Abstract
Experiments were designed to determine whether anandamide affects cytosolic Ca2+ concentrations in endothelial cells and, if so, whether CB1 cannabinoid receptors are involved. To this effect, human umbilical vein-derived EA.hy926 endothelial cells were loaded with fura-2 to monitor changes in cytosolic Ca2+ using conventional fluorescence spectrometry methods.
Anandamide induced an increase in Ca2+ in endothelial cells which, in contrast to histamine, developed slowly and was transient. Anandamide caused a concentration-dependent release of Ca2+ from intracellular stores without triggering capacitative Ca2+ entry, contrary to histamine or the endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin.
Anandamide pretreatment slightly reduced the mobilization of Ca2+ from intracellular stores that was evoked by histamine. The mobilization of Ca2+ from intracellular stores evoked by anandamide was impaired by 10 mM caffeine.
Anandamide and histamine each significantly increased NO synthase activity in EA.hy926 cells, as determined by the enhanced conversion of L-[3H]-arginine to L-[3H]-citruline.
The CB1 cannabinoid receptor antagonist SR141716A (1 μM) only produced a marginal reduction of the mobilization of Ca2+ produced by 5 μM anandamide. However, at 5 μM SR141716A elicited the release of Ca2+ from intracellular stores. This concentration strongly impaired the mobilization of cytosolic Ca2+ evoked by either anandamide, histamine or thapsigargin.
Pretreatment of the cells with either 200 μM phenylmethylsulphonyl fluoride (to inhibit the conversion of anandamide into arachidonic acid) or 400 ng ml−1 pertussis toxin (to uncouple CB1 cannabinoid receptors from Gi/o proteins) had no significant effect on the mobilization of cytosolic Ca2+ evoked by either anandamide, or histamine.
Taken together the results demonstrate that anandamide mobilizes Ca2+ from a caffeine-sensitive intracellular Ca2+ store that functionally overlaps in part with the internal stores mobilized by histamine. However, a classical CB1 cannabinoid receptor-mediated and pertussis toxin-sensitive mechanism does not mediate this novel effect of anandamide in endothelial cells.
The mobilization of cytosolic Ca2+ in endothelial cells may account for the endothelium-dependent and NO-mediated vasodilator actions of anandamide. Due to its non-specific inhibition of Ca2+ signalling in endothelial cells, SR141716A may not be used to assess the physiological involvement of endogenous cannabinoids to endothelium-dependent control of vascular smooth muscle tone.
Keywords: Cannabinoids, endothelium-derived hyperpolarizing factor, blood vessels, vasodilation, fatty acids
Introduction
Anandamide (arachidonylethanolamide), an endogenous ligand to cannabinoid (CB) receptors, is produced in various organs and found in plasma (see Felder & Glass, 1998; Guiffrida & Piomelli, 1998 and references therein). In addition to its neurobehavioural actions, anandamide induces hypotension in vivo. This action is inhibited by the CB1 receptor antagonist SR141716A (Lake et al., 1997; see Felder & Glass for review). Activation of peripheral CB receptors and the generation of anandamide by blood cells may contribute to haemorraghic hypotension (Wagner et al., 1997). The synthesis of anandamide has been demonstrated in cultured endothelial cells (Deutsch et al., 1994) and in the isolated perfused rat mesentery (Randall et al., 1996). CB1 receptors are expressed in both endothelial and vascular smooth muscle cells (Deutsch et al., 1994; Sugiura et al., 1998). Hence, it is possible that, under physiological conditions, endogenous cannabinoids, such as anandamide, modulate arterial tone by stimulating endothelial cells to release vasodilator substances or by acting directly on vascular smooth muscle.
In rat cerebral arterioles, anandamide releases vasodilator cyclo-oxygenase products (Ellis et al., 1995). In rat juxtamdedullary arterioles, the vasodilator response to anandamide is blocked after nitric oxide synthase inhibition (Deutsch et al., 1994). However, in bovine coronary arteries the relaxation induced by anandamide results from its conversion into arachidonic acid, which is then converted to vasodilator cyclo-oxygenase and cytochrome P450 metabolites (Pratt et al., 1998). In rat hepatic arteries, anandamide causes endothelium-dependent hyperpolarization as well as a direct relaxation of vascular smooth muscle (Zygmunt et al., 1997). However, in this artery, the relaxation seems independent of hyperpolarization and K+ channel activation in vascular smooth muscle (Zygmunt et al., 1997). In rat mesenteric arteries, anandamide also elicits a hyperpolarization of vascular smooth muscle that is strictly endothelium-dependent (Chataigneau et al., 1998). Besides the rat juxtamedullary arterioles study (Deutsch et al., 1994), the concentrations of SR141716A required to block the vasodilator actions of anandamide in these in vitro models are beyond its selectivity range for CB1 receptors (Felder et al., 1995; Felder & Glass, 1998). Therefore, both the mechanism of the vasodilator action of anandamide and the involvement of CB1 receptors are still highly controversial.
Since, in certain arteries, anandamide may stimulate the release of either NO (Deutsch et al., 1997), of prostaglandins (Ellis et al., 1995) or of an endothelium-derived hyperpolarizing factor(s) (Zygmunt et al., 1997; Chataigneau et al., 1998), it is possible that anandamide mobilizes cytosolic Ca2+ in endothelial cells. Indeed, the secretion of these vasodilator mediators by the endothelium is largely dependent on the elevation of cytosolic Ca2+ concentration (Adams et al., 1989; Graier et al., 1994b). Synthetic cannabinoid agonists induce the mobilization of Ca2+ in fibroblasts as well as neural cell lines (Felder et al., 1995). In the latter, the effect is mediated by CB1 receptors. Whereas, in fibroblasts this action is not dependent on the expression of CB1 receptors, since the mobilization of Ca2+ could be obtained in cells with no demonstrable specific binding sites (Felder et al., 1992). Furthermore, the concentrations of agonists required for this effect were orders of magnitude greater than their potency at CB1 receptors (Felder et al., 1992). In the DDT1-MF-2 vas deferens-derived smooth muscle cell line, the mobilization of cytosolic Ca2+ evoked by cannabinoids is partially blocked by concentrations of SR141716A within its selectivity for CB1 receptors (Filipeanu et al., 1997). Also, cannabinoids evoke capacitative Ca2+ entry (Filipeanu et al., 1997). In cultured rat striatal astrocytes, the stable anandamide analogue (R)-methanandamide depletes intracellular Ca2+ stores through a pertussis toxin-sensitive mechanism (Venance et al., 1997). In the present study, experiments were designed to determine whether or not anandamide elicits the mobilization of Ca2+ in cultured endothelial cells. Since, such was the case, we also assessed the effects of SR141716A, as a probe for the involvement of CB1 receptors (Rinaldi-Carmona et al., 1995).
Methods
Cell culture
A human umbilical vein-derived endothelial cell line EA.hy926 (a generous gift from Dr Edgell, University of North Carolina, Chapell Hill, NC, U.S.A.) was subcultured in the laboratory in Dulbecco's minimum essential medium (DMEM), containing 10% foetal calf serum and supplemented with 1% HAT (hypoxanthine 5 mM, aminopterin 20 μM, thymidine 0.8 mM).
Ca2+ measurements
Determination of changes in intracellular Ca2+ levels was performed spectrofluorimetrically using fura-2 as a probe (Graier et al., 1995). Cell suspensions were obtained after moderate trypsinization of subconfluent endothelial cells. The cell suspensions were incubated for 30 min at 37°C in DMEM containing 2.5 μmol l−1 fura-2/AM to load the fluorescent probe. To study the effects of pertussis toxin, the cells were treated for 3 h with 400 ng ml−1 of the toxin before putting them in suspension. After loading with fura-2/AM, the cells were centrifuged, washed and resuspended in 2 ml of Ca2+-free physiological salt solution (composition in mM): NaCl 135, MgCl2 1, KCl 5, HEPES 10, D-Glucose 10, EGTA 0.1, adjusted to pH 7.4 with NaOH. The cell suspension was transferred to a cuvette, and stirred continuously. To study the effects of the aminohydrolase inhibitor phenylmethylsulphonyl fluoride (PMSF, 200 μM), or the CB1-receptor antagonist SR141716A, the cell suspension were first treated with either solvent (dimethyl sulphoxide 0.1% v v−1) or the drugs for 4 min before the start of the experiments. All experiments were carried out at room temperature following a 4 min equilibration period. Initial experiments were carried out in physiological salt solution containing CaCl2 2.5 mM. In a second set of experiments, the cell suspensions were first equilibrated in Ca2+-free physiological salt solution for 4 min. Afterwards, they were challenged (or not) with either anandamide (0.1–10 μM), histamine (100 μM) or thapsigargin (2 μM) to release Ca2+ from intracellular stores. Thereafter, CaCl2 2.5 mM was added to the suspension to record the elevation of cytosolic Ca2+ which is caused by Ca2+ entry from the extracellular milieu.
Measurement of nitric oxide synthase activity
Activity of endothelial nitric oxide synthase was monitored by measuring the conversion of L-[3H]-arginine into L-[3H]-citrulline as previously described (Graier et al., 1996). Briefly, EA.hy926 cells were cultured in 6 well plastic dishes. At confluence, culture medium was removed, the cells were washed twice with phosphate buffer (in mM: NaCl 137, K 2.7, Na2HPO4 8, KH2PO4 1.5, pH adjusted at 7.4). The experiment was started by the addition of 900 μl HEPES buffer plus 2.5 mM Ca2+, containing 2×106 d.p.m. L-[3H]-arginine and 100 μl of either buffer, anandamide (to achieve 5 μM final concentration) or histamine (100 μM final). In parallel series, experiments were repeated in the presence of 300 μM L-NG-nitro-arginine were performed for each conditions. After 15 min, incubation buffer was discarded, cells were washed three times with chilled Ca2+-free HEPES buffer and were finally lysed with 1 ml 0.01 M HCl. After 1 h at 4°C, the total incorporated radioactivity was measured in a 100 μl aliquot. The remaining 0.9 ml HCl were buffered with 100 μl of 200 mmol l−1 sodium acetate buffer (pH=13.0) containing 10 mM L-citrulline. The amino acids, L-[3H]-arginine and L-[3H]-citrulline were separated using column chromatography as described by Mayer et al. (1991).
Data collection and statistical analysis
Due to the uncertainties inherent to measurement of intracellular Ca2+ with fluorescent probes (Morgan, 1993; Paltauf-Doburzynska & Graier, 1997; Graier et al., 1998), the relative cytosolic concentration of Ca2+ is expressed as the ratio of Fura-2 fluorescence that is due to excitation at 340 nm relative to that due to excitation at 380 nm (F340/F380). Activity of endothelial nitric oxide synthase was calculated by the L-NG-nitro-arginine-sensitive percentage of the conversion of L-[3H]-arginine to L-[3H]-citrulline. Unless otherwise stated, evaluation of the statistical significance between treatments was performed either by a two-tailed Student's t-test for unpaired observations or by One-way analysis of variance when more than two treatment groups were assessed. P<0.05 was considered to represent a statistically significant difference.
Drugs
Medium and supplements used for cell culture were purchased from Gibco BRL (Vienna, Austria), SR141716A (N-piperidino - 5 - (4-chlorophenyl) -1-(2,4 -dichlorophenyl)-4-methyl-3-pyrazole-carboxamide) was a generous gift from Dr Brelière and Sanofi-Recherche (Montpellier, France), anandamide (Biomol, Plymouth Meeting, PA, U.S.A.), histamine hydrochloride, phenylmethylsulphonyl fluoride (PMSF), pertussis toxin, thapsigargin (Sigma, Vienna, Austria), fura-2/AM (Molecular Probes, Leiden, Netherlands).
Results
In Ca2+-free physiological salt solution, anandamide (0.1–10 μM) evoked concentration-dependent mobilization of cytosolic Ca2+ (Figure 1A). Higher concentrations of anandamide were not tested due to solubility complications, hence, the potency of anandamide (in terms of half-maximally effective concentration) could not be determined. Under similar conditions, histamine elicited an initial short-lived peak of cytosolic Ca2+ increase, which was followed by a second slowly developing peak (Figure 1B). Introduction of CaCl2 2.5 mM into the extracellular milieu evoked an increase in the average cytosolic Ca2+ concentration both in the absence and in the presence of endothelial stimulants that is due to Ca2+ entry. The influx of Ca2+ that was obtained in the absence of stimulant was comparable to that obtained in the presence of anandamide (Figure 1B). Whereas, in the presence of histamine, Ca2+ entry was significantly enhanced (Figure 1B). In physiological salt solution already containing CaCl2 2.5 mM, anandamide induced an elevation of cytosolic Ca2+ that still developed slowly and was transient (Figure 1C). By comparison the Ca2+ signal elicited by 100 μM histamine comprised a sharp rise, followed by a decrease to a sustained plateau phase (Figure 1C).
Figure 1.
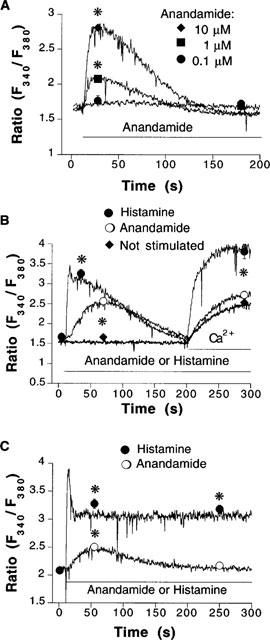
(A) Concentration-dependent mobilization of cytosolic Ca2+ induced by anandamide in Ca2+-free physiological salt solution. Representative recordings that were obtained in fura 2-loaded EA.hy926 endothelial cell suspensions challenged with 0.1 μM (circles, n=5), 1 μM (squares, n=6) and 10 μM (diamonds, n=7) anandamide, respectively. 0.1 μM anandamide did not produce a significant release of Ca2+ from intracellular stores. (B) Representative recordings from EA.hy926 endothelial cell suspensions that were either not stimulated (bottom trace, diamonds, n=10) or stimulated with 100 μM histamine (top trace, squares, n=5) or 5 μM anandamide (middle trace, circles, n=11) in nominally Ca2+-free physiological. In all panels, horizontal bars indicate application of stimulants or readmission of Ca2+. (C) Representative recordings obtained in cells that were incubated with physiological salt solution containing 2.5 mM Ca2+, followed by a stimulation with either 5 μM anandamide (open circles, n=4) or 100 μM histamine (closed circles, n=4). Symbols represent mean±s.e.mean of fluorescence ratio values obtained at indicated times. *P<0.05 between changes in cytosolic Ca2+ in stimulated versus non-stimulated cells. Error bars not visible when the size of the symbol exceeds the spread of s.e.mean.
As shown in Figure 2A, prior treatment of the cells with anandamide significantly attenuated the mobilization of Ca2+ from intracellular stores evoked by histamine. The stimulation of Ca2+ release from intracellular stores, without activation of capacitative Ca2+ entry is reminiscent of the effects of caffeine in endothelial cells (Graier et al., 1994a). Application of caffeine (10 mM) did not cause any significant increase of average cytosolic Ca2+ under the present experimental conditions (Figure 2B). However, the mobilization of cytosolic Ca2+ evoked by anandamide was significantly impaired after caffeine (Figure 2B).
Figure 2.
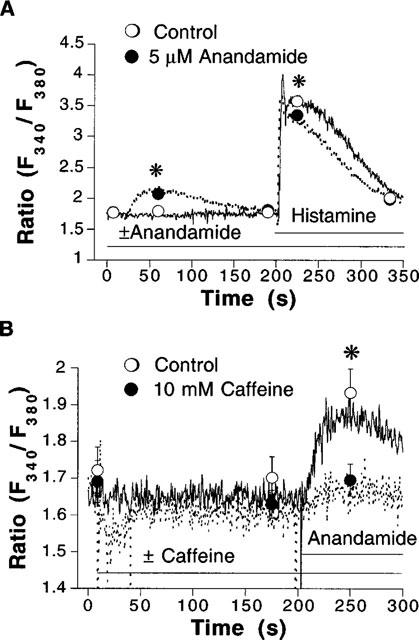
(A) Representative recordings from fura 2-loaded EA.hy926 endothelial cell suspensions that were stimulated with 100 μM histamine in the absence (continuous lines and open circles, n=4) and in the presence (dotted lines and closed circles, n=5) of 5 μM anandamide, applied as indicated by horizontal bars. Experiments were performed in Ca2+-free physiological salt solution. (B) Recordings obtained in cells that were stimulated with 5 μM anandamide in the absence (continuous lines and open circles, n=7) and in the presence (dotted lines and closed circles, n=7) of caffeine. Experiments were performed in Ca2+-free physiological salt solution. Symbols represent mean±s.e.mean of fluorescence ratio values obtained at indicated times. *P<0.05 between treatments. Error bars not visible when the size of the symbol exceeds the spread of s.e.mean.
The mobilization of Ca2+ contributes to the stimulation of endothelium-derived vasodilator mediators such as NO. Therefore, to assess this hypothesis the action of anandamide was determined on EA.hy926 cell NO synthase activity. Anandamide (5 μM) caused a 2 fold increase in L-[3H]-arginine conversion to L-[3H]-citrulline, relative to basal NO synthase activity (Figure 3). In parallel tests, 100 μM histamine provoked a 5 fold enhancement of NO synthase activity (Figure 3).
Figure 3.
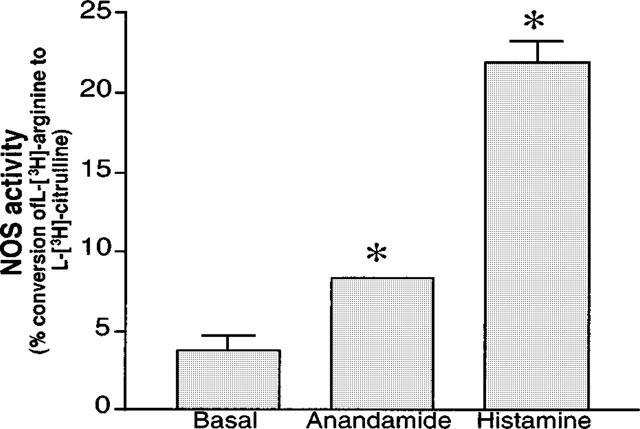
Endothelial NOS activity was determined in confluent EA.hy926 endothelial cells cultured in 6 well plastic dishes. The cells were challenged or not (basal) with 5 μM anandamide or 100 μM histamine, in the absence and in the presence of 300 μM L-NG-nitro-arginine, as detailed in methods. Activity of endothelial nitric oxide synthase was expressed as the mean±s.e.mean of the percentage of the L-NG-nitro-arginine-sensitive conversion of L-[3H-]-arginine to L-[3H]-citrulline. Error bar for anandamide was too small to be apparent. *P<0.05 between control and anandamide- or histamine-treated groups (n=6 each).
The mechanism of action of anandamide was further characterized by use of the CB receptor antagonist SR141716A. At 1 μM SR141716A had no significant effect on Ca2+ (data not shown, n=2). However, the mobilization of cytosolic Ca2+ evoked by anandamide was slightly, but significantly, reduced (Figure 4A). Increasing the concentration of SR141716A to 5 μM, further reduced the mobilization of Ca2+ by anandamide, and depressed the Ca2+ entry obtained after addition of 2.5 mM CaCl2 (Figure 4B). However, at this concentration, SR141716A also elicited the mobilization of Ca2+ from intracellular stores (0.56±0.02 Δratio units above basal Ca2+ levels, n=5). Five μM SR141716A did not significantly affect the Ca2+ influx obtained after addition of 2.5 mM CaCl2 (0.87±0.14 ratio units above basal Ca2+levels in the absence, n=10, as compared to 0.56±0.16, n=5 in the presence of SR141716A). At this concentration, SR141716A significantly depressed the mobilization of Ca2+ evoked by histamine from both intra- and extracellular pools (Figure 5A). While SR141716A had no effect on the depletion of Ca2+ from intracellular stores that was elicited by thapsigargin, it significantly diminished subsequent influx of Ca2+ obtained after addition of 2.5 mM CaCl2 (Figure 5B). Five μM SR141716A by itself also stimulated NO synthase activity, as determined using the L-[3H]-citrulline conversion assay (data not shown).
Figure 4.
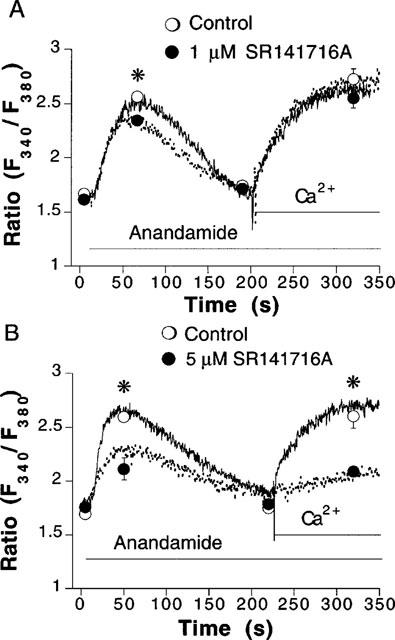
Inhibition by SR141716A of anandamide-induced mobilization of cytosolic Ca2+. Recordings from fura 2-loaded endothelial cell suspensions that were stimulated with anandamide in the absence (continuous lines and open circles) and in the presence (dotted lines and closed circles) of either 1 μM (A) or 5 μM SR141716A (B). The cells were first equilibrated for 5 min with or without SR141716A in Ca2+-free physiological salt solution. Circles represent mean±s.e.mean of fluorescence ratio values obtained at indicated times. Error bars not visible when the size of the symbol exceeds the span of the s.e.mean. *P<0.05 between control and SR141716A-treated groups (n=4–6).
Figure 5.
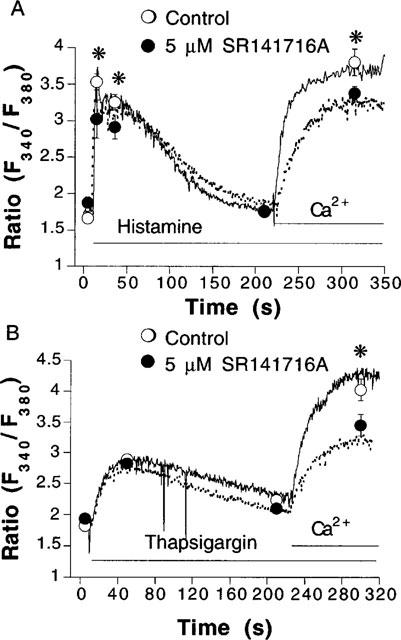
Inhibition by SR141716A of histamine- and thapsigargin-induced mobilization of cytosolic Ca2+. Recordings from fura 2-loaded endothelial cell suspensions that were stimulated with 100 μM histamine (A) or 2 μM thapsigargin (B) in the absence (continuous lines and open circles) and in the presence (dotted lines and closed circles) of 5 μM SR141716A. The cells were first equilibrated for 5 min with or without SR141716A in Ca2+-free physiological salt solution. Circles represent mean±s.e.mean of fluorescence ratio values obtained at indicated times. Error bars not visible when the size of the symbol exceeds the spread of s.e.mean. *P<0.05 between control and SR141716A-treated groups (n=5–6).
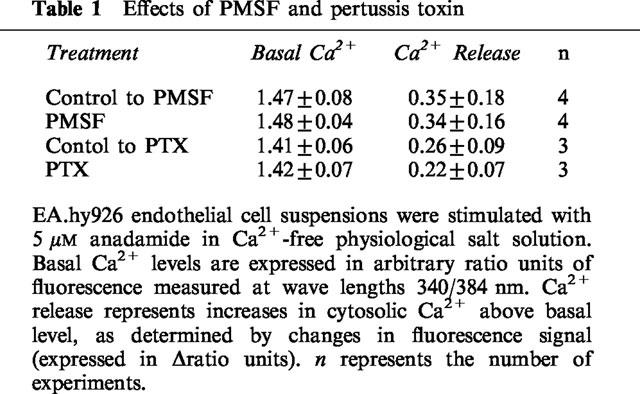
In addition to inhibition by CB1 receptor antagonist SR141716A, the actions of anandamide can be affected by amidohydrolase activity and by the uncoupling of the receptors to GTP binding proteins after treatment with pertussis toxin. In the presence of the amidohydrolase inhibitor PMSF (200 μM), the mobilization of cytosolic Ca2+ evoked by 5 μM anandamide was not affected (Table 1). Likewise incubation of the cells with pertussis toxin (400 ng ml−1 for 3 h) prior to testing, did not significantly change the Ca2+ signals induced by anandamide (Table 1). Neither PMSF nor pertussis toxin significantly modified spontaneous Ca2+ influx (data not shown).
Table 1.
Effects of PMSF and pertussis toxin
Discussion
The present study demonstrates that anandamide mobilizes cytosolic Ca2+ from intracellular stores in the human umbilical vein-derived endothelial cells EA.hy926. However, it does not apparently stimulate capacitative Ca2+ entry. The mobilization of cytosolic Ca2+ evoked by anandamide, which was caffeine-sensitive, somewhat reduced that elicited by histamine. This suggests that functional pools of Ca2+ recruited by both agonists may partially overlap. The putative CB1 receptor antagonist SR141716A inhibited this action. However, SR141716A rather behaved as agonist by stimulating the release of Ca2+ from intracellular stores by itself. The actions of anandamide were not affected by either the Gi/o protein uncoupler pertussis toxin or the amidohydrolase inhibitor PMSF.
Anandamide mobilized Ca2+ from intracellular stores, without stimulating capacitative Ca2+ entry. Synthetic cannabinoids mobilize Ca2+ from intracellular stores in human fibroblasts and rat mesenteric arteries without activation of Ca2+ influx (Felder et al., 1992; 1995; White & Hiley, 1998a). Whereas, in DDT1-MF-2 smooth muscle cells, cannabinoids stimulate capacitative Ca2+ entry (Filipeanu et al., 1997). The latter mechanism is responsible for the Ca2+ influx that is induced following the depletion of intracellular stores (for review see Adams et al., 1989; Graier et al., 1994b). It accounts for the sustained elevation of cytosolic Ca2+ evoked by agonists (such as histamine), or by inhibitors of endoplasmic reticulum Ca2+ pumps (e.g. thapsigargin), which deplete intracellular Ca2+ stores independently of receptor-dependent transduction mechanisms (Graier et al., 1995).
Anandamide slightly, but significantly reduced the mobilization of Ca2+ from intracellular stores evoked by histamine. Hence, the functional pools of Ca2+ used by anandamide and histamine overlap in part. Intracellular Ca2+ stores in endothelial cells can be functionally divided into inositol, 1,4,5-triphosphate (IP3) and caffeine-sensitive pools (Graier et al., 1994a; Sasajima et al., 1997). Caffeine blocked the anandamide-induced mobilization of Ca2+ from intracellular pools. Caffeine has been shown to deplete certain endothelial Ca2+ stores without accompanied elevation of cytosolic Ca2+ (Graier et al., 1994a). Thus, we suggest that the lack of anandamide to elevate endothelial Ca2+ in caffeine-treated cells is due to a ‘quiet' depletion of anadamide-sensitive Ca2+ stores by caffeine. In rat arterial smooth muscle, caffeine and anandamide also seem to neutralize each other (Zygmunt et al., 1997; White & Hiley, 1998a). Taken together with the observations presented herein, we conclude that caffeine and anandamide compete for the same functional Ca2+ pool in these vascular tissues.
The results obtained with the NO synthase assay confirm that anandamide may trigger the release of endothelium-derived vasodilator mediators, since it induces Ca2+ signalling. It elicited a 2 fold increase in NO synthase activity, while histamine induced a 5 fold stimulation. These results are commensurate with the ability of the compounds to mobilize Ca2+ in EA.hy926 endothelial cells. Deutsch et al. (1994) reported that anandamide stimulates the production of NO in perfused renal arteries in the nanomolar range, an effect abolished by selective concentrations of SR141716A. The differences in drug potency may be due to differences in the mechanism of action of anandamide which acts through CB1 receptors in the renal endothelial cells.
The putative CB receptor antagonist SR141716A evoked the release of Ca2+ from intracellular stores by itself. At 1 μM, which is a maximally effective concentration at blocking the actions of agonists at CB1 receptors (Felder & Glass, 1998; see e.g. Filipeanu et al., 1997). SR141716A marginally inhibited the mobilization of Ca2+ elicited by anandamide, without evoking any Ca2+ signal by itself. At a higher concentration, SR141716A impaired significantly the mobilization of Ca2+ from intracellular stored induced by anandamide and histamine, respectively. Even more intriguing, capacitative Ca2+ entry stimulated either by histamine or thapsigargin was impaired by 5 μM SR141716A. By contrast, spontaneous Ca2+ influx obtained in the absence of endothelial stimulants was not altered. These findings can not be interpreted on the assumption that SR141716A is acting as a neutral competitive antagonist at CB1 receptors.
Actually, SR141716A is an inverse agonist at both CB1 and CB2 receptors (Bouaboula et al., 1997; MacLennan et al., 1998). This implies that SR141716A induces a conformation of CB receptors that disrupts coupling to certain G proteins; however, it could eventually promote formation of complexes with others (for review on receptor promiscuity see Kenakin, 1996). Hence, the mobilization of Ca2+ by SR141716A could possibly reflect agonistic actions at CB-like receptors. Also, SR141716A was able to enhance NO synthase activity like anandamide. In CB receptor-transfected chinese hamster ovarian cells, submicromolar concentrations SR141716A impair cannabinoid-induced inhibition of the accumulation of cyclic AMP stimulated by forskolin (Felder et al., 1995). On the contrary, at micromolar concentrations, SR141716A enhances cyclic AMP elevation (Felder et al., 1995). In this model, CB1 receptors inhibit adenylate cyclase via pertussis toxin-sensitive G proteins, and stimulate the same enzyme through pertussis toxin-resistant G proteins (Felder & Glass, 1998. Accordingly, in the present study pertussis toxin did not influence the mobilization of Ca2+ induced by anandamide. Therefore, this finding is consistent with the interpretation that SR141716A preferentially disrupts coupling to pertussis toxin-sensitive transducer mechanisms, and may promote some alternative pathways.
In the context of agonistic properties of SR141716A, the depression of anandamide-induced mobilization of Ca2+ could result from the depletion by SR141716A of a common functional pool of Ca2+ prior to the addition of anandamide. An agonistic effect of SR141716A can also explain the non-specific actions on the capacitative Ca2+ entry. Pilot experiments confirm that anandamide, like SR141716A, attenuates Ca2+ entry evoked by histamine (unpublished observations). Indeed, the cytosolic fura-2 signal generated after induction of capacitative Ca2+ entry is due in part to the mobilization of Ca2+ through the Ca2+-induced Ca2+ release mechanism (Mozhayeva, 1996). Hence, depletion of the caffeine-sensitive stores would eliminate this component of the bulk cytosolic Ca2+ increase. Alternatively, these compounds may directly or indirectly have non-specific actions on Ca2+ channels that mediate the capacitative Ca2+ entry. Venance et al. (1997) have shown in rat astrocytes that (R)-methanandamide depletes intracellular stores and prevents the subsequent release of Ca2+ from intracellular stores that is evoked agonists.
SR141716A depressed Ca2+ entry in the presence of anandamide. This effect was unexpected since anandamide alone did not stimulate capacitative Ca2+ entry. These results can be explained by assuming anandamide facilitates Ca2+ extrusion or inhibits Ca2+ influx (e.g. Venance et al. (1997)). As an agonist, SR141716A would reinforce Ca2+ extrusion or inhibit Ca2+ influx. Also, the severe depletion of peripheral anandamide-sensitive stores may induce a steal effect. In such a case, the Ca2+ entering the bulk cytoplasm would be shunted in order to first refill the depleted anandamide-sensitive stores. Further investigation are warranted to test these possibilities.
Alternatively to the activation of CB-receptors, anandamide may be acting on some intracellular targets. Thus, the mobilization of Ca2+ in human fibroblasts (Felder et al., 1995), the impairment by anandamide of astrocyte junctional coupling (Venance et al., 1997) and the stimulation of mitogen-activated protein kinase in haematopoietic cells (Derocq et al., 1998) occur independently of CB1/2 receptors (for review see Hillard & Campbell, 1997). Anandamide may be converted to arachidonic acid, which would in turn be processed into cyclo-oxygenase, lipoxygenase or cytochrome P450 mono-oxygenase metabolites, as shown in bovine coronary endothelial cells (e.g. Pratt et al., 1998; see Felder & Glass, 1998 for review). However, the amidohydrolase inhibitor PMSF (Goparaju et al., 1998) had no effect suggesting that, unless some alternative pathway resistant to PMSF exists in EA.hy926 cells, anandamide is not acting as a precursor. By protecting anandamide from inactivation, PMSF potentiates activation of CB receptors by in numerous tissues. Since, this was not the case in the present study, it is possible that EA.hy926 cells lack amidohydrolase activity, as has been shown in other tissues (Felder et al., 1995).
The proposal that endogenous cannabinoids participate in endothelium-dependent vasodilatation is largely based on the use of SR141716A (see Randall & Kendall, 1998 for review). Inhibition by SR141716A of the mobilization of Ca2+ in endothelial cells may account in part for the impairment of endothelium-dependent relaxations (e.g. Randall et al., 1996; White & Hiley, 1997; Randall & Kendall, 1997). In addition, SR141716A may possess other non-specific effects. For example, it inhibits endothelium-independent relaxations evoked by the KATP channel activator levcromakalim (White & Hiley, 1998b). Taken together, the inverse agonism and its actions not related to CB receptors make SR141716A an inappropriate tool to assess the role of endogenous cannabinoids in the regulation of vascular tone.
At the present stage, it is not clear whether or not receptors are involved in the mobilization of cytosolic Ca2+ in endothelial cells, although the involvement of the CB1 receptor/pertussis toxin-sensitive G protein transduction pathway may be ruled out. It is possible that anandamide acts independently of a receptor to induce mobilization of Ca2+ from caffeine-sensitive pools. Nevertheless, the present study raises the intriguing possibility that anandamide may participate in the regulation of the release of Ca2+ from caffeine-sensitive pools in cells that synthesize the endogenous cannabinoid upon activation.
Acknowledgments
The authors are greatly indebted to Dr Edgell (University of North Carolina, Chapel Hill, NC, U.S.A.) for providing us with the human umbilical vein-derived endothelial cell line EA.hy926 and to Dr Breliere and Sanofi-Recherche (Montpellier, France) for the generous gift of SR141716A (N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazolecarboxamide). These studies were funded through Austrian Science Funds Lise Meitner-Visiting Scientist Fellowship No. M00442 (awarded to J.V.Mombouli) and research grants No P-12341-Med and SFB714, the Austrian Heart Foundation and the Franz Lanyar and Stiftung (in support to W.F.Graier).
References
- ADAMS D.J., BARAKEH J., LASKEY R., VAN BREEMEN C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- BOUABOULA M., PERRACHON S., MILLIGAN L., CANAT X., RINALDI-CARMONA M., PORTIER M., BARTH F., CALANDRA B., PECCEU F., LUPKER J., MAFFRAND J.P., LE-FUR G., CASELLAS P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- CHATAIGNEAU T., FELETOU M., THOLLON C., VILLENEUVE N., VILAINE J.P., DUHAULT J., VANHOUTTE P.M. Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br. J. Pharmacol. 1998;123:968–974. doi: 10.1038/sj.bjp.0701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEROCQ J.M., BOUABOULA M., MARCHAND J., RINALDI-CARMONA M., SEGUI M., CASELLAS P. The endogenous cannabinoid anandamide is a lipid messenger activating cell growth via a cannabinoid receptor-independent pathway in hematopoietic cell lines. FEBS Lett. 1998;425:419–425. doi: 10.1016/s0014-5793(98)00275-0. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY, SCHMID P.C., KREBSAQCH R.J., SCHMID H.H.O., DAS S.K., KEY S.K., ARREAZA G., THORUP C., STEFANO G., PIOMELLI D. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1994;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS E.F., MOORE S.F., WILLOUGHBY K.A. Anandamide and delta 9-THC dilation of cerebral arterioles is blocked by indomethacin. Am. J. Physiol. 1995;269:H1859–H1864. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- FELDER C.C., GLASS M. Cannabinoid receptors and their endogenous agonists. Ann. Rev., Pharmacol. Toxicol. 1988;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- FELDER C.C., VELUZ J.S., WILLIAMS H.L., BRILEY E.M., MATSUDA L.A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol. Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- FILIPEANU C.M., DE ZEEUW D., NELEMANS S.A. Δ9-Tetrahydrocannabinol activates [Ca2+]i increases partly sensitive to capacitative store refilling. Eur. J. Pharmacol. 1997;336:R1–R3. doi: 10.1016/s0014-2999(97)01254-5. [DOI] [PubMed] [Google Scholar]
- GOPARAJU S.K., UEDA N., YAMAGUCHI H., YAMAMOTO S. Anandamide amidohydrolase reacting with 2-arachidonylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- GRAIER W.F., PALTAUF-DOBURZYNSKA J., HILL B., FLEISCHHACKER E., HOEBEL B.G., KOSTNER G.M., STUREK S. Submaximal stimulation of procine endothelial cells causes focal Ca2+ elevation beneath the cell membrane. J. Physiol. Lond. 1998;506:109–125. doi: 10.1111/j.1469-7793.1998.109bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAIER W.F., SIMECEK S., BOWLES D.K., STUREK M. Heterogeneity of caffeine- and bradykinin-sensitive Ca2+ stores in vascular endothelial cells. Biochem. J. 1994a;300:637–641. doi: 10.1042/bj3000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAIER W.F., STUREK M., KUKOVETZ W.R. Ca2+ regulation and endothelial vascular function. Endothelium. 1994b;1:223–236. [Google Scholar]
- GRAIER W.F., SIMECEK S., KUKOVETZ W.R., KOSTNER G.M. High D-glucose-induced changes in endothelial Ca2+/EDRF signalling is due to generation of superoxide anions. Diabetes. 1996;45:1386–1395. doi: 10.2337/diab.45.10.1386. [DOI] [PubMed] [Google Scholar]
- GRAIER W.F., SIMECEK S., STUREK M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J. Physiol. 1995;482:259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIFFRIDA A., PIOMELLI D. Isotope dilution GC/MS determination of anandamide and other fatty acylethanolamides in rat blood plasma. FEBS Lett. 1998;422:373–376. doi: 10.1016/s0014-5793(98)00046-5. [DOI] [PubMed] [Google Scholar]
- HILLARD C.J., CAMPBELL W.B. Biochemistry and pharmacology of arachidonylethanolamide, a putative endogenous cannabinoid. J. Lipid Res. 1997;38:2383–2398. [PubMed] [Google Scholar]
- KENAKIN T. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol. Rev. 1996;48:413–463. [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Therap. 1997;281:1030–1037. [PubMed] [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., KWAN J., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;123:153P. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER B., JOHN M., BÖHME E. Partial purification and characterization of a Ca2+/calmodulin-dependent endothelium-derived relaxing factor-forming enzyme from porcine cerebellum. J. Cardiovasc. Pharmacol. 1991;17 Suppl 3:S46–S51. [Google Scholar]
- MORGAN K. Ca2+i versus [Ca2+]i. Biophys. J. 1993;65:561–562. doi: 10.1016/S0006-3495(93)81087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOZHAYEVA M.G. [Ca2+]i elevation evoked by Ca2+ readdition to the medium after agonist-induced Ca2+ release can involve both IP3-, and ryanodine-sensitive Ca2+ release. Pflügers Arch. 1996;433:180–187. doi: 10.1007/s004240050265. [DOI] [PubMed] [Google Scholar]
- PALTAUF-DOBURZYNSKA J., GRAIER W.F. Temperature dependence of agonist-stimulated Ca2+ signalling in cultured endothelial cells. Cell Calcium. 1997;21:43–51. doi: 10.1016/s0143-4160(97)90095-6. [DOI] [PubMed] [Google Scholar]
- PRATT P.F., HILLARD C.J., EDGEMONT W.S., CAMPBELL W.B. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am. J. Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. Eur. J. Pharmacol. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A. Endocannabinoids: a new class of vasoactive substances. Trends Pharmacol. Sci. 1998;19:55–58. doi: 10.1016/s0165-6147(97)01161-9. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., ALONSO R., SHIRE D., CONGY C., SOUBRIE P., BRELIKERE J.C., LE-FUR G. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- SASAJIMA H., WANG X., VAN BREEMEN C. Fractional Ca2+ release from the endoplasmic reticulum activates Ca2+ entry in freshly isolated rabbit aortic endothelial cells. Biochem. Biophys. Res. Commun. 1997;241:471–475. doi: 10.1006/bbrc.1997.7844. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KODAKA T., NAKANE S., KISHIMOTO S., KONDO S., WAKU K. Detection of an endogenous cannabimimetic molecule, 2-arachidonylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonylglycerol a possible vasomodulator. Biochem. Biophys. Res. Commun. 1998;243:838–843. doi: 10.1006/bbrc.1998.8187. [DOI] [PubMed] [Google Scholar]
- VENANCE L., PIOMELLI D., GLOWINSKI J., GIAUME C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- VENANCE L., SAGAN S., GIAUME C. (R)-methanandamide inhibits receptor-induced calcium responses by depleting internal calcium stores in cultured astrocytes. Pflügers Arch. 1997;434:147–149. doi: 10.1007/s004240050376. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., ELLIS E.A., RZIGALINSKI B.A., MARTIN B.R., KUNOS G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998a;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of the cannabinoid receptor antagonist, SR141716A, in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998b;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTATT, WALDECK K., EDWARDS G., KIRKUP A.J., WESTON A. Studies on the effects of anandamide in rat hepatic artery. Br. J. Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]


