Abstract
Our previous studies suggest that in addition to the cerebral dopaminergic systems the noradrenergic ones have a crucial role in the morphine-induced behavioural sensitization in mice. Therefore the effects of α2-adrenoceptor antagonist, idazoxan (1 and 3 mg kg−1, i.p.) on morphine-induced locomotor hyperactivity as well as on morphine-induced changes in cerebral noradrenaline (NA) and striatal dopamine (DA) metabolism were studied in mice withdrawn for 3 days from 5 day repeated morphine treatment. The concentrations of NA, free 3-methoxy-4-hydroxyphenylethylene glycol (MOPEG), DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 3-methoxytyramine (3-MT) were determined.
Acute morphine (10 mg kg−1, s.c.) increased locomotor activity in control and in morphine-withdrawn mice; idazoxan alone did not alter the activity. Idazoxan pretreatment did not alter the locomotor hyperactivity induced by acute morphine in control mice but potentiated it in morphine-withdrawn mice.
Acute morphine elevated MOPEG less but increased DOPAC and HVA more clearly in morphine-withdrawn mice than in controls, and decreased 3-MT only in controls. Idazoxan alone did not alter the NA or DA metabolite concentrations in control mice, but elevated MOPEG as well as DOPAC in morphine-withdrawn mice.
In control mice idazoxan enhanced acute morphine's elevating effect on MOPEG. In withdrawn mice idazoxan counteracted the tolerance so that acute morphine elevated MOPEG in these mice to about similar level as in controls.
Idazoxan pretreatment abolished the HVA increasing effect of acute morphine both in control and withdrawn mice. In control mice idazoxan enhanced morphine's elevating effect on DOPAC and abolished morphine's decreasing effect on 3-MT. Idazoxan did not alter morphine's effects on DOPAC or 3-MT concentrations in withdrawn mice.
Our results show that in morphine-withdrawn mice idazoxan pretreatment reveals the morphine-induced locomotor sensitization. This most probably occurs by overcoming the tolerance towards the acute morphine-induced increase of cerebral NA turnover and release. It is suggested that in mice the cerebral noradrenergic in addition to the dopaminergic systems are major determinants of the behavioural sensitization to morphine.
Keywords: Morphine challenge, morphine withdrawal, locomotor activity, striatal dopamine release, brain noradrenaline release, α2-adrenoceptors, behavioural sensitization
Introduction
The motor stimulation induced by acute morphine and other opioids in rats and mice increases progressively after repeated administration (Babbini & Davis, 1972; Ahtee, 1978; Havemann & Kuschinsky, 1982; Fernandes et al., 1977; Kuribara, 1995; Airio & Ahtee, 1997). In rats on withdrawal from opioids acute opioid challenge accelerates striatal and limbic dopamine (DA) turnover and release more than in naïve controls (Clouet & Ratner, 1970; Ahtee, 1973; Attila & Ahtee, 1983; 1984; Acquas & DiChiara, 1992; Ahtee et al., 1989; 1990). Similar sensitization to morphine-induced increase of striatal DA turnover and release during withdrawal has been found in mice (Ahtee et al., 1987; Airio et al., 1994; Airio & Ahtee, 1997).
In addition to the limbic and striatal DA, cerebral noradrenaline (NA) has been suggested to be involved in the morphine-induced changes in locomotor activity in mice (Estler, 1973; Kempf et al., 1976; Ayhan, 1978; Michaluk et al., 1991). Indeed, we recently found that when mice are tolerant to morphine's cerebral NA turnover and release increasing effect, they are not sensitized to morphine's locomotor stimulant effect, in spite of a clear sensitization in the morphine-induced increase of striatal DA turnover and release. The locomotor sensitization is seen first when the tolerance towards NA turnover and release increasing effect of morphine has disappeared, provided that morphine's increasing effect on DA turnover and release is sensitized (Airio & Ahtee, 1997). This temporal coupling of the effects of morphine thus suggests that in addition to the cerebral dopaminergic systems the noradrenergic ones have a crucial role in the morphine-induced behavioural sensitization in mice.
The activity of cerebral noradrenergic neurons is regulated by α2-adrenoceptors which are located presynaptically on noradrenergic nerve terminals and which modulate the release of NA via negative feedback mechanism (Starke et al., 1975; Cedarbaum & Aghajanian, 1977; Freedman & Aghajanian, 1984). α2-Adrenoceptor antagonist idazoxan increases NA release in cortex and hippocampus of rats; this effect is suggested to be mediated primarily by α2-adrenoceptors located on noradrenergic nerve terminals, rather than those located postsynaptically, somatodendritically or on the terminals of other neuronal inputs (Dennis et al., 1987; Thomas et al., 1994). Idazoxan also increases the concentration of NA metabolites in the mouse whole brain (Heal et al., 1989) and in various rat brain areas (Curet et al., 1987).
In the present experiments we studied if an increase of NA release induced by blocking presynaptic α2-adrenoceptors with idazoxan could restore the responsiveness of cerebral noradrenergic systems to a morphine challenge dose, and thereby reveal the morphine-induced locomotor hyperactivity during morphine withdrawal. Thus, we gave idazoxan pretreatment as well as an acute morphine challenge dose to mice withdrawn for 3 days. At this time point acute morphine increases the locomotor activity of the morphine-withdrawn mice to the same degree as in the controls but they still are tolerant to the acute morphine-induced increase of NA turnover and release. Furthermore, at this time our mice are already sensitized to the acute morphine-induced increase of DA turnover and release (Airio & Ahtee, 1997).
To estimate NA turnover and release we measured the concentrations of NA and free MOPEG, the main metabolite of NA and thus a functional index of cerebral NA turnover and release in mice (Sharman, 1969; Ceasar et al., 1974; Heal et al., 1989), in various brain areas. We also measured the striatal concentrations of DA and its metabolites 3-methoxytyramine (3-MT), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). 3-MT is generated extraneuronally by catechol-O-methyl transferase subsequent to DA release and is used as an index of DA release (Kehr, 1976; Wood, 1993). DOPAC is formed by monoamine oxidase mainly intraneuronally and is used as an index of intraneuronal DA synthesis and metabolism, and HVA is produced by the action of both of these enzymes, and is considered to indicate the sum of DA synthesis, metabolism and release (Westerink & Spaan, 1982a,1982b).
Methods
Animals
Male NMRI mice, weighing 21–32 g at the beginning of the experiments, were housed 6–10 to a cage at an ambient temperature of 21–23°C under 12 l : 12 d cycle (lights on at 0600 h). The mice had free access to fresh tap water and standard pellet food. They were weighed daily at 0800–0900 h. At the beginning of the repeated treatment the mean weight±s.e.mean of the control mice was 23.2±0.4 g, n=90, and that of the morphine treated mice 26.2±0.4 g, n=96.
Drugs and drug treatment
Morphine HCl (Ph. Eur., 2nd ed.) was dissolved in 0.9% NaCl (saline) and given subcutaneously (s.c.) in a volume of 0.01 ml g−1. Idazoxan HCl (RBI, Natick, MA, U.S.A.) was dissolved in saline and given intraperitoneally (i.p.) in a volume of 0.01 ml g−1. All doses refer to the free base. During the repeated treatment (Ahtee et al., 1987) the mice were given morphine three times daily, at 0800–0900, 1400–1500 and 2200–2300 h, for 5 days. The dose of morphine was increased from 100 mg kg−1×3 on day 1 to 150 mg kg−1×3 on day 2, and to 200 mg kg−1×3 on days 3–5. On day 5 the third daily morphine dose was omitted. This schedule of morphine administration induces weight loss on the first day of withdrawal (Airio & Ahtee 1997), indicating physical dependence (Stolerman et al., 1975). The control mice were given similar volumes of 0.9% NaCl solution s.c. After a withdrawal period of 3 days the acute treatments (saline, idazoxan, morphine) were given both in the behavioural and biochemical experiments between 1030 and 1330 h. In the biochemical experiments the mice were killed by decapitation 1 h after acute morphine administration, between 1200 and 1500 h. The dose of acute morphine was 10 mg kg−1. In our preliminary experiments using 0.3–10 mg kg−1 of idazoxan, doses smaller than 10 mg kg−1 did not significantly alter the acute morphine-induced locomotor hyperactivity in control mice. Thus, the idazoxan doses 1 and 3 mg kg−1 which do not stimulate locomotor activity in the control mice were used in the present experiments to determine the effects of idazoxan pretreatment in the morphine-withdrawn mice. Furthermore, based on these preliminary experiments studying the onset and duration of the effect of idazoxan on the locomotor activity, idazoxan was administered 30 min before acute morphine.
Dissection of the brain
After decapitation the brains were rapidly excised and dissected on an ice-cooled glass plate into four parts: (1) striatum, (2) hypothalamus, (3) lower brain stem and (4) area designated ‘rest of forebrain+midbrain', consisting mainly of cortical areas, hippocampus and thalamus, as described earlier (Attila et al., 1987). The brain parts were frozen on dry ice immediately after dissection, weighed and stored at −80°C, until the concentrations of NA, DA or their metabolites were estimated.
After decapitation, DA is released and metabolized rapidly. In rats, post-mortem 3-MT content is increased particularly quickly and therefore microwave irradiation is the preferred method of sacrifice (Westerink & Spaan, 1982a,1982b). However, in mouse striatum the steady state content of 3-MT is several times larger and the turnover as well as the post-mortem formation of 3-MT is clearly slower than in rats (Haikala, 1986; Wood et al., 1988). Thus, microwave irradiation is not necessary in studies of mouse striatal 3-MT content, provided that the dissection time is constant and sufficiently short. In the present experiments the striata were dissected and frozen within 90–120 s after decapitation when the post-mortem elevation of 3-MT concentration is about 20% (Haikala, 1986; Wood et al., 1988).
Biochemical experiments
After 3 days' withdrawal the mice were given an i.p. injection of idazoxan (1 or 3 mg kg−1) or saline. Thirty minutes later a challenge dose of morphine (10 mg kg−1) or saline was given s.c., the mice were killed 1 h later by decapitation, and the brains were dissected as described above. NA and its metabolite free MOPEG, and DA and its metabolites DOPAC, 3-MT and HVA were purified and isolated on Sephadex gel columns as described by Haikala (1987). Briefly, the proteins from the tissue samples were precipitated by 0.2 M perchloric acid. The samples were then purified and isolated using Sephadex G-10 gel chromatography. The biogenic amines and their metabolites were collected from the Sephadex G-10 eluates in three individual fractions containing (1) DA, 3-MT, NA and MOPEG; (2) DOPAC and HVA; and (3) 5-hydroxytryptamine. Fractions 1 and 2 of the Sephadex G-10 eluates were injected onto C18 reversed phase chromatographic columns for estimation of DA, NA and their metabolites. The detector used was the ESA Coulochem Model 5100, in some experiments the Model II detector was used.
Locomotor activity
After 3 days' withdrawal the mice were given an i.p. injection of idazoxan (1 or 3 mg kg−1) or saline. Thirty minutes later a challenge dose of morphine (10 mg kg−1) or saline was given s.c. and the mice were placed in groups of three mice (5–10 groups) in cages 18×33×15 cm. Groups of three mice, taken from the same home cage, were used in order not to affect the social behaviour of the mice. The interruptions of photocell beams (40 photocells per cage) were registered by a computerized counter. Locomotor activity was measured immediately after morphine or saline administration at 5 min intervals for 2 h between 1030 and 1530 h. Each mouse was used only once. The magnitude and duration of the exploration phase, when compared with the onset and magnitude of the effect of acute morphine, was found to be small in our previous experiments (Airio et al., 1995). Therefore, mice were not habituated before measurement.
Statistical analysis
A two tailed Student's t-test was used when comparing the means of body weight changes. In the locomotor activity experiments analysis of variance (ANOVA) with repeated measures was used both for the effects of acute and repeated treatments and for the overall effect; the effect of treatment as well as the overall effect were significant (P<0.001). In addition, two-way ANOVA was used to estimate the effects of acute (saline+saline, saline+morphine, idazoxan 1 mg kg−1+saline, idazoxan 1 mg kg−1+morphine, idazoxan 3 mg kg−1 +saline, idazoxan 3 mg kg−1+morphine) and repeated (saline, morphine) treatments (6×2 groups) and acute×repeated treatment interaction at each 15 min time point separately. When significant (P<0.05) main effects (acute or repeated treatment) were found comparisons of the group means at each time point were performed using Student-Newman-Keuls multiple range test. The two doses of idazoxan were compared together in the statistical analyses. In addition, to estimate the effects of acute and repeated saline and morphine treatment and their interaction only, two-way ANOVA was performed on 2×2 groups (acute saline, acute morphine, repeated saline, repeated morphine) at each 15 min time point. NA, DA and their metabolite data was analysed by two-way ANOVA to estimate the effects of acute and repeated treatments (6×2 groups) and acute×repeated treatment interaction as well as to estimate the effect of acute and repeated saline and morphine treatment and their interaction only (2×2 groups). When significant (P<0.05) main effects (acute or repeated treatment) were found comparisons of the group means were performed using Student's t-test with pooled variance. All ANOVAs were calculated using BMDP Statistical Software.
Results
Body weight during treatment and withdrawal
During the 5 day repeated treatment the control mice given three daily saline injections gained weight 3.6±0.2 g (mean±s.e.mean, n=90) whereas the mice given repeatedly morphine lost 2.8±0.2 g (n=94, P<0.001). During the following 3 day period when no injections were given the control mice continued to gain weight, gaining 1.4±0.2 g (n=90). However, during this period the morphine-withdrawn mice gained significantly more weight than the corresponding control mice (2.4±0.2 g, n=94, P<0.001), so that on the experimental day the weight of control mice was 28.2±0.3 g (n=90) and that of the morphine treated mice 26.1±0.3 g (n=94).
Locomotor activity
Withdrawal
At 3 days after withdrawal from the repeated morphine treatment the locomotor activity of mice was not altered (Figures 1 and 2, groups E vs A).
Figure 1.
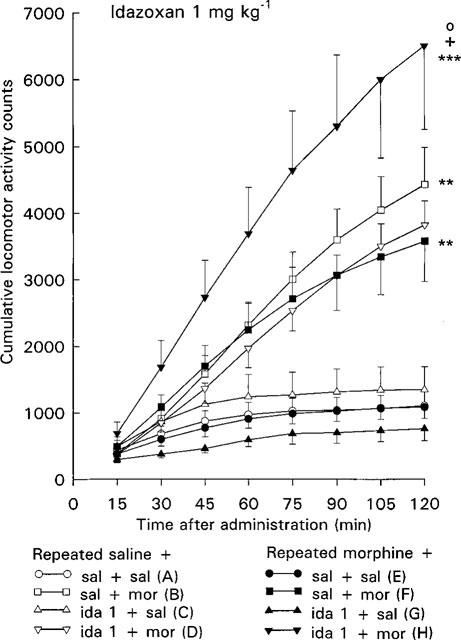
Effect of acute morphine challenge dose (10 mg kg−1 s.c.) on the locomotor activity of mice withdrawn for 3 days from 5 day repeated morphine treatment and pretreated with idazoxan (1 mg kg−1 i.p., 30 min before morphine challenge). Each point gives the mean±s.e.mean cumulative locomotor activity counts of groups of three mice and represent the following treatments: (A) repeated saline+acute saline+acute saline (n=10 groups of three mice); (B) repeated saline+acute saline+acute morphine (n=10); (C) repeated saline+acute idazoxan+acute saline (n=5); (D) repeated saline+acute idazoxan+acute morphine (n=5); (E) repeated morphine+acute saline+acute saline (n=10); (F) repeated morphine+ acute saline+acute morphine (n=10); (G) repeated morphine+acute idazoxan+acute saline (n=5); (H) repeated morphine+acute idazoxan+acute morphine (n=5). Statistical significances: effect of acute morphine treatment: **P<0.01, ***P<0.001 as compared with corresponding group given acute saline (B vs A, F vs E, H vs G); effect of idazoxan pretreatment: +P<0.05 as compared with corresponding group given acute saline (H vs F); effect of repeated morphine treatment: °P<0.05 as compared with corresponding repeated saline group (H vs D) (repeated measures ANOVA).
Figure 2.
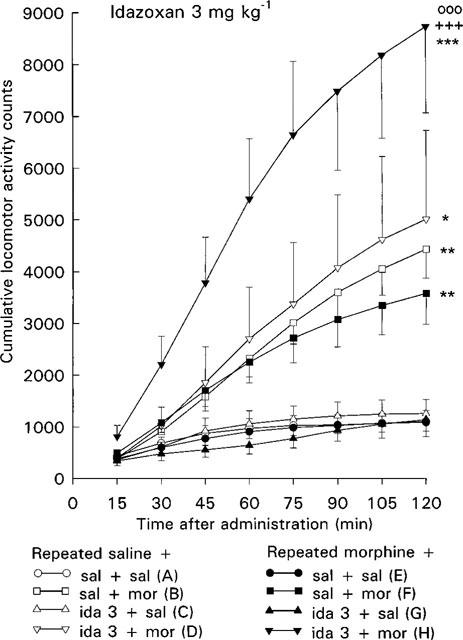
Effect of acute morphine challenge dose (10 mg kg−1 s.c.) on the locomotor activity of mice withdrawn for 3 days from 5 day repeated morphine treatment and pretreated with idazoxan (3 mg kg−1 i.p., 30 min before morphine challenge). Each point gives the mean±s.e.mean cumulative locomotor activity counts of groups of three mice and represent the following treatments: (A) repeated saline+acute saline+acute saline (n=10 groups of three mice); (B) repeated saline+acute saline+acute morphine (n=10); (C) repeated saline+acute idazoxan+acute saline (n=5); (D) repeated saline+acute idazoxan+acute morphine (n=5); (E) repeated morphine+acute saline+acute saline (n=10); (F) repeated morphine+ acute saline+acute morphine (n=10); (G) repeated morphine+acute idazoxan+acute saline (n=5); (H) repeated morphine+acute idazoxan+acute morphine (n=5). Statistical significances: effect of acute morphine treatment: *P<0.05, **P<0.01, ***P<0.001 as compared with corresponding group given acute saline (B vs A, D vs C, F vs E, H vs G); effect of idazoxan pretreatment: +++P<0.001 as compared with corresponding group given acute saline (H vs F); effect of repeated morphine treatment: °88P<0.001 as compared with corresponding repeated saline group (H vs D) (repeated measures ANOVA).
Morphine challenge
In the control mice the acute morphine challenge dose 10 mg kg−1 increased the locomotor activity (P<0.01, repeated measures ANOVA, Figures 1 and 2, groups B vs A). Two-way ANOVA also showed that the locomotor activity counts of control mice given acutely morphine were larger at each individual 75–120 min time point (P<0.05–P<0.01) than those of control mice given acutely saline.
In the morphine-withdrawn mice the morphine challenge dose increased the locomotor activity (P<0.01, repeated measures ANOVA, Figures 1 and 2, groups F vs E) to about similar degree as in corresponding control mice. Thus, the effect of acute morphine on mice treated repeatedly with morphine did not differ from that on corresponding control mice treated repeatedly with saline (Figures 1 and 2, groups F vs B). Furthermore, a similar response to morphine challenge in control and morphine-withdrawn mice was shown by two-way ANOVA for acute and repeated saline and morphine treatment, as no significant effect for repeated treatment or acute×repeated treatment interaction was found at any time point.
Idazoxan pretreatment
In control mice idazoxan alone (1 or 3 mg kg−1) did not alter locomotor activity (Figures 1 and 2, groups C vs A). Also pretreatment with 1 or 3 mg kg−1 of idazoxan was without effect on the morphine challenge-induced locomotor hyperactivity in control mice (Figures 1 and 2, groups D vs B).
In the morphine-withdrawn mice idazoxan alone did not alter the locomotor activity (Figures 1 and 2, groups G vs E). However, idazoxan (1 or 3 mg kg−1) pretreatment clearly enhanced the acute morphine-induced locomotor hyperactivity in the morphine-withdrawn mice. Thus, acute morphine increased the locomotor activity of morphine-withdrawn mice pretreated with idazoxan more than that of morphine-withdrawn mice pretreated with saline (P<0.05 and P<0.001, repeated measures ANOVA, Figures 1 and 2, groups H vs F). Two-way ANOVA also showed that the locomotor activity counts of morphine-withdrawn mice pretreated with idazoxan and given acutely morphine were larger than those of morphine-withdrawn mice pretreated with saline (P<0.01 and P<0.05 at individual 105 and 120 min time points after 1 mg kg−1 of idazoxan and P<0.05–P<0.001 at each individual 30–120 min time point after 3 mg kg−1 of idazoxan). Furthermore, acute morphine in combination with idazoxan increased the locomotor activity of morphine-withdrawn mice more than that of corresponding control mice treated repeatedly with saline (P<0.05 and P<0.001, repeated measures ANOVA, Figures 1 and 2, groups H vs D). Two-way ANOVA also showed that acute morphine increased the locomotor activity more in idazoxan-pretreated morphine-withdrawn mice than in idazoxan-pretreated control mice (P<0.05 at 120 min time point after 1 mg kg−1 of idazoxan and P<0.05–P<0.001 at each individual 30–120 min time point after 3 mg kg−1 of idazoxan). These findings indicate that unlike the controls, in the morphine-withdrawn mice idazoxan pretreatment potentiates the acute morphine-induced locomotor hyperactivity.
Cerebral NA
Withdrawal
At 3 days after withdrawal from the repeated morphine treatment NA and free MOPEG concentrations were not altered in any brain area studied (Figures 3 and 4, columns E vs A).
Figure 3.
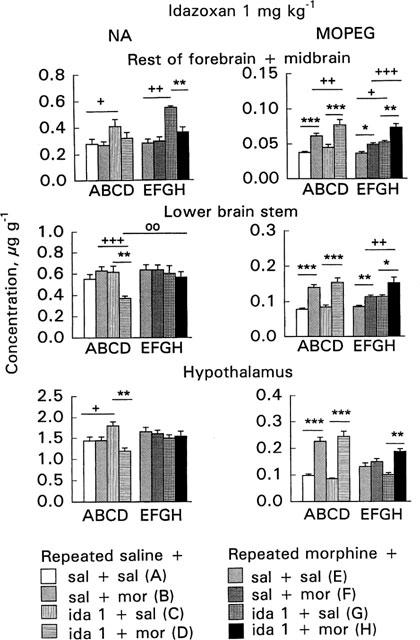
Effect of acute morphine challenge dose (10 mg kg−1 s.c., 1 h before decapitation) on noradrenaline (NA) and free 3-methoxy-4-hydroxyphenylethylene glycol (MOPEG) concentrations in the area ‘rest of forebrain+midbrain', lower brain stem and hypothalamus in mice withdrawn for 3 days from 5 day repeated morphine treatment and pretreated with idazoxan (1 mg kg−1 i.p., 30 min before morphine challenge). The columns give the mean±s.e.mean of NA and free MOPEG concentrations (μg g−1) and represent the following treatments: (A) repeated saline+acute saline+acute saline (n=13–18); (B) repeated saline+acute saline+acute morphine (n=15–20); (C) repeated saline+acute idazoxan+acute saline (n=6); (D) repeated saline+acute idazoxan+acute morphine (n=12); (E) repeated morphine+acute saline+acute saline (n=16–20); (F) repeated morphine+acute saline+acute morphine (n=16–20); (G) repeated morphine+acute idazoxan+acute saline (n=6); (H) repeated morphine+acute idazoxan+acute morphine (n=12). Statistical significances: effect of acute morphine treatment: *P<0.05, **P<0.01, ***P<0.001 as compared with corresponding group given acute saline (B vs A, D vs C, F vs E, H vs G); effect of idazoxan pretreatment: +P<0.05, ++P<0.01, +++P<0.001 as compared with corresponding group given acute saline (C vs A, D vs B, G vs E, H vs F) and effect of repeated morphine treatment: °8P<0.01 as compared with corresponding repeated saline group (H vs D) (two-way ANOVA followed by Student's t-test with pooled variance).
Figure 4.
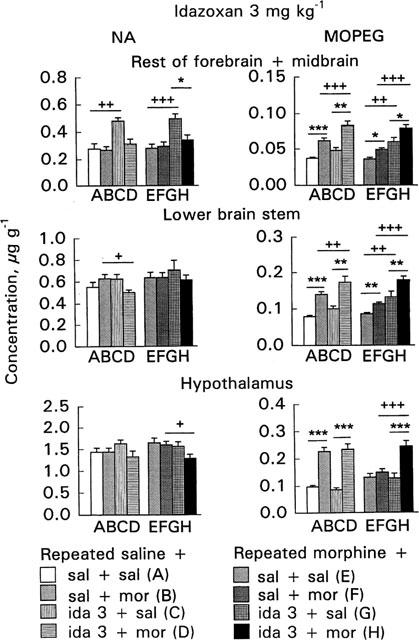
Effect of acute morphine challenge dose (10 mg kg−1 s.c., 1 h before decapitation) on noradrenaline (NA) and free 3-methoxy-4-hydroxyphenylethylene glycol (MOPEG) concentrations in the area ‘rest of forebrain+midbrain', lower brain stem and hypothalamus in mice withdrawn for 3 days from 5 day repeated morphine treatment and pretreated with idazoxan (3 mg kg−1 i.p., 30 min before morphine challenge). The columns give the mean±s.e.mean of NA and free MOPEG concentrations (μg g−1) and represent the following treatments: (A) repeated saline+acute saline+acute saline (n=13–18); (B) repeated saline+acute saline+acute morphine (n=15–20); (C) repeated saline+acute idazoxan+acute saline (n=6); (D) repeated saline+acute idazoxan+acute morphine (n=12); (E) repeated morphine+acute saline+acute saline (n=16–20); (F) repeated morphine+acute saline+acute morphine (n=16–20); (G) repeated morphine+acute idazoxan+acute saline (n=6); (H) repeated morphine+acute idazoxan+acute morphine (n=12). Statistical significances: effect of acute morphine treatment: *P<0.05, **P<0.01, ***P<0.001 as compared with corresponding group given acute saline (B vs A, D vs C, F vs E, H vs G); effect of idazoxan pretreatment: +P<0.05, ++P<0.01, +++P<0.001 as compared with corresponding group given acute saline (C vs A, D vs B, G vs E, H vs F) (two-way ANOVA followed by Student's t-test with pooled variance).
Cerebral NA, the area ‘rest of forebrain+midbrain'
Morphine challenge
The 10 mg kg−1 morphine challenge dose did not alter the NA concentrations in the area ‘rest of forebrain+midbrain' of either the control or morphine-withdrawn mice (Figures 3 and 4, columns B vs A or F vs E). However, morphine challenge increased the free MOPEG concentration in the control (P<0.001, Figures 3 and 4, columns B vs A) as well as in the morphine-withdrawn mice (P<0.05, Figures 3 and 4, columns F vs E). The increase was, however, smaller in the morphine-withdrawn mice. The reduced response in the morphine-withdrawn mice was shown by two-way ANOVA for the effects of acute and repeated saline and morphine treatment; the free MOPEG concentration in the morphine-withdrawn mice after challenge with morphine was smaller than in corresponding control mice (P<0.01) and furthermore, a significant acute×repeated treatment interaction (P<0.05) was found.
Idazoxan pretreatment
In the control mice idazoxan alone (1 or 3 mg kg−1) increased the NA concentration (P<0.05–P<0.01, Figures 3 and 4, columns C vs A). However, when idazoxan was administered before the morphine challenge dose the NA concentration was not increased (Figures 3 and 4, columns D vs B). Idazoxan alone did not alter the free MOPEG concentration in the control mice (Figures 3 and 4, columns C vs A). However, idazoxan pretreatment enhanced the morphine-induced increase of free MOPEG concentration in the control mice (P<0.01–P<0.001, Figures 3 and 4, columns D vs C) so that it was larger in control mice given acutely idazoxan and morphine than in the control mice given acutely saline and morphine (P<0.01–P<0.001, Figures 3 and 4, columns D vs B).
In the morphine-withdrawn mice idazoxan alone, as in controls, increased the NA concentration (P<0.01–P<0.001, Figures 3 and 4, columns G vs E). However, when idazoxan was administered before morphine challenge the NA concentration was not increased (Figures 3 and 4, columns H vs F). In the morphine-withdrawn mice idazoxan alone, in contrast to control mice, increased the free MOPEG concentration (P<0.05–P<0.01, Figures 3 and 4, columns G vs E); and in combination with morphine it further increased the free MOPEG concentration (P<0.05–P<0.01, Figures 3 and 4, columns H vs G). Thus, in the morphine-withdrawn mice pretreated with idazoxan the free MOPEG concentration was elevated highly significantly more than in withdrawn mice given acutely only morphine (P<0.001, Figures 3 and 4, columns H vs F).
Cerebral NA, lower brain stem
Morphine challenge
The morphine challenge dose did not alter the NA concentrations in the lower brain stem of either the control or morphine-withdrawn mice (Figures 3 and 4, columns B vs A or F vs E). However, morphine challenge increased the free MOPEG concentration in the control mice (P<0.001, Figures 3 and 4, columns B vs A), and also in the morphine-withdrawn mice (P<0.01, Figures 3 and 4, columns F vs E), but less than in the corresponding controls. The reduced response to morphine challenge in the morphine-withdrawn mice was shown by two-way ANOVA for the effects of acute and repeated saline and morphine treatment (P<0.01) and also by a significant acute×repeated treatment interaction (P<0.05).
Idazoxan pretreatment
In the control mice idazoxan (1 or 3 mg kg−1) did not alter the NA concentration (Figures 3 and 4, columns C vs A). However, the NA concentration was decreased in idazoxan-pretreated mice given acutely morphine (P<0.05–P<0.001, Figures 3 and 4, columns D vs B). Idazoxan (1 or 3 mg kg−1) alone did not alter the free MOPEG concentration in the control mice (Figures 3 and 4, columns C vs A). However, in the control mice pretreated with idazoxan 3 mg kg−1 morphine challenge elevated the free MOPEG concentration more than in corresponding mice given acutely only morphine (P<0.01, Figure 4, column D vs B).
In the morphine-withdrawn mice idazoxan, morphine challenge or both combined did not alter the NA concentrations (Figures 3 and 4). In the morphine-withdrawn mice idazoxan (3 mg kg−1) alone increased the free MOPEG concentration (P<0.01, Figure 4, column G vs E). In the idazoxan-pretreated mice morphine challenge further increased the free MOPEG concentration (P<0.05–P<0.01, Figures 3 and 4, columns H vs G). Thus, in the morphine-withdrawn mice pretreated with idazoxan the free MOPEG concentration was larger than in corresponding mice given acutely morphine (P<0.01–P<0.001, Figures 3 and 4, columns H vs F).
Cerebral NA, hypothalamus
Morphine challenge
The morphine challenge dose did not alter the hypothalamic NA concentrations either in the control or morphine-withdrawn mice (Figures 3 and 4, columns B vs A or F vs E). Morphine challenge increased the free MOPEG concentration in the control mice (P<0.001, Figures 3 and 4, columns B vs A) but did not alter it in the morphine-withdrawn mice (Figures 3 and 4, columns F vs E). This reduced response to morphine challenge was shown by a significant acute×repeated treatment interaction (P<0.001), found also in two-way ANOVA for the effects of acute and repeated saline and morphine treatment (P<0.001).
Idazoxan pretreatment
In the control mice idazoxan 1 mg kg−1 but not 3 mg kg−1 increased the NA concentration (P<0.05, Figures 3 and 4, columns C vs A). However, when idazoxan (1 mg kg−1) was given in combination with morphine the NA concentration was significantly decreased (P<0.01, Figure 3, column D vs C). Idazoxan alone did not alter the free MOPEG concentration or modify acute morphine's effect on that in the control mice (Figures 3 and 4, columns C vs A or D vs B).
In the morphine-withdrawn mice idazoxan alone did not alter the NA or free MOPEG concentrations (Figures 3 and 4, columns G vs E). The NA concentration tended to be smaller in morphine-challenged mice pretreated with 3 mg kg−1 of idazoxan as compared with mice given acutely only morphine (P<0.05, Figure 4, column H vs F). In the morphine-withdrawn mice pretreated with idazoxan, morphine challenge clearly increased the free MOPEG concentration so that it was larger than in corresponding mice given acutely idazoxan (P<0.01–P<0.001, Figures 3 and 4, columns H vs G). Furthermore, in combination with 3 mg kg−1 of idazoxan, morphine elevated the free MOPEG concentration significantly more than when given alone (P<0.001, Figure 4, column H vs F).
Striatal DA
Withdrawal
At 3 days after withdrawal from the repeated morphine treatment the striatal DA, DOPAC, HVA or 3-MT concentrations were not altered (Figures 5 and 6, columns E vs A).
Figure 5.
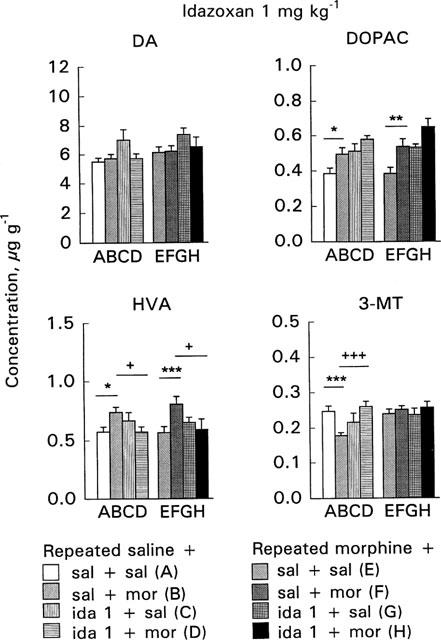
Effect of acute morphine challenge dose (10 mg kg−1 s.c., 1 h before decapitation) on striatal dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine (3-MT) and homovanillic acid (HVA) concentrations in mice withdrawn for 3 days from 5 day repeated morphine treatment and pretreated with idazoxan (1 mg kg−1 i.p., 30 min before morphine challenge). The columns give the mean±s.e.mean of DA, DOPAC, HVA and 3-MT concentrations (μg g−1) and represent the following treatments: (A) repeated saline+acute saline+acute saline (n=25–26); (B) repeated saline+acute saline+acute morphine (n=28–29); (C) repeated saline+acute idazoxan+acute saline (n=6); (D) repeated saline+ acute idazoxan+acute morphine (n=12); (E) repeated morphine+ acute saline+acute saline (n=25–27); (F) repeated morphine+acute saline+acute morphine (n=27–28); (G) repeated morphine+acute idazoxan+acute saline (n=6); (H) repeated morphine+acute idazoxan+acute morphine (n=12). Statistical significances: effect of acute morphine treatment: *P<0.05, **P<0.01, ***P<0.001 as compared with corresponding group given acute saline (B vs A, F vs E); effect of idazoxan pretreatment: +P<0.05, +++P<0.001 as compared with corresponding group given acute saline (C vs A, D vs B, G vs E, H vs F) (two-way ANOVA followed by Student's t-test with pooled variance).
Figure 6.
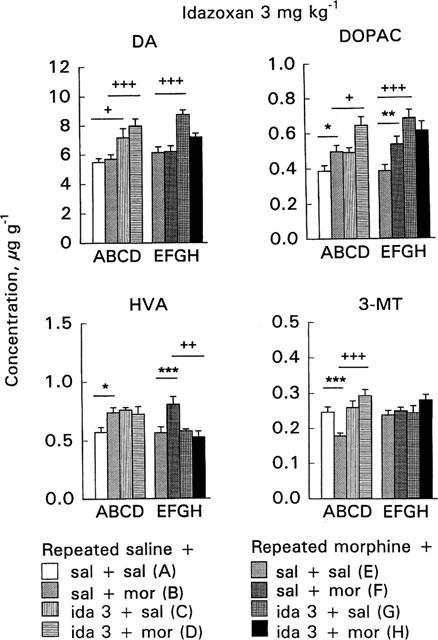
Effect of acute morphine challenge dose (10 mg kg−1 s.c., 1 h before decapitation) on striatal dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine (3-MT) and homovanillic acid (HVA) concentrations in mice withdrawn for 3 days from 5 day repeated morphine treatment and pretreated with idazoxan (3 mg kg−1 i.p., 30 min before morphine challenge). The columns give the mean±s.e.mean of DA, DOPAC, HVA and 3-MT concentrations (μg g−1) and represent the following treatments: (A) repeated saline+acute saline+acute saline (n=25–26); (B) repeated saline+acute saline+acute morphine (n=28–29); (C) repeated saline+acute idazoxan+acute saline (n=6); (D) repeated saline+ acute idazoxan+acute morphine (n=12); (E) repeated morphine+ acute saline+acute saline (n=25–27); (F) repeated morphine+acute saline+acute morphine (n=27–28); (G) repeated morphine+acute idazoxan+acute saline (n=6); (H) repeated morphine+acute idazoxan+acute morphine (n=12). Statistical significances: effect of acute morphine treatment: *P<0.05, **P<0.01, ***P<0.001 as compared with corresponding group given acute saline (B vs A, F vs E); effect of idazoxan pretreatment: +P<0.05, ++P<0.01, +++P<0.001 as compared with corresponding group given acute saline (C vs A, D vs B, G vs E, H vs F) (two-way ANOVA followed by Student's t-test with pooled variance).
Morphine challenge
The 10 mg kg−1 morphine challenge dose did not alter DA concentrations either in the control or morphine-withdrawn mice (Figures 5 and 6, columns B vs A or F vs E). In control mice morphine challenge increased DOPAC and HVA concentrations and decreased 3-MT concentration (P<0.05, P<0.05 and P<0.001, respectively, Figures 5 and 6, columns B vs A).
In the morphine-withdrawn mice morphine challenge clearly increased DOPAC and HVA concentrations (P<0.01 and P<0.001, respectively, Figures 5 and 6, columns F vs E). This effect tended to be larger than in controls; morphine challenge in the control mice elevated the DOPAC and HVA concentrations by 28.5 and 29%, respectively, the corresponding elevations in the morphine-withdrawn mice were 39.4 and 42.5%, respectively. Furthermore, in contrast to control mice morphine challenge did not alter 3-MT concentration in morphine-withdrawn mice. The different responses to morphine challenge in the control and morphine-withdrawn mice were shown by a significant acute×repeated treatment interaction on 3-MT concentration (P<0.05). Furthermore, in two-way ANOVA for the effects of acute and repeated saline and morphine treatment a significant acute×repeated treatment interaction (P<0.001) was found and 3-MT concentration was larger in morphine-withdrawn mice given acutely morphine than in corresponding control mice (P<0.001).
Idazoxan pretreatment
In control mice idazoxan 3 mg kg−1 alone and in combination with morphine increased DA concentration (P<0.05 and P<0.001, respectively, Figure 6, column C vs A and D vs B). Idazoxan (1 or 3 mg kg−1) alone did not alter DOPAC, HVA or 3-MT concentrations in control mice (Figures 5 and 6, columns C vs A). However, in combination with idazoxan 3 mg kg−1, the morphine challenge further increased DOPAC concentration so that it was larger than in mice given acutely only morphine (P<0.05, Figure 6, column D vs B). In control mice pretreatment with idazoxan 1 mg kg−1 abolished the morphine challenge-induced elevation of HVA concentration (P<0.05, Figure 5, column D vs B), but pretreatment with 3 mg kg−1 of idazoxan did not alter acute morphine's effect on HVA (Figure 6, column D vs B). Moreover, pretreatment with either dose of idazoxan abolished the morphine-induced decrease of 3-MT (P<0.001, Figures 5 and 6, columns D vs B).
In the morphine-withdrawn mice, idazoxan 3 mg kg−1 alone increased DA and DOPAC concentrations (P<0.001, Figure 6, columns G vs E). However, these increases were not apparent when idazoxan was given before morphine challenge (Figure 6, columns H vs F). Idazoxan (1 or 3 mg kg−1) alone did not alter HVA concentration in the withdrawn mice (Figures 5 and 6, columns G vs E) but idazoxan pretreatment abolished the morphine-induced increase of HVA (P<0.05–P<0.01, Figures 5 and 6, columns H vs F). Idazoxan (1 or 3 mg kg−1) did not alter 3-MT concentration in the withdrawn mice either when given alone or before morphine challenge (Figures 5 and 6, columns G vs E or H vs F).
Discussion
The present results emphasize the role of the cerebral noradrenergic transmission in the expression of locomotor sensitization to morphine in mice. In the mice withdrawn from morphine for 3 days, but not in the control mice, idazoxan pretreatment clearly potentiated the acute morphine-induced locomotor hyperactivity. In the withdrawn mice idazoxan counteracted the tolerance-induced reduction in the acute morphine's effect to increase cerebral NA metabolism and release, but idazoxan did not potentiate the enhanced striatal DA release induced by morphine challenge. In the control mice idazoxan somewhat increased the acute morphine-induced increase of cerebral NA release but did not enhance morphine's effects on DA release.
In mice 1 day withdrawal from repeated morphine treatment induces modest regional changes of the NA turnover in the forebrain area and the lower brain stem but not in the hypothalamus (Airio et al., 1995) while after longer withdrawal periods the α-methyl-p-tyrosine-induced depletion of cerebral NA or the cortical MOPEG concentration are not altered (Etemadzadeh, 1993; Funada et al., 1993; Airio & Ahtee, 1997). Furthermore, the striatal DA metabolism and turnover are weakened in mice after 1 day but no longer after 3 day withdrawal from repeated morphine treatment (Ahtee et al., 1987; Airio et al., 1994; Airio & Ahtee, 1997). In agreement with our earlier findings in the present experiments the cerebral NA or free MOPEG concentrations were not altered in any brain area of mice withdrawn for 3 days from the repeated morphine treatment. Neither were the concentrations of striatal DA or its metabolites altered.
Acute morphine elevated the free MOPEG concentration in all brain areas of the control mice but in the morphine-withdrawn mice there was clear tolerance towards this effect. This agrees with reports (Etemadzadeh, 1993; Airio et al., 1995; Airio & Ahtee, 1997) indicating that in mice withdrawn from repeated morphine treatment there is tolerance to the cerebral NA turnover and release increasing effect of acute morphine at 1 or 3 days after the withdrawal and suggests that although withdrawal from repeated morphine treatment may not affect the basal NA turnover and release in mice, it alters the response of the cerebral noradrenergic systems to a morphine challenge dose.
In the present experiments the acute 10 mg kg−1 morphine challenge dose increased the striatal HVA and DOPAC concentrations both in the control and morphine-withdrawn mice; the increases were larger in the withdrawn mice. Furthermore, acute morphine decreased the striatal 3-MT concentration in the control mice, but not in the morphine-withdrawn mice. Although the main effect of systemic morphine on cerebral DA in mice is to increase DA synthesis and turnover (Ahtee et al., 1987; Attila et al., 1987; Etemadzadeh, 1993; Airio et al., 1994; Funada et al., 1994), acute morphine administration has been reported to decrease the striatal 3-MT concentration in certain mouse strains including NMRI mice (Racagni et al., 1979; Airio and Ahtee, 1997). Further, using in vivo microdialysis it has been shown in rats that morphine when given locally inhibits the release of striatal DA (Rossetti et al., 1990; Piepponen et al., 1995). Thus it is likely that morphine has a dual effect on nigrostriatal DA release, a stimulatory effect on the somatodendritic region of dopaminergic neurons and an inhibitory effect on the terminal area of these neurons. Furthermore, tolerance develops more readily to the inhibitory effect of morphine which may contribute to the sensitization of DA release (Mikkola et al., 1995; Piepponen et al., 1995). All in all our results suggest that in our control mice acute morphine increases the functional utilization of DA; and the morphine-induced changes of HVA, DOPAC and 3-MT concentrations in the striata we found in morphine-withdrawn mice can be interpreted as an enhanced turnover and release of DA (see Introduction). Thus, as in our earlier studies (Airio et al., 1994; Airio & Ahtee, 1997), mice were sensitized to the acute morphine-induced increase of striatal DA turnover and release after 5 day repeated morphine treatment followed by 3 day withdrawal.
Idazoxan 1–20 mg kg−1 has been found to increase NA release in various rat brain areas (Scatton et al., 1983; Curet et al., 1987; Dennis et al., 1987; Thomas et al., 1994). Based on our preliminary experiments (see Methods), the doses of idazoxan (1 and 3 mg kg−1) used in the present study did not increase the locomotor activity of our NMRI mice. When given alone to control mice idazoxan evoked a dose-dependent increase in the NA concentration of the ‘rest of forebrain+ midbrain' only and did not significantly elevate free MOPEG in any brain area studied. However, in the C57/B1/6 mice idazoxan at the doses of 1 and 5 mg kg−1 elevated the whole brain MOPEG concentration (Heal et al., 1989) suggesting that the magnitude of response to idazoxan may somewhat vary in mice belonging to different strains. In combination with morphine idazoxan slightly enhanced the morphine-induced increase of free MOPEG in the lower brain stem and in the area ‘rest of forebrain+midbrain', but the NA concentrations in mice given both idazoxan and morphine did not differ from those of saline+morphine-treated controls. Thus, at the doses used the effects of idazoxan on the brain NA turnover in our control mice were minor.
In contrast to the control mice in the morphine-withdrawn mice idazoxan alone and in combination with morphine clearly enhanced the NA turnover. Thus, in the withdrawn mice idazoxan dose-dependently and significantly elevated the free MOPEG in the lower brain stem and in the area ‘rest of forebrain+midbrain'. In the forebrain area idazoxan alone elevated the NA concentration, too. However, in the hypothalamus of the morphine-withdrawn mice idazoxan alone did not alter the free MOPEG or NA. Thus, it seems that idazoxan alone does not affect the hypothalamic NA turnover in the morphine-withdrawn mice indicating that the NAergic systems in the hypothalamus during morphine-withdrawal function differently from those in other brain areas. Indeed, we showed previously that tolerance to the effects of acute morphine on NA metabolism persisted in the hypothalamus longer after withdrawal from repeated morphine administration than in the other two brain areas studied (Airio & Ahtee, 1997). Also, as discussed above, in contrast to other brain areas in the hypothalamus of mice withdrawn for 1 day from morphine the NA turnover was not enhanced (Airio et al., 1995). However, in combination with morphine idazoxan clearly enhanced the morphine-induced increase of the free MOPEG in all brain areas of the morphine-withdrawn mice elevating the MOPEG concentration to about same degree as in controls. Thus, idazoxan pretreatment counteracted the reduction induced by the tolerance towards the acute morphine-induced increase of free MOPEG concentration of morphine-withdrawn mice. As the effect of idazoxan on the cerebral NA release is suggested to be mediated primarily by α2-adrenoceptors located on noradrenergic nerve terminals (see Introduction), our results suggest that the reduced responsiveness of the cerebral noradrenergic systems to a morphine challenge brought about by tolerance can be overcome by blocking the presynaptic α2-adrenoceptors by idazoxan.
There are differences in the selectivity of available α2-adrenoceptor antagonists. RX821002 (2-methoxy-idazoxan), BRL 44408 and ARC 239 have affinity for non-adrenoceptor imidazoline and 5-hydroxytryptamine 5-HT1A-autoreceptors (Callado et al., 1996, Meana et al., 1996), and atipamezole can modulate the effects of striatal DA receptor activation in rats, increasing motor activity (MacDonald et al., 1996). Idazoxan binds to non-adrenoceptor imidazoline I2 binding sites as well (Hussain et al., 1993; Miralles et al., 1993). However, this may not contribute to the altered NA release since idazoxan labels imidazoline binding sites in the rat, guinea-pig and human but not in the mouse cerebral cortex membranes (Hussain et al., 1993). On the other hand, idazoxan is an agonist at cerebral 5-HT1A–autoreceptors and decreases cerebral 5-HT metabolism at doses higher than 5 mg kg−1 in rats (Llado et al., 1996). Thus, it cannot fully be excluded that receptors other than α2-adrenoceptors on noradrenergic nerve terminals may contribute to the alteration of NA metabolism by idazoxan and may thus modulate the behaviour of mice.
At the doses used in our study idazoxan's effects on striatal DA metabolism were only minor. At 3 mg kg−1 idazoxan elevated the striatal DA concentration both in control and morphine-withdrawn mice, elevated DOPAC in the morphine-withdrawn mice as well as enhanced the acute morphine-induced increase of DOPAC concentration in the control but not in the morphine-withdrawn mice. Pretreatment with idazoxan abolished the acute morphine-induced decrease of striatal 3-MT concentration in the control mice. These findings agree with the reports that in rats idazoxan when given alone stimulates cerebral DA metabolism as well as DA cell firing (Tian et al., 1991; Grenhoff & Svensson, 1993; Nisenbaum & Abercrombie, 1993; Paez & Leibowitz, 1993; Chen & Reith, 1995). In rats the alteration of adrenergic tone from the locus coeruleus may result in an alteration of the firing rate of mesolimbic and striatal DA neurons (Grenhoff & Svensson, 1993) or α2-adrenoceptors located on cerebral dopaminergic neurons may exert a direct influence on mesolimbic and nigrostriatal dopaminergic neurons (Biegon et al., 1992; Trendelenburg et al., 1994). Also in the mouse brain α2-adrenoceptors have been suggested to be involved in the regulation of dopaminergic neurons (Yavich et al., 1997; Sallinen et al., 1997). However, in our control mice 1 mg kg−1 but not 3 mg kg−1 of idazoxan and in the morphine-withdrawn mice both idazoxan doses prevented the acute morphine-induced increase of striatal HVA. Thus, it is apparent that at the doses used in our study idazoxan did not elevate the concentrations of HVA or 3-MT, the dopamine metabolites which are considered to indicate the release of DA. All in all, our biochemical findings indicate that at the doses used idazoxan did not potentiate the effects of acute morphine on cerebral DA release in the morphine-withdrawn mice, and thus its effects on DA clearly differ from its effects on cerebral NA, the morphine-induced release of which was clearly enhanced in the morphine-withdrawn mice as indicated by the elevated MOPEG concentration after combined administration of acute morphine and idazoxan.
In our control or morphine-withdrawn mice idazoxan alone did not alter the locomotor activity agreeing with studies in which equal doses of idazoxan were used (Chojnacka-Wojcik, 1992; Jackson et al., 1992). Neither did idazoxan pretreatment alter the acute morphine-induced locomotor hyperactivity in the control mice but clearly potentiated it in the morphine-withdrawn mice. In the present study as in our previous study (Airio & Ahtee, 1997) the increases of the locomotor activity induced by the morphine challenge dose were about similar in the mice withdrawn from morphine for 3 days and in the control mice. In our previous study we found locomotor sensitization at 5 day withdrawal, when mice no longer were tolerant to the acute morphine-induced increase of free MOPEG concentration (Airio & Ahtee, 1997). Further, we now report that in mice withdrawn from morphine for 3 days and pretreated with idazoxan the morphine challenge elevates the free MOPEG concentration to the same degree as in control mice thus counteracting the effect of tolerance on cerebral NA metabolism. These results suggest that the morphine-induced sensitization of locomotor activity cannot be expressed in mice until the responsiveness of the cerebral noradrenergic systems to morphine challenge is regained, either after longer withdrawal period or by modulating the function of the cerebral noradrenergic systems by idazoxan.
However, the cerebral dopaminergic systems are also important in the mediation of the morphine-induced locomotor hyperactivity in mice (Carroll & Sharp, 1972; Racagni et al., 1979; Longoni et al., 1987; Funada et al., 1994). Mice are similarly sensitized to the morphine challenge-induced increase of striatal DA turnover and release at 3 and 5 day withdrawal from 5 day repeated morphine treatment (Airio & Ahtee, 1997). In the present study idazoxan pretreatment had only minor effects on the morphine-induced increase of striatal DA metabolism but it revealed the locomotor sensitization already at 3 day withdrawal, when it counteracted the tolerance-induced changes in morphine's effects on cerebral NA. The coupling between morphine-induced changes in locomotor activity and in cerebral NA and DA metabolism suggests that the morphine-induced sensitization of locomotor activity is expressed in mice only when they are sensitized to the striatal DA turnover and release increasing effect of acute morphine and when morphine increases the NA turnover and release. As the morphine-induced changes in striatal DA metabolism are similar in mice and rats (Ahtee et al., 1987; Attila et al., 1987; Airio et al., 1994; Airio & Ahtee, 1997) it is suggested that, as in rats, the sensitization of morphine-induced locomotor hyperactivity in morphine-withdrawn mice is brought about by hyperactive dopaminergic systems. However, in the withdrawn mice this effect can be masked by tolerance of their cerebral noradrenergic systems. Only when the noradrenergic tone is restored morphine-withdrawn mice, like rats, react to morphine challenge with enhanced locomotor activity.
In conclusion, the current findings suggest that morphine-withdrawn mice are sensitized to acute morphine-induced locomotor hyperactivity only when they are sensitized to the striatal DA turnover and release increasing effect of acute morphine and when simultaneously morphine enhances the activity of the cerebral noradrenergic system to the same extent as it does in naïve mice. Thus, in mice the expression of the behavioural sensitization to morphine is determined by the extent of the morphine-induced enhancement of cerebral noradrenergic systems in addition to sensitization of their dopaminergic systems.
Finally, the morphine-induced behavioural sensitization has been implicated in its reinforcing effects (Shippenberg et al., 1996). Therefore, it cannot be excluded that the noradrenergic systems are of importance in the morphine-seeking behaviour. Furthermore, there is some evidence that in rats the activation of brain noradrenergic transmission is involved in the control of locomotor activity (Salmi et al., 1998). Thus, cerebral noradrenergic mechanisms might be involved in the sensitization of the locomotor response and related phenomena after repeated morphine administration not only in mice but in other species as well. There is plenty of clinical data showing the treatment of opioid withdrawal syndrome with α2- adrenoceptor agonists like clonidine (Cooper et al., 1996). Our findings suggest that the effects of drugs affecting the cerebral noradrenergic systems should also be investigated in the treatment of compulsive opioid abuse.
Acknowledgments
We wish to thank Ms Marjo Vaha for skilful assistance.
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- MOPEG
3-methoxy-4-hydroxyphenylethylene glycol
- 3-MT
3-methoxytyramine
- NA
noradrenaline
References
- ACQUAS E., DI CHIARA G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate withdrawal. J. Neurochem. 1992;58:1620–1625. doi: 10.1111/j.1471-4159.1992.tb10033.x. [DOI] [PubMed] [Google Scholar]
- AHTEE L. Catalepsy and stereotyped behaviour in rats treated chronically with methadone: relation to brain homovanillic acid content. J. Pharm. Pharmacol. 1973;25:649–651. doi: 10.1111/j.2042-7158.1973.tb10653.x. [DOI] [PubMed] [Google Scholar]
- AHTEE L.Effects of drugs affecting extrapyramidal motor functions on catalepsy and stereotypies induced by narcotic analgesics Factors affecting the action of narcotics 1978New York: Raven Press; 479–494.ed. Adler, M.L., Manara, L., Samanin, R. pp [Google Scholar]
- AHTEE L., ATTILA L.M.J., CARLSON K.R. Augmentation of morphine-induced changes in brain monoamine metabolism after chronic naltrexone treatment. J. Pharmacol. Exp. Ther. 1990;255:803–808. [PubMed] [Google Scholar]
- AHTEE L., ATTILA L.M.J., CARLSON K.R., HAIKALA H. Changes in brain monoamine metabolism during withdrawal from chronic oral self-administration of morphine and in response to a morphine challenge dose in the withdrawn state. J. Pharmacol. Exp. Ther. 1989;249:303–310. [PubMed] [Google Scholar]
- AHTEE L., ATTILA P., LAUHAKANGAS V., SOLKINEN A., SIPILÄ J. The fall of homovanillic acid and 5-hydroxyindoleacetic acid concentrations in brains of mice withdrawn from repeated morphine treatment and their restoration by acute morphine administration. J. Neural Transmission. 1987;68:63–78. doi: 10.1007/BF01244640. [DOI] [PubMed] [Google Scholar]
- AIRIO J., AHTEE L. Role of cerebral dopamine and noradrenaline in the morphine-induced locomotor sensitisation in mice. Pharmacol. Biochem. Behav. 1997;58:379–386. doi: 10.1016/s0091-3057(97)00252-9. [DOI] [PubMed] [Google Scholar]
- AIRIO J., ATTILA M., AHTEE L. Regional differences in cerebral noradrenaline turnover in mice withdrawn from repeated morphine treatment and tolerance to the effects of acute morphine. Pharmacol. Toxicol. 1995;77:196–203. doi: 10.1111/j.1600-0773.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- AIRIO J., ATTILA M., LEIKOLA-PELHO T., AHTEE L. Withdrawal from repeated morphine treatment sensitises mice to the striatal dopamine release enhancing effect of acute morphine. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:548–554. doi: 10.1007/BF00173025. [DOI] [PubMed] [Google Scholar]
- ATTILA L.M.J., AHTEE L. Cerebral dopamine and noradrenaline turnover and effects of morphine test dose in rats withdrawn from 20 days' morphine treatment. Med. Biol. 1983;61:249–257. [PubMed] [Google Scholar]
- ATTILA L.M.J., AHTEE L. Retardation of cerebral dopamine turnover after morphine withdrawal and its enhanced acceleration by acute morphine administration in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1984;327:201–207. doi: 10.1007/BF00502450. [DOI] [PubMed] [Google Scholar]
- ATTILA L.M.J., ETEMADZADEH E., AHTEE L. Differences in the effects of morphine on the α-methyl-p-tyrosine-induced depletion of dopamine and noradrenaline in various areas of the mouse brain. Pharmacol. Toxicol. 1987;61:26–32. doi: 10.1111/j.1600-0773.1987.tb01767.x. [DOI] [PubMed] [Google Scholar]
- AYHAN I.H.Morphine-induced behavior of mice and its relation to brain catecholamines Characteristics and function of opioids 1978Amsterdam: Elsevier/North-Holland Biomedical Press; 349–350.ed. Van Ree, J.M., Terenius, L. pp [Google Scholar]
- BABBINI M., DAVIS W.M. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br. J. Pharmacol. 1972;46:213–224. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIEGON A., MATHIS C.A., BUDINGER T.F. Quantitative in vitro and ex vivo autoradiography of the α2-adrenoceptor antagonist [3H]atipamezole. Eur. J. Pharmacol. 1992;224:27–38. doi: 10.1016/0014-2999(92)94814-c. [DOI] [PubMed] [Google Scholar]
- CALLADO L.F., GABILONDO A.M., MEANA J.J. [3H]RX821002 (2-methoxyidazoxan) binds to alpha 2-adrenoceptor subtypes and a non-adrenoceptor imidazoline binding site in rat kidney. Eur. J. Pharmacol. 1996;316:359–368. doi: 10.1016/s0014-2999(96)00692-9. [DOI] [PubMed] [Google Scholar]
- CARROLL B.J., SHARP P.T. Monoamine mediation of the morphine-induced activation in mice. Br. J. Pharmacol. 1972;46:124–139. doi: 10.1111/j.1476-5381.1972.tb06855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEASAR P.M., HAGUE P., SHARMAN D.F., WERDINIUS B. Studies on the metabolism of catecholamines in the central nervous system of the mouse. Br. J. Pharmacol. 1974;51:187–195. doi: 10.1111/j.1476-5381.1974.tb09646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEDARBAUM J.M., AGHAJANIAN G.K. Catecholamine receptors on locus coeruleus neurons: pharmacological characterization. Eur. J. Pharmacol. 1977;44:375–385. doi: 10.1016/0014-2999(77)90312-0. [DOI] [PubMed] [Google Scholar]
- CHEN N.-H., REITH M.E.A. Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (±)-8-hydroxy-2-(di-n-propylamino)tetralin. J. Neurochem. 1995;64:1585–1597. doi: 10.1046/j.1471-4159.1995.64041585.x. [DOI] [PubMed] [Google Scholar]
- CHOJNACKA-WOJCIK E. Involvement of dopamine autoreceptors in the hypoactivity induced by 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in mice. Pol. J. Pharmacol. Pharm. 1992;44:135–146. [PubMed] [Google Scholar]
- CLOUET D.H., RATNER M. Catecholamine biosynthesis in brains of rats treated with morphine. Science. 1970;168:854–855. doi: 10.1126/science.168.3933.854. [DOI] [PubMed] [Google Scholar]
- COOPER J.R., BLOOM F.E., ROTH R.H. The biochemical basis of neuropharmacology. New York/Oxford: Oxford University Press; 1996. pp. 226–292. [Google Scholar]
- CURET O., DENNIS T., SCATTON B. Evidence for the involvement of presynaptic alpha-2 adrenoceptors in the regulation of norepinephrine metabolism in the rat brain. J. Pharmacol. Exp. Ther. 1987;240:327–336. [PubMed] [Google Scholar]
- DENNIS T., L'HEREUX R., CARTER C., SCATTON B. Presynaptic alpha-2 adrenoceptors play a major role in the effects of idazoxan on cortical noradrenaline release (as measured by in vivo dialysis) in the rat. J. Pharmacol. Exp. Ther. 1987;241:642–649. [PubMed] [Google Scholar]
- ESTLER C.-J. Effect of α- and β-adrenergic blocking agents and para-chlorphenylalanine on morphine- and caffeine-stimulated locomotor activity in mice. Psychopharmacol. 1973;28:261–268. doi: 10.1007/BF00429306. [DOI] [PubMed] [Google Scholar]
- ETEMADZADEH E. Cerebral catecholamine depletion in mice withdrawn from repeated morphine treatment and development of tolerance to the enhancing effect of morphine on noradrenaline depletion. J. Pharmacol. Exp. Ther. 1993;266:749–755. [PubMed] [Google Scholar]
- FERNANDES M., KLUVE S., COPER H. Quantitative assessment of tolerance to and dependence on morphine in mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 1977;297:53–60. doi: 10.1007/BF00508810. [DOI] [PubMed] [Google Scholar]
- FREEDMAN J.E., AGHAJANIAN G.K. Idazoxan (RX 781094) selectively antagonizes α2-adrenoceptors on rat central neurons. Eur. J. Pharmacol. 1984;105:265–272. doi: 10.1016/0014-2999(84)90618-6. [DOI] [PubMed] [Google Scholar]
- FUNADA M., NARITA M., SUZUKI T., MIASAWA M. Effect of pretreatment with pertussistoxin on the development of physical dependence on morphine. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:88–95. doi: 10.1007/BF00168542. [DOI] [PubMed] [Google Scholar]
- FUNADA M., SUZUKI T., MIASAWA M. The role of dopamine D1-receptors in morphine-induced hyperlocomotion in mice. Neurosci. Lett. 1994;169:1–4. doi: 10.1016/0304-3940(94)90342-5. [DOI] [PubMed] [Google Scholar]
- GRENHOFF J., SVENSSON T.H. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur. J. Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- HAIKALA H. Different changes in striatal dopamine metabolism induced by nicotine in mice kept at different ambient temperatures. Evidence for partly separate metabolic routes of dopamine derived from separate compartmentations. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;334:3873–3876. doi: 10.1007/BF00569372. [DOI] [PubMed] [Google Scholar]
- HAIKALA H. Use of a novel type of rotating disc electrode and a flow cell with laminar flow pattern for the electrochemical detection of biogenic monoamines and their metabolites after Sephadex gel chromatographic purification and high-performance liquid chromatographic isolation from rat brain. J. Neurochem. 1987;49:1033–1041. doi: 10.1111/j.1471-4159.1987.tb09991.x. [DOI] [PubMed] [Google Scholar]
- HAVEMANN U., KUSCHINSKY K. Neurochemical aspects of the opioid-induced ‘catatonia'. Neurochem. Int. 1982;4:199–215. doi: 10.1016/0197-0186(82)90055-9. [DOI] [PubMed] [Google Scholar]
- HEAL D.J., PROW M.R., BUCKETT W.R. Measurement of 3-methoxy-4-hydroxyphenylglycol (MHPG) in mouse brain by h.p.l.c. with electrochemical detection, as an index of noradrenaline utilisation and presynaptic α2-adrenoceptor function. Br. J. Pharmacol. 1989;96:547–556. doi: 10.1111/j.1476-5381.1989.tb11852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSSAIN J.F., KENDALL D.A., WILSON V.G. Species-selective binding of [3H]-idazoxan to α2-adrenoceptors and non-adrenoceptor, imidazoline binding sites in the central nervous system. Br. J. Pharmacol. 1993;109:831–837. doi: 10.1111/j.1476-5381.1993.tb13650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON H.C., GRIFFIN I.J., NUTT D.J. α2-adrenoceptor antagonists block the stimulant effects of cocaine in mice. Life Sci. 1992;50:PL155–PL159. doi: 10.1016/0024-3205(92)90267-s. [DOI] [PubMed] [Google Scholar]
- KEHR W. 3-methoxytyramine as an indicator of impulse-induced dopamine release in rat brain in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 1976;293:209–215. doi: 10.1007/BF00507343. [DOI] [PubMed] [Google Scholar]
- KEMPF E., GILL M., MACK G., MANDEL P. Effects of acute morphine administration on the catecholamine metabolism of three strains of mice. Psychopharmacology Communications. 1976;2:241–250. [PubMed] [Google Scholar]
- KURIBARA H. Modification of morphine sensitization by opioid and dopamine receptor antagonists; evaluation by studying ambulation in mice. Eur. J. Pharmacol. 1995;275:251–258. doi: 10.1016/0014-2999(94)00787-8. [DOI] [PubMed] [Google Scholar]
- LLADO J., ESTEBAN S., GARCIA-SEVILLA J.A. The α2-adrenoceptor antagonist idazoxan is an agonist at 5-HT1A autoreceptors modulating serotonin synthesis in the rat brain in vivo. Neurosci. Lett. 1996;218:111–114. doi: 10.1016/s0304-3940(96)13132-3. [DOI] [PubMed] [Google Scholar]
- LONGONI R., SPINA L., DI CHIARA G. Dopaminergic D-1 receptors: essential role in morphine-induced hypermotility. Psychopharmacol. 1987;93:401–402. doi: 10.1007/BF00187265. [DOI] [PubMed] [Google Scholar]
- MACDONALD E., VIRTANEN S., SIRVIÖ J., HAAPALINNA A.Potentiation by the α2-adrenoceptor antagonist, atipamezole, of apomorphine-induced circling in rats with combined unilateral substantia nigra and DSP-4 lesions Brit. J. Pharmacol. 1996119344PProceedings Supplement [Google Scholar]
- MEANA J.J., CALLADO L.F., PAZOS A., GRIJALBA B., GARCIA-SEVILLA J.A. The subtype-selective alpha 2-adrenoceptor antagonists BRL 44408 and ARC 239 also recognize 5-HT1A receptors in the rat brain. Eur. J. Pharmacol. 1996;312:385–388. doi: 10.1016/0014-2999(96)00598-5. [DOI] [PubMed] [Google Scholar]
- MICHALUK J., ANTKIEWICZ-MICHALUK L., ROKOSZ-PELC A., VETULANI J. Opiate and α1 adrenergic receptors in mice responding to morphine with sedation or with running fit. Pol. J. Pharmacol. Pharm. 1991;43:115–119. [PubMed] [Google Scholar]
- MIKKOLA J., PIEPPONEN T.P., AHTEE L. Repeated morphine treatment induces tolerance to the inhibitory effect of morphine on striatal dopamine release. Analgesia. 1995;1:582–585. [Google Scholar]
- MIRALLES A., OLMOS G., SASTRE M., BARTUREN F., MARTIN I., GARCIA-SEVILLA J.A. Discrimination and pharmacological characterization of I2-imidazoline sites with [3H]idazoxan and alpha-2 adrenoceptors with [3H]RX821002 (2-methoxy idazoxan) in the human and rat brains. J. Pharmacol. Exp. Ther. 1993;264:1187–1197. [PubMed] [Google Scholar]
- NISENBAUM L.K., ABERCROMBIE E.D. Presynaptic alterations associated with enhancement of evoked release and synthesis of norepinephrine in hippocampus of chronically cold-stressed rats. Brain Res. 1993;608:280–287. doi: 10.1016/0006-8993(93)91469-9. [DOI] [PubMed] [Google Scholar]
- PAEZ X., LEIBOWITZ S.F. Changes in extracellular PVN monoamines and macronutrient intake after idazoxan or fluoxetine injection. Pharmacol. Biocem. Behav. 1993;46:933–941. doi: 10.1016/0091-3057(93)90225-i. [DOI] [PubMed] [Google Scholar]
- PIEPPONEN T.P., MIKKOLA J., RUOTSALAINEN M., AHTEE L. Dose-dependent decrease of dopamine release in the striatum by intrastriatal morphine. Analgesia. 1995;1:643–646. [Google Scholar]
- RACAGNI G., BRUNO F., IULIANI E., PAOLETTI R. Differential sensitivity to morphine-induced analgesia and motor activity in two inbred strains of mice: Behavioral and biochemical correlations. J. Pharmacol. Exp. Ther. 1979;209:111–116. [PubMed] [Google Scholar]
- ROSSETTI Z.L., CARBONI S., MELIS F., NEFF N.H., GESSA G.L.Locally perfused morphne inhibits striatal DA release: reversal by systemic morphine Soc. Neurosci. Abstr. 1990161047part 2 [Google Scholar]
- SALLINEN J., LINK R.E., HAAPALINNA A., VIITAMAA T., KULATUNGA M., SJÖHOLM B., MACDONALD E., PELTO-HUIKKO M., LEINO T., BARSCH G.S., KOBILKA B.K., SCHEININ M. Genetic alteration of α2C-adrenoceptor expression in mice: influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a subtype-nonselective α2-adrenoceptor agonist. Mol. Pharmacol. 1997;51:36–46. doi: 10.1124/mol.51.1.36. [DOI] [PubMed] [Google Scholar]
- SALMI P., MALMGREN K., SVENSSON T.H., AHLENIUS S. Stimulation of forward locomotion by SCH-23390 and raclopride in d-amphetamine-treated rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:593–599. doi: 10.1007/pl00005213. [DOI] [PubMed] [Google Scholar]
- SCATTON B., DEDEK J., ZIVKOVIC B. Lack of involvement of α2-adrenoceptors in the regulation of striatal dopaminergic transmission. Eur. J. Pharmacol. 1983;86:427–433. doi: 10.1016/0014-2999(83)90192-9. [DOI] [PubMed] [Google Scholar]
- SHARMAN D.F. Glycol metabolites of noradrenaline in brain tissue. Br. J. Pharmacol. 1969;36:523–534. doi: 10.1111/j.1476-5381.1969.tb08008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIPPENBERG T.S., HEIDBREDER C.H., LEFEVOUR A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur. J. Pharmacol. 1996;299:33–39. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- STARKE K., BOROWSKI E., ENDO T. Preferential blockade of presynaptic α-adrenoceptors by yohimbine. Eur. J. Pharmacol. 1975;34:385–388. doi: 10.1016/0014-2999(75)90268-x. [DOI] [PubMed] [Google Scholar]
- STOLERMAN I.P., JOHNSON C.A., BUNKER P., JARVIK M.E. Weight loss and shock-elicited aggression as indices of morphine abstinence in rats. Psychopharmacol. 1975;45:157–161. doi: 10.1007/BF00429054. [DOI] [PubMed] [Google Scholar]
- THOMAS D.N., NUTT D., HOLMAN R.B. Regionally specific changes in extracellular noradrenaline following chronic idazoxan as revealed by in vivo microdialysis. Eur. J. Pharmacol. 1994;261:53–57. doi: 10.1016/0014-2999(94)90299-2. [DOI] [PubMed] [Google Scholar]
- TIAN Y., LOOKINGLAND K.J., MOORE K.E. Contribution of noradrenergic neurons to 3,4-dihydroxyphenylacetic acid concentrations in the regions of the rat brain containing incertohypothalamic dopaminergic neurons. Brain Res. 1991;555:135–140. doi: 10.1016/0006-8993(91)90869-w. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG A.-E., STARKE K., LIMBERGER N. Presynaptic α2A-adrenoceptors inhibit the release of endogenous dopamine in rabbit caudate nucleus slices. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:473–481. doi: 10.1007/BF00173016. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H.C., SPAAN S.J. Estimation of the turnover of 3-methoxytyramine in the rat striatum by HPLC with electrochemical detection: Implications for the sequence in the cerebral metabolism of dopamine. J. Neurochem. 1982a;38:342–347. doi: 10.1111/j.1471-4159.1982.tb08634.x. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H.C., SPAAN S.J. On the significance of endogenous 3-methoxytyramine for the effects of centrally acting drugs on dopamine release in the rat brain. J. Neurochem. 1982b;38:680–686. doi: 10.1111/j.1471-4159.1982.tb08685.x. [DOI] [PubMed] [Google Scholar]
- WOOD P.L.Interrelationships of opioid, dopaminergic, cholinergic, and GABAergic pathways in the central nervous system Handbook of experimental pharmacology, vol 104/I; Opioids I 1993Berlin/Heidelberg: Springer; 624–643.ed. Herz, A. pp [Google Scholar]
- WOOD P.L., KIM H.S., STOCKLIN K., RAO T.S. Dynamics of the striatal 3-MT pool in rat and mouse: species differences as assessed by steady-state measurements and intracerebral dialysis. Life Sci. 1988;42:2275–2282. doi: 10.1016/0024-3205(88)90380-3. [DOI] [PubMed] [Google Scholar]
- YAVICH L., LAPPALAINEN R., SIRVIÖ J., HAAPALINNA A., MACDONALD E. α2-adrenergic control of dopamine overflow and metabolism in mouse striatum. Eur. J. Pharmacol. 1997;339:113–119. doi: 10.1016/s0014-2999(97)01375-7. [DOI] [PubMed] [Google Scholar]


