Abstract
The effects of two new synthetic compounds showing in vitro catechol-O-methyl transferase (COMT) inhibitor properties were studied in vivo and compared with the effects of nitecapone and Ro-41-0960.
QO IA (3-(3-hydroxy-4-methoxy-5-nitrobenzylidene)-2,4-pentanedione), QO IIR ([2-(3,4-dihydroxy-2-nitrophenyl)vinyl]phenyl ketone), nitecapone and Ro-41-0960 (30 mg kg−1, i.p.) were given to reserpinized rats 1 h before the administration of L-DOPA/carbidopa (LD/CD, 50 : 50 mg kg−1, i.p.). Locomotor activity was assessed 1 h later. All the COMT inhibitors (COMTI), with the exception of QO IA, markedly potentiated LD/CD reversal of reserpine-induced akinesia. Similar results were obtained when the COMTI were coadministered with LD/CD. The effect of compound QO IIR was dose-dependent (7.5–30 mg kg−1, i.p.).
The COMTI (30 mg kg−1, i.p.) potentiated LD/CD reversal of both catalepsy and hypothermia of reserpinized mice.
QO IIR, nitecapone and Ro-41-0960 (30 mg kg−1, i.p.) reduced striatal 3-methyl-DOPA (3-OMD) levels and increased dopamine (DA) and dihydroxyphenylacetic acid (DOPAC) levels. Compound QO IA was devoid of any effect on striatal amine levels. In contrast to the other inhibitors, Ro-41-0961 reduced HVA levels as well. The effect of QO IIR on striatal amine levels was dose-dependent (7.5–60 mg kg−1, i.p.)
These results suggest that the new compound QO IIR is an effective peripherally acting COMT inhibitor in vivo.
Keywords: Amines, antiparkinsonian therapy, COMT, L-DOPA, Parkinson's disease
Introduction
Catechol-O-methyl transferase (COMT; EC 2.1.1.6) is one of the enzymes involved in the deactivation of cathecolamines and drugs with a catechol structure (Guldberg & Marsden, 1975). Levodopa (LD) is widely used in the treatment of Parkinson's disease (PD) (Papavasilous et al., 1972; Bartholini & Pletscher, 1975), which is characterized by a marked reduction of dopamine (DA) levels in the striatum. LD is converted by dopa decarboxylase (DDC) to DA, both in peripheral tissues and in brain. To allow higher concentrations of LD to reach the brain and to reduce peripheral side effects of the drug, it is coadministered in combination with a peripheral DDC inhibitor (eg. carbidopa or benserazide). In this situation LD is largely converted into 3-O-methyl-DOPA (3-OMD; Sharpless et al., 1972; Sandler et al., 1974) by COMT. Accumulation of 3-OMD may be detrimental for PD patients since it competes with LD for the active transport through the intestinal mucosa and the blood-brain-barrier (Muenter et al., 1973; Reches et al., 1982). Thus, selective COMT inhibition improves the bio-availability of LD (Cedarbaum et al., 1991; Nissinen et al., 1992; Jorga et al., 1997) and allows a decrease in its dose as shown in recent clinical trials (Rajput et al., 1997; Yamamoto et al., 1997; Adler et al., 1998; Baas et al., 1998).
During the last years, new catechol derivatives that induce potent and selective COMT inhibition both in vitro and in vivo have been synthesized (Männistö et al., 1988; Bäckstrom et al., 1989; Borgulya et al., 1989; Nissinen et al., 1988; 1992). Most importantly, these compounds have been shown to be effective in experimental animals models of PD, following their administration with LD/CD (Lindén et al., 1988; Maj et al., 1990). Furthermore, clinical trials have demonstrated that they may be succesfully used as coadjuvants in antiparkinsonian therapy (Rajput et al., 1997; Yamamoto et al., 1997; Adler et al., 1998; Baas et al., 1998). We have previously reported the synthesis and in vitro COMT inhibitor (COMTI) properties of several new synthetic compounds (Pérez et al., 1993; 1994). In this work we have studied the effects of 3-(3-hydroxy-4-methoxy-5-nitrobenzylidene)-2,4-pentanedione (QO IA) and [2-(3,4-dihydroxy-2-nitrophenyl)vinyl]phenyl ketone (QO IIR), which showed appropriate in vitro potency. The behavioural and neurochemical effects of these new compounds and two known COMTI (nitecapone and Ro-41-0960) were investigated after their administration in combination with LD/CD to rodents. Taken together our results show that QO IIR is an effective COMTI following its in vivo administration, showing great similarities with nitecapone. However, QO IA at the dose used (30 mg kg−1) was ineffective in the different assays.
Methods
Animals
Male Sprague-Dawley rats weighing 350±50 g were housed four per cage and male CD1 albino mice weighing 27±3 g were housed 15 per cage (Animal facilities of the University of Santiago de Compostela) at 21±1°C in 12 : 12 h dark:light cycle. The animals had free access to water and food. All experiments were performed according to the guidelines of the EC.
Drugs
D-amphetamine sulphate, reserpine and LD (3,4-dihydroxyphenylalanine) were from SIGMA; Ro-41-0960 (2-fluoro-3,4-dihydroxy-5-nitrobenzophenone) from Research Biochemicals International (RBI) and S(−)-carbidopa (CD) from Dupont Pharma; while the other COMTI were synthesized at the Institute of Organic Chemistry (CSIC):QO IA, QO IIR and nitecapone (OR-462: 3-(3,4-dihydroxy-5-nitrobenziliden)-2,4-pentanedione).
Amine standards used in the HPLC measurements were purchased from SIGMA; all biochemical reagents, analytical or HPLC grade, and the carboxy-methyl-cellulose were from MERCK.
Reserpine was solubilized in water containing lactic acid (1% v v−1); D-amphetamine sulphate in saline (0.9% NaCl) and all other drugs were suspended in sodium carboxy-methylcellulose (1% w v−1). Drugs were prepared immediately before use and were administered intraperitoneally (i.p.) in a volumen of 0.01 ml g−1.
LD (50 mg kg−1, i.p.), CD (50 mg kg−1, i.p.) and the inhibitors (30 mg kg−1, i.p.) were given to rats 2 h before sacrifice. In another experiment the inhibitors were given 1 h before the administration of LD/CD.
Behavioural studies
Locomotor activity
Twenty-four hours prior the experiment rats were placed in individual cages, where the locomotor activity was going to be measured and they had free access to food and water. Rats received reserpine 5 mg kg−1 i.p. 18 h before measurements. Locomotor activity was measured in activity cages (Panlab Actisystem DAS 16 V.1), which contained an electromagnetic field sensitive to any motion within it. All the experiments were done at the same time of the day (09.00–13.00 h) to avoid alterations due to circadian rythms. Results are accumulated counts during 1 h (mean±s.e.mean).
Reserpine-induced hypothermia
Reserpine 5 mg kg−1 was given to mice 18–20 h before drug administration. Rectal body temperature was measured with a rectal probe connected to a thermometer. Three measurements (30 min apart) were averaged and considered as basal temperature. After drug administration (LD/CD alone or coadministered with the COMTI) measurements were done every 30 min for 2 h. Results are expressed as change in temperature (Δ°C) over basal temperature.
Reserpine-induced catalepsy
To assess catalepsy after reserpine treatment (see above), mice were placed with their forepaws on one horizontal wire and their hindpaws on another 6 cm apart and 2 cm lower. Time (s) spent in this position was recorded and the cut-off time was 30 s. Catalepsy was scored every 30 min for 2 h after the administration of LD/CD alone or in combination with the COMTI.
Amines and their metabolites measurements
The same animals used in the locomotor activity studies were sacrificed at the end of the experiments for amine measurements. Both striata were removed on ice and homogenized (Ultraturrax, set 5, 10 s) in 20 volumes of 0.1 M perchloric acid containing 40 μM sodium bisulphite. Following centrifugation of samples (10,000×g) aliquots of the supernatants were filtered (Millipore HV 0.45 μm) and analysed or stored at −80°C until analysis. Amines and their metabolites were measured by a standard HPLC with coulometric detection method. In brief, samples were injected onto a reverse phase column (15×0.4 cm, Spherisorb ODS 2, 5 μm) and eluted with 0.1 M citric acid-0.1 M sodium citrate buffer pH 4.0 containing 12% methanol and 0.83 mM octane sulphonic acid, at a flow rate of 1 ml min−1. Results are expressed in μg g−1 of tissue. Detection limit was 20 pg for DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxyindoleacetic acid (5-HIIA) and 50 pg for 3-OMD and homovanillic acid (HVA).
Statistical analysis
Statistical analyses and graphs were carried out with GraphPad Prism software (v2.1, Sorrento, U.S.A.). Statistical significant differences were determined by one-way (treatment) analysis of variance (ANOVA), followed by Dunnett's post hoc test or by repeated measures ANOVA followed by Dunnett's multiple comparison test (catalepsy and hypothermia).
Results
Effects of COMTI on locomotor activity of reserpinized rats treated with LD/CD
The structures of the reference COMTI and the two new compounds shared some similarities, as shown in Figure 1.
Figure 1.
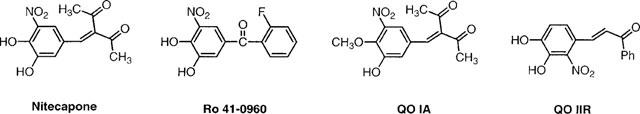
Chemical structures of the COMTI.
The doses of LD/CD (50 : 50 mg kg−1, i.p.) and the COMTI (30 mg kg−1, i.p.) used in this study were based in those previously shown to be effective by other authors (Männistö et al., 1992; Lindén et al., 1988). In one experiment the COMTI were administered 1 h before LD/CD administration and locomotor activity was evaluated 1 h later for 1 h. LD/CD (50 : 50 mg kg−1, i.p.) reversed reserpine-induced akinesia (P<0.01, Figure 2A). All the COMTI, with the exception of QO IA, markedly potentiated (6- fold increase, P<0.01) LD/CD reversal of reserpine-induced akinesia, being equally effective at the dose used (30 mg kg−1, i.p.). When the inhibitors were coadministered with LD/CD similar results were obtained (Figure 2B, P<0.01). Again QO IA was without effect, while QO IIR and the reference COMTI markedly potentiated the effect of LD/CD on locomotor activity of reserpinized rats. The effect of increasing doses of the new compound QO IIR on locomotor activity induced by LD/CD is shown in Figure 3. QO IIR dose-dependently potentiated (11.25–30 mg kg−1, P<0.01) the LD/CD reversal of reserpine-induced akinesia. The lowest dose assessed was without effect, as was the dose of 60 mg kg−1, the highest dose tested.
Figure 2.
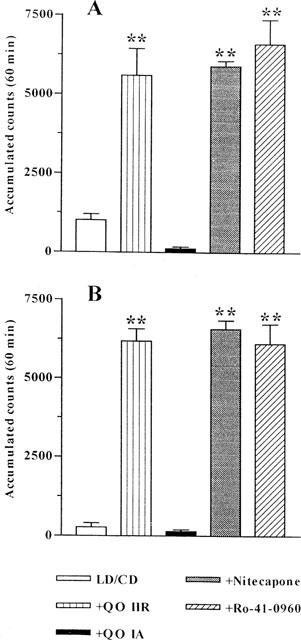
Effect of COMTI on locomotor activity of reserpinized rats treated with LD/CD. (A) COMTI (30 mg kg−1, i.p.) were administered to rats 1 h before LD/CD (50 : 50 mg kg−1, i.p.) and locomotor activity was measured 1 h later for 1 h. (B) COMTI (30 mg kg−1, i.p.) were coadministered to rats with LD/CD (50 : 50 mg kg−1, i.p.) and locomotor activity was measured 1 h later for 1 h. Reserpinized rats showed akinesia (accumulated counts during 1 h : 36.33±9.49). Results are mean±s.e.mean (n=5–8) of accumulated counts during 1 h. **P<0.01 significant difference versus LD/CD treated rats (one-way ANOVA followed by Dunnett's test).
Figure 3.
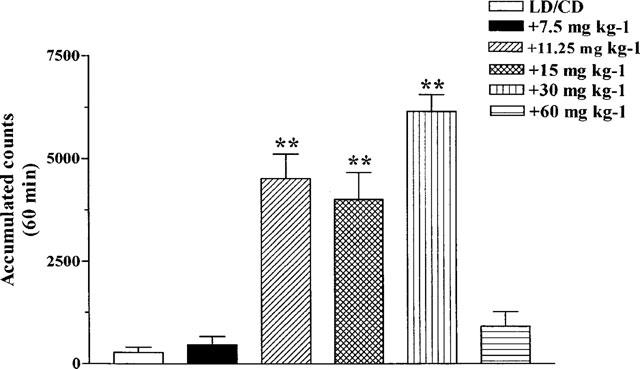
Dose-response curve of the effect of QO IIR coadministered with LD/CD on locomotor activity of reserpinized rats. Increasing doses (7.5–60 mg kg−1, i.p.) of QO IIR were coadministered to rats with LD/CD (50 : 50 mg kg−1, i.p.) and locomotor activity was measured 1 h later for 1 h. Reserpinized rats showed akinesia (accumulated counts during 1 h : 36.33±9.49) Results are mean±s.e.mean (n=5–8) of accumulated counts during 1 h. **P<0.01 significant difference versus LD/CD treated rats (one-way ANOVA followed by Dunnett's test).
Effect of COMTI on reserpine-induced catalepsy
The effect of COMTI on catalepsy displayed by reserpinized mice is shown in Figure 4. LD/CD induced a significant (P<0.01), although transient, reversal of reserpine-induced catalepsy, observed 30 min post-administration. In contrast, the COMTI markedly potentiated the LD/CD effect up to 2 h after their administration. However, while nitecapone only induced a partial reversal (around a 40% reduction in time spent in the forced posture, P<0.01), Ro-41-0960 and compound QO IIR fully reversed catalepsy (90% reduction, P<0.01).
Figure 4.
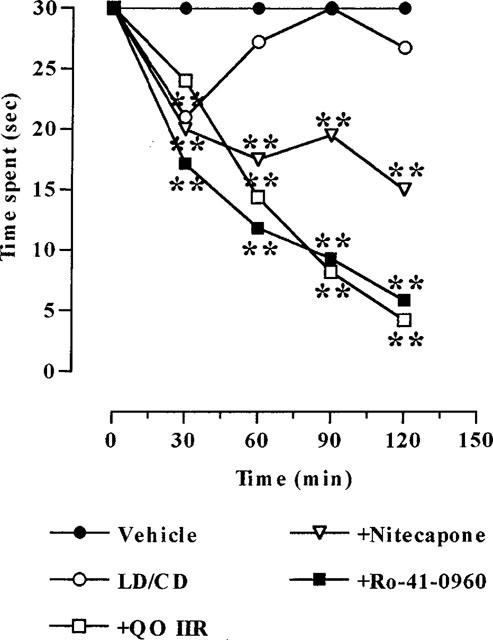
Effects of COMTI (30 mg kg−1, i.p.) coadministered with LD/CD (50 : 50 mg kg−1, i.p.) on reserpine-induced catalepsy. Results (time spent in the forced posture) are mean (n=5–8); s.e.mean which ranged between 0.00–5.55, were omitted for the sake of clarity. **P<0.01 versus reserpinized vehicle-treated animals (repeated measures ANOVA followed by Dunnett's multiple comparison test).
Effect of COMTI on reserpine-induced hypothermia
Reserpinized mice showed a marked decrease in body temperature, approximately 4°C. LD/CD non-significantly increased the body temperature (around 1°C) of reserpinized rats. This effect was significantly potentiated (4–5 fold increase, P<0.01) by all the COMTI to the same extent (Figure 5).
Figure 5.
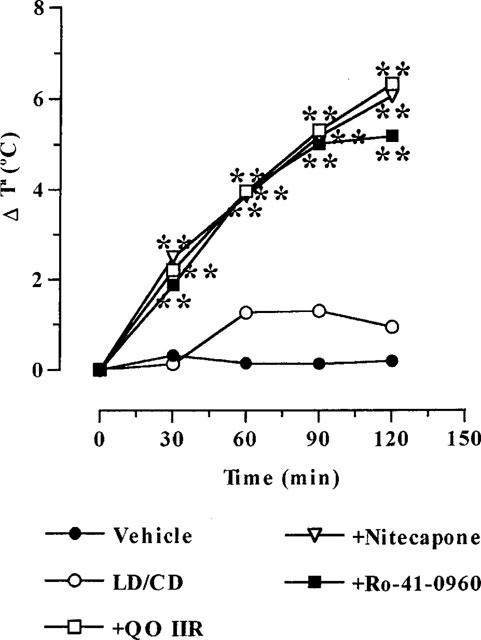
Effects of COMTI (30 mg kg−1, i.p.) coadministered with LD/CD (50 : 50 mg kg−1, i.p.) on reserpine-induced hypothermia. Results (increments in temperature: Δ°C) are mean (n=5–8); s.e.mean, which ranged between 0.12–1.73, were omitted for the sake of clarity. **P<0.01 significant difference versus reserpinized vehicle-treated animals (repeated measures ANOVA followed by Dunnett's multiple comparison test).
Effect of COMTI on striatal amine levels of reserpinized rats treated with LD/CD
Treatment of reserpinized rats with LD/CD resulted in high 3-OMD striatal levels (Table 1). Striatal levels of 3-OMD were significantly reduced by nitecapone, Ro-41-0960 and QO IIR (30 mg kg−1, i.p.). This dose of QO IA was devoid of any effect. The 3-OMD/LD ratio obtained for the different treatments is shown in Table 1. The high ratio observed following LD/CD treatment was not modified by compound QO IA, but was effectively reduced (P<0.01) by the other three COMTI under study. The decrease (to 20%) induced by QO IIR was similar to that induced by nitecapone. However, Ro-41-0960 showed a higher effect (to 5%). The decrease in 3-OMD/LD ratio induced by QO IIR treatment tended to be dose-dependent (Table 1), reflecting the dose dependent decrease in 3-OMD striatal levels (data not shown).
Table 1.
Effect of COMTI on 3-OMD/LD ratio in reserpinized rats treated with LD/CD

As shown in Figure 6, treatment of reserpinized mice with the COMTI 1 h before LD/CD administration induced significant increments in striatal DA (3–6 fold, P<0.05–0.01) and DOPAC levels (2–3 fold, P<0.01), with the exception of QO IA, which had no effect. HVA levels were only modified by Ro-41-0960, that markedly reduced its levels (to 5%, P<0.01).
Figure 6.
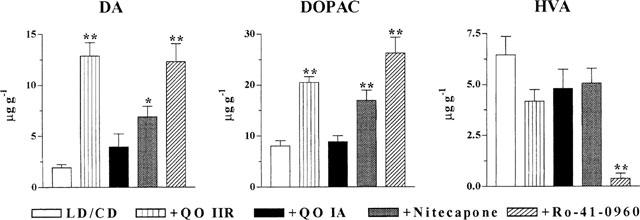
Striatal amine levels following COMTI and LD/CD administration to reserpinized rats. COMTI (30 mg kg−1, i.p.) were administered 1 h before LD/CD (50 : 50 mg kg−1, i.p.) and rats were sacrificed 2 h later. DA: dopamine; DOPAC: dihydrophenylacetic acid; HVA: homovanillic acid. Results expressed in μg g−1 are mean±s.e.mean (n=5–8). *P<0.05 **P<0.01 significant difference versus LD/CD treated rats (ANOVA followed by Dunnett's test).
At 2 h after LD/CD administered in combination with QO IIR a dose-dependent increase in DA (3–9 fold) and DOPAC (52–98%) levels was observed (Figure 7) compared to LD/CD treatment. However, the dose-response curve in the case of DA levels was bell-shaped. Indeed the highest dose of QO IIR tested (60 mg kg−1, i.p.) induced a much lower effect (only 3 fold increase) compared with the dose of 30 mg kg−1. HVA levels were only decreased (to 34%) after the dose of 60 mg kg−1. Levels of 5-HIAA, the major serotonin metabolite, were unaffected by QO IIR treatment (data not shown).
Figure 7.
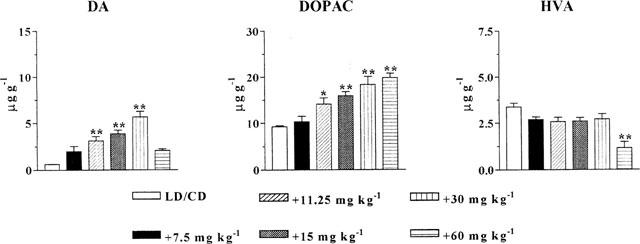
Striatal amine levels following increasing doses of QO IIR (7.5–60 mg kg−1, i.p.) coadministered with LD/CD (50 : 50 mg kg−1, i.p.) to reserpinized rats. Rats were sacrificed 2 h after drug administration. DA: dopamine; DOPAC: dihydroxyphenylacetic acid; HVA: homovanillic acid. Results expressed in μg g−1 are mean±s.e.mean (n=5–8). *P<0.05; **P<0.01 significant difference versus LD/CD treated rats (ANOVA followed by Dunnett's test).
Discussion
The aim of the present work was to study the possible in vivo COMT inhibition of two new synthetic compounds that inhibited COMT in vitro. Both compounds are modified nitrocatechols (Figure 1), showing different in vitro profiles. While compound QO IIR is a reversible tight-binding inhibitor, moderately potent (IC50=3.2 10−7 M; Pérez et al., 1993; 1994), compound QO IA is an irreversible weak COMTI (IC50=5.5 10−5 M; Pérez et al., 1993). The behavioural and neurochemical effects of both compounds in reserpinized rats treated with LD/CD have been compared with nitecapone and Ro-41-0960, effective COMTI in vivo.
In agreement with previous studies, nitecapone and Ro-41-0960 (30 mg kg−1) markedly potentiated LD/CD reversal of reserpine induced akinesia (Lindén et al., 1988; Maj et al., 1990). Compound QO IIR, at the same dose, potentiated to a similar extent the LD/CD effect. However, compound QO IA, was devoid of any effect on locomotor activity. The effect of QO IIR was dose-dependent, its maximal effect being observed at the dose of 30 mg kg−1. Reversal of reserpine-induced akinesia could be adscribed to effective in vivo COMT inhibition. In fact, striatal levels of 3-OMD, the major LD metabolite when administered with a peripheral DDC inhibitor (Sharpless et al., 1972; Sandler et al., 1974), were significantly reduced following the administration of the active COMTI (Männistö et al. 1988; 1992, Männistö & Tuomainen, 1991; Lindén et al., 1988; Zürcher et al., 1990), but not modified by QO IA, that neither modified locomotor activity. COMT inhibition may allow higher amounts of LD to enter the brain, that in turn is converted to DA. The COMTI also increased DA and DOPAC striatal levels in reserpinized rats treated with LD/CD, as has been shown in intact rats (Männistö et al., 1988; Männistö & Tuomainen, 1991; Lindén et al., 1988). As expected, the centrally acting inhibitor Ro-41-0960 was able to decrease HVA levels (Männistö & Tuomainen, 1991). While 3-OMD readily penetrates into the brain (Kopin et al., 1985), HVA shows very low, if any, penetration across the blood brain barrier. Thus, a decrease in striatal HVA levels is an index of brain COMT inhibition. However, HVA levels were reduced by the dose of 60 mg kg−1 of QO IIR. Similar effects of entacapone, another peripherally acting COMTI, has been occasionally found (Männistö & Tuomainen, 1991; Törnwall et al., 1993). Therefore a very high dose of QO IIR would be necessary to attain central COMT inhibition, or, alternatively, now it is able to reach the brain.
It should be mentioned that the highest dose of QO IIR tested (60 mg kg−1) was without effect on locomotor activity. This result is correlated with striatal DA levels, that were only modestly increased in comparison with levels after LD/CD alone. It could be speculated that this high dose of the compound may inhibit DDC activity, resulting in a lower increase in DA striatal levels compared with the other doses tested. Since LD and DOPAC levels were both increased following the administration of 60 mg kg−1 of QO IIR, these results suggest that, in this case, LD might be directly converted into DOPAC.
It has been reported that COMTI potentiates LD/benzeraside reversal of catalepsy and hypothermia induced by reserpine administration (Maj et al., 1990). Similarly, in the present work a significant potentiation of the LD/CD effect was observed with the active COMTI.
Since the discovery of COMT one of the major goals has been the development of inhibitors that may be clinically useful. However, most of them were ineffective in vivo, poorly selective towards COMT or toxic (for review see Männistö & Kaakkola, 1990). Tolcapone (Ro-40-7592: 3,4-dihydroxy-4′-methyl-5-nitrobenzophenone) and entacapone (OR-611: N,N-diethyl -2 - cyano-3-(3,4-dihydroxy-4′-methyl-5-nitrobenzophenone) are two commercially available COMTI. However, while entacapone, like nitecapone, does not cross the blood-brain barrier (Nissinen et al., 1988; Männistö et al., 1992), tolcapone, which is closely related to Ro-41-0960, inhibits brain COMT as well (Borgulya et al., 1989; Männistö et al., 1992). Although peripheral versus central inhibition differentially alters amine levels after COMTI administration, behavioural effects are quite similar. Indeed, as shown in the present work, the novel compound QO IIR potentiated to a similar extent LD/CD reversal of reserpine-induced akinesia when compared to the two reference COMTI.
In summary, compound QO IA, an irreversible and weak COMT inhibitor in vitro, at the dose tested, did not show any behavioural or neurochemical effect suggestive of in vivo COMT inhibition. However, the new synthetic compound QO IIR, a more potent (170 fold versus QO IA) and reversible tight-binding COMT inhibitor in vitro, has been shown to be an effective COMTI following its in vivo administration, with a similar potency to other well known COMTI.
Acknowledgments
We gratefully acknowledge the generous gift of carbidopa to Dupont Pharma (Spain). This work was supported by the Comisión Asesora de Investigación Científica y Técnica (Far88-0194/1), the Consejeria de Educación de la Comunidad de Madrid (C 126/91) and the Xunta de Galicia (XUGA 20308B96). E. Rivas had a predoctoral grant of the Xunta de Galicia.
References
- ADLER C.H., SINGER C., O'BRIEN C., HAUSER R.A., LEW M.F., MAREK K.L., DORFLINGER E., PEDDER S., DEPTULA D., YOO K. Randomized, placebo-controlled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa-carbidopa. Tolcapone Fluctuator Study Group III. Arch. Neurol. 1998;55:1089–1095. doi: 10.1001/archneur.55.8.1089. [DOI] [PubMed] [Google Scholar]
- BÄCKSTRÖM R., HONKANEN E., PIPPURI A., KAIRISALO P., PYSTYNEN J., HEINOLA K., NISSINEN E., LINDÉN I.-B., MÄNNISTO P.T., PHTO P. Synthesis of some novel potent and selective catechol-O-methyltransferase inhibitors. J. Med. Chem. 1989;32:841–846. doi: 10.1021/jm00124a017. [DOI] [PubMed] [Google Scholar]
- BARTHOLINI G., PLETSCHER A. Decarboxylase inhibitors. Pharmacol. Ther. 1975;1:407–421. doi: 10.1016/0306-039x(75)90047-1. [DOI] [PubMed] [Google Scholar]
- BAAS H., BEISKE A.G., GHIKA J., JACKSON M., OERTEL W.H., POEWE W., RANSMAYR G. Catechol-O-methyltransferase inhibition with tolcapone reduces the ‘wearing off' phenomenon and levodopa requirements in fluctuating parkinsonian patients. Neurology. 1998;50 Suppl. 5:S46–S53. doi: 10.1212/wnl.50.5_suppl_5.s46. [DOI] [PubMed] [Google Scholar]
- BORGULYA J., BRUDERER H., BERNAUER K., ZÜRCHER G., DA PRADA G. Catechol-O-methyltransferase-inhibiting pyrocatechol derivatives: synthesis and structure-activity studies. Helv. Chim. Acta. 1989;72:952–968. [Google Scholar]
- CEDARBAUM J.M., LEGER G., GUTTMAN M. Reduction of circulating 3-O-methyldopa by inhibition of catechol-O-methyltransferase with OR-611 and OR-462 in cynomolgus monkeys: Implications for the treatment of Parkinson's disease. Clin. Pharmacol. 1991;14:330–342. doi: 10.1097/00002826-199108000-00005. [DOI] [PubMed] [Google Scholar]
- GULDBERG H.C., MARSDEN C.A. Catechol-O-methyl-transferase: pharmacological aspects and physiological role. Pharmacol. Rev. 1975;27:135–206. [PubMed] [Google Scholar]
- JORGA K.M., SEDEK G., FOTTELER B., ZÜRCHER G., NIELSEN T., AITKEN J.W. Optimizing levodopa pharmacokinetics with multiple tolcapone doses in the elderly. Clin. Pharmacol. Ther. 1997;62:300–310. doi: 10.1016/S0009-9236(97)90033-3. [DOI] [PubMed] [Google Scholar]
- KOPIN I. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol. Rev. 1985;37:333–363. [PubMed] [Google Scholar]
- LINDÉN I.-B., NISSINEN E., ETEMADZADEH E., KAAKKOLA S., MÄNNISTÖ P., POHTO P. Favorable effect of catechol-O-methyltransferase inhibition by OR-462 in experimental models of Parkinson's disease. J. Pharmacol. Exp. Ther. 1988;247:289–293. [PubMed] [Google Scholar]
- MAJ J, , ROGÓZ Z., SKUZA G., SOWINSKA H., SUPERATA J. Behavioural and neurochemical effects of Ro 40-7592, a new COMT inhibitor with potential therapeutic activity in Parkinson's disease. J. Neural. Trans. [P–D Sect] 1990;2:101–112. doi: 10.1007/BF02260898. [DOI] [PubMed] [Google Scholar]
- MÄNNISTÖ P.T., KAAKKOLA S. Rationale for selective COMT inhibitors as adjuncts in the drug treatment of Parkinson's disease. Pharmacol. Toxicol. 1990;66:317–323. doi: 10.1111/j.1600-0773.1990.tb00756.x. [DOI] [PubMed] [Google Scholar]
- MÄNNISTÖ P.T., KAAKKOLA S., NISSINEN E., LINDÉN I.-B., POHTO P. Properties of novel effective and highly selective inhibitors of catechol-O-methyltransferase. Life Sci. 1988;43:1465–1471. doi: 10.1016/0024-3205(88)90258-5. [DOI] [PubMed] [Google Scholar]
- MÄNNISTÖ P.T., TUOMAINEN P. Effect of high single doses of levodopa and carbidopa on brain dopamine and its metabolites: modulation by selective inhibitors of monoamine oxidase and/or catechol-O-methyltransferase in the male rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:412–418. doi: 10.1007/BF00172580. [DOI] [PubMed] [Google Scholar]
- MÄNNISTÖ P.T., TUOMAINEN P., TUOMINEN R.K. Different in vivo properties of three new inhibitors of catechol-O-methyltransferase in the rat. Brit. J. Pharmacol. 1992;105:569–574. doi: 10.1111/j.1476-5381.1992.tb09020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUENTER M.D., DINAPOLI R.P., SHARPLESS N.S., TYCE G.M. 3-O-methyldopa, L-DOPA and trihexyphenidyl in the treatment of Parkinson's disease. Mayo Clin. Proc. 1973;48:173–178. [PubMed] [Google Scholar]
- NISSINEN E., LINDÉN I.-B., SCHULTZ E., KAAKKOLA S., MÄNNISTÖ P.T., POHTO P. Inhibition of catechol-O-methyltransferase activity by two novel disubstituted catechols in the rat. Eur. J. Pharmacol. 1988;153:263–269. doi: 10.1016/0014-2999(88)90614-0. [DOI] [PubMed] [Google Scholar]
- NISSINEN E., LINDÉN I.-B., SCHULTZ E., POHTO P. Biochemical and pharmacological properties of a peripherally acting catechol-O-methyltransferase inhibitor Entacapone. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;346:262–266. doi: 10.1007/BF00173538. [DOI] [PubMed] [Google Scholar]
- PAPAVASILOUS P.S., COTZIAS G.C., DUBY S.E. Levodopa in parkinsonism: Potentiation of central effects with a peripheral inhibitor. N. Engl. J. Med. 1972;285:8–14. doi: 10.1056/NEJM197201062860102. [DOI] [PubMed] [Google Scholar]
- PÉREZ R.A., FERNÁNDEZ-ALVÁREZ E., NIETO O., PIEDRAFITA F.J. Inhibition of catechol-O-methyltransferase by 1-vinyl derivatives of nitrocatechols and nitroguaiacols. Kinetics of the irreversible inhibition by 3-(3-hydroxy-4-methoxy-5-nitrobenzylidene)-2,4-pentanedione. Biochem. Pharmacol. 1993;45:1973–1981. doi: 10.1016/0006-2952(93)90006-i. [DOI] [PubMed] [Google Scholar]
- PÉREZ R.A., FERNÁNDEZ-ALVÁREZ E., NIETO O., PIEDRAFITA F.J. Kinetics of the reversible tight-binding inhibition of pig liver catechol-O-methyltransferase by [2-(3,4-dihydroxy-2-nitrophenyl)vinyl]phenyl ketone. J. Enzyme Inhib. 1994;8:123–131. doi: 10.3109/14756369409020195. [DOI] [PubMed] [Google Scholar]
- RAJPUT A.H., MARTIN W., SAINT-HILAIRE M.H., DORFLINGER E., PEDDER S. Tolcapone improves motor function in parkinsonian patients with the ‘wearing-off' phenomenon: a double-blind, placebo-controlled, multicenter trial. Neurology. 1997;49:1066–1071. doi: 10.1212/wnl.49.4.1066. [DOI] [PubMed] [Google Scholar]
- RECHES A., MIELKE C.R., FAHN S. 3-O-methyldopa inhibits rotation induced by levodopa in rats after unilateral destruction of the nigrostriatal pathway. Neurology. 1982;32:887–888. doi: 10.1212/wnl.32.8.887. [DOI] [PubMed] [Google Scholar]
- SANDLER M., JOHNSON R., RUTHVEN D, , RIED J., CALNE D. Transamination is a major pathway of L-DOPA metabolosm following peripheral decarboxylase inhibition. Nature. 1974;247:364–366. doi: 10.1038/247364b0. [DOI] [PubMed] [Google Scholar]
- SHARPLESS N.S., MUENTER M.D., TYCE G.M., OWEN C.A. 3-methoxy-4-hydroxyphenylalanine (3-O-methyldopa) in plasma during L-dopa therapy of patients with Parkinson's disease. Clin. Chim. Acta. 1972;37:359–369. doi: 10.1016/0009-8981(72)90456-1. [DOI] [PubMed] [Google Scholar]
- TÖRNWALL M., TUOMAINEN P., MÄNNISTÖ P.T. Modulation of rat brain endogenous dopamine metabolism by new inhibitors of catechol O-methyltransferase. Eur. J. Pharmacol. 1993;239:239–245. doi: 10.1016/0014-2999(93)90973-l. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO M., YOKOCHI M., KUNO S., HATTORI Y., TSUKAMOTO Y., NARABAYASHI H., TOHGI H., MIZUNO Y., KOWA H., YANAGISAWA N., KANAZAWA I. Effects of tolcapone, a catechol-O-methyltransferase inhibitor, on motor symptoms and pharmacokinetics of levodopa in patients with Parkinson's disease. J. Neural. Trans. 1997;104:229–236. doi: 10.1007/BF01273183. [DOI] [PubMed] [Google Scholar]
- ZÜRCHER G., COLZI A., DA PRADA M. Ro 40-7592: inhibition of COMT in rat brain and extracerebral tissues. J. Neural. Transm. 1990;32:375–380. doi: 10.1007/978-3-7091-9113-2_51. [DOI] [PubMed] [Google Scholar]


