Abstract
Intracerebral microdialysis was used to examine the function of the terminal 5-hydroxytryptamine (5-HT) autoreceptor in the anterior hypothalamus of anaesthetized rats at two points in the light phase of the light–dark cycle.
Infusion of the 5-HT1A/1B agonist 5-methoxy-3-(1,2,3,6-tetrahydro-4-pyridyl)-1H-indole (RU24969) 0.1, 1.0 and 10 μM through the microdialysis probe led to a concentration-dependent decrease (49, 56 and 65% respectively) in 5-HT output. The effect of RU24969 (1 and 5 μM) was prevented by concurrent infusion of methiothepin (1 and 10 μM) into the anterior hypothalamus via the microdialysis probe. Infusion of methiothepin alone (1.0 and 10 μM) increased (15 and 142% respectively) 5-HT output.
Infusion of RU24969 (5 μM) through the probe at mid-light and end-light resulted in a quantitatively greater decrease in 5-HT output at end-light compared with mid-light.
Following treatment with either paroxetine hydrochloride (10 mg kg−1 i.p.) or desipramine hydrochloride (10 mg kg−1 i.p.) for 21 days the function of the terminal 5-HT1B autoreceptor was more markedly attenuated at end-light.
The data show that, as defined by the response to RU24969, the function of the 5-HT1B receptors that control 5-HT output in the anterior hypothalamus is attenuated following chronic desipramine or paroxetine treatment in a time-of-day-dependent manner.
Keywords: 5-HT release, 5-HT1B receptors, hypothalamus, intracerebral microdialysis, paroxetine, desipramine
Introduction
In the brain, 5-hydroxytryptamine (5-HT) is a promiscuous neurotransmitter, exerting its effects through a wide range of distinct receptors divided into 5-HT1 to 5-HT7 subtypes (Hoyer et al., 1994). The 5-HT1 division is perhaps the most well documented and is further subdivided into 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E and 5-HT1F receptors (Hoyer et al., 1994). Neuronal release of 5-HT is under the control of two types of presynaptically located autoreceptors (Cerrito & Raiteri, 1979; Verge et al., 1985), which have been classified as 5-HT1A receptors on the cell body and 5-HT1B receptors on the nerve terminal in the rat brain (Martin & Sanders-Bush, 1982; Middlemiss, 1984; 1985; Verge et al., 1985; Martin & Marsden, 1986). There is some evidence that 5-HT1B/1D receptors are also found on 5-HT cell bodies, where they may be involved in controlling dendritic release of 5-HT (Starkey & Skingle, 1994; Davidson & Stamford, 1995).
For over 40 years 5-HT has been primarily implicated in the aetiology of depression, and there is clinical evidence to support this contention. For example, there is a significant decrease in plasma tryptophan levels in depressives. Cowen et al. (1989); Pietraszek et al. (1991) and Delgado et al. (1990) have shown that rapid tryptophan depletion precipitated a relapse in those depressed inpatients who were in remission. The number of 5-HT transporter sites is decreased in the cortex and hippocampus of depressed suicide victims (Leake et al., 1991; Little et al., 1993) and the density of 5-HT2 and 5-HT1D receptors in the frontal cortex has been reported to be increased in depressed suicide victims (Stanley & Mann, 1983; Lowther et al., 1991).
Since the discovery of the terminal 5-HT autoreceptor, and given the fact that many clinically effective antidepressant drugs target the 5-HT system, several authors have investigated the effect of chronic antidepressant drug treatment on terminal 5-HT autoreceptor function. However, both in vivo and in vitro results of these studies have been conflicting. Sleight et al. (1989), using in vivo microdialysis, found no evidence for autoreceptor down-regulation in the hippocampus after prolonged administration of either amitriptyline or MDL72394 (a putative monoamine oxidase inhibitor, MAOI). Similarly, Blier et al. (1988), using an indirect measure of receptor function and a non-specific antagonist, reported that terminal 5-HT autoreceptor function in the hippocampus was unaffected by chronic clorgyline treatment. By contrast, Blier et al. (1988) and Chaput et al. (1986), using the same methodology, strain of animal, experimental protocol and brain region, have shown that the 5-HT autoreceptor is down-regulated after either repeated fluoxetine or citalopram treatment. On the other hand, in vitro studies have demonstrated autoreceptor down-regulation in the hippocampus (Blier & Bouchard, 1994), hypothalamus (Moret & Briley, 1990; Blier & Bouchard, 1994) but not the frontal cortex (Blier & Bouchard, 1994) of rats and guinea-pigs following treatment with the selective serotonin reuptake inhibitors (SSRI) citalopram or paroxetine. Conversely, befloxamine (a reversible inhibitor of monoamine oxidase, RIMA) administration had no effect on 5-HT autoreceptor function in the hypothalamus, hippocampus or frontal cortex of the guinea-pig (Blier & Bouchard, 1994). The results of these studies illustrate that different classes of antidepressant drugs do not appear to have a common effect on terminal 5-HT autoreceptor function.
One important factor which all the studies cited above have overlooked is the marked circadian rhythm in the 5-HT system (for a comprehensive review see Martin & Redfern, 1997). Therefore, we have determined whether the terminal 5-HT1B autoreceptor function varies with the time of day and whether this influences the effects of either chronic paroxetine or desipramine treatment in the rat hypothalamus.
Methods
Animals
Male Wistar rats (Olac, Bicester) weighing 240–260 g were used throughout the study. The animals were housed in pairs under a 12 : 12 light-dark cycle (lights on 06.00 h), at an ambient temperature of 21°C and had free access to food and tap water.
Implantation of dialysis probes
Rats were anaesthetized with chloral hydrate (600 mg kg−1 i.p.) and supplementary doses of anaesthetic (30 mg i.p.) were given as needed during the course of the experiment. The animals were placed in a stereotaxic frame and concentric microdialysis probes (CMA/12 microdialysis probe, 2 mm long membrane and 0.5 mm o.d., Carnegie Medicin, Biotech Instruments) implanted into the anterior hypothalamus (co-ordinates with reference to Bregma and the skull surface AP −1.3 mm, ML −0.6 mm and depth −9.3 mm, according to the atlas of Paxinos & Watson, 1982). The probe was continuously perfused, at a rate of 1 μl min−1 (model 22 Microinjection pump, Harvard Apparatus or CMA/100 Microinjection pump, Carnegie Medicin, Biotech Instruments) with artificial cerebrospinal fluid (aCSF) (composition in mM: NaCl 147; KCl 4; CaCl2 4 pH 7.4) containing the selective 5-HT reuptake inhibitor citalopram (1 μM), and the effluent collected onto ice. At the end of the experiment the brain was removed and the position of the probe was visually confirmed. Before implantation the recovery of 5-HT from the probe in the dialysis solution was checked in vitro at the flow rate used; probes with recoveries of below 15% were discarded.
Experimental protocol
Following a 90 min stabilization period dialysate samples were collected every 15 min. Two aliquots were taken to serve as pre-intervention controls and successive 15 min fractions collected throughout the experiment. For agonist studies the drug was infused via the probe for 15 min, immediately after the control samples, and six subsequent samples were collected. For antagonist studies, the antagonist alone was infused via the probe for 15 min, after the control samples, then the agonist and the antagonist were infused together via the probe for a further 15 min. Dialysate samples were assayed immediately for their 5-HT content using reverse phase high performance liquid chromatography coupled to an electrochemical detector (HPLC-ECD). The times at which experiments were carried out are indicated in the appropriate part of the Results section.
Perfusate analysis
The HPLC-ECD system used has been described previously (Martin et al., 1992). Briefly it consisted of a Hypersil ODS2 column (10 cm×2 mm i.d.) with 3 μm packing (HPLC Technology) linked to a Coulochem electrochemical detector with dual electrodes, electrode 1 set at +0.1 V and electrode 2 at +0.28 V (model 5011 analytical cell, ESA Inc.). Mobile phase (composition: NaH2PO4 0.1 M; sodium octane sulphonic acid 0.93 mM; 0.07% v v−1 dibutylamine and 12% v v−1 methanol) adjusted to pH 3.0 with orthophosphoric acid, was supplied to the system by a Severn Analytical solvent delivery system (SA6410B Severn Analytical) at a flow rate of 0.45 ml min−1. The chromatogram was plotted on a Shimadzu C-R6A chromatopac integrator (Dyson Instruments Ltd).
Statistical analysis
Values are expressed as a percentage of the control value i.e. average concentration of 5-HT in the two dialysate samples taken immediately prior to any drug infusion. Data was analysed by two-way analysis of variance (ANOVA) to detect differences between treatments, followed by Studentized range test to determine where differences lay between treatments; P⩽0.05 was considered significant.
Chronic antidepressant treatment
Rats were treated for 21 days with a once daily injection of either desipramine hydrochloride (10 mg kg−1 i.p.) or paroxetine hydrochloride (10 mg kg−1 i.p.) or an equivalent volume of saline. The precise time of injection was varied each day to avoid the animals taking the injection as a time cue, but injection was always performed in the afternoon during the rat's light phase. All experiments were performed after a 24 h washout period.
Drugs
All drugs which were used acutely were infused via the probe and were made up in aCSF including 1 μM citalopram on the day of use. Chloral hydrate was made up in 0.9% sterile saline every 2 days as required. Pilot studies have indicated that drug transferred out of the dialysis probe with similar efficiencies to the rate of entry (i.e. approximately 15%).
Drugs were purchased from suppliers as follows: NaCl, KCl, orthophosphoric acid and NaH2PO4 all Aristar quality (BDH Chemicals), octane sulphonic acid (Kodak Clinical Drugs Ltd.), HPLC grade methanol (Rathburn Chemicals), dibutylamine (Aldrich), chloral hydrate, 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) and desipramine (Sigma Chemical Co.).
The following drugs were donated by the companies indicated; RU24969 (Roussel-Uclaf), citalopram (Lundbeck), methiothepin (Hoffmann-LaRoche) and paroxetine (SmithKline Beecham).
Results
All experiments were performed during the early to middle stages of the light period (i.e. 0800 to 1300 h) unless otherwise stated.
Basal efflux of 5-HT
The output of 5-HT into the dialysate was stable over the collection period. The mean 5-HT level in the first 30 min of collection was 25.0±0.2 fmole 15 μl−1 sample (n=12).
When calcium ions were removed from the perfusing aCSF (composition in mM: NaCl 147, KCl 4, pH 7.4) for 60 min there was a rapid decrease in 5-HT output within the first 15 min of this intervention, reaching a maximum decrease of 98% (2% of control ±0.6%, P<0.01, n=4) at t=45 min. When calcium ions were re-introduced into the aCSF, 5-HT levels returned rapidly to baseline and increased to a maximum of 121±6.6% of preintervention control at t=150 min (Figure 1).
Figure 1.
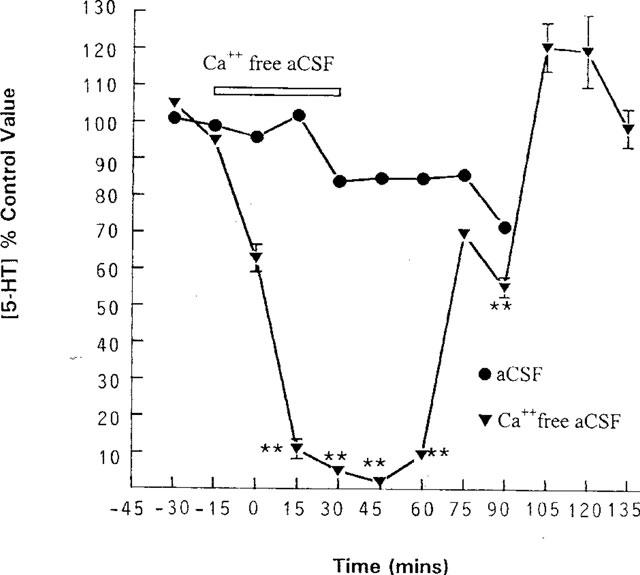
Effect of removing calcium ions from the perfusing medium on the level of 5-HT in dialysate collected from the anterior hypothalamus. Concentric microdialysis probes were perfused with aCSF containing (in mM): NaCl 147, KCl 4, CaCl2 4 and citalopram 1 μM and samples collected every 15 min. **P<0.01 vs pooled control (two way ANOVA). Data are expressed as a percentage of the concentration of 5-HT in the two dialysate samples taken immediately prior to any intervention (control value). Each point represents the mean value with s.e.mean shown by vertical bars. Control n=12, calcium free buffer n=4.
Modified aCSF containing 100 mM K+ was infused via the probe for 15 min. This led to an immediate and significant increase in 5-HT output of 456% of control ±11.5% (P<0.01, n=7) in the first fraction and 345% of control ±8.0% (P<0.01, n=6) in the second fraction; dialysate 5-HT content subsequently returned to baseline (data not shown).
Pharmacological characterization of the hypothalamic 5-HT autoreceptor
8-OH-DPAT is a selective agonist at the 5-HT1A receptor (Middlemiss & Fozard, 1983). It was used because subsequent experiments used RU24969 which is not completely selective for the 5-HT1B receptor, having an almost equal affinity for the 5-HT1A receptor (van Wijngaarden et al., 1990). 8-OH-DPAT was infused into the anterior hypothalamus to ensure that any effect of RU24969 produced was due to its activity as a 5-HT1B agonist. A 15 min infusion of 1 μM 8-OH-DPAT via the dialysis probe had no significant effect on 5-HT release compared to control animals, see Figure 2a.
Figure 2.
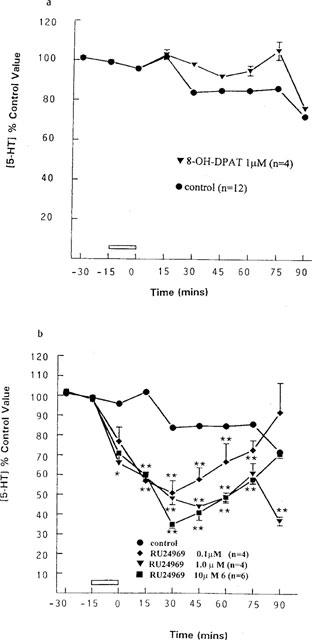
Effects of infusion of (a) the 5-HT1A agonist 8-OH-DPAT and (b) the 5-HT1A/1B agonist RU24969 on 5-HT output in the anterior hypothalamus. (a) 8-OH-DPAT, 1 μM (n=4) was infused for 15 min through the probe; pooled control, n=12. (b) RU24969, 0.1 μM n=4, 1 μM n=4, 10 μM n=6) was infused for 15 min; pooled control, n=12. *P<0.05, **P<0.01 vs pooled control value (two-way ANOVA). Data are expressed as a percentage of the concentration of 5-HT in the two dialysate samples taken immediately prior to any intervention (control value), each point represents mean with the s.e.mean indicated by vertical bars.
Increasing concentrations of RU24969 were infused for 15 min via the probe leading to a dose-dependent decrease in 5-HT output. At a concentration of 10 μM, there was a maximum inhibition of 65% (35±2% of control at t=30 min, P<0.01, n=6), 1 μM 56% (44±1.7% of control at t=45 min, P<0.01, n=4) and 0.1 μM had a maximum inhibition of 49% (51±6.2% of control at t=45 min, P<0.05, n=4), see Figure 2b.
Methiothepin (metitepin) has been shown to be a 5-HT1 receptor antagonist (Hilbert & Middlemiss, 1986); since no selective 5-HT1B receptor antagonist was available to us, methiothepin (1 μM), was infused for 15 min alone and for a further 15 min with 1 μM RU24969. Methiothepin reversed the RU24969-induced decrease in 5-HT output. Thus in the presence of 1 μM methiothepin, RU24969 (1 μM) caused a maximum inhibition of 5-HT output of 27% (73% of control ±7% of control at t=30 min, n=4), which was not significantly different from control animals (see Figure 3). Methiothepin (10 μM) also antagonized the effect of 5 μM RU24969, so that the maximum inhibition of 5-HT output was 17% (83 of control ±3.8% of control at t=30 min, n=4), which was not significantly different from controls, (see Figure 3).
Figure 3.
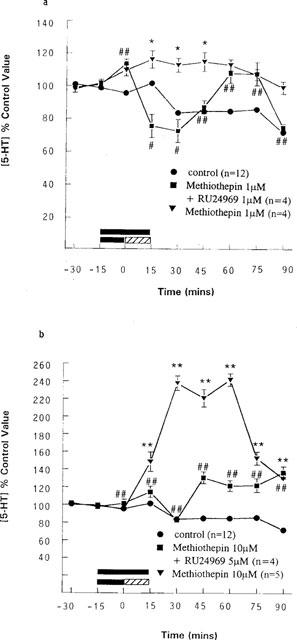
Effects of methiothepin infusion on the effects of RU24969 on 5-HT output in the anterior hypothalamus. Methiothepin was infused for 15 min and then either co-infused with RU24969 for a further 15 min (lined bar) or infused alone (solid bar) (a) 1 μM methiothepin+1 μM RU24969 (n=4) and 1 μM methiothepin (n=4), pooled control, n=12. *P<0.05 vs pooled control value, #P<0.05, ##P<0.01 vs 1 μM RU24969 (two-way ANOVA). (b) 10 μM methiothepin+5 μM RU24969 (n=4) and 10 μM methiothepin (n=5), pooled control, n=12. **P<0.01 vs pooled control value, ##P<0.01 vs 5 μM RU24969 (two-way ANOVA). Data are expressed as a percentage of the concentration of 5-HT in the two dialysate samples taken immediately prior to any intervention (control value), as mean with s.e.mean indicated by vertical bars.
Infusion of methiothepin (1 or 10 μM) alone for 30 min significantly increased 5-HT output, with maximum increases to 115% of preintervention levels (±5.8%) at t=45 min (P<0.05, n=4) and to 242% of preintervention controls (±7.1%) at t=60 min (P<0.01, n=5), (Figure 3) respectively.
Diurnal variation in 5-HT autoreceptor function
Basal 5-HT levels at mid-light and end-light were 38.7±0.3 fmole 15 μl−1 sample (n=4) and 28.4±0.1 fmole 15 μl−1 sample (n=6) respectively. There was no significant change in 5-HT release after lights off. The level of 5-HT in the dialysate was significantly higher at mid-light than end-light (P<0.05 unpaired Student's t-test).
Release of 5-HT from control animals was stable over the course of the experiment. When 5 μM RU24969 was infused at mid-light, i.e. 12.00 h, it produced a maximal decrease in 5-HT release of 65% (35% of control ±3.3% at t=45 min, P<0.01, n=4), with levels returning to basal by t=90 min (Figure 4).
Figure 4.
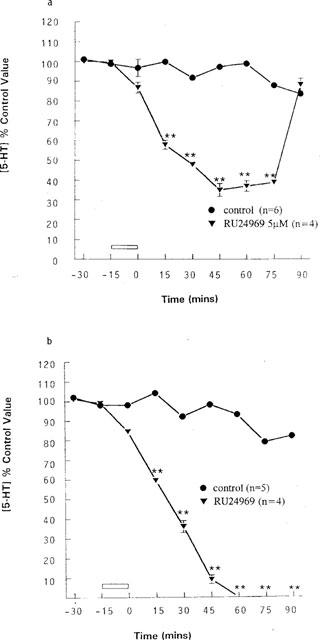
Effects of a 15 min infusion of 5 μM RU24969 at (a) mid-light and (b) end-light on 5-HT output in the anterior hypothalamus. (a) control (n=6) and 5 μM RU24969 (n=4). **P<0.01 vs control value for mid-light (two-way ANOVA). (b) control (n=5) and 5 μM RU24969 (n=4). **P<0.01 vs control value for end-light (two-way ANOVA). Data are expressed as a percentage of the concentration of 5-HT in the two dialysate samples taken immediately prior to any intervention (control value), as mean with the s.e.mean indicated by vertical bars.
By contrast, infusion of RU24969 at 18.00 h, the time lights went off in the animal colony, significantly attenuated 5-HT release to below the level of detection (1–2 fmole 15 μl−1), P<0.01 n=5. The effect was maximal 1 h post infusion and 5-HT levels did not return to basal values over the course of the experiment (Figure 4).
Antidepressant effects on 5-HT autoreceptor function at mid-light and end-light
Chronic antidepressant treatment did not affect basal levels of 5-HT (saline-treated 39±8.2 fmole 15 μl−1 sample, paroxetine 30.7±4.1 fmole 15 μl−1 sample, desipramine 34.4±2.2 fmole 15 μl−1 sample) measured at mid-light. When measured at end-light neither prolonged paroxetine nor desipramine treatment affected basal 5-HT levels compared to saline-treated animals (saline-treated 35±9 fmole 15 μl−1 sample, paroxetine 34.2±4.5 fmole 15 μl−1 sample, desipramine 41.3±5.6 fmole 15 μl−1 sample; one-way ANOVA with post hoc Studentized range test, P>0.05, n=4–5).
At mid-light, RU24969 (5 μM) infused into the hypothalamus of rats treated chronically with saline, produced a maximal inhibition of 5-HT release of 51% (49% of control ±1.7% at t=60 min, n=4). Infusion of RU24969 via the dialysis probe in desipramine-treated animals led to a maximal inhibition of 47% (53% of control ±1.7% at t=45 min, n=5). Paroxetine-treated animals gave a similar result, with a maximal inhibition of 46% (54% of control ±2.0% at t=60 min, n=4), (Figure 5). However in rats treated with either of the two antidepressant drugs (paroxetine or desipramine), the effects of RU24969 were significantly attenuated from t=75 min (Figure 5). Thus although the rate of onset and magnitude of the effect of RU24969 were not altered, the rate at which 5-HT levels returned to baseline was increased and the effects of RU24969 were short-lived in antidepressant-treated animals compared with control.
Figure 5.
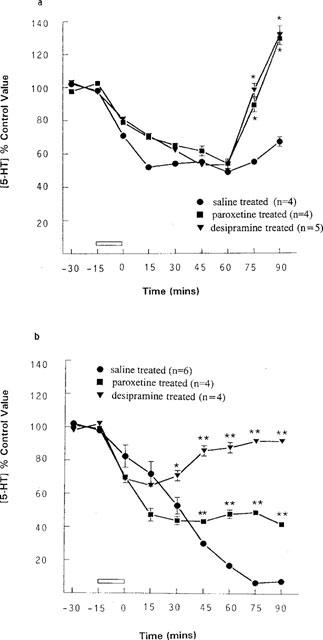
Rats were treated chronically with paroxetine hydrochloride (10 mg kg−1 i.p.), desipramine hydrochloride (10 mg kg−1 i.p.) or saline and the effect of a 15 min infusion of 5 μM RU24969 re-assessed at (a) mid-light and (b) end-light. (a) saline-treated+5 μM RU24969 (n=4), paroxetine-treated+5 μM RU24969 (n=4) and desipramine-treated+5 μM RU24969 (n=5). *P<0.05, vs saline-treated rats (two-way ANOVA). (b) saline-treated+5 μM RU24969 (n=6), paroxetine-treated+5 μM RU24969 (n=4) and desipramine-treated+5 μM RU24969 (n=4). *P<0.05, **P<0.01 vs saline-treated animals (two-way ANOVA). Data are expressed as a percentage of the concentration of 5-HT in the two dialysate samples taken immediately prior to any intervention (control value), as mean with s.e.mean indicated by vertical bars.
Infusion of RU24969 (5 μM) via the probe at end-light decreased 5-HT output significantly in saline-treated rats, with a maximal inhibition of 99% (1.0% of control ±1.8%, n=4, Figure 5). Chronic antidepressant treatment significantly attenuated this effect. Thus, the maximal inhibition of 5-HT output in desipramine-treated rats was 35% (65% of control ±2.0% at t=15 min, P<0.01, n=4), and paroxetine treatment led to a maximum decrease in 5-HT release of 66.5% (43.5% of control ±1.9% at t=45 min, P<0.01, n=5), (Figure 5). Therefore, at end-light the rate of onset and the magnitude of the response to RU24969 were significantly decreased after chronic antidepressant treatment (Figure 5).
Discussion and conclusions
Intracerebral microdialysis was used to investigate nerve terminal 5-HT1B autoreceptor function after chronic antidepressant treatment in the anterior hypothalamus of anaesthetized rats. The main finding of this study was that chronic antidepressant treatment attenuated the effect of RU24969 more markedly when infused locally at end-light, indicating that 5-HT1B autoreceptor function is attenuated in a time-of-day-dependant manner.
In order to confirm the origin of the 5-HT measured in the dialysate, two protocols were employed. Firstly, since exocytotic release is considered to be calcium dependent, calcium ions were removed from the aCSF for 1 h. This led to an immediate drop in 5-HT output which was reversed on the re-introduction of calcium ions into the aCSF. Secondly, aCSF with a high K+ content (100 mM) was perfused for 15 min which produced a 3–4 fold increase in 5-HT efflux. These two findings confirm the neuronal origin of the 5-HT measured in dialysate and are consistent with results reported by other authors in the caudate-putamen (Kalen et al., 1988), ventromedial hypothalamus (Auerbach et al., 1989) and ventral hippocampus (Sharp et al., 1989).
The terminal 5-HT autoreceptor has been extensively examined and characterized as a 5-HT1B receptor (Martin & Sanders-Bush, 1982; Middlemiss, 1984; 1985; Martin & Marsden, 1986; Price et al., 1997; Schlicker et al., 1997; Trillat et al., 1997). We carried out a limited series of experiments to confirm that we were able to replicate the published findings. Thus, infusion of increasing concentrations of the 5-HT1A/1B agonist RU24969 (0.1–10 μM) led to a dose-dependent decrease in the level of 5-HT in the dialysate. The inhibitory effect of RU24969 on 5-HT release has been extensively demonstrated before (Sleight et al., 1989; Hjorth & Tao, 1991; Martin et al., 1992); however the dose-dependent effect has not been reported, albeit Martin et al. (1992) have shown that 0.1 μM had no effect on 5-HT output in the ventral hippocampus whereas 1 μM RU24969 decreased output by 47%. Since 12 μM RU24969 is well known to have almost equal affinity for 5-HT1A and 5-HT1B receptors, to confirm that the overflow of 5-HT measured in dialysate was controlled by a 5-HT1B autoreceptor, and to ensure that any effect of RU24969 was mediated by the stimulation of a 5-HT1B receptor and not a 5-HT1A receptor, the selective 5-HT1A/7 receptor agonist, 8-OH-DPAT, was infused via the probe. 8-OH-DPAT (1 μM) had no effect on 5-HT output. Further corroboration could have been sought by examining the effect of RU24969 following treatment with WAY 100635; however it seems reasonable to conclude that hypothalamic 5-HT1A and 5-HT7 receptors do not affect 5-HT overflow in the anterior hypothalamus and the effects of RU24969 reported here were produced solely by stimulation of a 5-HT1B receptor.
Pre-infusion of methiothepin (10 μM) blocked the effects of RU24969 (5 μM) as has been described previously (O'Connor & Kruk, 1991; Martin et al., 1992). Infusion of methiothepin alone (1 μM and 10 μM) increased the overflow of 5-HT as has also been reported in hypothalamic slices in vitro (Pettibone & Pflueger, 1984) and in vivo (Baumann & Waldmeier, 1984; Martin & Marsden, 1986; O'Connor & Kruk, 1991; Roberts et al., 1997). Methiothepin is a 5-HT receptor antagonist which shows some selectivity for 5-HT1 receptors (Hibert & Middlemiss, 1986), however it is not entirely selective for 5-HT receptors. Methiothepin is also an antagonist at α2-adrenoceptors which are known to be present on 5-HT terminals and to have an inhibitory effect on 5-HT release in vivo (Tao & Hjorth, 1992) which is tonic (Marsden & Martin, 1986; Mongeau et al., 1993). The increased 5-HT output after methiothepin was concentration dependent (the increase after 1 μM was 15% and after 10 μM 142%). Thus the increased output of 5-HT may be attributable to inhibition of the autoinhibitory tone of both the 5-HT1B autoreceptor as well as the α2-adreno-heteroreceptor. However, since methiothepin (1 μM) attenuated the effects of RU24969 whilst having negligible effects on 5-HT output itself, it is reasonable to conclude that, in view of the data obtained here and extensively reported in the literature (see above discussion), RU24969 exerts its effects on 5-HT release in the rat via the 5-HT1B autoreceptor.
RU24969 administration caused a quantitatively greater inhibition of 5-HT output at end-light compared to mid-light. The greater effect of infusion of 5 μM RU24969 at end-light compared to mid-light could be due to a difference in the amount of 5-HT released and therefore receptor occupation by the endogenous ligand, or the sensitivity/number of autoreceptors at the two time points. A significant 24 h variation in the number of 5-HT1B binding sites has been demonstrated, but only in the cortex (Akiyoshi et al., 1989). The number of binding sites at mid-light was significantly higher than at end-light which does not correlate with the findings presented here which suggest that 5-HT1B receptor function is greatest at end-light. The differences could be because the receptor binding studies of Akiyoshi et al. (1989) measured both presynaptic and postsynaptic receptors. Alternatively, the cortical rhythm may be different from that in the hypothalamus. mRNA levels for the 5-HT1B receptor in the suprachiasmatic nuclei (SCN) of the hypothalamus have not been found to vary over 24 h (Roca et al., 1993), when measured at four equally spaced time points in the light-dark cycle. However since mRNA is not normally found in nerve terminals, in these experiments it can be taken as a measure of postsynaptic receptors in the SCN; this does not therefore preclude a rhythm in presynaptic receptors. Alternatively, there could be circadian rhythms in receptor-effector coupling, adenylate cyclase activity or the intracellular level of cyclic AMP. Prosser & Gillette (1991) have described a rhythm in phosphodiesterase activity in rat SCN, which is responsible for the circadian rhythm in cyclic AMP and noradrenaline observed.
In the experiments reported here, rats were treated chronically with two different types of antidepressant; viz. desipramine, a selective noradrenaline uptake inhibitor and paroxetine, a selective 5-HT uptake inhibitor (SSRI); no significant effects on basal 5-HT efflux were observed at either time point.
The effects of antidepressant treatment on 5-HT1B autoreceptor function at mid-light will be considered first because it is at this time point that most other studies assessing neurotransmitter receptor binding and function are carried out. When the effect of RU24969 (5 μM) was re-assessed after chronic antidepressant treatment at mid-light, there was no significant difference in the initial effect of RU24969 infusion between antidepressant- or saline-treated animals. Neither desipramine nor paroxetine significantly altered the maximal response of the autoreceptor to RU24969. However, there was a significant increase in 5-HT output over base line at the end of the experiment indicating that the effects of RU24969 were terminated more quickly in antidepressant pre-treated animals. These results are in agreement with the only comparable published study by Sleight et al. (1989), assuming that their experiments were performed at or near the middle of the light phase. These workers found that chronic treatment with either amitriptyline or MDL72394 (a MAOI) had no effect on the response to RU24969 (10 mg kg−1 i.p.) in the frontal cortex of the anaesthetized rat. Other in vivo studies, using microiontophoresis, have indirectly demonstrated autoreceptor down-regulation in the hippocampus after chronic fluoxetine or citalopram but not clorgyline treatment (Chaput et al., 1986; Blier et al., 1988). The results of studies performed in vitro are equivocal, reporting both a decrease in sensitivity after treatment with MAOI, selective 5-HT uptake inhibitors or non-selective 5-HT and noradrenaline uptake inhibitors (Maura & Raiteri, 1984; Moret & Briley, 1990) and a decrease (Johanning et al., 1992) or no change in the number of 5-HT1B binding sites (Montero et al., 1991) after treatment with a SSRI.
By contrast, at end-light prolonged antidepressant treatment significantly attenuated the effects of RU24969; desipramine appeared to down-regulate 5-HT1B autoreceptor function more effectively than paroxetine. This down-regulation of 5-HT1B autoreceptor function following chronic desipramine treatment might result from a desensitization of terminal α2-adrenoceptors as a result of an increase in noradrenaline around the 5-HT nerve terminal following blockade of noradrenaline reuptake. Down-regulation of the α2-adrenoceptor would decrease the feedback inhibition of this receptor on 5-HT release, the biophase level of 5-HT would then rise resulting in down-regulation of the 5-HT1B autoreceptor. Treatment with paroxetine would have a more direct effect. Blockade of 5-HT reuptake would directly increase 5-HT levels in the synaptic cleft thus allowing desensitization of the autoreceptor. Since this is the first observation that antidepressant drugs decrease 5-HT1B autoreceptor function in vivo it is pertinent to consider the possible mechanisms involved. The down-regulation may be attributable to changes in autoreceptor and/or α2-heteroadrenoceptor expression, expression of G proteins (Lesch et al., 1991), uncoupling of the receptor-effector mechanism (Okada et al., 1988; Rasenick & Wang, 1988) or changes in intracellular signal transduction pathways (Nestler et al., 1989; Perez et al., 1989).
Although the reason for the greater effect of desipramine is unclear, as outlined above the down-regulation in the α2-heteroadrenoreceptor may increase the terminal biophase concentration of 5-HT and perhaps expose the terminal autoreceptor to a greater level of 5-HT at end-light compared to mid-light, thus leading to greater down-regulation at this time point.
In conclusion, this study has demonstrated a significant difference in the function of the terminal 5-HT1B autoreceptor at two time points during the light phase of the light–dark cycle in vivo. More significantly, however, the data show that autoreceptor function is attenuated following prolonged antidepressant treatment and that this effect is time-of-day-dependent. This finding may explain why a down-regulation of 5-HT autoreceptor function has not been consistently reported in previous studies.
Acknowledgments
T.J.O. Sayer was supported by a SERC CASE studentship in conjunction with Knoll Pharmaceuticals.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- HPLC-ECD
high performance liquid chromatography with electrochemical detection
- 5-HT
5-hydroxytryptamine
- MAOI
monoamine oxidase inhibitor
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino) tetralin
- RU24969
5-methoxy-3(1,2,3,6-tetrahydro-4-pyridyl)-1H-indole
- SSRI
selective serotonin reuptake inhibitor
References
- AKIYOSHI I., KURANGA H., TSUCHIYAMA K., NAGAYAMA H. Circadian rhythm of serotonin receptor in rat brain. Pharmacol. Biochem. Behav. 1989;32:491–493. doi: 10.1016/0091-3057(89)90186-x. [DOI] [PubMed] [Google Scholar]
- AUERBACH S.B., MINZENBERG M.J., WILKINSON L.O. Extracellular serotonin and 5-hydroxyindoleacetic acid in hypothalamus of the unanaesthetized rat measured by in vivo dialysis coupled to high performance liquid chromatography with electrochemical detection: dialysate serotinin reflects neuronal release. Brain Res. 1989;499:157–203. doi: 10.1016/0006-8993(89)90776-2. [DOI] [PubMed] [Google Scholar]
- BAUMANN P.A., WALDMEIER P.C. Further evidence for negative feedback control of serotonin release in the central nervous system. Naunyn-Schmiedebergs Arch. Pharmacol. 1984;317:36–43. doi: 10.1007/BF00506254. [DOI] [PubMed] [Google Scholar]
- BLIER P., BOUCHARD C. Modulation of 5-HT release in the guinea-pig brain following long-term administration of antidepressant drugs. Br. J. Pharmacol. 1994;113:485–495. doi: 10.1111/j.1476-5381.1994.tb17015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIER P., CHAPUT Y., DE MONTIGNY C. Long-term 5-HT reuptake blockade, but not monoamine oxidase inhibition, decreases the function of terminal 5-HT autoreceptors: An electrophysiological study in the rat brain. Naunyn-Schmiedebergs Arch. Pharmacol. 1988;337:246–254. doi: 10.1007/BF00168834. [DOI] [PubMed] [Google Scholar]
- CERRITO F., RAITERI M. Serotonin release is modulated by presynaptic autoreceptors. Eur. J. Pharmacol. 1979;57:427–430. doi: 10.1016/0014-2999(79)90506-5. [DOI] [PubMed] [Google Scholar]
- CHAPUT Y., DE MONTIGNY C., BLIER P. Effects of a selective 5-HT uptake reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: Electrophysiological studies in the rat brain. Naunyn-Schmiedebergs Arch. Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- COWEN P.J., PARRY-BILLINGS M., NEWSHOLME E.A. Decreased plasma tryptophan levels in major depression. J. Aff. Disorders. 1989;16:27–33. doi: 10.1016/0165-0327(89)90051-7. [DOI] [PubMed] [Google Scholar]
- DAVIDSON C., STAMFORD J.A. Effects of endogenously released 5-HT on the action of 8-hydroxy-DPAT at 5-HT1A autoreceptors. Br. J. Pharmacol. 1995;114:P357. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELGADO P.L., CHARNEY D.S., PRICE L.H., AGHAJANIAN G.K., LANDIS H., HENINGER G.R. Serotonin function and the mechanism of antidepressant action. Arch. Gen. Psychiat. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- HIBERT M., MIDDLEMISS D.N. Stereoselective blockade at the 5-HT autoreceptor and inhibition of radioligand binding to central 5-HT recognition sites by the optical isomers of methiothepin. Neuropharmacoloy. 1986;25:1–4. doi: 10.1016/0028-3908(86)90050-x. [DOI] [PubMed] [Google Scholar]
- HJORTH S., TAO R. The putative 5-HT1B receptor agonist CP-9,129 suppresses rat hippocampal 5-HT release in vivo: Comparison with RU24969. Eur. J. Pharmacol. 1991;209:249–252. doi: 10.1016/0014-2999(91)90177-r. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.H., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Revs. 1994;46:157–203. [PubMed] [Google Scholar]
- JOHANNING H., PLENGE P., MELLERUP E. Serotonin receptors in the brain of rats treated chronically with imipramine or RU24969: Support for the 5-HT1B receptor being the 5-HT autoreceptor. Pharmacol. Toxicol. 1992;70:131–134. doi: 10.1111/j.1600-0773.1992.tb00442.x. [DOI] [PubMed] [Google Scholar]
- KALEN P., STRECKER R.E., ROSENGREN E., BJORKLUND A. Endogenous release of neuronal serotonin and 5-hydroxyindoleacetic acid in the caudate putamen of the rat as revealed by intracerebral dialysis coupled to high-performance liquid chromotography with fluorimetric detection. J. Neurochem. 1988;51:1422–1435. doi: 10.1111/j.1471-4159.1988.tb01107.x. [DOI] [PubMed] [Google Scholar]
- LEAKE A., FAIRBAIRN A.F., MCKEITH I.G., FERRIER I.N. Studies on the serotonin binding site in major depressive disorder and control post-mortem brain. Neurochemical and clinical correlates. Psychiatr. Res. 1991;39:155–165. doi: 10.1016/0165-1781(91)90084-3. [DOI] [PubMed] [Google Scholar]
- LESCH K.P., AULAKH C.S., TOLLIVER T.J., HILL J.L., MURPHY D.L. Regulation of G proteins by chronic antidepressant drug treatment in the rat brain: Tricyclics but not clorgyline increase Goα subunits. Eur. J. Pharmacol. Mol. Pharmacol. Sect. 1991;207:361–364. doi: 10.1016/0922-4106(91)90012-7. [DOI] [PubMed] [Google Scholar]
- LITTLE K.Y., CARROLL F.I., DUNCAN G.E. Decreased cortical 5-HT binding in depressed humans. Soc. Neurosci. Abs. 1993;19:769.3. [Google Scholar]
- LOWTHER K., DE PARMENTIER F., KATONA C., HORTON R. 5-HTID and 5-HTIE binding sites in post-mortem brain samples from suicides and controls. Br. J. Pharmacol. 1991;104:362P. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- MARSDEN C.A., MARTIN K.F. Involvement of 5-HT1A-receptors and α2-receptors in the decreased 5-hydroxytryptamine release and metabolism in rat suprachiasmatic nucleus after intravenous 8-hydroxy-2 (normal-dipropylamino) tetralin. Br. J. Pharmacol. 1986;89:277–286. doi: 10.1111/j.1476-5381.1986.tb10257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN K.F., HANNON S., PENLIPS I., HEAL D.J. Opposing roles for 5-HT1B and 5-HT3 receptors in the control of 5-HT release in rat hippocampus in vivo. Br. J. Pharmacol. 1992;106:139–142. doi: 10.1111/j.1476-5381.1992.tb14306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN K.F., MARSDEN C.A. In vivo voltammetry in the suprachiasmatic nucleus of the rat: Effects of RU24969, methiothepin and ketanserin. Eur. J. Pharmacol. 1986;121:135–139. doi: 10.1016/0014-2999(86)90403-6. [DOI] [PubMed] [Google Scholar]
- MARTIN K.F., REDFERN P.H.5-Hydroxytryptamine and noradrenaline synthesis, release and metabolism in the central nervous system: circadian rhythms and central mechanisms Physiology and Pharmacology of Biological Rhythms. Handbook of Experimental Pharmacology 1997Berlin: Springer; 157–176.eds Redfern, P.H. & Lemmer, B. pp [Google Scholar]
- MARTIN L.L., SANDERS-BUSH E. Comparison of the pharmacological characteristics of 5-HT, and 5-HT2 binding sites with those of serotonin autoreceptors which modulate serotonin release. Naunyn-Schmiedebergs Arch. Pharmacol. 1982;321:165–170. doi: 10.1007/BF00505480. [DOI] [PubMed] [Google Scholar]
- MAURA G., RAITERI M. Functional evidence that chronic drugs induce adaptive changes of central autoreceptors regulating serotonin release. Eur. J. Pharmacol. 1984;97:309–313. doi: 10.1016/0014-2999(84)90466-7. [DOI] [PubMed] [Google Scholar]
- MIDDLEMISS D.N. 8-Hydroxy-2-(di-n-propylamino) tetralin is devoid of activity at the 5-hydroxytryptamine autoreceptor. Implications for the proposed link between the autoreceptor and the [3H]5-HT recognition site. Naunyn-Schmiedebergs Arch. Pharmacol. 1984;327:18–22. doi: 10.1007/BF00504986. [DOI] [PubMed] [Google Scholar]
- MIDDLEMISS D.N. The putative 5-HT1 receptor agonist RU24969 inhibits the efflux of 5-hydroxytryptamine from rat frontal cortex slices by stimulation of the 5-HT autoreceptor. J. Pharm. Pharmacol. 1985;37:434–437. doi: 10.1111/j.2042-7158.1985.tb03032.x. [DOI] [PubMed] [Google Scholar]
- MIDDLEMISS D.N., FOZARD J.R. 8-Hydroxy-2-(di-n-propylamino)tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur. J. Pharmacol. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- MONGEAU R., BLIER P., DE MONTIGNY C. In vivo electrophysiological evidence for tonic activation by endogenous noradrenaline of α2-adrenoceptors on 5-hydroxytryptamine terminals in the rat hippocampus. Naunyn-Schmiedebergs Arch. Pharmacol. 1993;347:266–272. doi: 10.1007/BF00167444. [DOI] [PubMed] [Google Scholar]
- MONTERO D., DE FELIPE M.C., DEL RIO J. Acute or chronic antidepressants do not modify [125I]cyanopindolol binding to 5-HT1B receptors in rat brain. Eur. J. Pharmacol. 1991;196:327–329. doi: 10.1016/0014-2999(91)90448-y. [DOI] [PubMed] [Google Scholar]
- MORET C., BRILEY M. Serotonin autoreceptor subsensitivity and antidepressant activity. Eur. J. Pharmacol. 1990;180:351–356. doi: 10.1016/0014-2999(90)90320-6. [DOI] [PubMed] [Google Scholar]
- NESTLER E.J., TERWILLIGER R.Z., DUMAN P.S. Chronic antidepressant administration alters the subcellular distribution of cyclic AMP-dependent protein kinase in rat frontal cortex. J. Neurochem. 1989;53:1644–1647. doi: 10.1111/j.1471-4159.1989.tb08564.x. [DOI] [PubMed] [Google Scholar]
- O'CONNOR J.J., KRUK Z.L. Frequency dependence of 5-HT autoreceptor function in rat dorsal raphe and suprachiasmatic nuclei using fast cyclic voltammetry. Brain Res. 1991;568:123–130. doi: 10.1016/0006-8993(91)91387-g. [DOI] [PubMed] [Google Scholar]
- OKADA F., TOKUMITSU Y., UI M. Possible involvement of pertussis toxin substrates (Gi, Go) in desipramine-induced refractoriness of adenylate cyclase in cerebral cortices of rats. J. Neurochem. 1988;51:194–199. doi: 10.1111/j.1471-4159.1988.tb04855.x. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. Sydney: Academic Press; 1982. The rat brain in stereotaxic co-ordinates. [Google Scholar]
- PEREZ J., TINELLI D., BRUNELLO N., RACAGNI G. cAMP-dependent phosphorylation of soluble and crude microtubule fractions of rat cerebral cortex after prolonged desmethylimipramine treatment. Eur. J. Pharmacol. Mol. Pharmacol. Sect. 1989;172:305–316. doi: 10.1016/0922-4106(89)90060-6. [DOI] [PubMed] [Google Scholar]
- PETTIBONE D.J., PFLUEGER A.B. Effects of methiothepin and lysergic acid diethylamide on serotonin release in vitro and serotonin synthesis in vivo: Possible interaction with serotonin autoreceptor function. J. Neurochem. 1984;43:83–90. doi: 10.1111/j.1471-4159.1984.tb06681.x. [DOI] [PubMed] [Google Scholar]
- PIETRASZEK M.H., TAKAHASHI S. , TAKADA Y., INATOMI H., KONDO N., OHARA K.E., TAKADA A. Diurnal patterns of serotonin, 5-hydroxyindoleacetic acid, tryptophan and fibrinolytic activity in blood of depressive patients and healthy volunteers. Thromb. Res. 1991;64:243–252. doi: 10.1016/0049-3848(91)90123-e. [DOI] [PubMed] [Google Scholar]
- PRICE G.W., BURTON M.J., COLLIN I.J., DUCKWORTH M., GASTON L., GOTHERT M., JONES B.J., ROBERTS C., WATSON J.M., MIDDLEMISS D.M. SB-216641 and BRL-15572 compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn-Schmeidebergs Arch. Pharmacol. 1997;356:312–320. doi: 10.1007/pl00005056. [DOI] [PubMed] [Google Scholar]
- PROSSER R.A., GILLETTE M.U. Cyclic changes in cAMP concentration and phosphodiesterase activity in a mammalian circadian clock studied in vitro. Brain Res. 1991;568:185–192. doi: 10.1016/0006-8993(91)91396-i. [DOI] [PubMed] [Google Scholar]
- RASENICK M.M., WANG N. Exchange of guanine nucleotides between tubulin and GTP-binding proteins that regulate adenylate cyclase: Cytoskeletal modification of neuronal signal transduction. J. Neurochem. 1988;51:300–311. doi: 10.1111/j.1471-4159.1988.tb04870.x. [DOI] [PubMed] [Google Scholar]
- ROBERTS C., PRICE G.W., JONES B.J. The role of 5-HT1B/1D receptors in the modulation of 5-hydroxytryptamine levels of the frontal cortex of the conscious guinea-pig. Eur. J. Pharmacol. 1997;326:23–30. doi: 10.1016/s0014-2999(97)00156-8. [DOI] [PubMed] [Google Scholar]
- ROCA A.L., WEAVER D.R., REPPERT S.M. Serotonin receptor gene expression in the rat suprachiasmatic nuclei. Brain Res. 1993;608:159–165. doi: 10.1016/0006-8993(93)90789-p. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., FINK K., MOLDERINGS G.J., PRICE G.W., DUCKWORTH M., GASTER L., MIDDLEMISS D.N., ZENTNER J., LIKUNGA J., GOTHERT M. Effects of selective h5-HT1B (SB-216641) and h5-HT1D (BRL-15572) receptor ligands on guinea-pig and human 5-HT auto- and heteroreceptors. Naunyn-Schmeidebergs Arch. Pharmacol. 1997;356:321–327. doi: 10.1007/pl00005057. [DOI] [PubMed] [Google Scholar]
- SHARP T., BRAMWELL S.R., CLARK D., GRAHAME-SMITH D.G. In vivo measurement of extracellular 5-hydroxytryptamine in hippocampus of the anaesthetised rat using microdialysis: Changes in relation to 5-hydroxytryptaminergic neuronal activity. J. Neurochem. 1989;53:234–240. doi: 10.1111/j.1471-4159.1989.tb07319.x. [DOI] [PubMed] [Google Scholar]
- SLEIGHT A.J., SMITH R.J., MARSDEN C.A., PALFREYMAN M.G. The effects of chronic treatment with amitriptyline and MDL 72394 on the control of 5-HT release in vivo. Neuropharmacology. 1989;28:477–480. doi: 10.1016/0028-3908(89)90082-8. [DOI] [PubMed] [Google Scholar]
- STANLEY M., MANN J.J. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- STARKEY S.J., SKINGLE M. 5-HT1D as well as 5-HT1A autoreceptors modulate 5-HT release in the guinea-pig dorsal raphe nucleus. Neuropharmacology. 1994;33:393–402. doi: 10.1016/0028-3908(94)90069-8. [DOI] [PubMed] [Google Scholar]
- TAO R., HJORTH S. α2-Adrenoceptor modulation of rat ventral hippocampal 5-hydroxytryptamine release in vivo. Naunyn-Schmiedebergs Arch Pharmacol. 1992;345:137–143. doi: 10.1007/BF00165728. [DOI] [PubMed] [Google Scholar]
- TRILLAT A.C., MALAGIE I., SCEARCE K., PONS D., ANMELLA M.C., JACQUOT C., HEN R., GARDIER A.M. Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: In vivo microdialysis study. J. Neurochem. 1997;69:2019–2025. doi: 10.1046/j.1471-4159.1997.69052019.x. [DOI] [PubMed] [Google Scholar]
- VAN WIJNGAARDEN I., TULP M.T.M., SOUDIJN W. The concept of selectivity in 5-HT receptor research. Eur. J. Pharmacol. 1990;188:301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]
- VERGE D., DAVAL G., PATEY A., GOZLAN H., EL MESTIKAWY S., HAMON M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites, but not terminals are of the 5-HT1A subtype. Eur. J. Pharmacol. 1985;113:463–464. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]


