Abstract
The antimycotic agent clotrimazole (CLT) is a promising potential therapeutic agent for a variety of diseases including cancer. Although it is known that CLT alters calcium homeostasis in many cell types, its cardiac effects are virtually unknown. We investigated the effects of CLT on L-type calcium current (ICa,L) and action potentials in guinea-pig ventricular myocytes. CLT (5, 25 and 50 μM) inhibited basal ICa,L by 16, 59 and 93%, respectively. The inhibitory effect of CLT was rapid and the peak effect was attained within 3 min. At a concentration of 25 μM, the inhibitory effect of CLT was partially reversible whereas the response to 50 μM CLT persisted following drug withdrawal. CLT abbreviated action potential duration at 50 and 90% of repolarization and suppressed the plateau significantly. These results indicate that CLT may have important cardiac effects at concentrations used to induce the antiproliferative action of the drug.
Keywords: Clotrimazole, calcium current, myocyte
Introduction
The imidazole derivative clotrimazole (CLT) is commonly used as a topical antifungal antibiotic. Although it derives its antifungal property from its ability to inhibit cytochrome P-450 mediated reactions (Sheets et al., 1986; Ayub & Levell, 1988), recent studies have shown that CLT also exerts marked ability to inhibit the proliferation of normal and cancerous cells in vitro and in vivo (Benzaquen et al., 1995). This finding and the potential clinical role of this agent in the treatment of cancer have made CLT the focus of intense investigations. The ability of CLT to interfere with cellular calcium homeostasis is considered to contribute to its antiproliferative properties. CLT has been shown to affect calcium influx and intracellular Ca2+ stores in several cell types (Alvarez et al., 1992; Villalobos et al., 1992; Benzaquen et al., 1995). As the cardiac calcium current plays a critical role in excitation-contraction coupling, we considered it of importance to examine the effects of this drug on the L-type calcium current (ICa,L) and action potentials in guinea-pig ventricular myocytes.
Methods
Ventricular myocytes from male Charles River albino guinea-pigs (300–350 g) were isolated as described previously (Thomas et al., 1997). Briefly, guinea-pig hearts were perfused retrogradely through the aorta with oxygenated calcium-free solution of the following composition (mM): NaCl 120, KCl 3.8, KH2PO4 1.2, MgSO4 1.2, N-[2-Hydroxyethyl] piperazine-N′-[2-ethane sulphonic acid] (HEPES) 10, glucose 11 along with collagenase Type-2 and protease. After dissociation, the myocytes were suspended in Kraft-Brühe solution of the following composition (mM): KOH 80, glutamic acid 50, KCl 30, KH2PO4 30, taurine 20, HEPES 10, glucose 10, MgSO4 3, EGTA 0.5 (pH was adjusted to 7.4 with KOH). During the experiment, cells were superfused with a solution containing (in mM), NaCl 145, HEPES 10, glucose 10, KCl 4, CaCl2 1.8 and MgCl2 1.
The pipette solution contained (in mM) ethylene glycol-bis (-aminoethyl ether)-N, N, N′, N′-tetra acetic acid (EGTA) 1, CsCl 130, HEPES 20, MgCl2 1, tetraethylammonium (TEA)-Cl 10, CaCl2 0.4 and ATP Na2 4. Recordings were initiated 15 min after rupturing the membrane to allow complete dialysis of the cytoplasm. Two experiments were also done using nystatin perforated patch configuration (ATP replaced by nystatin) to examine the effect in the absence of cell dialysis.
ICa,L recordings were made at room temperature using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA, U.S.A.). Na+ channels were inactivated by holding cells at −40 mV and K+ currents were blocked by using cesium instead of potassium. ICa,L (measured as the peak inward current) was elicited by a step depolarization from −40 to 10 mV every 20 s. Currents were filtered at 1 kHz and sampled at 5 kHz. The pCLAMP software program (Axon Instruments) was used for the acquisition and analysis of currents. Percentage values (log transformed) were analysed using two-way analysis of variance followed by Student-Newman-Keuls test to assess the significance level. CLT (dissolved in dimethyl sulphoxide (DMSO)) was added to the bath after 5 min exposure to DMSO alone in order to test the direct effect of the vehicle.
Action potentials were recorded at a 2 s basic cycle length in contracting cells using the current-clamp mode of an Axopatch 200A amplifier. The external solution contained (in mM): CaCl2 2, KCl 4, MgCl2 1, glucose 10, NaCl 140, HEPES 10, and pH was adjusted to 7.4 with NaOH. Pipette solution contained (mM): potassium aspartate 135, NaCl 10, KCl 10, HEPES 10, MgCl2 1, MgATP 5, and pH was adjusted to 7.1 with KOH. Changes in action potential duration were analysed using Student's t-test (paired) and the significance was established when P value was less than 0.05.
Results
A time- and voltage-dependent inward current with all of the characteristics of ICa,L was elicited by a step depolarization from −40 to 10 mV. CLT (5 and 25 μM) showed a concentration-dependent inhibition of the ICa,L. Figure 1A demonstrates the individual recordings obtained from an experiment in which the myocyte was exposed to CLT (50 μM) after 5 min of superfusion with DMSO. CLT (50 μM) caused a rapid decrease in the ICa,L. The effect of CLT was evident within a minute and the peak effect was attained by about 3 min. Concentrations less than 5 μM did not have any noticeable effect on ICa,L. Figure 1B demonstrates the time response and the reversibility of CLT action on ICa,L. The exposure of ventricular myocyte to CLT (25 μM) caused a rapid decrease in ICa,L. Withdrawal of the drug from the superfusion medium resulted in a slow and partial reversal of the effect which was observed approximately 5 min after restoration of the drug-free superfusion. However, ICa,L was still significantly inhibited after 15 min of washout demonstrating incomplete reversibility within this time period. With 50 μM CLT, the effect was not reversible for up to 15 min. In two experiments under nystatin perforated patch configuration, where dialysis of the cytoplasm is minimal, CLT responses were similar in magnitude compared to conventional whole cell experiments (data not shown).
Figure 1.
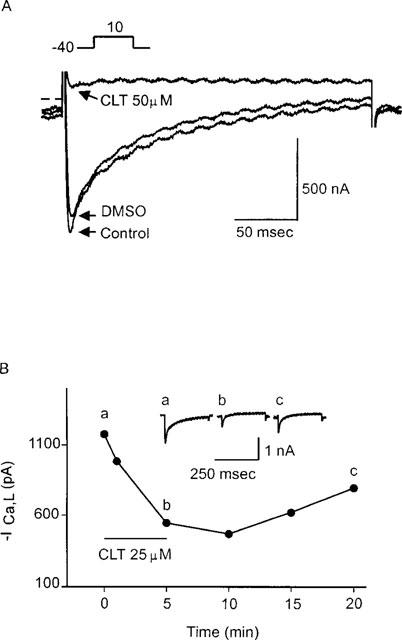
(A) Inhibition of ICa,L by clotrimazole (CLT) in a guinea-pig ventricular myocyte. ICa,L was elicited by 250 ms voltage step to 10 mV from a holding potential of −40 mV. Individual ICa,L traces were taken from a representative experiment in which the myocyte was superfused with the vehicle (DMSO) and CLT (50 μM). CLT (50 μM) almost completely blocked the ICa,L. (B) CLT (25 μM)-induced inhibition of ICa,L and its partial recovery on washout in another guinea-pig ventricular myocyte. Individual currents (inset) and the time courses of ICa,L during DMSO (a), CLT (b) and washout (c). Individual current traces were obtained at time points indicated as a, b and c.
At concentrations of 5 (n=3), 25 (n=4) and 50 μM (n=5), CLT reduced ICa,L to 84, 41 and 6% of pre-drug values respectively (Figure 2A). Figure 2B shows the peak current voltage relationship in five ventricular myocytes showing the near complete inhibition of ICa,L by CLT (50 μM).
Figure 2.
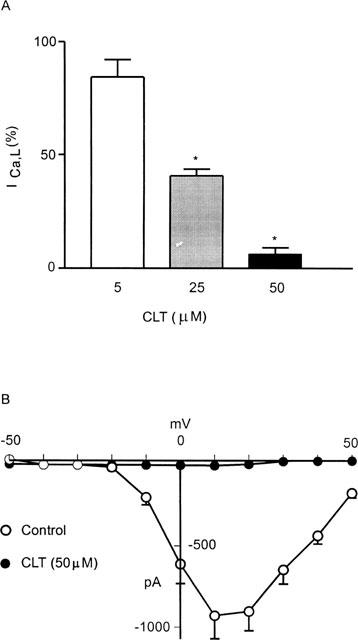
(A) Concentration-dependent inhibition of ICa,L by CLT in ventricular myocytes. n=3 for 5 μM, 4 for 25 μM and 5 for 50 μM CLT. Drug effects shown in this histogram were taken at 5 min post-CLT. Data are shown as ICa,L (%) of respective control values±s.e.mean. * P<0.05 compares to respective control values. (B) Mean current voltage relationship of peak ICa,L obtained from five ventricular myocytes showing the potent inhibition of ICa,L by CLT (50 μM).
CLT caused significant abbreviation of the action potential duration (Figure 3). CLT (25 μM) abbreviated the action potential duration at 50% repolarization from 184±14 to 133±14 ms (P<0.05). Action potential duration at 90% repolarization was also reduced by CLT from 210±13 to 180±13 ms (P<0.05) in five ventricular myocytes. CLT also suppressed the plateau voltage at APD10 from 110.7±3 to 89.2±4.3 mV (P<0.05).
Figure 3.
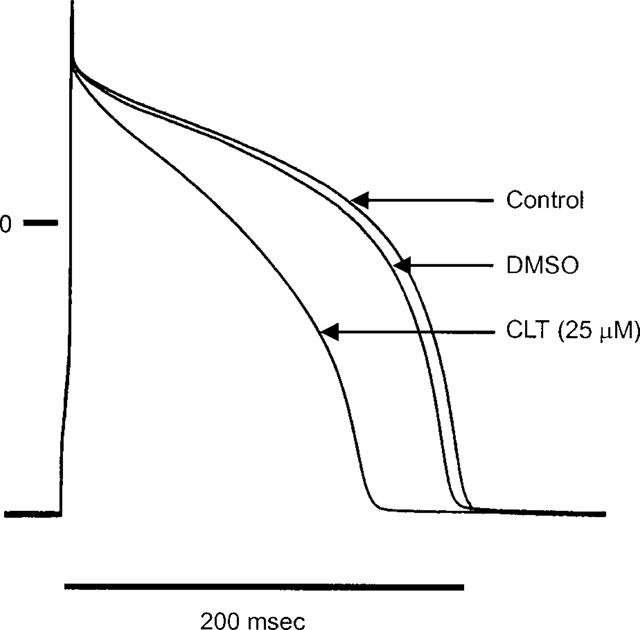
Effect of CLT on action potential in a guinea-pig ventricular myocyte. Individual traces were obtained from a representative experiment in which the myocyte was initially superfused with the vehicle (DMSO) followed by CLT (25 μM). CLT shortened the action potential duration significantly.
Discussion
Although CLT has been shown to modulate calcium levels in various cells, to our knowledge, this is the first study which examined the effect of CLT on ICa,L in cardiac cells or indeed any other cell type. The results of this study reveal a novel and highly potent inhibitory effect of CLT on cardiac ICa,L. CLT is a very widely used topical antimycotic with a potential use as oral medication for cancer and other diseases. The antiproliferative action is likely due to its ability to interfere with calcium homeostasis of the cell. It has been shown that CLT depletes intracellular Ca2+ stores (Benzaquen et al., 1995), inhibits voltage and ligand stimulated Ca2+ influx (Villalobos et al., 1992) and Ca2+ activated K+ channels (Alvarez et al., 1992; Brugnara et al., 1995) in different cell lines. The results demonstrated in this study showing inhibitory effects of CLT on ICa,L, suggest that these channels may represent a major site of action of CLT-induced modulation of intracellular calcium. Although the precise mechanisms for this effect need to be studied, it could be due to a direct effect of the drug on the channel protein or secondary to modulation of intracellular calcium stores. Indeed, with respect to the latter it has been suggested that the effects of CLT on Ca2+ influx and Ca2+ -activated channels in nucleated cells occur secondary to drug-induced depletion of intracellular Ca2+ stores (Benzaquen et al., 1995).
CLT also showed significant effects on the action potential characteristics of the guinea-pig ventricular myocyte. [The phases of repolarization of the action potential appears to be vulnerable to CLT action.] The action potential durations at various levels of repolarization as well as the plateau of the action potential were significantly reduced by CLT in all the myocytes exposed to it. CLT did not affect the resting membrane potential in these experiments. It appears that the inhibitory effect of CLT on ICa,L has contributed significantly to the suppression of the plateau and abbreviation of the action potential duration by this drug. However, it is not clear whether CLT has any effects on other repolarizing currents such as delayed rectifier potassium current and their relative contribution to the overall effect of the drug on repolarization.
Because of the increasing possibility for the systemic use of this drug in therapeutics, the results of the present study demonstrating inhibition of ICa,L and abbreviation of the action potential duration in guinea-pig ventricular myocytes are of potential importance. These results suggest that CLT may have potent effects on cardiac function, at concentrations which are in the same range as those used to induce antiproliferative action (Benzaquen et al., 1995).
Acknowledgments
This work was supported by Medical Research Council of Canada grant MT 9553 (N. Narayanan) and Heart and Stroke Foundation of Ontario (HSFO) grant T-3392 (M Karmazyn). M. Karmazyn is a Career Investigator of HSFO.
Abbreviations
- CLT
clotrimazole
- ICa,L
L-tpye calcium channel
References
- ALVAREZ J., MONTERO M., GARCIA-SANCHO J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J. Biol. Chem. 1992;267:11789–11793. [PubMed] [Google Scholar]
- AYUB M., LEVELL J. Structure-activity relationships of the inhibition of human placental aromatase by imidazole drugs including ketoconazole. J. Steroid Biochem. 1988;31:65–72. doi: 10.1016/0022-4731(88)90207-5. [DOI] [PubMed] [Google Scholar]
- BENZAQUEN L.R., BRUGNARA C., BYERS H.R., GATTONI-CELLI S., HALPERIN J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nature Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- BRUGNARA C., ARMSBY C.C., SAKAMOTO M., RIFAI N., ALPER S.L., PLATT O. Oral administration of clotrimazole and blockade of human erythrocyte Ca2+ activated K+ channel: the imidazole ring is not required for inhibitory activity. J. Pharmacol. Exp. Ther. 1995;273:266–272. [PubMed] [Google Scholar]
- SHEETS J.J., MASON J.I., WISE C.A., ESTABROOK R.W. Inhibition of rat liver microsomal cytochrome P-450 steroid hydroxylase reactions by imidazole antimycotic agents. Biochem. Pharmacol. 1986;35:487–491. doi: 10.1016/0006-2952(86)90224-8. [DOI] [PubMed] [Google Scholar]
- THOMAS G.P, , SIMS S.M., KARMAZYN M. Differential effects of endothelin-1 on basal and isoproterenol-enhanced Ca2+ current in guinea-pig ventricular myocytes. J. Physiol. (London) 1997;503:55–65. doi: 10.1111/j.1469-7793.1997.055bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLALOBOS C., FONTERIZ R., LOPEZ M., GARCIA A.G., GARCIA-SANCHO J. Inhibition of voltage-gated Ca++ entry into GH3 and chromaffin cells by imidazole antimycotics and other cytochrome P450 blockers. FASEB J. 1992;6:2742–2747. doi: 10.1096/fasebj.6.9.1319362. [DOI] [PubMed] [Google Scholar]


