Abstract
Pilocarpine, a muscarinic acetylcholine receptor (mAChR) agonist, is widely used for treatment of xerostomia and glaucoma. It can also cause many other cellular responses by activating different subtypes of mAChRs in different tissues. However, the potential role of pilocarpine in modulating cardiac function remained unstudied.
We found that pilocarpine produced concentration-dependent (0.1–10 μM) decrease in sinus rhythm and action potential duration, and hyperpolarization of membrane potential in guinea-pig hearts. The effects were nearly completely reversed by 1 μM atropine or 2 nM 4DAMP methiodide (an M3-selective antagonist).
Patch-clamp recordings in dispersed myocytes from guinea-pig and canine atria revealed that pilocarpine induces a novel K+ current with delayed rectifying properties. The current was suppressed by low concentrations of M3-selective antagonists 4DAMP methiodide (2–10 nM), 4DAMP mustard (4–20 nM, an ackylating agent) and p-F-HHSiD (20–200 nM). Antagonists towards other subtypes (M1, M2 or M4) all failed to alter the current.
The affinity of pilocarpine (KD) at mAChRs derived from displacement binding of [3H]-NMS in the homogenates from dog atria was 2.2 μM (65% of the total binding) and that of 4DAMP methiodide was 2.8 nM (70% of total binding), consistent with the concentration of pilocarpine needed for the current induction and for the modulation of the cardiac electrical activity and the concentration of 4DAMP to block pilocarpine effects.
Our data indicate, for the first time, that pilocarpine modulates the cellular electrical properties of the hearts, likely by activating a K+ current mediated by M3 receptors.
Keywords: Muscarinic acetylcholine receptor, M3 receptor subtype, pilocarpine, heart rate, action potential duration, resting membrane potential, K+ current, receptor binding assay
Introduction
Pilocarpine is a commonly used agent for the treatment of glaucoma and detached retina, as well as for the care of xerostomia (dry mouth) because it stimulates salivary secretion. The cellular mechanism for pilocarpine's efficacy is attributed to its ability to activate muscarinic acetylcholine receptors. Pilocarpine has also been indicated in a variety of other functions such as induction of purposeless chewing behaviour, seizures and contraction of trachea, etc. (Gabelt & Kaufman, 1992; Maslanski et al., 1994). Interestingly, although mAChRs play an important role in regulating heart functions such as heart rate, contractile force, excitation conduction, membrane repolarization and so forth, the potential effects of pilocarpine in cardiac tissues remained unstudied.
It is known that mAChRs consist of four functionally defined subtypes, designated M1, M2, M3 and M4, corresponding to the molecularly identified four isoforms: m1, m2, m3 and m4 (Hulme et al., 1990; Goyal, 1989; van Zwieten & Doods, 1995; Eglen & Whiting, 1990; Mutschler et al., 1995). While it has long been believed that the M2 receptor is the only functional subtype of mAChRs in cardiac tissues, this concept has been challenged by growing body of evidence suggesting the presence of other subtypes as well. Recently, the existence of M3 receptors in canine atrial myocytes has been convincingly demonstrated with both functional and molecular evidence in our laboratory. We found that the cardiac M3 receptors are functionally coupled to a novel type of delayed rectifier K+ channels (Shi et al., 1998a,1998b) and we named this K+ current IKM3 (M3 receptor-mediated K+ current). We have confirmed that the cardiac M3 receptors and IKM3 can be activated by tetramethylammonium (Shi et al., 1998b) and choline (Shi et al., 1998c). Preliminary data have been obtained in our laboratory indicating that pilocarpine can also activate a K+ current with properties almost identical to TMA- or choline-induced currents in dog atrial cells and the stimulation of mAChRs, likely the M3 receptor subtype, is required for the current activation (Wang et al., 1998).
It is known that the cardiac membrane repolarization is controlled by the delicate balance between the inward and the outward currents, particularly the phase 2 plateau repolarization. K+ currents are crucial in determining the rate of membrane repolarization thereby the action potential duration (APD). Activation of an outward K+ current would expect to accelerate repolarization (or shorten APD), to hyperpolarize membrane potential, and to weaken contraction by indirectly decreasing Ca2+ entry into the cells. It is also known that mAChRs play an important role in regulating the heart functions such as the heart rate, the membrane potential and repolarization, and the cardiac contraction. In the light of the ability of pilocarpine to activate mAChRs and to induce a K+ current, it is quite conceivable that pilocarpine could affect the cardiac electrical activity.
Based on these rationales, we designed experiments to answer the following questions: (1) whether pilocarpine can modulate the heart rate, membrane potentials and repolarization of cardiac myocytes and (2) what are the potential ionic and receptor mechanisms underlying pilocarpine actions in cardiac myocytes. Standard microelectrode techniques were applied to isolated guinea-pig heart preparations and whole-cell patch clamp techniques to dispersed myocytes from guinea-pig and dog atria. Preliminary results have been presented in an abstract form in Life Science.
Methods
Guinea-pig atrial preparations
Guinea-pig atrial preparation procedures have been described in detail elsewhere (Wang et al., 1990; 1991). Briefly, adult guinea-pigs of either sex weighing about 300 g were killed by a blow on the head. Their hearts were quickly removed, washed in cool, oxygenated Tyrode solution, then cannulated to a Langendorff perfusion device via aortic artery. The hearts were placed horizontally onto a Sylgard-covered bottom of a 20 ml Lucite chamber and perfused with Tyrode solution at 5 ml min−1. The superfusion solution contained (mM): NaCl 120, KCl 4, MgCl2 1.2, KH2PO4 1.2, NaHCO3 25, glucose 10 and CaCl2 2; pH was adjusted to 7.4 with NaOH. The superfusate was aerated with 95% O2 5% CO2, and the bath temperature was maintained at 36°C by a heating element and proportional power supply (Hanna Instruments, Philadelphia, PA, U.S.A.). One hour was allowed for heart equilibration before experiments were begun.
Standard microelectrode techniques
The microelectrode techniques used have been described in detail previously (Wang et al., 1990; 1991; 1993a). Glass microelectrodes filled with 3 M KCl and with tip resistances of 8–20 MΩ were coupled by a headstage to an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA, U.S.A.). Command pulses were generated with Axoptape in Tape software (Axon Instruments, Foster City, CA, U.S.A.) running on an IBM AT type computer interfaced with a D/A converter. The Bandwidth for recordings was set to 10 KHz.
Action potential characteristics, including resting potential, action potential duration to 50 and 90% repolarization (APD50 and APD90, respectively), were determined. To monitor the heart rate, action potentials were recorded at sinus rhythm. After base-line measurements, pilocarpine was added to the superfusate, and action potential characteristics were monitored over time. Different concentrations of pilocarpine (0.1, 1 and 10 μM) were given sequentially. The measurements made under control conditions were repeated after 20 min of drug perfusion at each concentration and after 30 min of washout. In experiments aimed at exploring the role of M3 subtype of mAChRs, atropine (1 μM) or 4DAMP (2 nM, and M3-selective antagonist) was co-applied with pilocarpine. Recordings were obtained from at least ten cells under each different situation (with or without drugs and washout) for comparison.
Isolation of atrial myocytes
Single canine atrial myocytes were isolated with techniques as previously described (Yue et al., 1997; Shi et al., 1998a). Briefly, the right atrium from adult mongrel dogs (18–23 kg) of either sex was quickly dissected and mounted via the right coronary artery to a Langendorff perfusion system. The preparation was perfused with Ca2+-containing Tyrode solution (composition same as the solution described below for whole-cell patch-clamp recording) at 37°C until the effluent was clear of blood, and then switched to Ca2+-free Tyrode solution for 20 min at a constant rate of 12 ml min−1, followed by perfusion with the same solution containing collagenase (110 U ml−1 CLS II collagenase; worthington Biochemical, Freehold, NJ, U.S.A.) and 0.1% bovine serum albumin (Sigma Chemicals, St. Louis, MO, U.S.A.). The dispersed cells were stored in KB medium at 4°C for later electrophysiological experiments. The KB medium for cell storage contained (mM): KCl 20, KH2PO4 10, glucose 25, potassium glutamate 70, β-hydroxybutyric acid 10, taurine 20, EGTA 10, 0.1% albumin and mannitol 40; pH was adjusted to 7.4 with KOH.
For isolation of guinea-pig atrial myocytes, hearts were quickly removed from adult guinea-pig of about 300 g and cannulated to a Langendorff perfusion system via an aortic artery. Cell isolation procedures were same as described above for canine atrial myocytes.
Whole-cell patch-clamp techniques
Patch-clamp recording techniques used have been described in detail elsewhere (Wang et al., 1993b; 1994; 1995; Shi et al., 1998a). Ionic currents were recorded with the whole-cell voltage-clamp methods, using an Axopatch-200B amplifier (Axon Instruments, Foster City, CA, U.S.A.). Borosilicate glass electrodes (1 mm O.D.) had tip resistances of 1–3 MΩ when filled with pipette solution of following composition (mM): GTP 0.1, potassium aspartate 110, KCl 20, MgCl2 1, Mg-ATP 5, HEPES 10, EGTA 10, phosphocreatine 5; pH adjusted to 7.3 with KOH. Junction potentials were zeroed before formation of the membrane-pipette seal in Tyrode solution containing (mM): NaCl 136, KCl 5.4, MgCl2 1, NaH2PO4 0.33, HEPES 5, glucose 10 and CaCl2 1; pH adjusted to 7.4 with NaOH. Mean seal resistance averaged 11±1 GΩ. Five minutes after seal formation, the membrane was ruptured by gentle suction to establish the whole-cell configuration.
Command pulses were generated with pCLAMP6 software running on an IBM AT type computer interfaced with a D/A converter. Recordings were low-pass filtered at 1 KHz. The capacitance and series resistance (Rs) were electrically compensated to minimize the duration of the capacitive surge on the current recording and the voltage drop across the clamped cell membrane. Rs along the clamp circuit was estimated by dividing the time constant obtained by fitting the decay of the capacitive transient by the calculated membrane capacitance (the time integral of the capacitive response to a 5 mV hyperpolarizing step from a holding potential of −60 mV divided by the voltage drop). Before Rs compensation, the decay of the capacitive surge was a single exponential function of time with a time constant of 465±29 μs (cell capacitance, 80±6 pF, n=37 cells). Precompensation Rs values averaged 4.9±0.5 MΩ. After compensation, the time constant was reduced to 104±5 μs (cell capacitance, 70±5 pF), and Rs was reduced to 1.3±0.1 MΩ. Currents recorded during the present study rarely exceeded 2.0 nA. The mean maximum voltage drop across the Rs was thus in the range of 3 mV. Cells with changing leak current (indicated by >10 pA changes in holding current at −50 mV) were rejected. Experiments were conducted at 36±1°C.
Contamination by sodium current was prevented by holding the cells at −50 mV. Potential contamination by other currents was minimized by including the following compounds in the bath solution: dofetilide (1 μM, to inhibit IKr), 293B (20 μM, to block IKs), glyburide (10 μM, to prevent ATP-sensitive K+ current), 4-aminopyridine (4AP, 200 μM to block transient outward K+ current and the ultra-rapid delayed rectifier K+ current), and Cd2+ (200 μM, to suppress calcium current). Chemicals used for microelectrode and patch-clamp recordings were purchased from SIGMA Chemical (St. Louis, MO, U.S.A.), except for 293B which was a kind gift from Hoechst Pharmaceuticals.
Membrane receptor binding assay
Methods for receptor binding used in this study were same as described in details elsewhere (Shi et al., 1998a). Fresh atrial tissues dissected from canine heart were minced and washed with ice-cold PBS buffer. The tissues were then homogenized with a polytron in 15 ml of ice-cold lysis buffer containing Tris-HCl (5 mM), EDTA (2 mM) (pH 7.4), plus a protease inhibitor cocktail consisting (in μg ml−1): phenylmethylsuphony fluoride 5, benzamidine 10, and soybean trypsin inhibitor 5. The homogenate was centrifuged at 500×g for 15 min at 4°C. The pellets were then homogenized as before, spun again and the supernatants pooled. The supernatants were centrifuged at 45,000×g for 15 min and the pellets washed twice in the same buffer. The membrane fractions were resuspended in a buffer containing (in mM): Tris-HCl 75 (pH 7.4), MgCl2 12.5, and EDTA 5. The protein content was determined with Bio-Rad Protein Assay Kit (Bio-Rad, Mississauga, ON, Canada) using bovine serum albumin as the standard.
Saturation binding assays were performed using eight concentrations of [3H]-NMS ([N-methyl-3H]-scopolamine methylchloride) ranging from 2–2500 pM. Nonspecific binding was measured in the presence of 1 μM atropine. Experiments were carried out in triplicate for each experiment with total of five individual preparations. Incubations (90 min at room temperature) were terminated by rapid filtration using GF/B filters (Xymotech, Montreal, PQ), and radioactivity was counted with an LS6500 Scintillation Counter (Beckman, Fullerton, CA, U.S.A.). with average efficiency of 58%.
Competition binding assays were carried out as follows: Homogenates were incubated with 400 pM of [3H]-NMS with pilocarpine (1 nM–1 mM) or 4DAMP methiodide (0.1 nM–30 μM), respectively. Fixed amounts of membrane protein (100 μg) were used for each sample in the binding study. Seven individual experiments were performed with each determination performed in duplicate for each compound. Chemicals and reagents for the binding study were purchased from Research Biochemicals International (Natick, MA, U.S.A.).
Data analysis
Group data are expressed as mean±s.e.mean. Statistical comparisons were performed on raw data with Student's t-test, with a two-tailed P<0.05 taken to indicate a statistically significant difference. Comparisons for multiple groups were performed with analysis of variance (ANOVA) with Scheffé's contrasts. Binding data were analysed using curve-fitting functions of the GraphPad Prism software (GraphPad Software, CA, U.S.A.). To assure validity and accuracy of displacement binding studies, linear regression was performed on the per cent bound versus the ratio of bound over free ligand and only data with a regression coefficient of ⩾0.9 was accepted for analyses. The F test was used to compare fits for the competition binding data, and the best fit (one-site binding versus two-site binding) was determined by the probability value for the F test and by the charge in the residual sum of squares for the two different fits. One- and two-site models were tested, and the model yielding the least residual sum of squares was taken to describe the data.
Results
Pilocarpine modulation of heart rate, APD and RMP in guinea-pig atrial preparations
Figure 1 illustrates concentration-dependent effects of pilocarpine on the heart rate (the rate of sinus rhythm) in a representative guinea-pig atrial preparation. The slowing of the HR was reversible and exchange of perfusate back to control solution without pilocarpine completely restored the HR to the pre-drug level. Pilocarpine slowing of the HR was nearly abolished by 1 μM atropine (data not shown), a non-selective mAChR antagonist. Since we had previously obtained data suggesting that pilocarpine can induce a K+ current via stimulating M3 receptors in canine hearts (Wang et al., 1998), we evaluated the effects of 4DAMP (an M3-selective antagonist, Barlow & Shepherd, 1986; Michel et al., 1989; Araujo et al., 1991; van Zwieten & Doods, 1995) on pilocarpine-induced changes of HR. To prevent potential contribution of M2 receptors, 100 nM methoctramine (Michel & Whiting 1988; van Zwieten & Doods, 1995) was present in the bathing solution throughout the experiments to inhibit the M2 receptors. 4DAMP (2 nM) considerably reversed pilocarpine slowing of HR, indicating a role of M3 receptors in mediating pilocarpine's action (Figure 1A). Mean data of per cent slowing of the HR are presented in Figure 1B. Appreciable effects were seen with 0.1 μM pilocarpine and statistically significant slowing was achieved at higher concentrations: 19% by 1 μM (P<0.05) and 30% by 10 μM (P<0.01).
Figure 1.
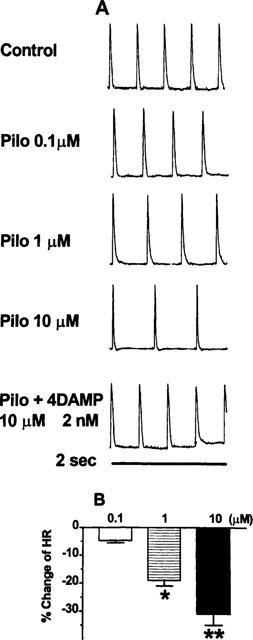
Pilocarpine modulation of sinus rate via stimulation of mAChRs in guinea-pig atria. Sinus rate was determined as the firing frequency of action potentials (AP) recorded in atrial preparations with intact sinus nodes. (A) Concentration-dependent slowing of sinus rate by pilocarpine. Shown are trains of action potentials recorded within 2 s time frame under control conditions, in the presence of varying concentrations of pilocarpine and following application of 4DAMP (2 nM, an M3-selective antagonist) to pilocarpine (10 μM)-containing solution. (B) Per cent changes of sinus rate from baseline recording, in the presence of pilocarpine, and after co-application of pilocarpine (10 μM) and 4DAMP (2 nM). *P<0.05 and **P<0.01 Student t-test, drug versus control values (n=5 hearts).
The effects of pilocarpine on APD were also evaluated and the results are illustrated in Figure 2. Remarkable changes in AP, particular the morphology and APD were consistently observed upon exposure of cells to pilocarpine. APD was strikingly shortened relative to baseline recordings. Again, the effects were clearly dependent on the concentrations of pilocarpine applied and the changes were more pronounced in APD50 relative to those in APD90, suggesting that pilocarpine affected the plateau phase more than it did the final phase of repolarization. But the difference was reduced with higher concentrations of pilocarpine. For example, 0.1 μM pilocarpine produced approximately 24 and 6% shortening of APD50 and APD90, respectively, and 10 μM pilocarpine briefed APD50 by 69% and APD90 by 60%. The effects of pilocarpine were readily restored by inclusion of 4DAMP (2 nM) in the perfusion solution (see Figure 2B and Figure 3).
Figure 2.
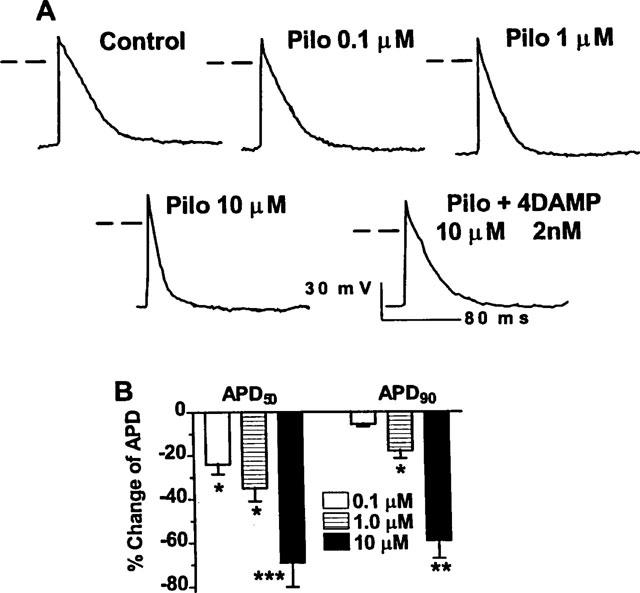
Pilocarpine modulation of action potential duration (APD) by activation of mAChRs in guinea-pig atria. (A) Concentration-dependent APD shortening by pilocarpine. Shown are typical examples of action potentials recorded from a same preparation before and after drugs. Dash lines indicate zero potential levels. (B) Averaged data on pilocarpine shortening of APD, expressed as per cent changes over control values (n=4 hearts). *P<0.05, **P<0.01 and ***P<0.001 Scheffe's contrast after F-test.
Figure 3.
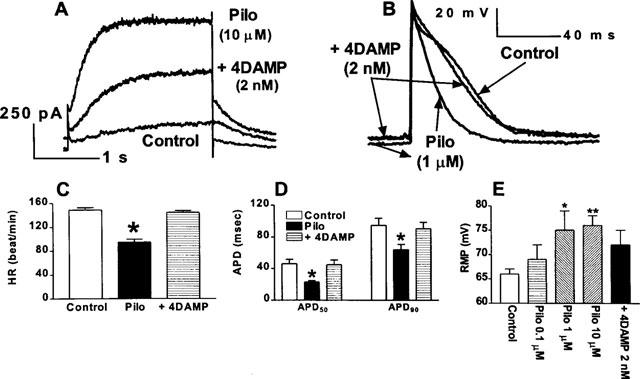
Comparison of pilocarpine induction of a novel K+ current and shortening of APD in guinea-pig atrial myocytes. (A) Superimposed current traces showing the blockade of pilocarpine-induced currents by 4DAMP (2 nM) in guinea-pig atrial myocytes. Only current traces recorded at +50 mV are shown. (B) Superimposed action potentials recorded before drugs, in the presence of pilocarpine and after co-application of pilocarpine and 4DAMP in guinea-pig atrium. (C) Slowing of heart rate. Shown are data averaged from five hearts. (D) Shortening of action potential duration (ADP50 and APD90). (E) Hyperpolarization of the resting membrane potential induced by pilocarpine and the inhibitory effects of 4DAMP. *P<0.05 and **P<0.01, compared to control.
Pilocarpine was also found to significantly hyperpolarize the membrane (see Figure 3B) in a concentration-dependent manner. The averaged resting membrane potential (RMP) was 67±1 mV under control conditions, 76±2 mV with 10 μM pilocarpine (P<0.01, n=14 cells of four hearts), and 71±3 mV in the presence of pilocarpine (10 μM) plus 4DAMP (2 nM). 4DAMP (2 nM) was able to partially reverse the hyperpolarizing effects produced by 10 μM pilocarpine. The data are illustrated in Figure 3B and E.
Pilocarpine induction of a novel K+ current in isolated guinea-pig atrial myocytes
Slowing of HR, shortening of APD and increase in RMP can all be explained by an increase in outward ion currents. Indeed, we have performed preliminary experiments and found that pilocarpine is able to induce a novel K+ current via stimulation of M3 receptors in canine atrial myocytes (Wang et al., 1998). In order to understand the potential molecular mechanisms by which pilocarpine modulates cardiac electrical activity, we performed whole-cell patch-clamp experiments in isolated guinea-pig atrial myocytes. Dofetilide (1 μM) and 293B (20 μM) were included in the superfusate to antecedently block the rapid and the slow components of the delayed rectifier K+ current (IKr and IKs), respectively. In some cells (20% of the cells tested), complete suppression of IKr and IKs was achieved and pilocarpine at a concentration of 10 μM was applied to the bath. In these cells, a delayed rectifier type of current was activated with pilocarpine by depolarizing voltage steps (Figures 3A and Figure 4). The current did not show any rundown within the entire recording period up to 2 h. This current is highly sensitive to the non-specific mAChR antagonist: abolished by 1 μM atropine. More importantly, 4DAMP at a concentration as low as 2 nM (an M3 subtype-selective antagonist) remarkably diminished the current, as shown in Figures 3A, 4A and B. For better comparison, the whole-cell recording, the action potentials, and mean data of HR, APD and RMP are compiled together in Figure 3.
Figure 4.
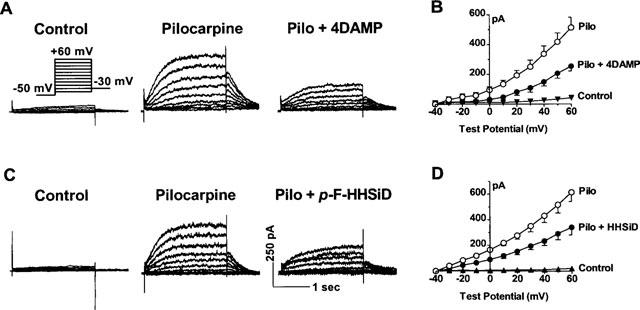
Pilocarpine induction of a novel K+ current via stimulation of M3 receptors in isolated single guinea-pig atrial myocytes. Currents were elicited by 2 s pulses to potentials ranging from −40 to +50 mV with 10 mV increment followed by a 1 s step to −30 mV. Voltage steps were delivered from a holding potential (HP) of −50 mV at an interpulse interval of 5 s. (A) Analogue data showing current induction by pilocarpine (10 μM) and suppression by 4DAMP (2 nM, an M3-selective antagonist). (B) I-V relationships under control conditions, in the presence of pilocarpine and after co-application of pilocarpine and 4DAMP (2 nM). (C) Examples showing the inhibitory effects of p-F-HHSiD (20 nM, an M3-selective antagonist) on pilocarpine-induced K+ currents. Calibration same for A and C. (D) I-V relationships under control conditions, in the presence of pilocarpine and after co-application of pilocarpine and p-F-HHSiD (20 nM). Data shown are mean±s.e.mean from four cells.
To ensure that 4DAMP really acts on mAChRs without direct effects on IKr and IKs or other non-specific effects, the experiments were carried out in the absence of dofetilide and 293B. In all five cells examined, 4DAMP up to 10 nM did not affect IKr or IKs, indicating that these two currents were not involved in the pilocarpine-induced currents. To further assure that 4DAMP specifically acts on M3 receptor but not on any other subtypes to block pilocarpine-induced current, effects of p-F-HHSiD, another M3-preferential antagonist (Lambrecht et al., 1988), were also evaluated. As shown in Figure 4C and D, 20 nM p-F-HHSiD, applied to the pilocarpine-containing bathing solution, caused around 50% current reduction equivalent to the effects of 2 nM 4DAMP.
The pilocarpine-induced currents in guinea-pig atrial myocytes resemble the novel K+ current mediated by M3 receptors first identified in canine atrial cells (Fermini & Nattel, 1994; Shi et al., 1998a). Since IKr and IKs might contaminate the currents of interest and confound the analysis and complete elimination of these two currents is hard in guinea-pig myocytes, if not impossible, as already stated above, we decided to perform the experiments with canine atrial cells to further characterize the pilocarpine-induced current. Based on our experience and previous experiments, removal of all contaminating currents is consistently achievable in dog myocytes.
Pilocarpine induction of a K+ current in isolated canine atrial myocytes
As in guinea-pig atrial cells, a delayed rectifier type of K+ current was consistently activated in the presence of pilocarpine when all other potential contaminating currents (such as IKr, IKs, Ito, IKur.d, IKATP, ICa and INa) had been effectively minimized. The minimal concentration of pilocarpine required for the current induction was 0.1 μM, and a maximal level of the current activation was observed with 10 μM pilocarpine. We therefore used 10 μM pilocarpine for the rest of our experiments.
Since IKr and IKs share some similarities with pilocarpine-activated K+ current, possible contribution of these two currents to pilocarpine-induced current was examined. Recordings were made with the solution which did not contain dofetilide and 293B. Following pilocarpine activation of the current to steady state levels (usually 10 min), dofetilide (1 μM) or 293B (20 μM) was added to the pilocarpine-containing solution and the whole-cell recordings were repeated 20 min after exposure. Our results showed that neither of the drugs produced any changes of pilocarpine-induced current, excluding the involvement of IKr and IKs in the pilocarpine-induced K+ current.
It seems that pilocarpine, like TMA (Shi et al., 1998a) and choline (Wang et al., 1998), activates the K+ currents via stimulation of M3 receptors. This was verified by the following experiments. Effects of atropine on the currents were first tested. Atropine at 1 μM eliminated the pilocarpine-induced currents, suggesting a role of mAChRs in the current induction. To explore whether M3 receptors were responsible for mediating pilocarpine activation of the K+ current, we assessed the effects of 4DAMP. As depicted in Figure 5A, B and C, 4DAMP, concomitantly applied with pilocarpine, substantially depressed the current at all test potentials studied (ranging from −40 to +50 mV). Like in guinea-pig myocytes, 2 nM of 4DAMP produced approximately 50% inhibition (45±4% at +50 mV, n=6). Greater percentage of block was observed with higher concentrations of the drug: 75% by 5 nM and 93% by 10 nM. Blockade of pilocarpine-induced K+ current by 4DAMP mustard (4 and 20 nM), an alkylating agent selective to M3 receptors over other subtypes (Ehlert & Griffin, 1988; Eglen et al., 1994), was also consistently observed (see Figure 5C). Furthermore, like in guinea-pig atrial myocytes, p-F-HHSiD at 20 nM or 200 nM substantially suppressed the current and the results are shown in Figure 5C.
Figure 5.
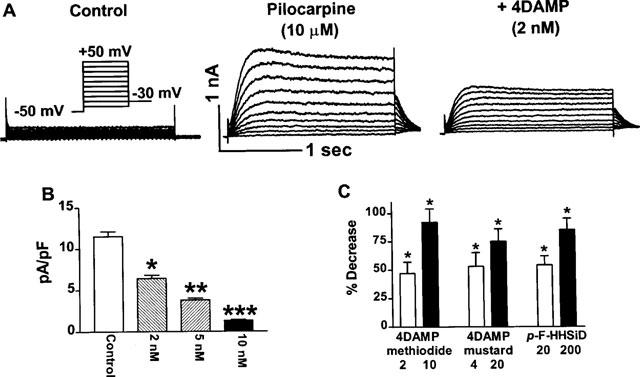
Effects of M3 mAChR subtype-selective antagonists on the pilocarpine-induced K+ current in canine atrial myocytes. Currents were elicited in the presence of 10 μM pilocarpine, with voltage protocols same as described in Figure 4. (A) A family of traces recorded before drug application, the K+ current induced in the presence of pilocarpine (10 mM), and inhibition of the currents by 4DAMP (2 nM). (B) Current density-voltage relationship of baseline current and after exposure of cells to 4DAMP at various concentrations indicated. Shown are averaged data from a total of eight cells. *P<0.05, **P<0.01, and ***P<0.001 comparison between pilocarpine and pilocarpine + 4DAMP. (C) Per cent decreases in the pilocarpine-induced K+ current by 4DAMP (2 and 10 nM, n=8), 4DAMP mustard (4 and 20 nM, n=4), and p-F-HHSiD (20 and 200 nM, n=5), respectively. *P<0.05 without antagonist vs with antagonist.
To investigate whether the pilocarpine-induced current also responds to the antagonists selective toward other subtypes (M1/M2/M4) of mAChRs, effects of pirenzepine (PZ, for M1), methoctramine (MA, for M2), and tropicamide (PA, for M4, Lazareno et al., 1990; Lazareno & Birdsall, 1993) were evaluated. The results are presented in Figure 6. None of these compounds exhibited any blocking actions on pilocarpine-induced K+ currents. To ensure that the failure of these compounds to inhibit the currents were not due to insufficient concentrations used, we further tested the effects of elevated concentrations of PZ (100 nM) and MA (100 nM) on the current. However, we still failed to observe any changes of the current. Higher concentrations of the drugs were not studied because they may cause cross-actions on different subtypes.
Figure 6.
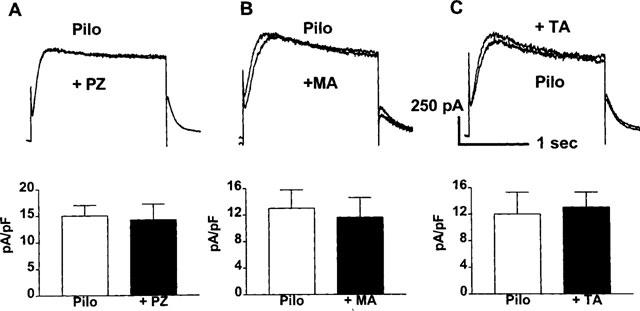
Lack of effects of mAChR subtype-selective antagonists on the pilocarpine-induced K+ current in canine atrial myocytes. Currents were elicited in the presence of 10 μM pilocarpine, with voltage protocols same as described in Figure 4. Upper panels: Analogue data from representative myocytes showing effects of pirenzepine (PZ, 100 nM (A)), methoctramine (MA, 20 nM (B)), and tropicamide (TA, 200 nM (C)). Only currents recorded at a TP of +50 mV are shown for the sake of clarity. Bottom panels: Current density of the pilocarpine-induced K+ current before and after antagonists, measured at +50 mV (n=3 for PZ, n=4 for MA, and n=3 for PA).
To ensure that the effects of pilocarpine were not the consequence of enhanced release of catecholamine from presynaptic gangolians, additional experiments were performed to evaluate the effects of α1- and β-adrenergic receptor antagonist prazosin (2 μM) and propralonol (2 μM). However, no changes were observed under such conditions and pilocarpine demonstrated the same potency for K+ current induction (data not shown), excluding the contribution of adrenoceptor activation. This was further precluded by our preliminary study showing that phenylephrine (an α1-adrenoceptor agonist) and isoproterelol (a β-adrenoceptor agonist) both cause significant decrease in the current, instead of increase as expected if these receptors are involved in pilocarpine induction of the K+ current.
Pilocarpine binding to mAChRs in membrane homogenates from canine atria
If, as our functional study suggested, pilocarpine modulates heart function by activating a K+ current via stimulation of an M3 receptor, then it should be able to displace [3H]-NMS binding in a competitive fashion. To clarify this issue and also to verify the validity of the pilocarpine concentrations used in our experiments, mAChR binding assay was carried out. Saturation binding of [3H]-NMS to the membrane homogenates from canine atria yielded a maximum binding value, or mAChR density of 282±26 fmol mg−1 protein and a dissociation constant KD of 223±24 pM (Figure 7A). The experiments for competition binding were performed with 400 pM [3H]-NMS and thirteen concentrations of pilocarpine ranging from 1nM–1 mM. For comparison, binding of 4DAMP was also analysed. The data are shown in Figure 7B. Pilocarpine and 4DAMP binding both yielded displacement curves best described with a two-site binding model. The KD values were 34.6±2.4 nM (30%, percentage of high affinity binding) and 2.2±0.1 μM (70%, percentage of low affinity binding) for pilocarpine, and 2.4±0.5 nM and 80.7±10.2 nM for 4DAMP. These values were quite compatible with the concentrations at which pilocarpine produced induction of the K+ current and alteration of the HR and APD in both canine cells and guinea-pig cells and the concentration of 4DAMP which produced 50% reduction of pilocarpine-induced current.
Figure 7.
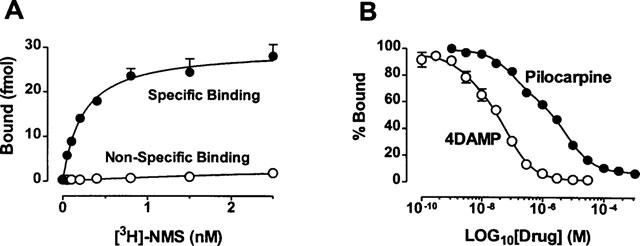
Radioligand binding with [3H]-NMS to homogenates from canine atrial tissues. (A) Saturation binding. Shown are the specific and non-specific data averaged from four individual preparations carried out in triplicate for each experiment. Symbols are experimental data and lines represent the fit with a one-site binding model. (B) Displacement binding of [3H]-NMS with 4DAMP 0.1 nM–30 μM and pilocarpine (1 nM–1 mM). Each point represents the mean±s.e.mean from six experiments assayed in duplicate. Curves are best fits of the experimental data to a two-site binding model.
Discussion
We have provided evidence, in the present study, in support of our hypothesis that pilocarpine can modulate the cellular electrical properties of cardiac tissues (heart rate, membrane potential and action potential duration), likely by activating a novel K+ current via stimulation of cardiac M3 receptors. Our study revealed a novel aspect of pharmacological property of pilocarpine.
Pilocarpine is administered orally for the treatment of xerostomia that follows head and neck radiation treatments or that is associated with Sjögren's syndrome. It is also applied topically for the treatment of glaucoma. The peak serum concentrations in patients receiving oral pilocarpine was found to be in the range of several hundreds of nanomolar (∼300 nM or ∼0.3 μM, Wiseman & Faulds, 1995). These concentrations are comparable with the concentrations required to produce significant modulation of cardiac electrical properties and M3 receptor stimulation and K+ current induction in the present study. For example, 0.1 μM pilocarpine caused ∼25% shortening of APD50 and 5% slowing of HR and hyperpolarizing of the membrane. Increasing the concentration to 1 μM strikingly enhanced the effects of pilocarpine (20% slowing of HR, 35% briefing of APD and 14% negative shift of the RMP). Similar concentrations of pilocarpine were needed for the induction of the K+ current. More interestingly, the affinity of pilocarpine at mAChRs (KD=2.2 μM) was in good agreement with the concentrations required to achieve its pharmacological effects. The concentrations of pilocarpine are lower than or comparable with those used for eliciting other responses such as stimulation of phosphoinositide turnover in the hippocampus (18 μM, Hoss et al., 1990), outflow facility (41 μM, Gabelt & Kaufman, 1992), miosis (4.1 μM, Gabelt & Kaufman, 1992), etc. Activation of the K+ current provides an explanation for the changes of the cardiac electrical properties induced by pilocarpine. Induction of an outward K+ current in a cell should accelerate the rate of membrane repolarization thereby shortening of APD and should also hyperpolarize the membrane which in turn should slow the rate of depolarization thereby the heart rate. It should be kept in mind, however, that pilocarpine may also affect other currents like Ca2+ and Na2+ to account for its modulation of the cardiac electrical activity. This notion absolutely awaits future investigation. On the other hand, slowing of HR and hyperpolarization of membrane are usually desired properties for the anti-arrhythmic efficacy. Acceleration of repolarization would expect to cause negative inotropy by indirectly reducing the Ca2+ influx because of shortened plateau duration of AP. Future studies are warranted to test these hypotheses and to establish the potential of pilocarpine in these purposes.
Although overall pilocarpine acts as a non-subtype-specific agonist of mAChRs, it demonstrates selectivity toward one or another depending on particular types of tissues to which it is applied. For example, the mAChR subtypes involved in the mediation of pilocarpine-induced purposeless chewing behaviour appear to be M2 and M3 receptors because both 4DAMP and AF-DX 116 (an M2-selective antagonist) inhibit the effects of pilocarpine, but pirenzepine did not. Stewart et al. concluded, based on the fact that methoctramine and PZ inhibited pilocarpine-induced seizures in their experimental study in mice, that M1 receptors play an important role in mediating pilocarpine-induced seizures and M2 in modulating neuronal activity (Stewart et al., 1989). p-F-HHSiD did not show any effects in their model of seizures. Another study demonstrated that pilocarpine is a partial agonist at both M1 and M2 receptors in the central nerve system. With respect to the memory task, M1 effects of pilocarpine apparently predominate (Hoss et al., 1990). Gabelt & Kaufman performed a study aimed at elucidating the subtypes of mAChRs responsible for outflow facility, accommodative and miotic responses to pilocarpine in rhesus monkeys and these three functional responses are all mediated through an M3 receptor subtype, because 4DAMP inhibited the effects of pilocarpine with a potency around 30 fold higher than PZ and 100 fold higher than AF-DX 116 (Gabelt & Kaufman, 1992). On the other hand, it was found that M3 receptor subtype predominates in the bovine iris sphincter smooth muscle and ciliary processes (Honkanen et al., 1990) and in rhesus monkey ciliary muscle (Poyer et al., 1994), suggesting pilocarpine acts primarily on M3 receptors for the treatment of glaucoma. In the present study, we found that the effects of pilocarpine on HR, RMP and APD were readily reversed by low concentrations of 4DAMP. Correspondingly, pilocarpine-induced K+ current was equally sensitive to 4DAMP in both guinea-pig and canine atrial myocytes. Furthermore, other M3-preferring inhibitors like 4DAMP mustard or p-F-HHSiD were also able to suppress the pilocarpine-induced K+ current. More importantly, the concentration of 4DAMP needed to block the current coincides well with the KD value of 4DAMP from binding assay. On the contrary, antagonists selective at M1 or M2 or M4 receptor subtypes did not alter the pilocarpine-induced K+ current. These results are unlikely due to insufficient concentrations that we used, because the concentrations of these inhibitors were chosen based on many previous studies as well as on our owned data from binding assays (Shi et al., 1998; Wang et al., 1998). For example, we have previously demonstrated that methoctramine at 20 nM significantly inhibited cardiac M2 receptor without affecting the M3 receptors in dog atrial myocytes. It is unclear, however, why pilocarpine shows selectivity at M3 over M2 in cardiac cells or in other words, why pilocarpine has M2 activity in other tissues but not in the heart. Interestingly, similar phenomenon has also been observed with other compounds. For example, methoctramine is known to be an M2-selective blocker. But its affinity to M2 is different in different tissues. Melchiorre et al. reported that methoctramine possesses a 275 fold greater affinity at cardiac than at ileal M2 receptors in functional studies (Melchiorre et al., 1987). It was also found that methoctramine while showing high affinity for cardiac M2 receptors has rather low affinity for vascular M2 receptors (Wess et al., 1988). Consistent with these findings, Michel & Whiting found 158 fold higher affinity of methoctramine at cardiac M2 receptors than at exocrine gland M2 receptors (Michel & Whiting, 1988). One possible explanation for these observations is there are structural differences of M2 receptors of different tissue origins. Yet, there is no evidence so far to support this idea in terms of molecular structure of M2 receptors. Another possible explanation is, as proposed by Hoss et al. based on their experimental data, that different confirmations of pilocarpine are active as agonists at different mAChR subtypes (Hoss et al., 1990). Obviously, more specific studies are needed to clarify the issue before a conclusion can be made.
Our data represent first evidence indicating that pilocarpine can modulate the cardiac electrical properties in isolated hearts and induce a K+ current in isolated single myocytes. However, effects on hearts have not been reported in patients receiving pilocarpine. It is possible that the blood pilocarpine never reaches levels sufficiently high to bring about its cardiac effects. Or probably, under in vivo situations, the cardiac effects of pilocarpine seen under in vitro conditions are balanced out by reflex. More detailed and aimed studies are required to clarify this issue. Nonetheless, pilocarpine should at least be considered an effective pharmacological probe for studying the cardiac M3 receptors and the novel K+ current.
Acknowledgments
This work was supported in part by the Medical Research Council of Canada, the Heart and Stroke Foundation of Quebec, an Establishment Grant for young investigators from the Fonds de Recherche en Sante de Quebec awarded to Dr Wang, and the Fonds de la Recherche de l'Institut de Cardiologie de Montreal. Dr Wang is a research scholar of the Heart and Stroke Foundation of Canada. The authors wish to thank XiaoFan Yang for excellent technical assistance and Nathalie Ethier for her professional help with the radioligand binding assay.
Abbreviations
- mAChR
muscarinic acetylcholine receptor
- HR
heart rate
- AP
action potential
- APD50 and APD90
action potential duration at 50 and 90%repolarization, respectively
- PZ
pirenzepine
- MA
methoctramine
- 4DAMP
4-Diphenylacetoxy-N-methylpiperidine methiodide
- TA
tropicamide
- [3H]-NMS
[N-methyl-3H]-scopolamine methyl chloride
- KD
dissociation constant
- HP
holding potential
- TP
test potential
- IKr
rapid component of delayed rectified K+ current
- IKs
slow component of delayed rectifier K+ current
References
- ARAUJO D.M., LAPCHAK P.A., QUIRION R. Heterogeneous binding of [3H]4-DAMP to muscarinic cholinergic sites in the rat brain: evidence from membrane binding and autoradiographic studies. Synapse. 1991;9:165–176. doi: 10.1002/syn.890090303. [DOI] [PubMed] [Google Scholar]
- BARLOW R.P., SHEPHERD M.K. A further search for selective antagonists at M2-muscarinic receptors. Br. J. Pharmacol. 1986;89:837–843. doi: 10.1111/j.1476-5381.1986.tb11189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., REDDY H., WATSON N. Selective inactivation of muscarinic receptor subtypes. Int. J. Biochem. 1994;26:1357–1368. doi: 10.1016/0020-711x(94)90178-3. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., WHITING R.L. Heterogeneity of vascular muscarinic receptors. J. Auton. Pharmacol. 1990;10:233–245. doi: 10.1111/j.1474-8673.1990.tb00023.x. [DOI] [PubMed] [Google Scholar]
- EHLERT F.J., GRIFFIN M.T. The use of irreversible ligands to inactivate receptor subtypes: 4-DAMP mustard and muscarinic receptors in smooth muscle. Life Sci. 1988;62:1659–1664. doi: 10.1016/s0024-3205(98)00124-6. [DOI] [PubMed] [Google Scholar]
- FERMINI B., NATTEL S. Choline chloride activates time-dependent and time-independent K+ currents in dog atrial myocytes. Am. J. Physiol. 1994;266:C42–C51. doi: 10.1152/ajpcell.1994.266.1.C42. [DOI] [PubMed] [Google Scholar]
- GABELT B.T., KAUFMAN P.L. Inhibition of outflow facility and accommodative and miotic responses to pilocarpine in rhesus monkeys by muscarinic receptor subtype antagonists. J. Pharmacol. Exp. Ther. 1992;263:1133–1139. [PubMed] [Google Scholar]
- GOYAL R.K. Muscarinic receptor subtypes. Physiology and clinical implications. N. Engl. J. Med. 1989;321:1022–1028. doi: 10.1056/NEJM198910123211506. [DOI] [PubMed] [Google Scholar]
- HEDLUND B., BARTFAI T. Binding of [3H]-pilocarpine to membranes from rat cerebral cortex. Naunyn. Schmiedebergs. Arch. Pharmacol. 1981;317:126–130. doi: 10.1007/BF00500067. [DOI] [PubMed] [Google Scholar]
- HONKANEN R.E., HOWARD E.F., ABDEL-LATIF A.A. M3-muscarinic receptor subtype predominates in the bovine iris sphincter smooth muscle and ciliary processes. Invest. Ophthalmol. Visual. Sci. 1990;31:590–593. [PubMed] [Google Scholar]
- HOSS W., WOODRUFF J.M., ELLERBROCK B.R., PERIYASAMY S., GHODSI-HOVSEPIAN S., STIBBE J., BOHNETT M., MESSER W.S., Jr Biochemical and behavioral responses of pilocarpine at muscarinic receptor subtypes in the CNS. Comparison with receptor binding and low-energy conformations. Brain Res. 1990;533:232–238. doi: 10.1016/0006-8993(90)91344-g. [DOI] [PubMed] [Google Scholar]
- HUMLE E.C., BIRDSALL N.J., BUCKLEY N.J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- LAMBRECHT G., FEIFEL R., FORTH B., STROHMANN C., TACKE R., MUTSCHLER E. p-Fluoro-hexahydro-sila-difenidol: The first M2β-selective muscarinic antagonist. Eur. J. Pharmacol. 1988;152:193–194. doi: 10.1016/0014-2999(88)90856-4. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., BIRDSALL N.J.M. Pharmacological characterization of acetylcholine-stimulated [35S]-GTP S binding mediated by human muscarinic m1-m4 receptors: antagonist studies. Br. J. Pharmacol. 1993;109:1120–1127. doi: 10.1111/j.1476-5381.1993.tb13738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZERENO S., BUCKLEY N.J., ROBERTS F.F. Characterization of muscarinic M4 binding sites in rabbit lung, chicken heart, and NG108-15 cells. Mol. Pharmacol. 1990;38:805–815. [PubMed] [Google Scholar]
- MASLANSKI J.A., POWELT R., DEIRMENGIANT C., PATELT J. Assessment of the muscarinic receptor subtypes involved in pilocarpine-induced seizures in mice. Neurosci. Lett. 1994;168:225–228. doi: 10.1016/0304-3940(94)90456-1. [DOI] [PubMed] [Google Scholar]
- MELCHIORRE C., CASSINELLI A., QUAGLIA W. Differential blockade of muscarinic receptor subtypes by polymethylene tetraamines. Novel class of selective antagonists of cardiac M2 muscarinic receptors. J. Med. Chem. 1987;30:201–204. doi: 10.1021/jm00384a034. [DOI] [PubMed] [Google Scholar]
- MICHEL A.D., STEFANICH E., WHITING R.L. Direct labeling of rat M3-muscarinic receptors by [3H]4-DAMP. Eur. J. Pharmacol. 1989;166:459–466. doi: 10.1016/0014-2999(89)90359-2. [DOI] [PubMed] [Google Scholar]
- MICHEL A.D., WHITING R.L. Methoctramine, a polymethylene tetraamine, differentiates three subtypes of muscarinic receptor in direct binding studies. Eur. J. Pharmacol. 1988;145:61–66. doi: 10.1016/0014-2999(88)90349-4. [DOI] [PubMed] [Google Scholar]
- MUTSCHLER E., MOSER U., WESS J., LAMBRECHT G. Muscarinic receptor subtypes-pharmacological, molecular biological and therapeutical aspects. Pharm. Acta Helv. 1995;69:243–258. doi: 10.1016/0031-6865(94)00045-w. [DOI] [PubMed] [Google Scholar]
- POYER J.F., GABELT B.T., KAUFMAN P.L. The effect of muscarinic agonists and selective receptor subtype antagonists on the contractile response of the isolated rhesus monkey ciliary muscle. Exp. Eye Res. 1994;59:729–736. doi: 10.1006/exer.1994.1159. [DOI] [PubMed] [Google Scholar]
- SHI H., WANG H., WANG Z.Identification and characterization of multiple subtypes of muscarinic acetylcholine receptors and their physiological functions in canine hearts Mol. Pharmacol. 1998a. in press [PubMed]
- SHI H., WANG H., WANG Z. Functional and molecular identification and characterization of cardiac M3 and M4 subtypes of muscarinic acetylcholine receptors (mAChRs) Circulation. 1998b;98:1694. [Google Scholar]
- SHI H., WANG H., WANG Z. Choline is a selective agonist of M3 subtype of muscarinic acetylcholine receptors (mAChRs) in cardiac myocytes. Can. J. Cardiol. 1998c;14:144F. [Google Scholar]
- STEWART B.R., JENNER P., MARSDEN C.D. Assessment of the muscarinic receptor subtype involved in the mediation of pilocarpine-induced purposeless chewing behaviour. Psychopharmacology. 1989;97:228–234. doi: 10.1007/BF00442255. [DOI] [PubMed] [Google Scholar]
- VAN ZWIETEN P.A., DOODS H.N. Muscarinic receptors and drugs in cardiovascular medicine. Cardiovasc. Drugs Ther. 1995;9:159–167. doi: 10.1007/BF00877757. [DOI] [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Repolarization differences between guinea-pig atrial endocardium and epicardium - evidence for a role of the transient outward current (Ito) Am. J. Physiol. 1991;260:H1501–H1506. doi: 10.1152/ajpheart.1991.260.5.H1501. [DOI] [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Mechanism of flecainide's rate-dependent actions on action potential duration in canine atrial tissue. J. Pharmacol. Exp. Ther. 1993a;267:575–581. [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ. Res. 1993b;73:276–285. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc. Res. 1994;28:1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- WANG Z., FERMINI B., NATTEL S. Effects of flecainide, quinidine, and 4-aminopyridine on transient and sustained Outward current in human atrial myocytes. J. Pharmacol. Exp. Ther. 1995;272:84–196. [PubMed] [Google Scholar]
- WANG Z., PELLETIER L.C., TALAJIC M., NATTEL S. Effects of flecainide and quinidine on human atrial action potentials: Role of rate-dependence and comparison with guinea-pig, rabbit, and dog tissues. Circulation. 1990;82:274–283. doi: 10.1161/01.cir.82.1.274. [DOI] [PubMed] [Google Scholar]
- WANG H., SHI H., WANG Z. Pilocarpine modulates heart functions via activation of M3 subtype muscarinic acetylcholine receptors and a novel K+ current. Can. J. Cardiol. 1998;14:144F. [Google Scholar]
- WESS J., ANGELI P., MELCHIORRE C., MOSER U., MUTSCHLER E., LAMBRECHT G. Methoctramine selectively blocks cardiac muscarinic M2 receptors in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 1988;338:246–249. doi: 10.1007/BF00173395. [DOI] [PubMed] [Google Scholar]
- WISEMAN L.R., FAULDS D. Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerostomia. Drugs. 1995;49:143–155. doi: 10.2165/00003495-199549010-00010. [DOI] [PubMed] [Google Scholar]
- YUE L., FENG J., GASPO R., LI G.R., WANG Z., NATTEL S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]


