Abstract
Phosphodiesterase (PDE) 4 inhibitors have been shown to inhibit eosinophil PDE4 activity in vitro and accumulation of eosinophils in experimental airways inflammation. However, direct effects on eosinophil trafficking have not been studied in detail and it is not known if activity in vitro translates into efficacy in vivo. In the present study, we compared the activity of five PDE4 inhibitors in vitro and against trafficking of 111In-eosinophils in cutaneous inflammation in the guinea-pig.
The rank order of potency for inhibition of PDE4 activity in guinea-pig eosinophil, neutrophil and macrophage, and human neutrophil lysates was RP73401>SB207499>CDP840>rolipram>LAS31025. On TNFα production by human PBMC, all inhibitors with the exception of rolipram showed potency similar to their effect on neutrophil lysates.
In a brain cerebellum binding assay, the rank order of potency at displacing [3H]-rolipram was RP73401>rolipram>SB207499>CDP840>LAS30125.
Trafficking of 111In-eosinophils to skin sites injected with PAF, ZAP or antigen in sensitized sites was inhibited by oral administration of all PDE4 inhibitors. The rank order of potency was RP73401=rolipram>LAS31025>SB207499>CDP840.
With the exception was RP73401, which was the most potent compound in all assays, there was no clear relationship between activity of PDE4 inhibitors in vitro and capacity to inhibit eosinophil trafficking in vivo. Thus, we conclude that in vitro activity of PDE4 inhibitors does not predict in vivo efficacy in an experimental model of eosinophil trafficking.
Keywords: Eosinophil, eosinophil trafficking, phosphodiesterase, phosphodiesterase 4 inhibitors, rolipram, RP73401, LAS31025, SB207499, CDP840
Introduction
Eosinophils are considered to have important effector functions in chronic allergic diseases such as asthma (Venge, 1990), rhinitis (Klementsson, 1992) dermatitis (Bruijnzeel-Koonen et al., 1992) and conjunctivitis (Foster et al., 1991). They are often the predominant leukocyte type in these diseases and through secretion of a cocktail of lipid and protein mediators are thought to modulate bronchial smooth muscle tone in the airways, cause oedema formation and influence the function of other cells (Martin et al., 1996). Cationic proteins (e.g. major basic protein and eosinophil-cationic protein), stored in eosinophil granules and released upon activation, are important for host defence against parasites. Misdirected release of these proteins in allergic inflammation damages host epithelial cells thus contributing to disease pathology (Wardlaw et al., 1988; Montefort et al., 1992). Controlling accumulation and activation of eosinophils may offer therapeutic benefit in allergic diseases. However, a detailed understanding of the mechanisms underlying eosinophil accumulation in vivo is essential to the development of new and safe therapeutic strategies based on reduced recruitment of these cells (Teixeira et al., 1995).
One strategy to control tissue eosinophilia and eosinophil activation is to increase intracellular levels of cyclic AMP in eosinophils and other cells that participate in the inflammatory process (Teixeira et al., 1997). The intracellular levels of cyclic AMP are regulated by the rate of cyclic AMP production by receptor-coupled adenylate cyclase and the rate of cyclic AMP degradation by 3′,5′-cyclic nucleotide phosphodiesterases (PDE). Seven distinct families of PDEs have been described based on genetic, biochemical and pharmacological data (Beavo et al., 1994; Torphy, 1998). Of these families, PDE4 appears to be the most important for the regulation of cyclic AMP levels in eosinophils (Dent et al., 1991; Souness et al., 1991). In accordance with the importance of PDE4 for the control of cyclic AMP levels in eosinophils, inhibitors of this enzyme have been shown to raise cyclic AMP in eosinophils and suppress a range of functions, including the respiratory burst, enzyme release, chemotaxis, lipid mediator production, homotypic aggregation and elevation of intracellular Ca2+ (reviewed in Teixeira et al., 1997; Torphy, 1998). Moreover, several structurally different inhibitors of PDE4 have been shown to inhibit the accumulation of eosinophils in a range of animal models of allergic inflammation (reviewed in Teixeira et al., 1997). While a number of studies have compared the capacity of these structurally unrelated PDE4 inhibitors to inhibit leukocyte function in vitro (e.g. Barnette et al., 1996), much less is known about the comparative efficacy and potency of PDE4 inhibitors on eosinophil trafficking in vivo.
In guinea-pig skin, the intradermal injection of different known mediators of inflammation or of antigen, in sites previously sensitized with an antigen-specific (BGG) IgG1-rich anti-serum (passive cutaneous anaphylaxis, PCA reaction), leads to a dose-dependent rapid recruitment of intravenously injected 111In-labelled eosinophils (Faccioli et al., 1991; Teixeira et al., 1993a). In the PCA reaction, the mediators responsible for cell accumulation have not been fully characterized, but a 5-lipoxygenase product, probably LTB4, appears to play an important role (Teixeira & Hellewell, 1994). The mechanism by which 111In-eosinophils accumulate in guinea-pig skin has been demonstrated to be dependent on fucoidin-sensitive selectins and β1 and β2 integrins (Weg et al., 1993; Teixeira et al., 1994a; Teixeira & Hellewell, 1997b). In this model, the systemic (i.p. plus i.v.) administration of the PDE4 inhibitor rolipram, but not of inhibitors of PDE3 or PDE5, effectively suppressed the recruitment of eosinophils into inflamed skin sites (Teixeira et al., 1994b).
The aim of the present study was to compare the capacity of five selective PDE4 inhibitors, rolipram, RP73401 (Raeburn et al., 1994), SB207499 (Barnette et al., 1998), LAS31025 (Beleta et al., 1996) and CDP840 (Perry et al., 1998) to modulate eosinophil trafficking in guinea-pig skin. As our earlier studies showed that rolipram had no effect on neutrophil trafficking in guinea-pig skin (despite abrogating eosinophil trafficking under similar conditions), we took the opportunity to re-examine the effects of these inhibitors on neutrophil accumulation. To determine whether activities of these compounds on PDE4 in vitro would predict activity in vivo, we first compared the ability of these drugs to inhibit human and guinea-pig PDE4 isoenzyme activity in whole cells and cell extracts.
Methods
Induction, harvesting and purification of guinea-pig eosinophils, neutrophils and macrophages
Eosinophils were elicited in the peritoneal cavity as described previously (Teixeira et al., 1993a). Briefly, female guinea-pigs (Harlan, Bicester; 500–600 g) were treated with horse serum (1 ml i.p.) every other day for 2 weeks and the cells collected by peritoneal lavage with heparinized saline (10 iu ml−1) 2 days after the last injection. The cells obtained were layered onto a discontinuous Percoll-HBSS (Ca2+- and Mg2+-free) gradient followed by centrifugation (1500×g, 25 min at 20°C). Eosinophils (>95% pure, >98% viable as assessed by trypan blue exclusion) were collected from the 1.090/1.095 and 1.095/1.100 g ml−1 density interfaces.
Neutrophils were elicited in the peritoneal cavity of female guinea-pigs (500–600 g) by the i.p. injection of 15 ml of a 5% (w v−1) solution of casein as previously described (Teixeira et al., 1993b). After 12 h, the animals were sacrificed and the peritoneal cavity washed with heparinized saline (10 iu ml−1). The rest of the procedure was followed as described for the eosinophils. The cells were also collected from the 1.090/1.095 and 1.095/1.100 g ml−1 interfaces. The purity of the preparation was greater than 98% and the rare contaminants were eosinophils and occasional mononuclear cells. Viability was greater than 98%.
Macrophages were elicited in the peritoneal cavity of male guinea-pigs (400–450 g) by a single i.p. injection of horse serum followed by lavage 7 days later. Cells were layered onto a discontinuous Percoll gradient and centrifuged at 1600×g for 20 min at 20°C according to the method of Gartner (1980). Macrophages, >98% pure, were collected from the 1.070/1.075 g ml−1 interface.
Purification of human neutrophils
Buffy coats from human blood were obtained from the Blood Transfusion Service (Cambridge) and mixed with an equal volume of 3% dextran to allow sedimentation of red blood cells. The leukocyte rich supernatant was layered on to an equal volume of Ficoll and centrifuged at 1000×g for 30 min at 20°C. Neutrophils (>95% pure) were recovered in the pellet and remaining red cells were lysed using ammonium chloride lysis buffer (in mM: NH4Cl2 155, KHCO3 10 and EDTA 0.1).
Preparation of cell lysates
Neutrophils, eosinophils or macrophages were lysed for 30 min on ice at a concentration of 3.2×107 cell ml−1 in solution containing 70% lysis buffer (in mM: MOPS 10, EGTA 1, magnesium acetate 1 and dithiothreitol 5, pH 7.4) and 30% ethylene glycol. Cell lysates were stored at −80°C.
Measurement of cyclic AMP PDE activity
PDE4 activity of cell lysates was assayed using a high throughput variation of the method of Thompson et al. (1979). The reaction is based on the breakdown of [3H]-cyclic AMP by PDE4 to the corresponding 5′-monophosphate, which is subsequently dephosphorylated by snake venom nucleotidase (Ophiphagus hannah).
The assay was carried out in 96-well Millipore filtration plates (Durapore, Millipore Ltd., Watford, Herts, U.K.) that were prewashed in ice cold 0.9% saline. The reaction was buffered to pH 7.5 in Tris-HCl (80 mM), MgCl2 (20 mM), mercaptoethanol (12 mM) and 10 mg ml−1 BSA and each well contained the following: [3H]-cyclic AMP (approximately 30,000 c.p.m.), cyclic AMP (12.5 pmol) and 10 μg nucleotidase. The reaction was started by the addition of 10 μl cell lysate to produce 10–20% substrate hydrolysis. PDE4 inhibitors were solubilized in DMSO, diluted in assay buffer and added to duplicate wells at a range of concentrations (final DMSO concentration 0.5%). Plates were incubated for 30 min at 30°C and the reaction terminated by addition of 80 μl 30% Dowex AX resin. After mixing, the plates were filtered on a Millipore filtration system and the supernatants collected into a 96-well optiplate. Two hundred μl Microscint 40 (Canberra Packard, Pangbourne) was added to each well, plates sealed, mixed and counted on a Packard TopCount scintillation counter (Canberra Packard).
TNFα production by peripheral blood mononuclear cells
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats by centrifugation on a density gradient of Ficoll-Paque. PBMC were harvested, washed three times, resuspended at 2×106 cell ml−1 in RPMI 1640 medium containing 2% FBS and plated in 48-well tissue culture plates. PDE4 inhibitors were solubilized in DMSO, diluted in RPMI and added to duplicate wells at a range of concentrations (final DMSO concentration 0.5%). Cells were stimulated with lipolysaccharide at a final concentration of 100 ng ml−1 and incubated for 22 h at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were pelleted by centrifugation and TNFα in the supernatant assayed using ELISA (R&D Systems, Abingdon). Cell viability was not significantly affected by any of the PDE4 inhibitors when tested up to five times their IC50 concentrations (data not shown). The concentration of TNFα in control supernatants was below detection limits and in supernatants from LPS stimulated cells was 2.59±0.5 ng ml−1 (n=3).
Rolipram binding assay
Rat brain membranes were used as a source of high affinity rolipram binding protein (RBP). The binding assay was a high throughput version based upon a method described by Schneider et al. (1986). Briefly, assay buffer (in mM: Tris-HCl 20, MgCl2 2, dithiothreitol 0.1, pH 7.5). PDE4 inhibitor and [3H]-rolipram (approximately 300,000 d.p.m.) were pipetted into 96-well Millipore microtitre plates. One hour after addition of RBP (100 μg per well), reactions were terminated by filtration (Millipore), filtered protein washed and dried followed by addition of Microscint 0 and counting on a Packard TopCount scintillation counter.
Radiolabelling of guinea-pig eosinophils and neutrophils for in vivo trafficking studies
The purified eosinophils and neutrophils were radiolabelled by incubation with 111InCl3 (100 μCi in 10 μl) chelated to 2-mercaptopyridine-N-oxide (40 μg in 0.1 ml of 50 mM PBS, pH 7.4) for 15 min at room temperature. The cells were then washed twice in HBSS (calcium- and magnesium-free) containing 10% guinea-pig platelet-poor plasma and resuspended at a final concentration of 107 cells ml−1 prior to injection.
Preparation of zymosan-activated plasma
Zymosan-activated plasma (ZAP) was used as a source of guinea-pig C5a des Arg. Guinea-pig heparinized (10 iu ml−1) plasma was incubated with zymosan (5 mg ml−1) at 37°C. After 30 min, zymosan was removed by centrifugation (2×10 min at 3000×g) and the ZAP stored in aliquots at −20°C.
Preparation of passive cutaneous anaphylaxis sera and reactions
Details of the preparation of sera and doses of antigen are described elsewhere (Weg et al., 1991). Briefly, male guinea-pigs (Harlan, Oxon; 350–400 g) were immunized with bovine gamma-globulin (BGG) in Freund's complete adjuvant followed by a boost on day 21 and serum collected on day 30. Recipient animals received an i.d. injection of 50 μl of a 1/50 dilution of the anti-serum followed, 16–20 h later, by the injection of antigen (BGG, 0.01–1 μg per site). Most of the tissue-fixing antibody was of the IgG1 isotype (Weg et al., 1991).
Measurement of leukocyte trafficking in guinea-pig skin
Radiolabelled eosinophils or neutrophils were injected i.v. (2.5×106 cells per animal) into recipient guinea-pigs (Harlan; 350–400 g) sedated with Hypnorm. PDE4 inhibitors were solubilized in 10% DMSO (v v−1 in sterile water) and administered by oral gavage. Fifty-five minutes later, radiolabelled leukocytes were injected i.v. via an ear vein and, 5 min after this, inflammatory mediators or antigen were injected i.d. in 0.1 ml volumes into the dorsal skin of the shaved animals. Thus, the total time between oral administration and induction of cutaneous inflammation was 1 h. Each animal received a duplicate of each i.d. treatment following a randomized injection plan and 111In-labelled cell accumulation was assessed after 1 h. At this time, blood was obtained by cardiac puncture and the animals were sacrificed by an overdose of sodium pentobarbitone. The dorsal skin was removed, cleaned free of excess blood and the skin sites punched out with a 17 mm punch. The samples were counted in an automatic 5-head gamma-counter (Canberra Packard) and the number of leukocyte accumulating in each site expressed as 111In-labelled cells per skin site.
Reagents
The following compounds were purchased from Sigma Chemical Company (Poole, Dorset, U.K.): 2-mercaptopyridine-N-oxide, DMSO, casein, bovine gamma globulin (BGG), dithiothreitol, ethylene glycol, Freund's complete adjuvant, zymosan, cyclic AMP and snake venom (Ophiphagus hannah). Hanks solutions, HEPES and horse serum were purchased from Life Technologies Limited (Paisley, Scotland). Dextran, Ficoll, Ficoll-Paque and Percoll were from Pharmacia (Milton Keynes, Bucks, U.K.) and C16 PAF from Bachem (Saffron Walden, Essex, U.K.). 111InCl3 and [3H]-cyclic AMP (25 Ci mmol−1), [methyl-3H]-rolipram (21 Ci mmol−1) and 111InCl3 were purchased from Amersham International plc, Amersham. The following selective PDE4 isoenzyme inhibitors were synthesized by the Chemistry Department at Chiroscience: rolipram [4-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidi-none], RP73401 [N-(3,5-dichloropyrid-4-yl)-3-cyclopentyloxy-4-methoxybenzamide] (Raeburn et al., 1994), LAS31025 [1-propyl-3-(4-chlorophenyl)-xanthine] (Beleta et al., 1996), SB207499 [c-4-cyano-4-(3-cyclopentlyoxy-4-methoxyphenyl)-r-1-cyclohexanecarboxylic acid] (Barnette et al., 1998) and the sulphate salt of CDP840 (R-[+]-4-[2-3-cyclopentyloxy-4-methoxyphenyl-2-phenylethyl]pyridine) Perry et al., 1998).
Statistical analysis
For the neutrophil experiments, results were compared using analysis of variance and P values assigned using Student-Newman-Keuls (Instat Software). Per cent inhibition was calculated after subtracting background (saline) values. Results were presented as the mean±s.e.mean for the number of animals given and were considered significant when P<0.05.
Results
Effects of PDE4 inhibitors on guinea-pig and human leukocyte PDE4 activity
Before conducting the in vivo studies, we wished to confirm the activity of the PDE4 inhibitors against guinea-pig and, for comparison, human PDE4 in whole cells and cell lysates. Figure 1 shows the dose-inhibition curves for all five compounds on the PDE4 activity isolated from guinea-pig eosinophils. Whereas all agents almost abrogated guinea-pig eosinophil PDE4 activity at the highest concentrations tested, RP73401 was the most potent. The rank order of potency for inhibition of the guinea-pig eosinophil PDE4 activity was RP73401>SB207499>CDP840>rolipram>LAS31025 (Table 1). A similar rank order of potency for inhibition of PDE4 was observed when these compounds were tested against the enzyme activity in lysates of guinea-pig neutrophils and macrophages (Table 1).
Figure 1.
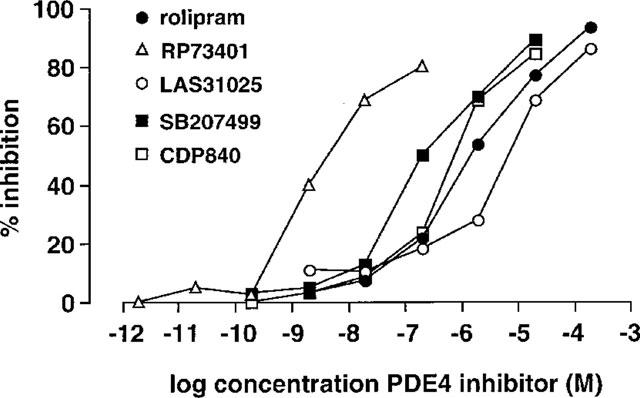
Inhibition of PDE4 activity in lysates of guinea-pig eosinophils by PDE4 inhibitors. Values are means of at least five different experiments. For clarity, error bars have been omitted.
Table 1.
Comparison of the ability of PDE4 inhibitors to suppress PDE4 activity in vitro
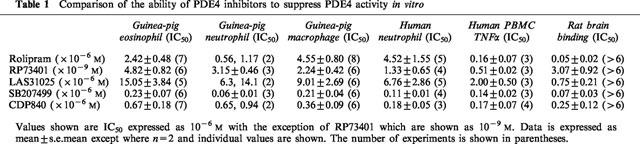
On human neutrophil lysates, the compounds also inhibited PDE4 activity with a similar rank order of potency to that seen with guinea-pig cells (Table 1). When tested against TNFα production by human PBMC, all inhibitors with the exception of rolipram showed potency similar to their effect on neutrophil lysates (Table 1). In contrast, rolipram was approximately 30 times more potent as an inhibitor of TNFα production by PBMC than it was as an inhibitor of enzyme activity in neutrophil lysates.
Rolipram binding assay
In our hands, RP73401 was the most potent in this assay and was approximately 10 fold more potent than rolipram. SB207499 showed similar potency to rolipram, CDP840 was 5 fold less potent than rolipram and LAS30125 was the least potent.
Effects of PDE4 inhibitors on eosinophil trafficking in guinea-pig skin
We have previously shown that systemic treatment with rolipram (5 mg kg−1 i.p. plus 0.5 mg kg−1 i.v.), but not with PDE3 or PDE5 inhibitors, effectively inhibited 111In-eosinophil recruitment induced by several inflammatory mediators and in a PCA reaction in guinea-pig skin (Teixeira et al., 1994b). In the present study, oral treatment with rolipram effectively and dose-dependently inhibited 111In-eosinophil recruitment induced by PAF (10−9 mol per site), ZAP (30%) and in the PCA reaction (1 μg of BGG per site) (Figure 2). Maximal inhibition of 111In-eosinophil recruitment was achieved at a dose of 2 mg kg−1 of rolipram such that the response in the PCA reaction was virtually abolished (Figure 2). The IC50 values (mg kg−1) for inhibition of 111In-eosinophil recruitment induced by PAF, ZAP and in the PCA reaction in guinea-pig skin by oral treatment with rolipram are shown in Table 2.
Figure 2.
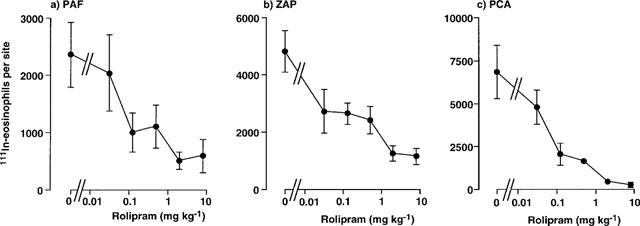
Effect of oral administration of rolipram on the trafficking of 111In-eosinophils to skin sites injected with PAF, ZAP or in the PCA reaction. Rolipram was administered p.o. 1 h before i.v. injection of 111In-eosinophils and i.d. injection of PAF (1 nmol), ZAP (30%) or antigen (1 μg BGG, shown as PCA). Accumulation of 111In-eosinophils in sites was assessed after 1 h. Values are mean±s.e.mean of experiments in 3–10 animals at each dose and have been subtracted for saline values (189±22 111In-eosinophils per site).
Table 2.
IC50 values for inhibition of 111In-eosinophil recruitment in guinea-pig skin by oral administration of PDE4 inhibitors
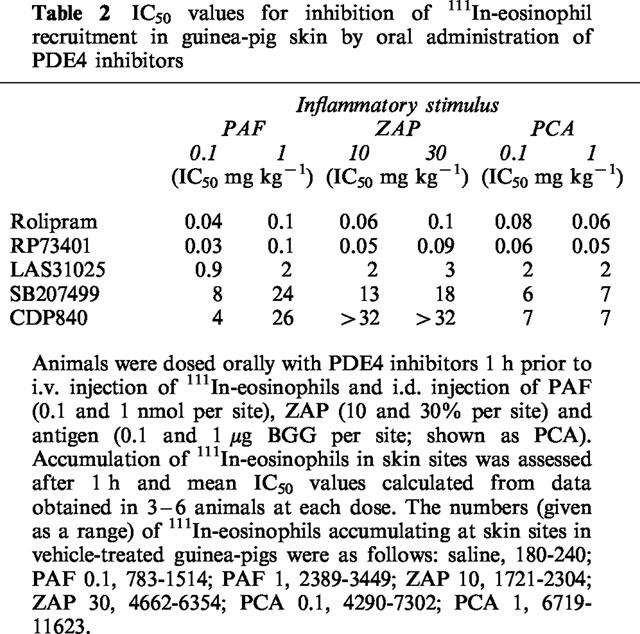
Figure 3 shows the effects of oral administration of RP73401 on 111In-eosinophil recruitment in guinea-pig skin. As found with rolipram, RP73401 abrogated 111In-eosinophil recruitment induced by PAF and ZAP and in the PCA reaction. Inhibition was maximal at 0.5 mg kg−1 and, similarly to the effects of rolipram, the trafficking of radiolabelled eosinophils in the PCA reaction was virtually abolished (Figure 3). RP73401 was of similar potency to rolipram but substantially more potent than the other PDE4 inhibitors tested (Table 2). Both LAS31025 (Figure 4) and SB207499 (Figure 5) abolished 111In-eosinophil recruitment in the PCA reaction in guinea-pig skin at the highest doses tested (8 and 32 mg kg−1, respectively). The 111In-eosinophil recruitment induced by PAF and ZAP was also effectively inhibited, although the maximum inhibition by SB207499 of the response to PAF (10−9 mol per site) was 58% (Figure 5).
Figure 3.
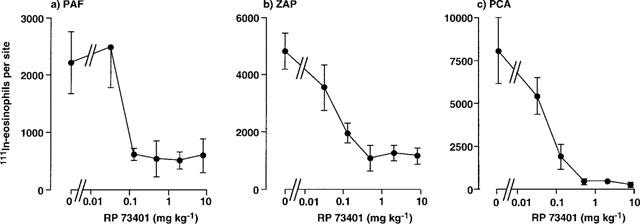
Effect of oral administration of RP73401 on the trafficking of 111In-eosinophils to skin sites injected with PAF, ZAP or in the PCA reaction. RP73401 was administered p.o. 1 h before i.v. injection of 111In-eosinophils and i.d. injection of PAF (1 nmol), ZAP (30%) or antigen (1 μg BGG, shown as PCA). Accumulation of 111In-eosinophils in sites was assessed after 1 h. Values are mean±s.e.mean of experiments in 3–9 animals at each dose and have been subtracted for saline values (180±20 111In-eosinophils per site).
Figure 4.
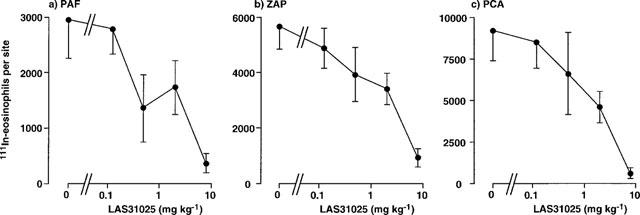
Effect of oral administration of LAS31025 on the trafficking of 111In-eosinophils to skin sites injected with PAF, ZAP or in the PCA reaction. LAS31025 was administered p.o. 1 h before i.v. injection of 111In-eosinophils and i.d. injection of PAF (1 nmol), ZAP (30%) or antigen (1 μg BGG, shown as PCA). Accumulation of 111In-eosinophils in sites was assessed after 1 h. Values are mean±s.e.mean of experiments in 4–7 animals at each dose and have been subtracted for saline values (240±46 111In-eosinophils per site).
Figure 5.
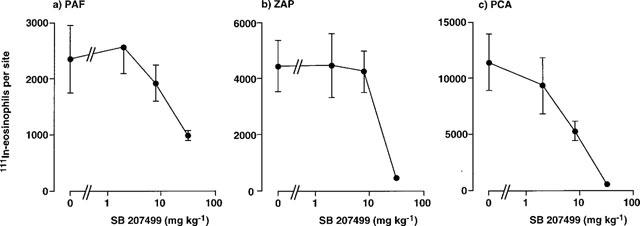
Effect of oral administration of SB207499 on the trafficking of 111In-eosinophils to skin sites injected with PAF, ZAP or in the PCA reaction. SB207499 was administered p.o. 1 h before i.v. injection of 111In-eosinophils and i.d. injection of PAF (1 nmol), ZAP (30%) or antigen (1 μg BGG, shown as PCA). Accumulation of 111In-eosinophils in sites was assessed after 1 h. Values are mean±s.e.mean of experiments in 4–6 animals at each dose and have been subtracted for saline values (233±58 111In-eosinophils per site).
In contrast to the inhibitory effects of the PDE4 inhibitors described above, CDP840 only partially inhibited the 111In-eosinophil recruitment in the PCA reaction (maximal inhibition was 57% at 32 mg kg−1) (Figure 6). Moreover, the maximal inhibition of ZAP- and PAF-induced was 39 and 52%, respectively (Figure 6). Of the compounds tested, CDP840 was the least potent and the least effective (Figure 6 and Table 2).
Figure 6.
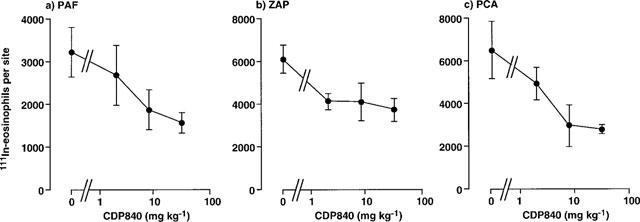
Effect of oral administration of CDP840 on the trafficking of 111In-eosinophils to skin sites injected with PAF, ZAP or in the PCA reaction. CDP840 was administered p.o. 1 h before i.v. injection of 111In-eosinophils and i.d. injection of PAF (1 nmol), ZAP (30%) or antigen (1 μg BGG, shown as PCA). Accumulation of 111In-eosinophils in sites was assessed after 1 h. Values are mean±s.e.mean of experiments in 4–6 animals at each dose and have been subtracted for saline values (239±33 111In-eosinophils per site).
Thus, the overall rank order of potency for inhibition of 111In-eosinophil recruitment in guinea-pig skin by oral administration of PDE4 inhibitors was RP73401=rolipram>LAS31205>SB207499⩾CDP840. In addition, eosinophil trafficking in the PCA reaction was inhibited to a greater extent than were responses to PAF and ZAP. No toxic effects of compounds were observed over the 2 h duration of the experiments.
Effects of PDE4 inhibitors on neutrophil trafficking in guinea-pig skin
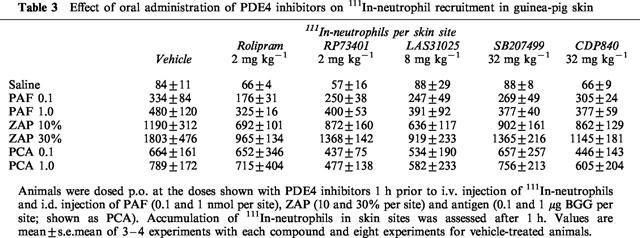
In contrast to its marked inhibitory effect on 111In-eosinophil recruitment in guinea-pig skin, we have previously reported that rolipram failed to suppress the 111In-neutrophil recruitment induced by several inflammatory mediators and in a PCA reaction (Teixeira et al., 1994b). Table 3 shows the effects of rolipram, RP73401, LAS31205, SB207499 and CDP840 on 111In-neutrophil recruitment induced by PAF, ZAP and in the PCA reaction in guinea-pig skin. The doses of the drugs used were chosen based on their ability to inhibit maximally the 111In-eosinophil recruitment induced by the same mediators. At these doses, none of the drugs had any significant effect on the recruitment of 111In-neutrophil induced any of the stimuli used (Table 3).
Table 3.
Effect of oral administration of PDE4 inhibitors on 111In-neutrophil recruitment in guinea-pig skin
Discussion
There has been much interest in the development of PDE4 inhibitors for the treatment of allergic diseases, especially asthma. In laboratory animals these drugs have potent anti-inflammatory effects under diverse situations and, in the context of allergic diseases, have been shown to inhibit recruitment and function of eosinophils and other leukocytes and the release and action of several inflammatory mediators and cytokines (Teixeira et al., 1997; Torphy, 1998). In addition, in the context of asthma, inhibitors of PDE4 may act synergistically with β2-adrenoceptor agonists to induce significant bronchodilation (Giembycz & Dent, 1992). In the present study, we have evaluated the effects of five structurally different inhibitors of PDE4 (rolipram, RP73401, SB207499, CDP840 and LAS31205) for their ability to suppress PDE4 activity in vitro and eosinophil and neutrophil trafficking in vivo. In contrast to other studies that have evaluated the effects of PDE4 inhibitors on eosinophil migration in lung models where PDE4 inhibitors may affect eosinophil migration indirectly (for example by effects on T cells or macrophages), here we are addressing the effects of these drugs on eosinophil trafficking directly.
Rolipram appears to bind to purified PDE4 with two distinct affinities; a binding site to which rolipram has μM affinity and one to which rolipram has nM affinity. The former is usually referred to as the PDE4 catalytic site and the latter as the `rolipram-binding site'. Recently, it has been proposed that these two sites represent different conformational states of PDE4; LPDE4 is the conformer to which rolipram binds with low affinity and HPDE4 is the conformer to which rolipram binds with high affinity (Barnette et al., 1995b; Jacobitz et al., 1996; Kelly et al., 1996). All five PDE4 inhibitors tested here completely suppressed the catalytic activity of the enzyme obtained from leukocytes with the following rank order of potency: RP73401>SB207499>CDP840>rolipram>LAS31025. We also tested the ability of these drugs to interact with the HPDE4 in rat cerebellum. The rank order of potency for inhibition in this assay was RP73401>rolipram>SB207499>CDP840>LAS31205.
Several studies have shown that the ability of PDE4 inhibitors to interact with LPDE4 usually correlates with the ability of these drugs to inhibit several leukocyte functions in vitro (Barnette et al., 1995b; 1996; 1998). For example, in guinea-pig eosinophils, the ability of PDE4 inhibitors to suppress superoxide production in vitro correlates with inhibition of the catalytic activity of PDE4 isolated from these cells (Barnette et al., 1995b). In the present study, we found that inhibition of TNFα production by LPS-stimulated PBMC also correlates with inhibition of PDE4 catalytic activity (see Table 1). In contrast, there is a poor correlation between the ability of PDE4 inhibitors to suppress some leukocyte functions and their ability to bind to the HPDE4 (Barnette et al., 1996). The capacity of PDE4 inhibitors to have anti-inflammatory activity has therefore been attributed to inhibition of LPDE4 while side effects have been attributed to binding to HPDE4 (Barnette et al., 1995a; Duplantier et al., 1996). The importance of these different conformational states of the enzyme to the anti-inflammatory activity of PDE4 inhibitors in vivo is not yet known. Here, we have tested a range of structurally unrelated PDE4 inhibitors given orally for their ability to suppress eosinophil trafficking in guinea-pig skin. Oral treatment with all five PDE4 inhibitors reduced eosinophil recruitment with the following rank order of potency RP73401=rolipram>LAS31205>SB207499>CDP840. There was a poor correlation between inhibition of eosinophil trafficking in guinea-pig skin and inhibition of the LPDE4 (see Tables 1 and 2). In addition, inhibition of PDE4 in whole cells (PBMC) did not predict in vivo activity. There was also a poor correlation between inhibition of eosinophil trafficking and binding of PDE4 inhibitors to HPDE4. The exception was RP73401 that showed highest potency against LPDE4, HPDE4 and in vivo. Despite the range of potencies, all inhibitors displayed similar efficacy in vitro (see Figure 1) but this did not necessarily translate into effectiveness in vivo. Thus, while rolipram, RP73401, LAS31025 and SB207499 inhibited 111In-eosinophil trafficking by a maximum of 92–98%, the maximum inhibition achieved by CDP840 was 59%. Inasmuch as the drugs were given by the oral route, the pharmacokinetic characteristics of each compound (absorption, distribution, half-life) may have significantly affected their capacity to inhibit eosinophil trafficking in vivo. Our studies suggest that in vitro activity on cell lysates or whole cells (at least human PBMC) is not a reliable predictor of potency or effectiveness in vivo.
The mechanisms by which inhibitors of PDE4 suppress the trafficking of eosinophils in guinea-pig skin are not entirely known. We have previously suggested that several cellular sites of action could account for the inhibitory effects on eosinophil recruitment in vivo (Teixeira et al., 1994b). The possibility that mast cells were the main cellular target for the inhibitory action of rolipram was raised based on the ability of rolipram to inhibit 111In-eosinophil recruitment in a PCA reaction to a greater extent than in response to direct-acting mediators (Teixeira et al., 1994b). This finding was repeated in the present study, as was the lack of effect of PDE4 inhibitors on 111In-neutrophil trafficking (see Table 3) and oedema formation (data not shown) in the PCA reaction. Thus, while inhibition of mast cells has a minor contribution to the inhibition of 111In-eosinophil trafficking observed, mast cells do not appear to be the main cellular target for the inhibitory actions of PDE4 inhibitors in our model.
Inhibition of endothelial cell adhesion molecule expression at skin sites could also explain the ability of PDE4 inhibitors to suppress 111In-eosinophil trafficking in vivo. For example, these drugs could inhibit the expression of the VLA-4 ligand VCAM -1 in skin sites as has been demonstrated in vitro (Blease et al., 1998). Inasmuch as VLA-4 appears to be important for eosinophil, but not neutrophil, recruitment in guinea-pig skin (Weg et al., 1993), inhibition of the expression of VCAM-1 by PDE4 inhibitors could explain our observations on leukocyte recruitment. However, 111In-eosinophil trafficking induced by PAF, ZAP or antigen in sensitized skin is rapid (Faccioli et al., 1991) and protein-synthesis independent (Teixeira et al., 1996) and is, therefore, unlikely to rely on the upregulation of VCAM-1. Another cellular target for PDE4 inhibitors is the eosinophil itself. We have shown that pretreatment of eosinophils with salmeterol significantly inhibited the trafficking of 111In-eosinophils in guinea-pig skin (Teixeira & Hellewell, 1997a). The inhibitory effect of salmeterol was maintained even after eosinophils were washed prior to their infusion in vivo. We suggest, therefore, that the eosinophil is the main cellular target for the inhibitory effects of PDE4 inhibitors on 111In-eosinophil trafficking in our model.
Several studies have recently shown that the release of endogenous corticosteroids by PDE4 inhibitors may account for some of their anti-inflammatory effect in vivo (reviewed in Teixeira et al., 1997). Although this issue was not addressed in the present study, we believe that the release of endogenous steroids is unlikely to explain the ability of PDE4 inhibitors to suppress 111In-eosinophil trafficking in guinea-pig skin. This is because (i) contrary to the immediate inhibitory effects of PDE4 inhibitors on 111In-eosinophil trafficking, dexamethasone requires a 2.5 h pretreatment for inhibition to be observed (Teixeira et al., 1996) and (ii) dexamethasone, but not PDE4 inhibitors, significantly inhibited oedema formation in guinea-pig skin (Teixeira et al., 1996) suggesting a different mechanism of action.
Although PDE4 inhibitors are effective blockers of the PDE4 enzyme from neutrophils (see Table 1) and neutrophil function in vitro (e.g. Au et al., 1998), we failed to observe any inhibitory effect on 111In-neutrophil recruitment in vivo (see Table 3). Similarly, PDE4 inhibitors have been shown to be inactive against neutrophil recruitment in some, but not all, animal models (reviewed in Teixeira et al., 1997). As mentioned above, an effect on the expression of cell adhesion molecules is unlikely to explain this lack of effect. One possible alternative is that the cyclic AMP turnover in guinea-pig neutrophils is lower than that of guinea-pig eosinophils and it would be necessary to stimulate adenylate cyclase to observe an inhibitory effect. However, we failed to block 111In-neutrophil recruitment with intradermal injection of E-type prostaglandins, agents which also elevate cyclic AMP in leukocytes (Teixeira et al., 1993a). Clearly further studies are needed to explain the inability of PDE4 inhibitors to block 111In-neutrophil recruitment in guinea-pig skin.
Inhibitors of PDE4 are effective blockers of the recruitment of 111In-eosinophils into sites of allergic and mediator-induced inflammation in guinea-pig skin. We suggest that the main cellular target for the inhibitory effects of these agents is the eosinophils themselves. Contrary to in vitro studies of eosinophil function there was no correlation between inhibition of 111In-eosinophil recruitment and inhibition of the enzyme catalytic site or the rolipram-binding site. We suggest that this guinea-pig model of eosinophil trafficking will be useful in the screening and development of PDE4 inhibitors for the oral treatment of diseases where eosinophils are thought to play an important pathophysiological role.
Acknowledgments
The National Asthma Campaign, Novartis (Switzerland) and Chiroscience supported this work. We thank Joanna Gregory, Chris Palmer, Trevor Morgan and Lewis Gowers for their invaluable contributions.
Abbreviations
- PDE4
phosphodiesterase 4
- ZAP
zymosan activated plasma
References
- AU B.-T., TEIXEIRA M.M., COLLINS P.D., WILLIAMS T.J. Effect of PDE4 inhibitors on zymosan-induced IL-8 release form human neutrophils: Synergism with prostanoids and salbutamol. Br. J. Pharmacol. 1998;123:1260–1266. doi: 10.1038/sj.bjp.0701723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNETTE M.S., GROUS M., CIESLINSKI L.B., BURMAN M., CHRISTENSEN S.B., TORPHY T.J. Inhibitors of phosphodiesterase IV (PDE IV) increase acid secretion in rabbit isolated gastric glands: correlation between function and interaction with a high-affinity rolipram binding site. J. Pharmacol. Exp. Ther. 1995a;273:1396–1402. [PubMed] [Google Scholar]
- BARNETTE M.S., MANNING C.D., CIESLINSKI L.B., CHRISTENSEN S.B., TORPHY T.J. The ability of phosphodiesterase IV inhibitors to suppress superoxide production in guinea pig eosinophils is correlated with inhibition of phosphodiesterase IV catalytic activity. J. Pharmacol. Exp. Ther. 1995b;273:674–679. [PubMed] [Google Scholar]
- BARNETTE M.S., BARTUS J.O.'L., BURMAN M., CHRISTENSEN S.B., CIESLINSKI L.B., ESSER K.M., PRABHAKAR U.S., RUSH J.A., TORPHY T.J. Association of the anti-inflammatory activity of phosphodiesterase 4 (PDE4) inhibitors with either inhibition of PDE4 catalytic activity or competition for [3H]rolipram binding. Biochem. Pharmacol. 1996;51:949–956. doi: 10.1016/0006-2952(96)00053-6. [DOI] [PubMed] [Google Scholar]
- BARNETTE M.S., CHRISTENSEN S.B., ESSAYAN E.M., GROUS M., PRABHAKAR U., RUSH J.A., KAGEY-SOBOTKA A., TORPHY T.J. SB 207499 (Ariflo), a potent and selective second-generation phosphodiesterase 4 inhibitor: in vitro anti-inflammatory actions. J. Pharmacol. Exp. Ther. 1998;284:420–426. [PubMed] [Google Scholar]
- BEAVO J.A., CONTI M., HEASLIP R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994;46:399–405. [PubMed] [Google Scholar]
- BELETA J., BOU J., MIRALPEIX M., DOMENECH T., BERGA P., GRISTWOOD R.W., PALACIOS J.M.LAS 31025, a new compound with selective phosphodiesterase IV inhibitory activity 1996. Third International Conference on Cyclic Nucleotide Phosphodiesterases: From Genes to Therapies, Glasgow
- BLEASE K., BURKE-GAFFNEY A., HELLEWELL P.G. Modulation of cell adhesion molecule expression and function on lung microvascular endothelial cells by inhibition of phosphodiesterases 3 and 4. Br. J. Pharmacol. 1998;123:229–237. doi: 10.1038/sj.bjp.0701833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUIJNZEEL-KOOMEN C., STORZ E., MENZ G., BRUIJNZEEL P. Skin eosinophilia in patients with allergic and nonallergic asthma and atopic dermatitis. J. Allergy Clin. Immunol. 1992;89:52–59. doi: 10.1016/s0091-6749(05)80040-5. [DOI] [PubMed] [Google Scholar]
- DENT G., GIEMBYCZ M.A., RABE K.F., BARNES P.J. Inhibition of eosinophil cyclic nucleotide PDE activity and opsonised zymosan-stimulated respiratory burst by `type IV'-selective PDE inhibitors. Br. J. Pharmacol. 1991;103:1339–1346. doi: 10.1111/j.1476-5381.1991.tb09790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUPLANTIER A.J., BIGGERS M.S., CHAMBERS R.J., CHENG J.B., COOPER K., DAMON D.B., EGGLER J.F., KRAUS K.G., MARFAT A., MASAMUNE H., PILLAR J.S., SHIRLEY J.T., UMLAND J.P., WATSON J.W. Biarylcarboxylic acids and -amides: inhibition of phosphodiesterase type IV versus [3H]rolipram binding activity and their relationship to emetic behaviour in the ferret. J. Med. Chem. 1996;39:120–125. doi: 10.1021/jm9505066. [DOI] [PubMed] [Google Scholar]
- FACCIOLI L.H., NOURSHARGH S., MOQBEL R., WILLIAMS F.M., SEHMI R., KAY A.B., WILLIAMS T.J. The accumulation of 111In-eosinophils induced by inflammatory mediators in vivo. Immunology. 1991;73:222–227. [PMC free article] [PubMed] [Google Scholar]
- FOSTER C.S., RICE B.A., DUTT J.E. Immunopathology of atopic keratoconjunctivitis. Ophthalmology. 1991;98:1190–1196. doi: 10.1016/s0161-6420(91)32154-7. [DOI] [PubMed] [Google Scholar]
- GARTNER I. Separation of human eosinophils in density gradients of polyvinylypyrrolidone-coated silica gels (Percoll) Immunology. 1980;40:133–136. [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., DENT G. Prospects for selective cyclic nucleotide phosphodiesterase inhibitors in the treatment of bronchial asthma. Clin. Exp. Allergy. 1992;22:337–344. doi: 10.1111/j.1365-2222.1992.tb03095.x. [DOI] [PubMed] [Google Scholar]
- JACOBITZ S., MCLAUGHLIN M.M., LIVI G.P., BURMAN & TORPHY T.J. Mapping the functional domains of human recombinant phosphodiesterase 4A: structural requirements for catalytic activity and rolipram binding. Mol. Pharmacol. 1996;50:891–899. [PubMed] [Google Scholar]
- KELLY J.J., BARNES P.J., GIEMBYCZ M.A. Phosphodiesterase 4 in macrophages: relationship between cAMP accumulation, suppression of cAMP hydrolysis and inhibition of [3H]R-(−)-rolipram binding by selective inhibitors. Biochem. J. 1996;318:425–436. doi: 10.1042/bj3180425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEMENTSSON H. Eosinophils and the pathophysiology of allergic rhinitis. Clin. Exp. Allergy. 1992;22:1058–1064. doi: 10.1111/j.1365-2222.1992.tb00130.x. [DOI] [PubMed] [Google Scholar]
- MARTIN L.B., KITA H., LEIFERMAN K.M., GLEICH G.J. Eosinophils in Allergy: Role in Disease, Degranulation and Cytokines. Int. Arch. Allergy Immunol. 1996;109:207–215. doi: 10.1159/000237239. [DOI] [PubMed] [Google Scholar]
- MONTEFORT S., HERBERT C.A., ROBINSON C., HOLGATE S.T. The bronchial epithelium as a target for inflammatory attack in asthma. Clin. Exp. Allergy. 1992;22:511–520. doi: 10.1111/j.1365-2222.1992.tb00159.x. [DOI] [PubMed] [Google Scholar]
- PERRY M.J., O'CONNELL J., WALKER C., CRABBE T., BALDOCK D., RUSSELL A., LUMB S., HUANG Z., HOWAT D., ALLEN R., MERRIMAN M., WALLS J., DANIEL T., HUGHES B., LALIBERTE F., HIGGS G.A., OWENS R.J. CDP840. A novel inhibitor of PDE-4. Cell Biochem. Biophys. 1998;29:113–132. doi: 10.1007/BF02737831. [DOI] [PubMed] [Google Scholar]
- RAEBURN D., UNDERWOOD S.L., LEWIS S.A., WOODMAN V.R., BATTRAM C.H., TOMKINSON A., SHARMA S., JORDA R., SOUNESS J.E., WEBBER S.E., KARLSSON J.-A. Anti-inflammatory and bronchodilator properties of RP 73401, a novel and selective phosphodiesterase type IV inhibitors. Br. J. Pharmacol. 1994;113:1423–1431. doi: 10.1111/j.1476-5381.1994.tb17156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER H.H., SCHMIECHEN R., BREZINSKI M., SEIDLER J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur. J. Pharmacol. 1986;127:105–115. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- SOUNESS J.E., CARTER C.M., DIOCEE B.K., HASSALL G.A., WOOD L.J., TURNER N.C. Characterization of guinea-pig eosinophil phosphodiesterase activity. Biochem. Pharmacol. 1991;42:937–945. doi: 10.1016/0006-2952(91)90056-b. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., GRISTWOOD R.W., COOPER N., HELLEWELL P.G. Phosphodiesterase (PDE)4 inhibitors: anti-inflammatory drugs of the future. Trends Pharmacol. Sci. 1997;18:164–171. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. Effect of a 5-lipoxygenase inhibitor, ZM 230487, on cutaneous allergic inflammation in the guinea pig. Br. J. Pharmacol. 1994;111:811–818. doi: 10.1111/j.1476-5381.1994.tb14873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. Evidence that the eosinophil is a cellular target for the inhibitory action of salmeterol on eosinophil recruitment in vivo. Eur. J. Pharmacol. 1997a;323:255–260. doi: 10.1016/s0014-2999(97)00030-7. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. The selectin binding polysaccharide fucoidin inhibits eosinophil recruitment in vivo. Br. J. Pharmacol. 1997b;120:1059–1066. doi: 10.1038/sj.bjp.0701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., REYNIA S., ROBINSON M., SHOCK A., WILLIAMS T.J., WILLIAMS F.M., ROSSI A.G., HELLEWELL P.G. Role of CD18 in the accumulation of eosinophils and neutrophils and local oedema formation in inflammatory reactions in guinea pig skin. Br. J. Pharmacol. 1994a;111:1205–1211. doi: 10.1111/j.1476-5381.1994.tb14810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. E-type prostaglandins enhance local oedema formation and neutrophil accumulation but suppress eosinophil accumulation in guinea pig skin. Br. J. Pharmacol. 1993a;110:416–422. doi: 10.1111/j.1476-5381.1993.tb13826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. Role of prostaglandins and nitric oxide in acute inflammatory reactions in guinea pig skin. Br. J. Pharmacol. 1993b;110:1515–1521. doi: 10.1111/j.1476-5381.1993.tb13994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. Effect of phosphodiesterase isoenzyme inhibitors on cutaneous inflammation in the guinea pig. Br. J. Pharmacol. 1994b;112:332–340. doi: 10.1111/j.1476-5381.1994.tb13073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. Mechanisms and pharmacological modulation of eosinophil accumulation in vivo. Trends Pharmacol. Sci. 1995;16:418–423. doi: 10.1016/s0165-6147(00)89092-6. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. Effects of dexamethasone and cyclosporin A on the accumulation of eosinophils in acute inflammation in the guinea pig. Br. J. Pharmacol. 1996;118:317–324. doi: 10.1111/j.1476-5381.1996.tb15405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON W.J., TERASAKI W.L., EPSTEIN P.M., STRADA S.J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple forms of the enzyme. Adv. Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- TORPHY T.J. Phosphodiesterase isoenzymes. Molecular targets for novel antiasthma drugs. Am. J. Resp. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- VENGE P. The human eosinophil in inflammation. Agents Actions. 1990;29:122–126. doi: 10.1007/BF01964739. [DOI] [PubMed] [Google Scholar]
- WARDLAW A.J., DUNNETTE S., GLEICH G.J., COLLINS J.V., KAY A.B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am. Rev. Respir. Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- WEG V.B., WATSON M.L., CORDEIRO R.S.B., WILLIAMS T.J. Histamine, leukotriene D4 and platelet activating factor in guinea pig passive cutaneous anaphylaxis. Eur. J. Pharmacol. 1991;204:157–163. doi: 10.1016/0014-2999(91)90700-z. [DOI] [PubMed] [Google Scholar]
- WEG V.B., WILLIAMS T.J., LOBB R.R., NOURSHARGH S. A monoclonal antibody recognising very late activation antigen-4 (VLA-4) inhibits eosinophil accumulation in vivo. J. Exp. Med. 1993;177:561–566. doi: 10.1084/jem.177.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]


