Abstract
Some animal studies suggest that β-adrenoceptor-mediated vasorelaxation is in part mediated through nitric oxide (NO) release. Furthermore, in humans, we have recently shown that forearm blood flow is increased by infusion of β2-adrenergic agonists into the brachial artery, and the nitric oxide synthase (NOS) inhibitor NG-monomethyl-L-arginine (L-NMMA) inhibits this response.
The purpose of the present study was to determine whether stimulation of human umbilical vein endothelial β-adrenoceptors causes vasorelaxation and nitric oxide generation, and whether this might be mediated by cyclic adenosine-3′,5′-monophosphate (cyclic AMP).
Vasorelaxant responses were determined in umbilical vein rings to the nonselective β-adrenergic agonist isoprenaline and to the cyclic AMP analogue dibutyryl cyclic AMP, following precontraction with prostaglandin F2α.
NOS activity was measured in cultured human umbilical vein endothelial cells (HUVEC) by the conversion of [3H]-L-arginine to [3H]-L-citrulline, and adenylyl cyclase activity by the conversion of [α-32P]-ATP to [32P]-cyclic AMP.
Isoprenaline relaxed umbilical vein rings, and this vasorelaxation was abolished by β2- (but not β1-) adrenergic blockage, and by endothelium removal or 1 mM L-NMMA. In addition, vasorelaxant responses to dibutyryl cyclic AMP were inhibited by 1 mM L-NMMA, with a reduction in Emax from 90.0±9.3% to 50.5±9.9% (P<0.05).
Isoprenaline 1 μM increased NOS activity in HUVEC (34.0±5.9% above basal, P<0.001). Furthermore, isoprenaline increased adenylyl cyclase activity in a concentration-dependent manner; this response was inhibited by β2 (but not β1-) adrenergic blockade. Forskolin 1 μM and dibutyryl cyclic AMP 1 mM each increased NOS activity in HUVEC, to a degree similar to isoprenaline 1 μM. The increase in L-arginine to L-citrulline conversion observed with each agent was abolished by co-incubation with NOS inhibitors.
These results indicate that endothelial β2-adrenergic stimulation and cyclic AMP elevation activate the L-arginine/NO system, and give rise to vasorelaxation, in human umbilical vein.
Keywords: β-Adrenergic receptors, vascular endothelium, vasodilatation, nitric oxide, nitric oxide synthase, human umbilical vein
Introduction
Stimulation of β-adrenoceptors on vascular smooth muscle results in vasorelaxation, through activation of adenylyl cyclase and a consequent increase in cyclic adenosine-3′,5′-monophosphate (cyclic AMP) intracellularly (Kuriyama et al., 1982). Vascular endothelial cells also may express β-adrenoceptors (Steinberg et al., 1984; Buxton et al., 1987; Summers et al., 1987; Howell et al., 1988; Molenaar et al., 1988; Lefroy et al., 1993), although their physiological function, if any, remains unclear. Evidence is accumulating, however, that the vascular endothelium may facilitate or mediate β-adrenergic vasorelaxation. We have previously reported that β-adrenoceptor-mediated relaxation is in part endothelium dependent in the rat mesenteric artery (Graves & Poston, 1993) and pulmonary artery (Priest et al., 1997). This is in agreement with other reports which have demonstrated in vitro that relaxation to isoprenaline in systemic vessels is impaired by removal of the endothelium (Rubanyi & Vanhoutte, 1985; Kamata et al., 1989; Gray & Marshall, 1992) or inhibition of nitric oxide (NO) synthesis (Gray & Marshall, 1992; Blankesteijn & Thien, 1993). Furthermore, in vivo studies in cat hindlimb (Gardiner et al., 1991), newborn pig pial artery (Rebich et al., 1995) and canine coronary artery (Parent et al., 1993) support a role of the vascular endothelium in β-adrenergic vasorelaxation. In contrast, Moncada et al. (1991) have found no evidence of endothelium dependence on isoprenaline-induced vasorelaxation and two independent studies of isolated canine coronary arteries have also suggested that β-adrenoceptor-mediated vasorelaxation is endothelium-independent (Macdonald et al., 1987; Béa et al., 1994).
Few studies have investigated the function of endothelial β-adrenoceptors in human vasculature. Molenaar et al. (1988) found that isolated human internal mammary artery endothelium expresses β-adrenoceptors, as shown by autoradiography, but endothelial denudation did not affect relaxation to isoprenaline. We have recently reported that infusion of either isoprenaline or of the β2-adrenoceptor agonist salbutamol into the brachial artery of human subjects induces an increase in forearm blood flow, and that co-infusion of the nitric oxide synthase (NOS) inhibitor NG-monomethyl-L-arginine (L-NMMA) inhibits the response to either drug (Dawes et al., 1997).
In the present study, we examined whether in vitro stimulation of β-adrenoceptors in isolated human umbilical vein causes endothelium-dependent vasorelaxation, whether this occurs through activation of the L-arginine/NO pathway, and the mechanism involved. Human umbilical vein endothelial cells (HUVEC) are widely available and extensively used for biochemical and pharmacological studies of human endothelial cells. We examined vasodilatory responses of isolated rings of human umbilical vein to β-adrenoceptor stimulation or to cyclic AMP elevation by other means, and the effect on these responses of endothelium removal or NOS inhibition. We further investigated adenylyl cyclase and NOS activities in HUVEC in response to β-adrenoceptor activation or to other cyclic AMP-elevating agents. Experiments were also performed to determine whether β-adrenergic stimulation evoked changes in calcium concentration within the endothelial cells. This protocol provided a comparison of biochemical measures of β-adrenoceptor function in HUVEC with functional β-adrenergic responses in the intact vessel.
Methods
Fresh umbilical cords were obtained following delivery of healthy babies to healthy normotensive mothers, either by vaginal delivery or by elective Caesarean section. Approval was granted by the Research Ethics Committee, St Thomas' Hospital, London, U.K.
Functional responses of umbilical vein rings
Umbilical vein was dissected from the middle portion of the umbilical cord, cut into 2–3 mm rings and mounted in 3 mL organ baths containing Krebs buffer of the following composition (mM): NaCl 125, KCl 4.8, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, glucose 11, EDTA 0.3, CaCl2 2.5, pH 7.4 (gassed with 95% O2/5% CO2 37°C). Preliminary length-tension curves suggested that a resting tension of 2 g gave optimal contractile responses to KCl 45 mM, therefore this level of resting tension was used in all experiments.
Once stable and reproducible contractions were obtained with KCl 45 mM, rings were contracted with prostaglandin F2α 0.5–0.75 μM, the concentration chosen being that required to elicit approximately 70% of the KCl-induced tension. The presence of functional endothelium was confirmed by the demonstration of ⩾60% relaxation in response to acetylcholine 1 μM.
Following washout, rings were contracted once again with the same concentration of prostaglandin F2α, and relaxant concentration-effect curves were determined to isoprenaline (concentration range 10−9–10−4 M) or to the membrane-permeable non-hydrolysable cyclic AMP analogue dibutyryl cyclic AMP (concentration range 10−7–10−3 M), with incremental additions at 2 min intervals. After washout, rings were once again contracted with prostaglandin F2α, and repeat concentration-effect curves determined to isoprenaline or to dibutyryl cyclic AMP, following preincubation with one of the following: L-NMMA 1 mM; CGP 20712A 300 nM, a selective β1-adrenoceptor antagonist (Kaumann, 1986); ICI 118551 100 nM, a selective β2-adrenoceptor antagonist (Bilski et al., 1983; Lemoine et al., 1985); or the combination of CGP 20712A 300 nM and ICI 118551 100 nM. Preliminary experiments showed that repeated exposures of these rings to isoprenaline or to dibutyryl cyclic AMP in the absence of any antagonists did not give rise to tachyphylaxis. Concentration-effect curves to isoprenaline were determined simultaneously in rings of umbilical vein from the same cord whose endothelium had been removed by gently passing a mental rod along the lumen; endothelial denudation was confirmed in these rings by the absence of a relaxant response to acetylcholine 1 μM.
Isolation and culture of HUVEC
Following delivery, the umbilical vein was cannulated and flushed with 30 mL warm Dulbecco's phosphate-buffered saline (PBS), following which the cord was clamped at the distal end and the vessel filled with medium 199 with added collagenase (Type II, EC 3.4.24.3, 1 mg ml−1), until mildly distended. Following incubation at 37°C (15 min), the cord was unclamped and the digest drained. The vessel was gently massaged and flushed through with a further 30 mL Dulbecco's PBS, and the digests were pooled. The resultant endothelial cell suspension was centrifuged (400×g, 5 min), and the cell pellet was resuspended in medium 199 with Earle's salts supplemented with penicillin 500 IU ml−1, streptomycin 500 μg ml−1, fungizone 1.25 μg ml−1 glutamine 2 mM, foetal bovine serum 20%, heparin 0.01% (Grade 1A) and endothelial cell growth supplement 120 μg ml−1 (derived from bovine neural tissue). This suspension was seeded into a 25 mL gelatin-coated culture flask.
Monolayer cultures of cells were grown at 37°C in an atmosphere of 95% air and 5% CO2. At confluence, cells were detached from the substratum by brief exposure to trypsin-EDTA (2 min at 37°C), pelleted (400×g, 5 min) and passaged at a split ratio of 1 : 3. Confluent cells at passage 3 were used for all experiments. The presence of a pure population of HUVEC at confluence was confirmed both by their characteristic cobblestone morphology under phase-contrast microscopy and by positive direct immunofluorescence staining for von Willebrand factor and negative immunofluorescence staining for smooth muscle α-actin.
Evaluation of NOS activity
Conversion of radiolabelled L-arginine to L-citrulline was determined by a modification of the method of Brown et al. (1996). Confluent HUVEC at passage 3 in 24-well plates were washed twice with 1 mL balanced salt solution (BSS) buffer, of the following composition (mM): NaCl 125, KCl 5.4, NaHCO3 16.2, HEPES 15, NaH2PO4 1, MgSO4 0.8, CaCl2 1.8, glucose 5.5 (pH 7.4). Following incubation for 20 min with 3 μCi ml−1 [3H]-L-arginine (57 Ci mmol−1) in 200 μL of BSS at 37°C, in an atmosphere of 95% air and 5% CO2, L-NMMA 0.1 mM, or vehicle was added for a further 10 min. Agonists (isoprenaline 1 μM, forskolin 1 μM, dibutyryl cyclic AMP 1 mM or histamine 10 μM) or vehicle were then added and the incubation continued for another 10 min. Incubations were stopped by aspiration of the incubate, washing with 3×1 mL of ice-cold BSS without Ca2+ and addition of 0.5 mL of ice-cold 0.5 M trichloroacetic acid. After 2 h on ice, the extract was washed ×3 with 2 mL of water-saturated diethylether, 0.25 mL of the aqueous phase was removed and mixed with 0.75 mL 20 mM HEPES (pH 6.0). This extract was then applied to a 1 mL column of Dowex (Na+ form), citrulline was eluted with water (4 mL) and arginine with 0.1 M NaOH (4 mL). [3H]-L-arginine and L-citrulline in the different fractions were measured by liquid scintillation counting. Results were corrected for protein concentrations in the corresponding cellular extracts.
Measurement of cytoplasmic Ca2+
In order to determine whether β-adrenoceptor activation changes intracellular Ca2+ concentration in HUVEC and so, indirectly, NOS activity, experiments were performed to examine changes in cytoplasmic calcium concentration using the fluorescent dye fura-2-AM. The method was a modification of that described by Graier et al. (1990). HUVEC grown to confluence on 35 mm Petri dishes (n=4) were preincubated for 20 min at 37°C with 5 μM fura-2-AM in BSS. Cells were then washed with BSS and left at 37°C for a further 15 min. They were then perfused with BSS containing isoprenaline 1 μM, histamine 10 μM or vehicle, and fura-2 fluorescence was continuously measured (excitation at 340 and 380 nm, emission at 530 nm). The ratio of emission following excitation at 340 nm to that following excitation at 380 nm was taken as an index of intracellular Ca2+.
Cyclic AMP assay
Confluent HUVEC in 24-well plates were washed ×3 with BSS, then allowed to equilibrate for 20 min in 0.5 mL BSS at 37°C. Cells were then exposed to agonist or vehicle for 6 min, and the reaction stopped by removing the supernatant and adding 1 mL of 65% ice-cold ethanol. The residue was harvested using a cell scraper and the ethanol evaporated. Cyclic AMP was extracted and measured using a proprietary enzymeimmunoassay kit.
Adenylyl cyclase assay
Confluent HUVEC at passage 3 were harvested in Dulbecco's PBS (without Ca2+ or Mg2+) using a cell scraper, pelleted (400×g, 10 min) and homogenized at 4°C in 25 mM Tris-HCl buffer pH 7.4, containing sucrose 0.29 M and EDTA 0.25 mM. Homogenates were stored at −80°C until use.
Adenylyl cyclase activity was measured in HUVEC homogenates using a method modified from Salomon et al. (1974). Determinations were performed in triplicate at 37°C in 50 mM Tris-HCl buffer pH 7.4 containing sucrose 87 mM, magnesium acetate 5 mM, cyclic AMP 1 mM, GTP 4 μM, creatine phosphate 20 mM, creatine kinase 10 IU, Ro20-1724 0.25 mM (a cyclic AMP-specific phosphodiesterase inhibitor), [α-32P]-ATP 1 mM (3 μCi/reaction mixture) and 30 μg homogenate protein in a reaction volume of 100 μL. When indicated, forskolin 10 μM or isoprenaline (concentration 10−9–10−5 mM) with or without 300 nM CGP 20712A or 50 nM ICI 118551 were added. The reaction was terminated after 20 min by the addition of 800 μL 6.25% ice-cold trichloroacetic acid, and [3H]-cyclic AMP (approximately 10,000 c.p.m.) was added. ATP and cyclic AMP were separated chromatographically using Dowex and alumina columns, and 32P and 3H measured by liquid scintillation counting. The percentage fractional recovery of cyclic AMP (50–70%) was determined from the recovery of [3H]-cyclic AMP. Adenylyl cyclase activity (pmol cyclic AMP min−1 mg−1 protein) was derived from the 32P activity associated with the cyclic AMP fraction in the eluate from the columns, which was corrected for the fractional recovery of [3H]-cyclic AMP.
Materials
CGP 20712A was graciously provided by Ciba-Geigy Ltd (Basel, Switzerland), ICI 118551 by Zeneca Pharmaceuticals (Macclesfield, Cheshire, U.K.) and Ro20-1724 by Roche Research Centre (Welwyn Garden City, Hertfordshire, U.K.). Radiochemicals and the cyclic AMP enzymeimmunoassay kit were from Amersham International plc (Little Chalfont, Buckinghamshire, U.K.). Medium 199, antibiotics, antimycotics, trypsin-EDTA, Dulbecco's PBS and foetal bovine serum were from GIBCO–BRL (Paisley, U.K.). Protein measurements were performed using the DC Protein Assay kit, Bio-Rad Laboratories Ltd (Hemel Hempstead, Hertfordshire, U.K.). All other chemicals, including antibodies used for immunofluorescence staining, were from Sigma-Aldrich Company Ltd (Poole, Dorset, U.K.).
Statistical analysis
Relaxant responses to increasing concentrations of isoprenaline or dibutyryl cyclic AMP in umbilical vein rings, in the absence or presence of antagonists, were compared by two-way ANOVA. [3H]-L-arginine, [3H]-L-citrulline and cyclic AMP in HUVEC following different treatments were each compared by one-way ANOVA. Adenylyl cyclase activities in response to increasing concentrations of isoprenaline, in the absence or presence of antagonists, were compared by two-way ANOVA. Statistical significance was taken as P<0.05 (two-sided). Curves for relaxant responses in umbilical vein rings and for adenylyl cyclase responses in HUVEC were fitted by nonlinear regression using GraphPad Prism version 2.01 (GraphPad Software, Inc.). All data are expressed as mean±s.e.mean.
Results
Functional responses of umbilical vein rings
Isoprenaline elicited a concentration-dependent vasorelaxation in precontracted rings with intact endothelium; this was prevented by co-incubation with ICI 118551 100 nM either alone or in combination with CGP 20712A 300 nM, although CGP 20712A alone had no effect (Figure 1a). Relaxation was also abolished by prior denudation of the endothelium or by co-incubation with L-NMMA 1 mM (Figure 1b, Table 1). Dibutyryl cyclic AMP also elicited a concentration-dependent vasorelaxation in these rings; although log EC50 was not affected by L-NMMA 1 mM, Emax was significantly reduced in its presence (Figure 1c, Table 1). Contractile responses to PGE2α were not different in the absence or presence of L-NMMA (0.71±0.17 g and 0.79±0.22 g respectively). Relaxant responses to sodium nitroprusside 0.1 mM, an endothelium-independent vasodilator which degrades to form NO, were also unchanged in the absence or presence of L-NMMA (0.73±0.20 g and 0.75±0.24 g respectively).
Figure 1.

Mean±s.e.mean vasorelaxant responses in human umbilical vein rings. Responses are plotted as the percentage of the response to prostaglandin F2α. (a) Concentration-effect curves in vascular rings with intact endothelium, in response to isoprenaline alone (n=17), in the presence of CGP 20712A 300 nM (n=9), in the presence of ICI 118551 100 nM (n=8), and in the presence of CGP 20712A 300 nM+ICI 118551 100 nM (n=17). (b) Concentration-effect curves to isoprenaline, with intact endothelium (n=17) or following de-endothelialization (n=8), or to isoprenaline with intact endothelium in the presence of L-NMMA 1 mM (n=9). (c) Concentration-effect curves to dibutyryl cyclic AMP in vascular rings with intact endothelium, in the absence or presence of L-NMMA 1 mM (n=7).
Table 1.
Vaserelaxant potency and efficacy of isoprenaline and dibutyryl cyclic AMP, and the effect of L-NMMA 1 mM

Evaluation of NOS activity: conversion of [3H]-L-arginine to [3H]-L-citrulline
Basal [3H]-L-citrulline production was 205.3±26.3 d.p.m. μg−1 protein (n=39). This increased by 34.0±5.9% in the presence of isoprenaline 1 μM (P<0.001). In other experiments (n=8), cyclic GMP content as measured by radioimmunoassay increased from 1.97±0.12 pmol/106 cells in control HUVEC to 3.47±0.12 pmol/106 cells in HUVEC following 20 min treatment with isoprenaline 1 μM, in the presence of 1 μM 3-isobutyl-1-methylxanthine (P<0.001). This suggests that the increased conversion of [3H]-L-arginine to [3H]-L-citrulline observed reflects an increase in NO generation, since NO stimulates soluble guanylyl cyclase activity.
[3H]-L-citrulline production was increased also by forskolin 1 μM or by dibutyryl cyclic AMP 1 mM, to an extent similar to isoprenaline (Figure 2). As a positive control, histamine 10 μM increased [3H]-L-citrulline production to a greater degree (58.2±13.8%). There was no further increase when a combination of histamine 10 μM and isoprenaline 1 μM was used (49.2±14.0%), indicating a lack of any additive effect by the two agonists on NOS activity in HUVEC. L-NMMA 0.1 mM prevented the increase in [3H]-L-citrulline production in response to all agonists tested and, in many cases, reduced [3H]-L-citrulline production to below basal values (Figure 2).
Figure 2.

[3H]-L-citrulline production from [3H]-L-arginine in HUVEC, expressed as percentage change (mean±s.e.mean) from control values, either in the absence (open bars) or presence (shaded bars) of L-NMMA 0.1 mM. Responses are shown to isoprenaline 1 μM (Isop), forskolin 1 μM (Forsk), dibutyryl cyclic AMP 1 mM (DbcAMP) and histamine 10 μM (Hist). **P<0.01 and ***P<0.001 respectively, vs control. ‡‡‡P<0.001 vs corresponding agonist in the absence of L-NMMA. Numbers adjacent to bars indicate number of umbilical cords tested.
[3H]-L-arginine content of the cells represents the sum of the counts associated with L-arginine and those converted to L-citrulline. [3H]-L-arginine content in control HUVEC was 518.3±103.5 d.p.m. μg−1 protein (n=39), suggesting that approximately 40% of L-arginine taken up by these cells was converted to L-citrulline (Table 2). Accumulation of [3H]-L-arginine within HUVEC was significantly increased by isoprenaline 1 μM, forskolin 1 μM or dibutyryl cyclic AMP 1 mM (Figure 3). Moreover, the ratio of intracellular [3H]-L-citrulline to [3H]-L-arginine was significantly increased by each of these agonists (Table 2), suggesting that the observed increase in L-citrulline production was not caused solely by an increase in L-arginine uptake. By contrast, histamine 10 μM had no effect on intracellular [3H]-L-arginine content, despite its greater effect on [3H]-L-citrulline production than the other agonists. L-NMMA 0.1 mM prevented the increase in [3H]-L-arginine accumulation in response to isoprenaline, forskolin or dibutyryl cyclic AMP.
Table 2.
Intracellular [3H]–L-citrulline/[3H]–L-arginine ratio in HUVEC following different treatments
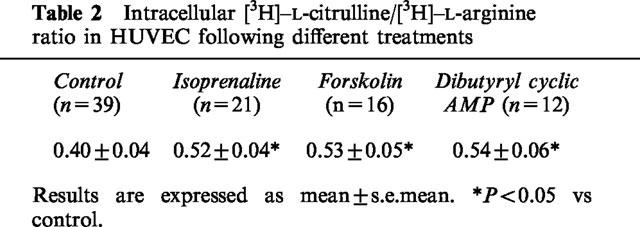
Figure 3.

[3H]-L-arginine content in HUVEC, following incubation with [3H]-L-arginine, expressed as percentage change (mean±s.e.mean) from control values, either in the absence (open bars) or presence (shaded bars) of L-NMMA 0.1 mM. Responses are shown to isoprenaline 1 μM (Isop), forskolin 1 μM (Forsk), dibutyryl cyclic AMP 1 mM (DbcAMP) and histamine 10 μM (Hist). *P<0.05 and ***P<0.001 respectively, vs control. ‡‡P<0.01 and ‡‡‡P<0.001 respectively, vs corresponding agonist in the absence of L-NMMA. Numbers adjacent to bars indicate number of umbilical cords tested.
L-NMMA inhibits both L-arginine uptake and NOS activity. In order to confirm that the inhibition of [3H]-L-citrulline production by L-NMMA observed in our experiments was not due simply to concomitant inhibition of L-arginine uptake, experiments were done also using NG-nitro-L-arginine methyl ester (L-NAME) 0.1 mM, which inhibits NOS with no effect on L-arginine uptake (Bogle et al., 1992). L-NAME abolished the increase in [3H]-L-citrulline production in response to isoprenaline, whereas the increase in [3H]-L-arginine content was unaffected (Figure 4).
Figure 4.
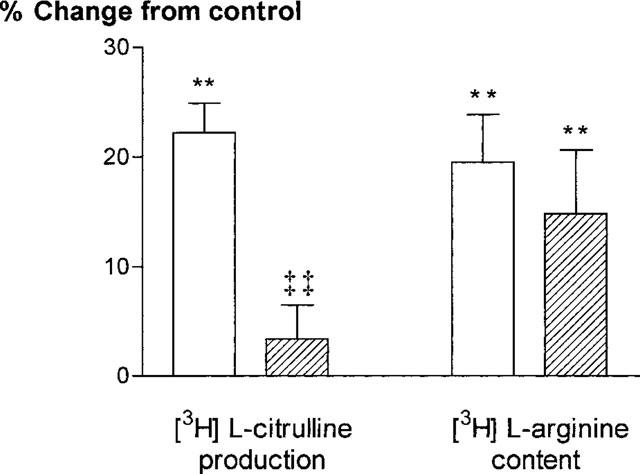
[3H]-L-citrulline production and [3H]-L-arginine content in HUVEC in response to isoprenaline 1 μM, expressed as percentage change (mean±s.e.mean) from control values, either in the absence (open bars) or presence (shaded bars) of L-NAME 0.1 mM. **P<0.01 vs control. ‡‡P<0.01 vs isoprenaline in the absence of L-NAME. Results are shown for HUVEC obtained from six different umbilical cords.
Measurement of cytoplasmic Ca2+
Treatment of HUVEC (n=4) with isoprenaline 1 μM consistently revealed no increase in intracellular Ca2+. By contrast, intracellular Ca2+ increased transiently in response to histamine 10 μM, used as a positive control, in all cases (Figure 5).
Figure 5.
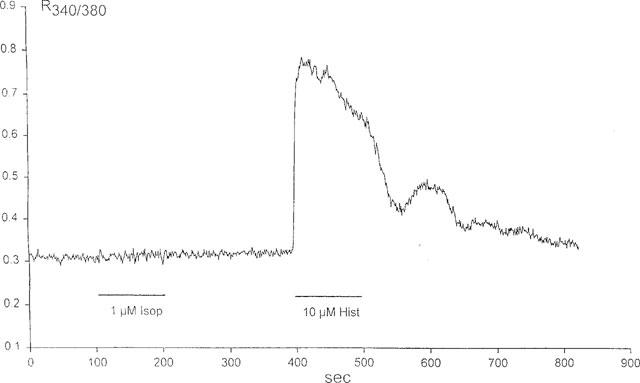
Ca2+ transients in a population of HUVEC in response to isoprenaline (Isop) 1 μM and to histamine (Hist) 10 μM, as determined by fura-2 fluorescence; the curve shows the ratio of emission at 530 nm following excitation at 340 nm to that following excitation at 380 nm.
Cyclic AMP assay
Cyclic AMP level in control HUVEC (n=5) was 4.1±0.3 pmol mg−1 protein. Treatment with isoprenaline 1 μM resulted in an increase to 7.3±0.9 pmol cyclic AMP mg−1 protein (P<0.01). Forskolin 1 μM elicited a larger increase, to 54.0±8.0 pmol cyclic AMP mg−1 protein (P<0.001).
Adenylyl cyclase activity in HUVEC
Basal adenylyl cyclase activity in HUVEC homogenates (n=6 cords, each assayed in triplicate) was 24.3±4.3 pmol cyclic AMP min−1 mg−1 protein. Treatment of HUVEC with isoprenaline elicited a concentration-dependent increase in adenylyl cyclase activity, with Emax 76.3±13.0 pmol cyclic AMP min−1 mg−1 protein (P<0.001). The increase in adenylyl cyclase activity in response to isoprenaline was inhibited by ICI 118551 but not by CGP 20712A, indicating involvement of β2- but not β1-adrenoceptors (Figure 6). Forskolin 10 mM, used as a positive control, caused a greater increase in adenylyl cyclase activity, to 143.4±25.5 pmol cyclic AMP min−1 mg−1 protein (P<0.001).
Figure 6.
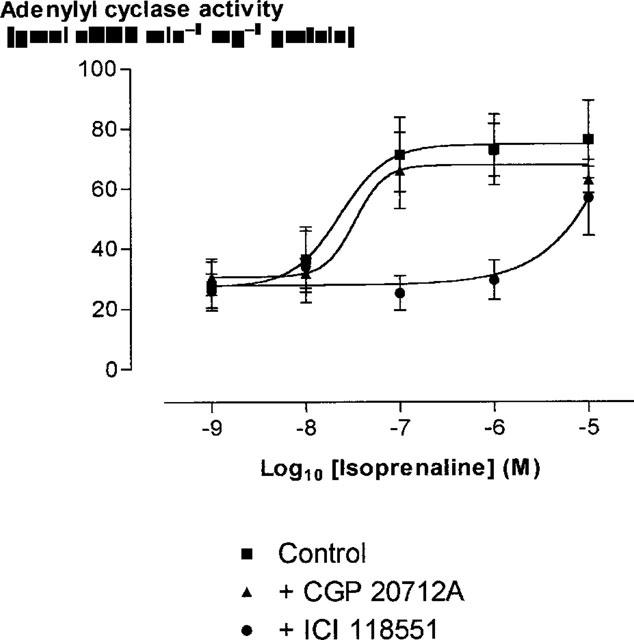
Mean±s.e.mean adenylyl cyclase activity in HUVEC homogenates (n=6) as a function of isoprenaline concentration. Responses are shown to isoprenaline alone, isoprenaline in the presence of CGP 20712A 300 nM, and isoprenaline in the presence of ICI 118551 50 nM.
Discussion
Previous in vitro and in vivo studies have suggested that β-adrenoceptor-mediated vasorelaxation may have an endothelium-dependent component in several animal species (Graves & Poston, 1993; Rubanyi & Vanhoutte, 1985; Kamata et al., 1989; Gardiner et al., 1991; Gray & Marshall, 1992; Blankesteijn & Thien, 1993; Parent et al., 1993; Rebich et al., 1995; Priest et al., 1997). The present results suggest that β-adrenoceptor-mediated vasorelaxation is completely endothelium-dependent in isolated human umbilical vein, at least at our chosen level of preconstriction. Isoprenaline induced a concentration-dependent relaxation of this vessel, which was abolished by removal of the endothelium or by co-incubation with L-NMMA. This vasorelaxation was attributable to β2-adrenoceptor subtype activation. Dibutyryl cyclic AMP also relaxed human umbilical vein, and the maximal degree of relaxation was higher than that achieved by isoprenaline; this response was partially, but not completely, inhibited by L-NMMA. This is consistent with an endothelium dependent component to vasorelaxation, caused by activation of the L-arginine/NO system in response to cyclic AMP elevation, although it is likely that there is also a substantial endothelium-independent component caused by direct elevation of cyclic AMP in vascular smooth muscle. Our studies of isolated HUVEC in culture confirmed the presence of functional β2-adrenoceptors, stimulation of which increased adenylyl cyclase activity. On the basis of these findings, we wished to determine whether β-adrenoceptor stimulation in isolated HUVEC could activate NOS, and the signal-transduction mechanism involved.
Stimulation of β-adrenoceptors in HUVEC with isoprenaline elicited an increase in the conversion of L-arginine to L-citrulline, indicating an increase in NOS activity. We have also demonstrated that isoprenaline activates adenylyl cyclase in HUVEC through stimulation of β2-adrenoceptors. The increase in NOS activity in response to β-adrenergic stimulation in HUVEC is likely to be mediated through cyclic AMP generation, since it was mimicked by forskolin, which elevates cytoplasmic cyclic AMP independently of β-adrenoceptors, and by dibutyryl cyclic AMP, a membrane-permeable cyclic AMP analogue. However, a maximal effect on NOS activity appears to be attained at relatively low levels of cyclic AMP, since forskolin increased NOS activity to a similar extent as isoprenaline, despite an approximately 10 fold greater effect on cyclic AMP.
Isoprenaline also increased intracellular [3H]-L-arginine content during incubation with [3H]-L-arginine in the medium, as did forskolin and dibutyryl cyclic AMP. Histamine, on the other hand, did not change HUVEC [3H]-L-arginine content, in agreement with a previous study (Sobrevia et al., 1995). Cyclic AMP may increase intracellular L-arginine, either through increased transport into the cell, decreased transport out of the cell or decreased metabolic conversion within the cell. An increase in intracellular L-arginine concentration might give rise to increased NOS activity, as observed, due to increased substrate availability. However, the intracellular L-arginine concentration in HUVEC has been reported as 100–800 μM (Gold et al., 1989; Baydoun et al., 1990; Mitchell et al., 1990), whilst the Km of the enzyme for L-arginine is <10 μM, with maximal stimulation detected at concentrations of L-arginine of 30–100 μM (Mayer et al., 1989; Palmer & Moncada, 1989). Nevertheless, despite saturating concentrations of intracellular L-arginine, extracellular L-arginine can increase endothelial NOS activity, and this so-called `arginine paradox' may be explained by caveolar co-localization of the y+ transporter and NOS in these cells, thus allowing direct transfer of extracellular L-arginine to the NOS enzyme (McDonald et al., 1997). However, we have found that the ratio of intracellular [3H]-L-citrulline to [3H]-L-arginine is significantly increased by β-adrenergic stimulation or cyclic AMP elevation in HUVEC, suggesting an increase in NOS activity above that which can be explained simply by an increase in L-arginine uptake. Our results also demonstrate that, even in the basal state, approximately 40% of radiolabelled L-arginine (present at an extracellular concentration of approximately 50 nM) taken up by HUVEC is converted to L-citrulline. This supports the view that extracellular L-arginine is transferred to endothelial NOS in preference to the general cytosolic pool of L-arginine, which is present at much higher concentration.
Although a rise in [3H]-L-citrulline in our system following activation of β-adrenoceptors or cyclic AMP is likely to reflect stimulation of NOS, it is possible that it reflects instead a decrease in argininosuccinate synthetase activity, since this enzyme is present in HUVEC and is involved in the conversion of L-citrulline back to L-arginine (MacAllister et al., 1994). However, since isoprenaline also increases cyclic GMP, which is an indicator of NO production, it is likely that the effect on [3H]-L-citrulline observed is due to an increase in synthesis rather than a decrease in disposal.
Graier et al. (1992) examined the possible role of cyclic AMP in NO formation in cultured porcine aortic endothelial cells; they found that, whilst forskolin, adenosine or isoprenaline (all of which stimulate cyclic AMP formation) alone did not increase NO synthesis, they amplified the formation of NO in response to bradykinin or ATP. This accords with the known ability of cyclic AMP to increase cyclic guanosine-3′,5′-monophosphate (cyclic GMP) levels. Our findings in HUVEC differ in that β-adrenergic stimulation or cyclic AMP elevation increased NOS activity, and isoprenaline 1 μM did not increase further the degree of activation of NOS induced by histamine 10 μM.
Agonists which have been shown previously to promote NO release from endothelium, including histamine, cause an influx of extracellular Ca2+ which, by promoting the binding of calmodulin to the endothelial constitutive isoform of NOS, leads to activation (Mayer et al., 1989; Mulsch et al., 1989). Indeed, we have confirmed that histamine caused a transient rise in Ca2+ in HUVEC. Isoprenaline was without demonstrable effect on Ca2+, suggesting that activation of NOS by β-adrenoceptor stimulation probably occurs by a novel mechanism not involving Ca2+ influx and calmodulin activation. It is unlikely to be due to activation of the inducible (Ca2+-independent) isoform of NOS, since cultured HUVEC have previously been found to express the endothelial isoform only, even following stimulation with proinflammatory cytokines (Rosenkranz-Weiss et al., 1994). Indeed, other workers in our laboratory have demonstrated that HUVEC in culture express the endothelial but not the inducible form of NOS, as determined by Western blotting using antibodies specific for each isoform (Campa, personal communication).
Sobrevia et al. (1997) have recently demonstrated that activation of A2-purinoceptors in HUVEC stimulates both L-arginine flux through the y+ transporter and NO synthesis. This also occurs with no change in intracellular Ca2+ concentration, but is attenuated by inhibition of tyrosine kinase activity. Since A2-purinoceptors activate adenylyl cyclase, and so increase cyclic AMP, it is possible that tyrosine kinase may also play a role in the signal-transduction pathway coupling β-adrenoceptor stimulation and NO biosysnthesis, possibly through direct phosphorylation of NOS (Corson et al., 1996). As in the case of A2-purinoceptors, such a mechanism may occur with no change in intracellular Ca2+. In addition, a possible role of cyclic AMP-dependent protein kinase (protein kinase A) in this process remains to be determined. Further studies are required to elucidate the mechanisms of β-adrenoceptor- and cyclic AMP-mediated activation of L-arginine accumulation and NO generation in these cells.
We have shown that endothelial β2-adrenoceptor activation stimulates NO production in HUVEC, and that this relaxes human umbilical vein. The physiological consequences of vascular endothelial NO production in response to β-adrenergic activation are at present unknown, but it is possible that this process is important in the normal control of vessel tone by the sympathoadrenal system. Furthermore, certain disease states, including atherosclerosis and heart failure, are associated both with endothelial dysfunction and with impaired β-adrenergic vasodilatation (Berkenboom et al., 1987; Bossaller et al., 1987; Drexler et al., 1992; Larosa & Forster, 1996). The present findings suggest that these abnormalities may be causally related.
In conclusion, we have shown that β2-adrenoceptors are present and functional in HUVEC, causing a rise in cyclic AMP production through adenylyl cyclase. We have also shown that β-adrenoceptor stimulation or cyclic AMP elevation by other means leads to an increase in NO production through the L-arginine/NOS system, and that this relaxes preconstricted umbilical vein. These findings clarify the mechanism of β-adrenoceptor action on human blood vessels and, if representative of other vascular beds, may be of physiological and pathophysiological importance.
Acknowledgments
This work was supported by project grants from the Medical Research Council and British Heart Foundation. B. Xu is a Wellcome Trust Travelling Research Fellow. We thank Amanda Wyatt and Professor Giovanni Mann for performing the cyclic GMP assays. We are grateful also to the obstetricians and midwives at St Thomas' Hospital for their assistance in providing umbilical cords.
Abbreviations
- ACh
acetylcholine
- BSS
balanced salt solution
- cyclic AMP
cyclic adenosine-3′,5′-monophosphate
- cyclic GMP
cyclic guanosine-3′,5′-monophosphate
- HUVEC
human umbilical vein endothelial cells
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- PBS
phosphate-buffered saline
References
- BAYDOUN A.R., EMERY P.W., PEARSON J.D., MANN G.E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- BÉA M.-L., GHALEH B., GIUDICELLI J.-F., BERDEAUX A. Lack of importance of NO in β-adrenoceptor-mediated relaxation of large epicardial canine coronary arteries. Br. J. Pharmacol. 1994;111:981–982. doi: 10.1111/j.1476-5381.1994.tb14839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERKENBOOM G., DEPIERREUX M., FONTAINE J. The influence of atherosclerosis on the mechanical responses of human isolated coronary arteries to substance P, isoprenaline and noradrenaline. Br. J. Pharmacol. 1987;92:113–120. doi: 10.1111/j.1476-5381.1987.tb11302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILSKI A., HALLIDAY S.E., FITZGERALD J.D., WALE J.L. The pharmacology of a β2-selective adrenoceptor antagonist (ICI 118,551) J. Cardiovasc. Pharmacol. 1983;5:430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- BLANKESTEIJN W.M., THIEN T. Effect of NG-monomethyl-L-arginine on the β-adrenoceptor-mediated relaxation of rat mesenteric resitance arteries. Life Sci. 1993;52:PL135–PL139. doi: 10.1016/0024-3205(93)90178-6. [DOI] [PubMed] [Google Scholar]
- BOGLE R.G., MONCADA S., PEARSON J.D., MANN G.E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br. J. Pharmacol. 1992;105:768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSSALLER C., HABIB G.B., YAMAMOTO H., WILLIAMS C., WELLS W., HENRY P.D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 3′,5′-monophosphate formation in the atherosclerotic human coronary artery and rabbit aorta. J. Clin. Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.A., PATEL V., WILKINSON G., BOARDER M.R. P2 purinoceptor-stimulated conversion of arginine to citrulline in bovine endothelial cells is reduced by inhibition of protein kinase C. Biochem. Pharmacol. 1996;52:1849–1854. doi: 10.1016/s0006-2952(96)00550-3. [DOI] [PubMed] [Google Scholar]
- BUXTON B.F., JONES C.R., MOLENAAR P., SUMMERS R.J. Characterization and autoradiographic localization of β-adrenoceptor subtypes in human cardiac tissues. Br. J. Pharmacol. 1987;92:299–310. doi: 10.1111/j.1476-5381.1987.tb11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORSON MA., JAMES N.L., LATTA S.E., NEREM R.M., BERK B.C., HARRISON D.G. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ. Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- DAWES M., CHOWIENCZYK P.J., RITTER J.M. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by β-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- DREXLER H., HAYOZ D., MUNZEL T., HORNIG B., JUST H., BRUNNER H.R., ZELIS R. Endothelial function in chronic congestive heart failure. Am. J. Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to acetylcholine, 5′-N-ethylcarboxamidoadenosine or salbutamol in conscious rats. Br. J. Pharmacol. 1991;103:1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD M.E., BUSH P.A., IGNARRO L.J. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem. Biophys. Res. Commun. 1989;164:714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- GRAIER W.F., GROSCHNER K., SCHMIDT K., KUKOVETZ W.R. Increases in endothelial cyclic AMP levels amplify agonist-induced formation of endothelium-derived relaxing factor (EDRF) Biochem. J. 1992;288:345–349. doi: 10.1042/bj2880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAIER W.F., SCHMIDT K., KUKOVETZ W.R. Effect of sodium fluoride on cytosolic free Ca2+ concentrations and cGMP levels in endothelial cells. Cell. Signalling. 1990;2:369–375. doi: 10.1016/0898-6568(90)90067-k. [DOI] [PubMed] [Google Scholar]
- GRAVES J., POSTON L. β-Adrenoceptor agonist mediated relaxation of rat isolated resistance arteries: a role for the endothelium and nitric oxide. Br. J. Pharmacol. 1993;108:631–637. doi: 10.1111/j.1476-5381.1993.tb12853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I. Novel signal transduction pathway mediating endothelium-dependent β-adrenoceptor vasorelaxation in rat thoracic aorta. Br. J. Pharmacol. 1992;107:684–690. doi: 10.1111/j.1476-5381.1992.tb14507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL R.E., ALBEDA S.M., DAISE M.L., LEVINE E.M. Characterization of β-adrenergic receptors in cultured human and bovine endothelial cells. J. Appl. Physiol. 1988;65:1251–1257. doi: 10.1152/jappl.1988.65.3.1251. [DOI] [PubMed] [Google Scholar]
- KAMATA K., MIYATA N., KASUYA Y. Involvement of endothelial cells in relaxation and contraction responses of the aorta to isoproterenol in naive and streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Therap. 1989;249:890–894. [PubMed] [Google Scholar]
- KAUMANN A.J. The β1-adrenoceptor antagonist CGP 20,712A unmasks β2-adrenoceptors activated by (−) adrenaline in the rat sino-atrial node. Naunyn Schmiedeberg's Arch. Pharmacol. 1986;333:73–76. doi: 10.1007/BF00500096. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., ITO Y., SUZUKI H., KITAMURA K., ITOH T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am. J. Physiol. 1982;243:H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- LAROSA G., FORSTER C. Coronary β-adrenoceptor function is modified by the endothelium in heart failure. J. Vasc. Res. 1996;33:62–70. doi: 10.1159/000159133. [DOI] [PubMed] [Google Scholar]
- LEFROY D.C., DONNELLY L.E., MCEWAN J.R., MACDERMOT J. Phorbol ester enhances activation of adenylate cyclase in bovine aortic endothelial cells. Life Sci. 1993;54:87–94. doi: 10.1016/0024-3205(94)00778-0. [DOI] [PubMed] [Google Scholar]
- LEMOINE H., EHLE B., KAUMANN A.J. Direct labelling of β2-adrenoceptors: comparisons of binding potency of 3H-ICI 118,551 and blocking potency of ICI 118,551. Naunyn Schmiedeberg's Arch. Pharmacol. 1985;331:40–51. doi: 10.1007/BF00498850. [DOI] [PubMed] [Google Scholar]
- MACALLISTER R.J., FICKLING S.A., WHITLEY G.S., VALLANCE P. Metabolism of methylarginines by human vasculature; implications for the regulation of nitric oxide synthesis. Br. J. Pharmacol. 1994;112:43–48. doi: 10.1111/j.1476-5381.1994.tb13026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD P.S., DUBBIN P.M., DUSTING G.J. β-Adrenoceptors on endothelial cells do not influence release of relaxing factor in dog coronary artries. Clin. Exp. Pharmacol. Physiol. 1987;14:525–534. doi: 10.1111/j.1440-1681.1987.tb01508.x. [DOI] [PubMed] [Google Scholar]
- MAYER B., SCHMIDT K., HUMBERT R., BOHME E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L-arginine into an activator of soluble guanylyl cyclase. Biochem. Biophys. Res. Commun. 1989;164:678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- MCDONALD K.K., ZHARIKOV S., BLOCK E.R., KILBERG M.S. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the `arginine paradox'. J. Biol. Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., HECKER M., ÄNGGÅRD E.E., VANE J.R. Cultured endothelial cells maintain their L-arginine level despite the continuous release of EDRF. Eur. J. Pharmacol. 1990;182:573–576. doi: 10.1016/0014-2999(90)90058-e. [DOI] [PubMed] [Google Scholar]
- MOLENAAR P., MALTA E., JONES C.R., BUXTON B.F., SUMMERS R.J. Autoradiographic localization and function of β-adrenoceptors on the human internal mammary artery and saphenous vein. Br. J. Pharmacol. 1988;95:225–233. doi: 10.1111/j.1476-5381.1988.tb16568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., REES D.D., SCHULZ R., PALMER R.M.J. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. USA. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULSCH A., BASSENGE E., BUSSE R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedeberg's Arch. Pharmacol. 1989;340:767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., MONCADA S. A novel citrulline forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;158:348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- PARENT R., AL-OBAIDI M., LAVALLÉE M. Nitric oxide formation contributes to β-adrenergic dilation of resistance coronary vessels in conscious dogs. Circ. Res. 1993;73:241–251. doi: 10.1161/01.res.73.2.241. [DOI] [PubMed] [Google Scholar]
- PRIEST R.M., HUCKS D., WARD J.P.T. Noradrenaline, β-adrenoceptor mediated vasorelaxation and nitric oxide in large and small pulmonary arteries of the rat. Br. J. Pharmacol. 1997;122:1375–1384. doi: 10.1038/sj.bjp.0701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBICH S., DEVINE J.O., ARMSTEAD W.M. Role of nitric oxide and cAMP in β-adrenoceptor-induced pial artery vasodilation. Am. J. Physiol. 1995;268:H1071–H1076. doi: 10.1152/ajpheart.1995.268.3.H1071. [DOI] [PubMed] [Google Scholar]
- ROSENKRANZ-WEISS P., SESSA W.C., MILSTIEN S., KAUFMAN S., WATSON C.A., POBER J.S. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells: elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J. Clin. Invest. 1994;93:2236–2243. doi: 10.1172/JCI117221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G., VANHOUTTE P.M. Endothelium-removal decreases relaxations of canine coronary arteries caused by β-adrenergic agonists and adenosine. J. Cardiovasc. Pharmacol. 1995;7:139–144. doi: 10.1097/00005344-198501000-00023. [DOI] [PubMed] [Google Scholar]
- SALOMON Y., LONDOS C., RODBELL M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- SOBREVIA L., CESARE P., YIDILEVICH D.L., MANN G.E. Diabetes-induces activation of system y+ and nitric oxide synthase in human endothelial cells: association with membrane hyperpolarization. J. Physiol. 1995;489:183–192. doi: 10.1113/jphysiol.1995.sp021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBREVIA L., YUDILEVICH D.L., MANN G.E. Activation of A2-purinoceptors by adenosine stimulates L-arginine transport (system y+) and nitric oxide synthesis in human fetal endothelial cells. J. Physiol. 1997;499:135–140. doi: 10.1113/jphysiol.1997.sp021916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG S.F., JAFFE E.A., BILEZIKIAN J.P. Endothelial cells contain beta adrenoceptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1984;325:310–313. doi: 10.1007/BF00504374. [DOI] [PubMed] [Google Scholar]
- SUMMERS R.J., MOLENAAR P., STEPHENSON J.A., JONES C.R. Autoradiographic localization of receptors in the mammalian cardiovascular system. Clin. Exp. Pharmacol. Physiol. 1987;14:437–447. doi: 10.1111/j.1440-1681.1987.tb00995.x. [DOI] [PubMed] [Google Scholar]


