless, it is possible that pathophysiological alterations in muscular types may, at least in part, account for the increased level of energy consumption in the rats with CHF.
In conclusion, the exercise capacity of rats with CHF deteriorated due to an increase in their energy consumption rate. Our results suggest that the increase in skeletal muscular Ca-ATPase activity may cause a decrease in exercise capacity of rats with CHF. Long-term trandolapril treatment of rats with CHF ameliorates the changes in energy utilization and thus enhances the exercise capacity at a dose that does not affect arterial blood pressure.
Abbreviations
- ACE
angiotensin converting enzyme
- CHF
chronic heart failure
- CP
creatinine phosphate
- MHC
myosin heavy chain
References
- BERNOCCHI P., CECCONI C., PEDERSINI P., PASINI E., CURELLO S., FERRARI R. Skeletal muscle metabolism in experimental heart failure. J. Mol. Cell. Cardiol. 1996;28:2263–2273. doi: 10.1006/jmcc.1996.0219. [DOI] [PubMed] [Google Scholar]
- BROQVIST M., DHALSTORM U., KARLSSON E., LARSSON J. Muscle water and electrolytes on severe chronic congestive heart failure before and after treatment with enalapril. Eur. Heart J. 1992a;13:243–250. doi: 10.1093/oxfordjournals.eurheartj.a060154. [DOI] [PubMed] [Google Scholar]
- BROQVIST M., DAHLSTROM U., KARLSSON E., LARSSON J. Muscle energy metabolism in severe chronic congestive heart failure – effect of treatment with enalapril. Eur. Heart J. 1992b;13:1217–1224. doi: 10.1093/oxfordjournals.eurheartj.a060340. [DOI] [PubMed] [Google Scholar]
- CROZIER I.G., NICHOLLS M.G., IKRAM H., ESPINER E.A., YANDLE P.G. Atrial natreuretic peptide levels in congestive heart failure in man before and during converting enzyme inhibition. Clin. Exp. Pharmacol. Physiol. 1989;16:417–424. doi: 10.1111/j.1440-1681.1989.tb01579.x. [DOI] [PubMed] [Google Scholar]
- COATS A.J. Symptoms and quality of life in heart failure: the muscle hypothesis. Br. Heart J. 1994;72 Suppl 2:536–539. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COATS A.J. Symptoms and quality of life in heart failure. J. Mol. Cell. Cardiol. 1994;28:2255–2262. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- DAVIES K.J., DONOVAN C.M., FEFINO C.J., BROOKS G.A., PACKER L., DALLMAN P.R. Distinguishing effects of anemia and muscle iron deficiency on exercise bioenergetics in the rat. Am. J. Physiol. 1984;246:E535–E543. doi: 10.1152/ajpendo.1984.246.6.E535. [DOI] [PubMed] [Google Scholar]
- DREXLER H., RIEDE U., MUNZEL T., KONIG H., FUNKE E., JUST H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- GUTMANN I., WAHLFELD A.W. Methods of enzymatic analysis 1974Verlag Chemie, Weinheim and Academic Press, New York, London; 1464–1468.2nd edition Bergmeyer, H.U., pp [Google Scholar]
- IZUMO S., NADAL-GINARD B., MAHDAVI V. All members of the MHC multi-gene family respond to thyroid hormone in a highly tissue specific manner. Science Wash. D.C. 1986;231:597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- KAWAHARA Y., HASEGAWA K., SAWAYAMA T., INOUE S., NAKAMURA T., KAKUMAE S., TADAOKA S., NAKAO M., NEZUO S. The effects of bunazosin vs. captopril on hemodynamic and neurohumoral parameters in patients with congestive heart failure. Kokyu-To-Junkan. 1989;37:1333–1340. [PubMed] [Google Scholar]
- KLEBER F.X., DOERING W. Prognosis of mild chronic heart failure: effects of the ACE inhibitor captopril. Herz. 1991;16:283–293. [PubMed] [Google Scholar]
- LOMPRE A.-M., NADAL-GINARD B., MAHDAVI V. Expression of the cardiac ventricular α- and β-myosin heavy chain genes is developmentally and hormonally regulated. J. Biol. Chem. 1984;259:6437–6446. [PubMed] [Google Scholar]
- LOWRY O.H., PASSONNEAU J.V. Academic Press, New York; 1972. A flexible system of enzymatic analysis; pp. 120–152. [Google Scholar]
- LOWRY O.H., ROSEBROUGH H.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MABEE S.W., METRA M., DOUGLAS E., REED B.S., DEI L., CODY R.J. Pulmonary hypertension and systemic hypotension as limitations to exercise in chronic heart failure. J. Card. Failure. 1994;1:27–33. doi: 10.1016/1071-9164(94)90005-1. [DOI] [PubMed] [Google Scholar]
- MAHDAVI V., STREHLER E.E., PERIASAMY M., WIECZOREK D., IZUMO S., GRUND S., STREHLER M.-A., NADAL-GINARD B.Sarcomeric myosin heavy chain gene family: Organization and pattern of expression Molecular Biology of Muscle Development 1986Liss, New York; 345–361.ed. Emerson, C., Fishman, D., Nadal-Ginard, B. and Siddiqui, M.A.Q. pp [DOI] [PubMed] [Google Scholar]
- MANCINI D.M., COYLE E., COGGAN A., BELTA J., FERRARO N., MONTAIN S., WILSON J.R. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- PFEFFER J.M., PFEFFER M.A., BRAUNWALD E. Hemodynamic benefits and prolonged survival with long-term captopril therapy in rats with myocardial infarction and heart failure. Circulation. 1987;75 Suppl. I:I149–I155. [PubMed] [Google Scholar]
- RAY S.G., PYE M., OLDROYD K.G., CHRISTIE J., NORTHRIDGE D.S., MORTON J.J., DARGIE H.J., COBBE S.M. Early treatment with captopril after acute myocardial infarction. Br. Heart J. 1993;69:215–222. doi: 10.1136/hrt.69.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABBAH H.N., HANSEN-SMITH F., SHAROV V.G., KONO T., LESCH M., GENGO P.J., STEFFEN R.P., LEVINE B.T., GOLDSTEIN S. Decreased muscle of type I myofibers in skeletal muscle of dogs with chronic heart failure. Circulation. 1993;87:1729–1737. doi: 10.1161/01.cir.87.5.1729. [DOI] [PubMed] [Google Scholar]
- SANBE A., TANONAKA K., HANAOKA Y., KATO T., TAKEO S. Regional energy metabolism of failing hearts following myocardial infarction. J. Mol. Cell. Cardiol. 1993;25:995–1013. doi: 10.1006/jmcc.1993.1113. [DOI] [PubMed] [Google Scholar]
- SANBE A., TANONAKA K., KOBAYASHI R., TAKEO S. Effects of long-term therapy with ACE inhibitors, captopril, enalapril and trandolapril, on myocardial energy metabolism in rats with chronic heart failure following myocardial infarction. J. Mol. Cell. Cardiol. 1995a;27:2209–2222. doi: 10.1016/s0022-2828(95)91551-6. [DOI] [PubMed] [Google Scholar]
- SANBE A., TSUKADA J., TAKEO S. Effects of trandolapril on cardiac angiotensin I converting enzyme activity in rats with chronic heart failure following myocardial infarction. Jpn. Heart J. 1995b;36:451–463. doi: 10.1536/ihj.36.451. [DOI] [PubMed] [Google Scholar]
- SAVE INVESTIGATORS Effects of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- SIMONIMI A., LONG C.S., DUDLEY G.A., YUE P., MCELIHINNY J., MASSIE B.M. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circ. Res. 1996a;79:128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- SIMONIMI A., MASSIE B.M., LONG C.S., QI M., SAMAREL A.M. Alterations in skeletal muscle gene expression in the rat with chronic congestive heart failure. J. Mol. Cell. Cardiol. 1996b;28:1683–1691. doi: 10.1006/jmcc.1996.0158. [DOI] [PubMed] [Google Scholar]
- SOLARO R.J., PANG D.C., BRIGGS F.N. The purification of cardiac myofibrils with Triton X-100. Biochem. Biophys. Acta. 1971;245:259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- SOLVD INVESTIGATORS Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- SULLIVAN M.J., GREEN H.J., COBB F.R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- TANONAKA K., MARUYAMA Y., TAKEO S. Beraprost, a prostacyclin mimetic agent, is beneficial for post-hypoxic recovery of cardiac function and metabolism in isolated rabbit hearts. Br. J. Pharmacol. 1991;104:779–786. doi: 10.1111/j.1476-5381.1991.tb12506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRACE STUDY GROUP A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- TAUSKY H.H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorous. J. Biol. Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- YOSHIMURA M., YASUE H., TANAKA H., KIKUTA K., SUMIDA H., KATO H., JOUGASAKI M., NAKAO K. Response of plasma concentrations of A type natriuretic peptide and B type natriuretic peptide on alacepril, an angiotensin-converting enzyme inhibitor, in patients with congestive heart failure. Br. Heart J. 1994;72:528–533. doi: 10.1136/hrt.72.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]


